| Research Article | ||
Open Vet. J.. 2025; 15(3): 1166-1177 Open Veterinary Journal, (2025), Vol. 15(3): 1166-1177 Research Article Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozeb-induced testicular toxicity in male ratsMohammed Fadhil K. Alrubaie and Sura Safi Khafaji*Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, Al-Qassim Green University, Ministry of Higher Education and Scientific Research, Al-Qassim City, Iraq *Corresponding Author: Sura Safi Khafaji. Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, Al-Qassim Green University, Ministry of Higher Education and Scientific Research, Al-Qassim City, Iraq. Email: sura.khafaji [at] vet.uoqasim.edu.iq Submitted: 10/11/2024 Accepted: 04/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
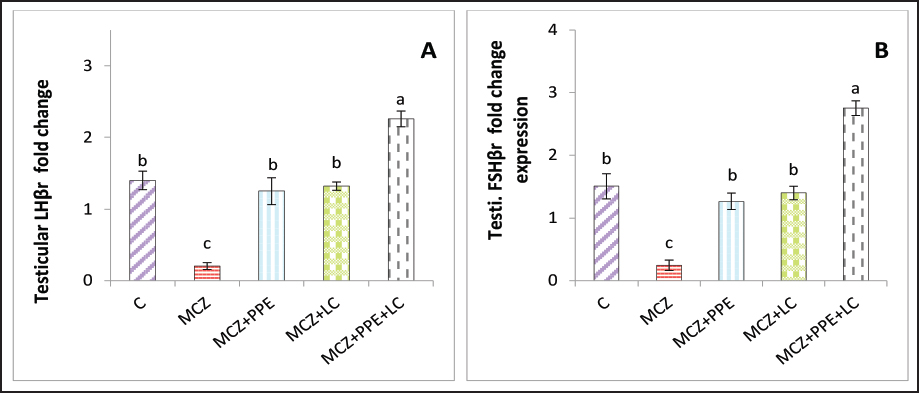
AbstractBackground: A mancozeb, belonging to a pesticide, can induce testicular injury via free radical generation and antioxidant suppression. Pinus pinaster extract (Pycnogenol®) and L. carnitine have potent antioxidant properties. Aim: This study aimed to compare and evaluate the therapeutic roles of P. pinaster extract (Pycnogenol®) and L. carnitine in eliminating and/or reducing the testicular toxicity of mancozeb in male rats, emphasizing their combined antioxidant. Methods: Testicular toxicity was induced by oral administration of mancozeb (MCZ) at a dose of 313.6 mg/Kg for 4 weeks. Fifty male rats were allocated randomly into 5 groups, each group contained 10 rats. The first group (control group; C), second group (mancozeb group; MCZ) was orally administered 313.6 mg/Kg of mancozeb as a single dose/day for 4 weeks, third group (MCZ+PPE) received MCZ as the second group then administered P. pinaster extract (Pycnogenol®) at dose 40 mg/ Kg/day/ orally for 4 weeks, fourth group (MCZ+LC) was treated as the second group then administered L. carnitine at dose 200 mg/Kg/day/orally for 4 weeks. The fifth group (MCZ+PPE+LC) was treated as the second group and then received PPE and LC at the same doses mentioned in the third and fourth groups for 4 weeks. Results: Testicular follicle-stimulating hormone receptor (FSHr) and luteinizing hormone receptor (LHr) gene expression was significantly upregulated in rats that received PPE + LC+ MCZ compared with the experimental rats. The seminal fluid quality was significantly elevated while abnormal morphology significantly declined in the MCZ+PPE, MCZ+LC, and MCZ+PPE+LC groups compared with the MCZ group. The serum FSH, testosterone, LH, and antioxidant enzymes, superoxide dismutase, and glutathione peroxidase, increased significantly, whereas malondialdehyde decreased significantly in rats that received PPE+ LC+ MCZ compared with all experimental rats. In addition, based on testicular-histopathological investigations, the administration of both PPE and LC, alone or in combination, can improve testicular disarrangement due to mancozeb-induced testicular damage. Conclusion: The administration of P. pinaster extract and L. carnitine, individually or in combination, has antioxidant effects against mancozeb-induced toxicity via improvement of seminal quality, regeneration of testicular tissues, and upregulation of FSHr and LHr genes positively influence reproductive hormones. This study is the first to demonstrate that the P. pinaster extract plays a therapeutic role in testicular atrophy caused by the fungicide, mancozeb. Keywords: L. carnitine, Mancozeb, Pinus pinaster, Therapeutic, Testicular atrophy. IntroductionPesticides are agents leading to intoxication. Pesticides are widely used in agriculture to produce human food in different parts of the world (Khan et al., 2023). Pesticides can be residues in different organs of the body, and extensive spraying on agricultural farms has frequently exposed humans to pesticides(Fernandes et al., 2023). The main source of persistent contamination is the use of contaminated foods and drinking water (Fernandes et al., 2023). Exposure to pesticides can damage health and disrupt endocrine functions, including carcinogenesis, neurotoxicity, disruption of immunological response, and reproductive efficacy (Lee et al., 2020). One of the fungicides used in pest control programs against human health, including in the reproductive system, is Mancozeb (MZB). Several studies have shown that Mancozeb can induce the overproduction of reactive oxygen species that are considered a threat to male fertility (Hashem et al., 2018; Saddein et al., 2019b), as well as its adverse effects on the gastrointestinal, neurological, and hematological. Further, recent research on animal models has revealed that MZB induces alterations in sperm motility, sperm morphology, and sperm count (Al-Khedhairy et al., 2012) and (Ashkanani et al., 2020). Interest in the health-promoting properties of Pinus pinaster, commonly called maritime pine, is increasing. The bark of P. pinaster contains many polyphenolic compounds, shows a wide variety of proanthocyanidins, and is recognized for its strong antioxidant activity (Bayer and Högger, 2024). This action occurs through the scavenging of free radicals, reduction in oxidative stress, and enhancement of the total antioxidant capacity of the body (Walke et al., 2023). It has been shown that the extract of P. pinaster expressed protective effects against a wide range of types of oxidative damage, even in the reproductive system, in some experimental studies (Ferreira-Santos et al., 2020b). Pinus pinaster extract protects testicular tissue against testicular-toxicant–induced oxidative damage by enhancing antioxidant defenses, such as Mancozeb (Ferreira-Santos et al., 2021). L. carnitine is a biologically active amino acid, a small water-soluble molecule of the chemical formula 3-hydroxy-4-N-trimethylaminobutyrate, which exists in meat and milk products. (Abd-Allah et al., 2009). L. carnitine is a strong antioxidant that plays an important role in energy production via long-chain fatty acids. It exerts numerous biological activities, including anti-inflammatory, anti-apoptotic, cardioprotective, and gastroprotective properties. In addition, it involves providing energy that enhances sperm metabolism, chemical maturity, mobility, and spermatogenesis (Ng et al., 2004). Previous studies exerted beneficial effects of L. carnitine and its acetylated form on sperm parameters and reproduction. Moreover, L. carnitine can ameliorate the mechanisms by which toxic compounds induce reproductive dysfunction largely by its antioxidant properties. (Abdelrazik and Agarwal, 2009, Abdel-Emam and Ahmed, 2021). However, the potential effects and mechanisms of P. pinaster extract and L. carnitine, alone or in combination, for eliminating the detrimental effects of mancozeb on testicular tissues have not been reported; based on these limitations, the current experiment aimed to evaluate the mechanisms by which P. pinaster extract and L. carnitine, alone or in combination, improve the hypothalamic-hypophyseal-testicular functions in Wister male rats exposed to mancozeb. Material and MethodsExperimental animalsWistar rats were used in the current study at the age of 15 weeks and weighed 210–220 g. All rats were reared under control laboratory conditions at 22°C ± 1°C with 12-hour light and 12-hour dark. They provided standard feed and ad libitum water. Before starting the experiment, all rats were allowed 14 days to acclimatize to the experimental conditions. The current experiment was carried out at Al-Qasim Green University/Veterinary Medicine from September 15, 2023, to December 15, 2023 (ESCVM, NO. 1872023.). Experimental designFifty Wistar male rats were divided into five groups (n =10). The control group received 4 ml of normal saline. The Mancozeb group (MCZ) was administered orally 313.6 mg/Kg of mancozeb (PVT, Ltd., Holand) single dose/day for 4 weeks to cause testicular toxicity, the Pine Bark extract group (PPE+MCZ) was given 313.6 mg /Kg of mancozeb for 4 weeks followed by administrating orally 40 mg/Kg.b.w/day of Pine Bark extract, P. pinaster, (pycnogenol®) (Puritan’s Pride, INC. USA) for 4 weeks. The L. carnitin group (LC+MZC) was administered orally 313.6 mg/Kg of mancozeb for 4 weeks followed by administered 200 mg/Kg of L. carnitine for 4 weeks. The last group (MCZ+ PPE + LC) was administered daily for 4 weeks, starting with 313.6 mg/Kg of mancozeb, followed by 40 and 200 mg/Kg.b.w. of pineapple bark extract and L. Carnitin for 4 weeks. Pine Bark extract and L. Carnitin were orally administered through a gavage tube on a daily basis for four consecutive weeks. The doses of pine bark extract, L. Carnitin, and mancozeb were used according to previous research (Sakr et al., 2009; Ko et al., 2018; Koohpeyma et al., 2022). After 4 weeks, blood samples were collected to estimate the concentrations of fertility hormones, oxidants, and antioxidant markers. After sacrifice, the rats’ testes were taken for histological study and fold changes in the levels of follicle-stimulating hormone receptor (FSHr) and luteinizing hormone receptor (LHr). Assessment of oxidant and antioxidant markersThe activities of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were assessed in rats’ serum according to the method illustrated by (Nishikimi et al., 1972; Kei, 1978; Mohandas et al., 1984). Quantification of gene expressionExtraction of RNA, cDNA synthesis, and RT-qPCR analysis Total RNA was extracted from tissue samples (testicular and pituitary tissues) by adding 1 µl of TRIzol reagent to 100 mg of the tissue sample. This was performed according to the manufacturer’s instructions for the Bioneer kit provided by Korea. The nano-drop-spectrophotometer “Thermos, USA” was used at absorbances of 260 and 280 nm to determine the quantity and purity of extracted RNA. The extracted RNA samples were treated with DNase-I-enzyme to remove any genome present; this was performed according to the manufacturer’s protocol for the Promega kit provided by the USA. The RNA samples were treated with cDNA synthesis depending on the protocol explained by AccuPower®, Korea kit. SYBER Green was used to detect gene amplification using a real-time PCR machine. The primers used in the present experiment were designed using the NCBI Gene bank database and primer 3 tool and are illustrated in Table 1. The relative expression “fold change” of target genes in testicular tissues was calculated by ∆∆Ct method explained by (Livak and Schmittgen, 2001), which depended on the target gene relative to GAPDH (housekeeping gene). Assessment of male fertility hormone concentrations The concentrations of testosterone, FSH, and LH were determined according to the manufacturer’s instructions using ELISA kits “Sunlong Biotech, Co., China”. The seminal fluid functionThe epididymal tail was cut, placed in saline (2 ml at 37°C), and cut into small pieces. One drop of the epididymal suspension was placed in the Neubauer chamber and manifested under a light microscope to calculate the count using Raji’s method (Raji et al., 2006). 10 µl of the epididymal suspension was placed on a warm slide and manifested under a light microscope to evaluate sperm motility according to Atashfaraz’s method with his colleagues’ method (Atashfaraz et al., 2013). Narayana’s method (Narayana et al., 2005) and Björndahl’s and his colleagues’ method (Björndahl et al., 2003) were used to evaluate sperm morphology and viability percentages, respectively. Histological StudyThe left testis was fixed in 10% buffered formaldehyde for 48 hours and processed histologically, depending on the method adopted by (Mescher, 2018), as follows: dehydration, clearing, embedded in paraffin, and staining with hematoxylin and eosin after sectioning into five-micrometer slices. Statistical analysisThe results of the present experiment were statistically analyzed using SPSS version 20. Levene’s test was used to test the homogeneity of variances. Shapiro–Wilk and Kolmogorov–Smirnov normality tests were performed to evaluate the normal distribution of data. The variances among the experimental groups were evaluated using one-way ANOVA, followed by Tukey’s post hoc test. The data are the mean (M) ± standard deviation (SD). The variances at p < 0.05 are significant statistically (Schefler, 1980). Table 1. The primer sequences used in real-time qPCR.
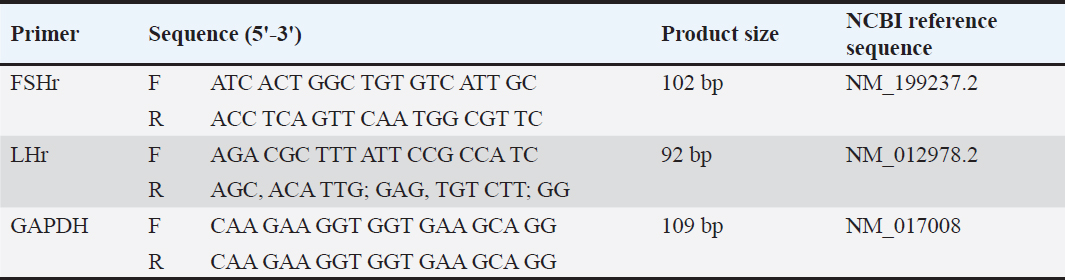
Fig. 1. Serum oxidative stress and antioxidant markers in rats. (A) MDA: malondialdehyde, (B) SOD: superoxide dismutase; (C) GPx: glutathione peroxidase. The different letters represent significant differences among groups at p < 0.05. C: control rats received distilled water, MCZ: rats received (313.6 mg/Kg/day) of mancozeb. MCZ+PPE: rats received (313.6 mg/Kg/day) of mancozeb and (40 mg/Kg/day) of Pinus pinaster extract. MCZ+LC: rats received (313.6 mg/Kg/day) of mancozeb and (200 mg/Kg/day) of L. carnitine. MCZ+PPE+LC: rats received (313.6 mg/Kg/day) of mancozeb, (40 mg/Kg/day) of Pinus pinaster extract, and (200 mg/Kg/day) of L. carnitine. Ethical approvalThe ethical scientific committee of Al-Qasim Green University/Veterinary Medicine approved the current experiment, ensuring proper care and use of laboratory animals (ESCVM, NO. 1872023.). ResultsComparative effects of P. pinaster Extract and L. carnitine on serum concentrations of oxidative stress and antioxidant markers against Mancozeb-induced reprorotoxicity in adult male ratsThe concentration of serum MDA was significantly elevated (p < 0.05) in MCZ rats compared with all experimental groups, Figure 1A. It was significantly decreased (p < 0.05) in the MCZ + PPE + LC group compared with the other experimental rats. The serum MDA concentration was significantly decreased (p < 0.05) in the MCZ+PPE and the MCZ+LC groups compared with the MCZ group, with non-significant differences (p > 0.05) among the MCZ+PPE, MCZ+LC, and control groups, Figure 1A. The serum concentration of superoxide dismutase, SOD, was significantly reduced (p < 0.05) in MCZ rats and elevated (p < 0.05) in MCZ+PPE+LC rats compared with all treatment groups, Figure 1B. The serum concentration of SOD was significantly increased (p < 0.05) in MCZ+PPE and the MCZ+LC groups compared with MCZ rats, with non-significant differences (p > 0.05) between the aforementioned groups, Figure 1B. The serum concentration of GPx was significantly decreased (p < 0.05) in the MCZ group and increased (p < 0.05) in the MCZ+PPE+LC group compared with all treatment groups, Figure 1C. The GPx concentration showed a significant increase (p < 0.05) in the MCZ+PPE and the MCZ+LC groups in comparison with MCZ rats, with non-significant differences (p > 0.05) among the MCZ+PPE, MCZ+LC, and control groups, Figure 1C. Comparative effects of P. pinaster Extract and L. carnitine on serum concentrations of male reproductive hormones and testicular genes in response to mancozeb-induced reprotoxicity in adult male ratsReproductive hormones The serum concentrations of LH, FSH, and testosterone are shown in Figure (2 A, B, and C, respectively). The serum testosterone, FSH, and LH levels were significantly decreased in the rats that received MCZ only compared with the experimental groups, Figure 2. The concentrations of FSH, LH, and testosterone in the MCZ+PPE+LC rats were significantly increased (p < 0.05) compared with all treatment groups, Figure 2. In comparison with the MCZ group, the concentrations of LH, FSH, and testosterone were significantly increased (p < 0.05) in the MCZ+PPE group and the MCZ+LC group, Figure (2 A, B, and C). The serum levels of testosterone, LH, and FSH in the MCZ+LC rats and the MCZ+PPE rats showed a significant decrement (p < 0.05) in comparison with the control group, with non-significant variation (p > 0.05) between both groups concerning LH and testosterone concentrations, Figure 2. Testicular genes The testicular gene expression levels of the luteinizing hormone-β receptor (LHβr) and follicle-stimulating hormone-β receptor (FSHβr) were significantly downregulated (p < 0.05) in mancozeb-exposed rats compared with all experimental groups, Figure 3. Interestingly, the cotreatment of P. pinaster extract with L. carnitine rats caused significant (p < 0.05) enhancement and upregulation of the testicular FSHβr, and LHβr gene expression levels compared with all experimental groups. The current results registered a significant (p < 0.05) upregulation in mancozeb co-administration of P. pinaster extract rats and in mancozeb-co-exposed to L. carnitine rats compared with mancozeb-treated male rats, whereas non-significant (p > 0.05) differences were observed among the MCZ+PPE, MCZ+LC, and control groups, Figure 3.
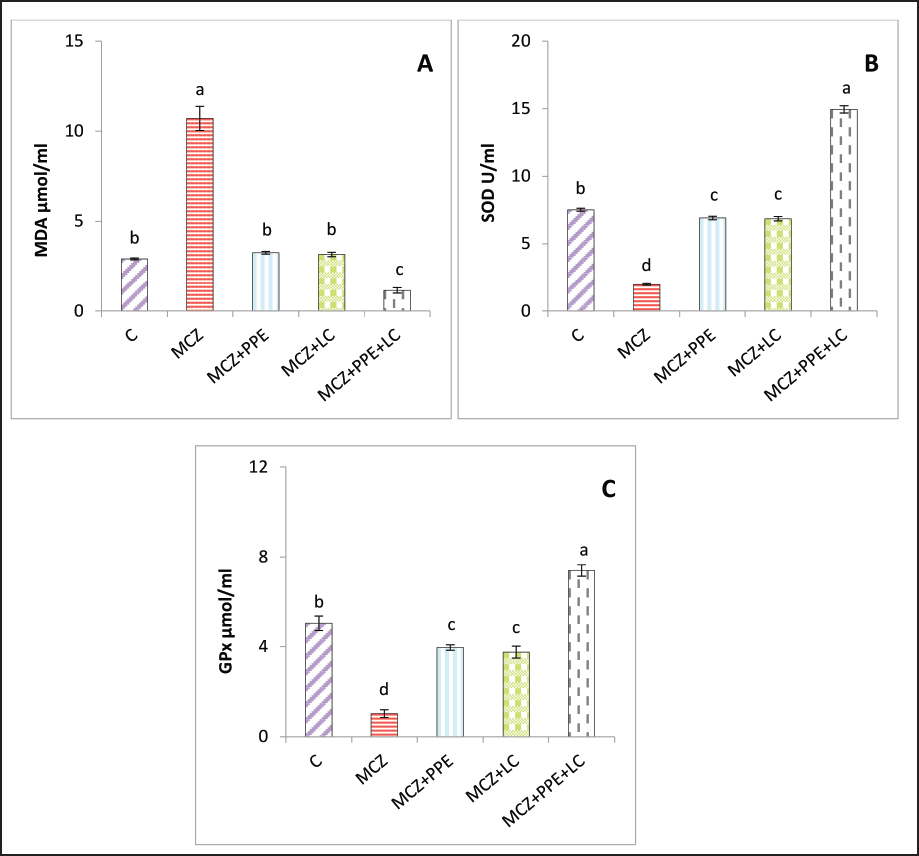
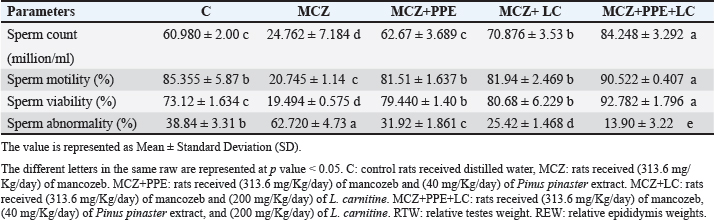
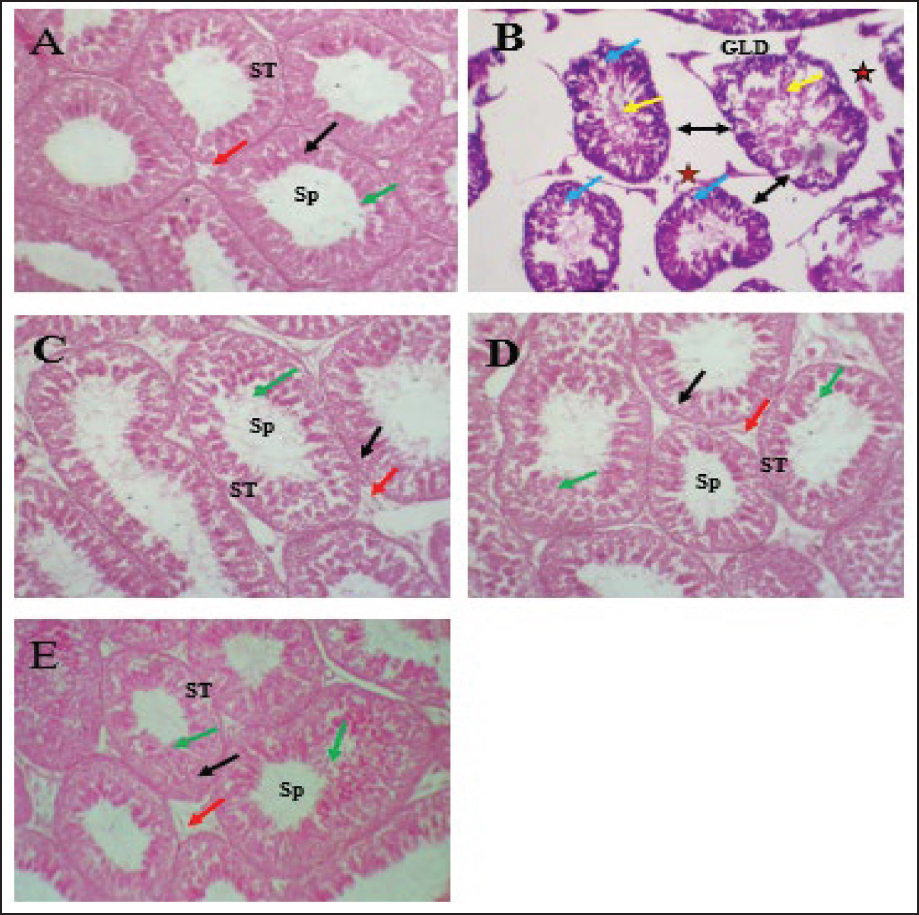
Fig. 2. Serum reproductive hormone levels in rats. (A) serum concentrations of luteinizing hormone (LH), (B) serum concentrations of follicle-stimulating hormone (FSH), and (C) serum concentrations of testosterone. The different letters represent significant differences among groups at p < 0.05. C: control rats received distilled water, MCZ: rats received (313.6 mg/Kg/day) of mancozeb. MCZ+PPE: rats received (313.6 mg/Kg/day) of mancozeb and (40 mg/Kg/day) of Pinus pinaster extract. MCZ+LC: rats received (313.6 mg/Kg/day) of mancozeb and (200 mg/Kg/day) of L. carnitine. MCZ+PPE+LC: rats received (313.6 mg/Kg/day) of mancozeb, (40 mg/Kg/day) of Pinus pinaster extract, and (200 mg/Kg/day) of L. carnitine. Comparative effects of P. pinaster Extract and L. carnitine on seminal analysis quality in response to mancozeb-induced reprotoxicity in adult male ratsTable 2 shows the effects of P. pinaster extract and L. carnitine on the seminal quality of rats exposed -to mancozeb. The semen count, sperm motility, and viability were significantly increased (p < 0.05) in the MCZ+PPE+LC males compared with all experimental rats, whereas they were significantly reduced in the MCZ-treated male rats compared with all experimental rats. Sperm count, motility, and viability were significantly elevated (p < 0.05) in MCZ+PPE males and MCZ+LC males compared with MCZ-treated rats and control rats, Table 2. The statistical analysis of sperm abnormality results in MCZ males revealed a significant elevation (p < 0.05) compared with all experimental rats. It was significantly reduced (p < 0.05) in MCZ+PPE+LC males compared with that in all treated rats. The sperm abnormalities of MCZ+PPE and MCZ+LC males registered a significant decrement (p < 0.05) compared with the control and MCZ males, Table 2. Comparative effects of P. pinaster Extract and L. carnitine on histological changes in response to mancozeb-induced reprotoxicity in adult male ratsHistological sections of the testes of mancozeb-treated rats showed necrosis and atrophy in the seminiferous tubules, distributed vacuolation among spermatogenic cells resulting from exfoliation germ cells, empty lumens from spermatozoa, and degeneration spermatogenic cells. Additionally, mancozeb caused germinal layer disintegration and interstitial space widening accompanied by loss of the quantities of interstitial cells (Figure 4B) compared with control rats that showed a typical histological appearance of Leydig cells and Sertoli cells with different stages of spermatogenesis (Figure 4A). Whereas the testicular section of either P. pinaster extract or L. carnitine-mancozeb-treated rats showed mild spermatogenic degeneration and normal architecture of Sertoli and Leydig cells, seminiferous tubules accompanied with the series stages of development of spermatogonia, spermatocytes, spermatid, and spermatozoa (Figure 4C, and D), while the testicular-histological sections of rats that received both P. pinaster extract or L. carnitine after being exposed to mancozeb showed a normal structure, same as that of control rats as normal germinal epithelia, typical spermatogenic cells with normal Sertoli and Leydig cells, seminiferous tubules filled with primary and secondary spermatocytes, spermatid, and spermatozoa (Figure 4E).
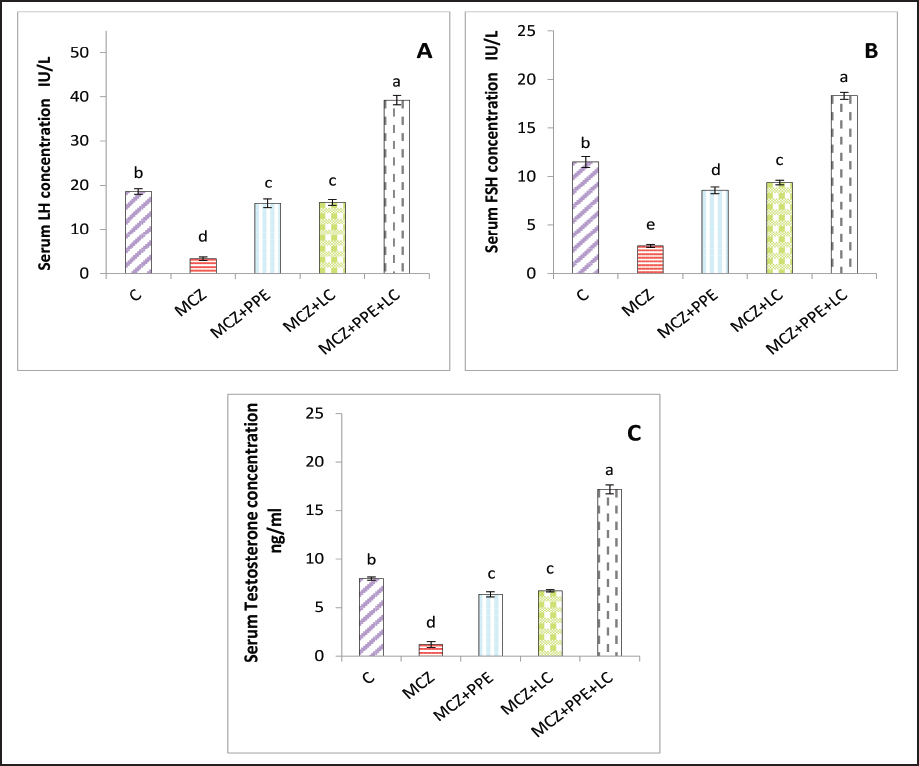
Fig. 3. The testicular fold change of male experimental rats. (A) testicular fold change of LHβr gene, and (B) testicular fold change of FSHβr gene. The different letters represent significant differences among groups at p < 0.05. C: control rats received distilled water, MCZ: rats received (313.6 mg/Kg/day) of mancozeb. MCZ+PPE: rats received (313.6 mg/Kg/day) of mancozeb and (40 mg/Kg/day) of Pinus pinaster extract. MCZ+LC: rats received (313.6 mg/Kg/day) of mancozeb and (200 mg/Kg/day) of L. carnitine. MCZ+PPE+LC: rats received (313.6 mg/Kg/day) of mancozeb, (40 mg/Kg/day) of Pinus pinaster extract, and (200 mg/Kg/day) of L. carnitine. Table 2. The effects of Pinus pinaster extract and L. carnitine on the seminal quality of male rats exposed to mancozeb.
DiscussionOxidative stress is the major cause of testicular damage associated with mancozeb exposure; mancozeb exposure exhibits oxidative stress by causing reactive oxygen species (ROS) like hydrogen peroxide, superoxide radicals, hydroxyl radicals, and lipid peroxide to be released from the cellular membrane (Ritchie and Ko, 2021). Consistent with previous studies (Saddein et al., 2019a; Yousuf et al., 2023), the administration of mancozeb to rats resulted in the elevation of testicular nitric oxide and MDA levels accompanied by a decrease in SOD, GSH, and total antioxidant activities, suggesting the presence of testicular oxidative stress. The current results revealed that there were dysfunction in antioxidant enzymes and elevated serum lipid peroxidation, MDA, in rats receiving only mancozeb that might be attributed to the mancozeb metabolites, ethylene thiourea, and carbon disulfide, abilities to generate ROS and reactive nitrogen species (RNS) through Fenton’s reaction (Kistinger and Hardej, 2022) with downregulation of expression of GPx and SOD (Mohammadi-Sardoo et al., 2018); therefore, these alterations could cause damage to the testicular cellular cytoplasmic organelles and sperm DNA (Dall’Agnol et al., 2021). Furthermore, mancozeb promotes lipid peroxidation and upregulates inducible NO synthase and NOX4, which are the main sources of superoxide radicals, nitric oxide (NO), and consequent peroxynitrite, which are considered potent genotoxicity and oxidative stress in testicular tissues, which caused elevated the MDA concentration(Mohammadi-Sardoo et al., 2021).
Fig. 4. Testicular-histological section of all experimental rats. (A) The testicular section of control rats showed a normal structure of interstitial cells, Sertoli cells, and seminiferous tubules with different stages of spermatogenesis. (B) The testicular sections of mancozeb-treated rats showed germinal layer degeneration (GLD), loss of interstitial cells (star), vacuolation among spermatogenic cells (blue arrow), widening the spaces among seminiferous tubules (bidirectional black arrow), and necrosis of seminiferous tubules (yellow arrow). (C) The testicular sections of P. pinaster extract-mancozebtreated rats showed that the germinal epithelia, Sertoli cells, interstitial cells, and seminiferous tubules return to the normal architecture with various phases of spermatogenesis. (D) The testicular section of L. carnitine-mancozeb-treated rats showed a normal structure as that of control rats. (E) Histologicaltesticular sections of both P. pinaster extract- and L. carnitine-mancozeb-treated rats showed that the seminiferous tubules returned to normal architecture. Hematoxylin and Eosin (H&E); 400X. The antioxidant role of PPE had been previously demonstrated (Atta et al., 2020), which were confirmed in the current results, whereby reduction in MDA and enhancement in the activities of superoxide dismutase and glutathione peroxidase in PPE co-administration to mancozeb-treated rats, which contributed to their biological components from procyanidin, quercetin, zinc, kaempferol, tricin, vitamin C and E (Singh et al., 2021; J. Bayer and P. Högger, 2024), which have potent antioxidant properties that protect cellular DNA and membranes against ROS, RNS, and oxidative damage (Liu et al., 2024). Besides, the statistical analysis of the current data revealed that there was a valuable enhancement in the antioxidant activities with reduced lipid peroxidation in L. carnitine co-exposure to mancozeb-treated rats, which was attributed to the ability of L. carnitine to scavenge free radicals and prevent the testicular oxidative stress (Salem et al., 2021). The present results of MNZ + LC-treated rats are in accordance with the findings (Abdel-Emam and Ahmed, 2021), who concluded that L. carnitine could reduce oxidative stress via diminished lipid peroxidation and improve antioxidant enzyme activities, including SOD and GPx. Intriguingly, the present results showed a significant elevation in GPx and SOD, accompanied by a lowering of MDA in mancozeb-treated rats that received both P. pinaster extract and L. carnitine (MCZ+PPE+LC group). This indicates the synergistic effects of both L. carnitine and the phytochemicals of P. pinaster extract, such as polyphenolic acid, flavonoids, and anthocyanins, which bolster the antioxidant defense in rats by inducing the synthesis of antioxidants and suppressing lipid peroxidation (Atta et al., 2020; Salem et al., 2021). Importantly, P. pinaster extract and L. carnitine can prevent oxidative stress by removing free radicals directly, maintaining the integrity of mitochondria, and chelating catalytic metals serve as promoters of ROS like copper and iron, suppressing the enzyme-generating ROS, NADPH oxidases, and xanthine oxidase, accompanied by further production of antioxidant enzymes (Surai, 2015; Ferreira-Santos et al., 2020a; Abdel-Emam and Ahmed, 2021). In the current investigation, exposure of the rats to mancozeb caused a decline in serum concentrations of FSH, testosterone, and LH as compared to the control group; this agrees with (Skalny et al., 2021), who observed that mancozeb and its metabolites have a toxic effect, as well as it can accumulate in the brain and testicular tissues, resulting in degeneration and apoptosis of the hypothalamic, gonadotropic, Leydig, and Sertoli cells, causing a decrement in serum FSH, and LH, in turn testosterone hormones. Moreover, the current results indicate the antiandrogenic and anti-steroidogenic effects of mancozeb, which inhibit and reduce the enzyme required to synthesize these hormones at the hypothalamus, pituitary, and testicular, HPT axis (Seshoka et al., 2021). The current results are consistent with (Elsharkawy et al., 2019), who reported a lower testosterone level upon exposure to mancozeb due to the suppression of testicular 3β-hydroxysteroid dehydrogenase, downregulation of steroidogenic acute regulatory protein, cytochrome P450 11A1 and 17A1 (CYP11A1 and CYP17A1), LHβ receptor and FSHβ receptor genes, resulting in a reduction in testosterone biosynthesis (Bhaskar et al., 2014; Skalny et al., 2021). Intriguingly, the serum levels of LH and FSH in the co-administration of PPE and L. carnitine in mancozeb-treated rats improved and enhanced due to the additive therapeutic role of both PPE and L. carnitine at the level HPT axis via their antioxidant, anti-inflammatory, and antiapoptotic activities (Fan et al., 2015; Alharbi, 2024) causing regeneration of the hypothalamic, and pituitary cells which induce the gene expression of hypothalamic GNRH and pituitary LHβ, and FSHβ resulting to elevate the levels of LH and FSH, which impact on Leydig to induce testosterone synthesis and on Sertoli cells to promote spermatogenesis, respectively (Sudjarwo et al., 2019; Hafezi et al., 2022). These findings align with the current results of the co-administration of PPE and L. carnitine (individually or in combination) in mancozeb-exposed rats. In addition, the present results are consistent with the findings of Atta et al., (2020), who reported that PPE, a natural compound, had a protective role and could potentially reverse the oxidative alterations in brain and testicular tissues in rats via upregulation CYP17A1, 17β-HSD, FSHβr, and LHβr and aromatase genes in testicular tissues, subsequently enhancing and improving the biosynthesis of testosterone. According to a previous study (Alyasari and Selman, 2023) that concluded that L. carnitine had antioxidant properties and was a positive regulator of testicular androgen production, L. carnitine could prevent testicular oxidative stress by blocking ROS production, upregulating the StRAR, steroidogenic enzyme (17β-HSD and 3β-HSD) genes, and inducing the transcriptional activators of the FSHβr, and LHβr gene in rats, resulting in elevated serum testosterone concentrations in male rats (Salem et al., 2021; Alyasari and Selman, 2023), these findings support the current results in Figures 2C and 3. The direct cytotoxic impact of mancozeb in testicular tissues accompanied by lowering testosterone levels could be the main reason for decreasing seminal quality and elevating dead and abnormal sperm in the current experiment, according to previous investigations (Mohammadi-Sardoo et al., 2018; Kumawat et al., 2024). Androgens and gonadotropins are vital in promoting spermatogenesis in male rats, so LH and FSH induce Leydig and Sertoli cells to synthesize testosterone and promote spermatogenesis, respectively; consequently, any lack of testosterone, FSH, and LH levels causes decreasing spermatogenesis, in turn, insufficient quantities of healthy sperm, and ultimately reduced fertility (Ramaswamy and Weinbauer, 2014; Elsharkawy et al., 2019). The negative effects of mancozeb administration on the seminal fluid in the current experiment might be attributed to several mechanisms (Mohammadi-Sardoo et al., 2021); one way was that the accumulation of mancozeb metabolites in testicles could damage and injure the Sertoli cells directly, in turn impairing and disrupting spermatogenesis and reducing sperm count and viability (Elsharkawy et al., 2019). Second, the drop in serum testosterone level investigated in the present experiment impairs spermatogenesis. Third, any change in gonadotropin levels can influence spermatogenesis (Ramaswamy and Weinbauer, 2014). Fourth, elevated testicular ROS levels directly affect the membrane polyunsaturated fatty acids of sperm, resulting in sperm dysfunction and reduced sperm viability, count, and motility of sperms (Ritchie and Ko, 2021). On the other hand, according to current findings, the administration of both P. pinaster extract with L. carnitine (PPE-LC) can play a major role collaboratively, rather than when they are performed individually, due to their synergistic abilities to improve seminal quality and eliminate the detrimental effects of mancozeb as reduced the abnormal and dead sperm with elevating the live, motility, and count sperm in mancozeb-PPE-LC treated rats that attributed to the quercetin, anthocyanin, and kaempferol abilities, the main natural antioxidants of P. pinaster extract, to prevent testicular oxidative stress (Yousuf et al., 2023) via inhibiting mancozeb metabolites’ accumulation in body organs, particularly testis, protecting spermatozoa membrane from free radicles, and preventing sperm deformities; as a result, enhancement the semen quality (Atta et al., 2020), all these findings align with current observation. Importantly, the current results were in line with the findings of (Abd-Elrazek and Ahmed-Farid, 2018), who concluded that the anti-apoptotic and antioxidant properties of L. carnitine can reduce the detrimental effects of mancozeb on mitochondrial, DNA, and plasma membrane integrity of sperm via lipid peroxidation reduction (Mardanshahi et al., 2019). L. carnitine had the ability to protect sperm membrane flexibility and maintain energy generation by removing the toxic acetyle-CoA and regulating fatty acid metabolism in sperm membrane that reflected positively in sperm viability and motility (Kooshesh et al., 2023), as well as, L. carnitine plays an avital role in incrementing the glial cells-derived neurotrophic factor, which enhanced the self-renewal of spermatogonia that influenced spermatogenesis (Cao et al., 2017). All the above facts may explain the current improvement in sperm quality in rats received L. carnitine after exposed to mancozeb. The testicular toxicity induced by mancozeb in current research might be due to the apoptotic properties of MCZ, which increased the permeability of Sertoli and Leydig mitochondria’ membrane and allowed the apoptotic factors to drain out (Mohammadi-Sardoo et al., 2018), concomitant with the generation of tumor necrosis factor is the major moderator of apoptosis (Gök and Deveci, 2022; Mesallam et al., 2023). In the current study, the severity of histopathological alterations of testicle tissues in the mancozeb- L. carnitine-treated rats as well as in the mancozeb-P. pinaster extract-treated rats was lower than that investigated in the mancozeb-treated rats. These enhancement changes might be attributed to the antioxidant and anti-inflammatory properties of P. pinaster extract, which reverse oxidative stress and avoid depletion of the endogenous SOD, GSH, CAT, and vitamins E and C, which support the integrity of the basement membrane of germinal epithelia, Sertoli and Leydig cells membrane via limiting lipid peroxidation induced by ROS in testicular tissues (Kim et al., 2012). Previous studies have shown that P. pinaster extract can restore and recover the structural components of seminiferous epithelia and Leydig cells via the anti-apoptotic and anti-inflammatory effects of its biological constituents like flavonoids, proanthocyanidin, and kaempferol (Sudjarwo et al., 2019; Khafaji, 2023). The current observation is consistent with (Ali et al., 2024) who found that the protective effects of L. carnitine and its derivatives could be defended against any testicular oxidative stress and damage via suppressing malondialdehyde and nitric oxide formations and modulating mancozeb-induced alterations in the antioxidant defense mechanisms in rat tissues (Dehghani et al., 2023). Additionally, a previous study had shown that L. carnitine can regulate the function of Sertoli cells and can protect the DNA, mitochondria, and membrane of sexual cells, Sertoli and Leydig cells, from ROS as well as facilitate carbohydrate and lipid intermediary metabolism for energy production required for sperm motility (Cao et al., 2017). Furthermore, the current observations are consistent with the finding of (Koohpeyma et al., 2022), who reported that when administering 200 mg/Kg of L. carnitine to male rats exposed to monosodium glutamate caused elevation in the testis weight and volume, enhanced the height of germinal epithelium, increment the volume, length, and diameter of seminiferous tubules, and reduction the apoptotic cells in seminiferous tubules, as well as, raising the quantities of spermatozoa and sexual lineage cells and sperm motility via the antioxidant properties of L. carnitine (Chang et al., 2023). LimitationsThe current experiment was conducted on male rats, and there was no evidence of humans due to species-specific differences in physiology and metabolism. The current study primarily focused on testicular oxidative stress and antioxidant activities without evaluating other potential mechanisms, such as anti-inflammatory and anti-apoptotic effects through which P. pinaster extract and L. carnitine might affect reproductive health. ConclusionThe findings highlight the antioxidant properties of Pinus pinaster and L. carnitine in mitigating oxidative stress via improving testicular health in rats exposed to Mancozeb. The current experiment first investigates the combined additive effects of P. pinaster extract and L. carnitine on testicular oxidative stress in male rats exposed to mancozeb due to their antioxidant activities. Importantly, mancozeb co-treated with L. carnitine and P. pinaster extract can act additively as a therapeutic substance at the level of hypothalamic-pituitary-testis to improve fecundity traits via their antioxidant activities. Further experiments are required to identify the effects of P. pinaster extract on prostatic hyperplasia. AcknowledgmentsThe author appreciates the efforts of the dean of Veterinary Medicine at Al-Qasim Green University in supporting the present experiment. Conflict of interestThe authors declare that they have no conflicts of interest. Authors’ contributionS.S. Khafaji designed the experiment, analyzed and interpreted the data, and Mohammed F. Kadhim wrote and organized the manuscript for publication. ReferencesAbd-Allah, A.R., Helal, G.K., Al-Yahya, A.A., Aleisa, A.M., Al-Rejaie, S.S. and Al-Bakheet, S.A. 2009. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. J. Oxid. Med. Longev. 2(2), 73–81. Abd-Elrazek, A. and Ahmed-Farid, O. 2018. Protective effect of L-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. Andrologia, 50(1), e12806; doi:10.1111/and.12806. Abdel-Emam, R.A. and Ahmed, E.A. 2021. Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet. Med. Sci. 7(4), 1426–1435; doi:10.1002/vms3.473. Abdelrazik, H. and Agarwal, A. 2009. L-carnitine and assisted reproduction. J. Androl. Med. Sci. 2009(1), 47–47. Al-Khedhairy, A.A., Srivastava, P.K., Musarrat, J. and Shukla, Y. 2012. Mancozeb-induced genotoxicity and apoptosis in cultured human lymphocytes. J. Lymph. Sci. 90, 815–824. Alharbi, F. 2024. Ameliorative effects of L-carnitine as an antioxidant against testicular toxicity induced by AlCl3 in male albino rats. Eur. Rev. Med. Pharmacol. Sci. 28(5), 1680–1694; doi:10.26355/eurrev_202403_35583. Ali, S.H., Elsyade, R.H., Abdel Wahab, K.H. and Al Badawi, M.H. 2024. The protective role of L-carnitine against cisplatin-induced testicular toxicity in the adult albino rat. Egypt. J. Histol. 47(1), 151–165; doi:10.21608/ejh.2022.172815.1803. Alyasari, N.K.H. and Selman, W.H. 2023. L-carnitine-loaded nanoparticle ameliorates cypermethrin-induced reproductive toxicity in adult male rats. J. Adv. Pharm. Technol. Res. 14(2), 147–154; doi:10.4103/japtr.japtr_46_23. Ashkanani, M., Farhadi, B., Ghanbarzadeh, E. and Akbari, H. 2020. Study on the protective effect of hydroalcoholic olive leaf extract (oleuropein) on the testis and sperm parameters in adult male NMRI mice exposed to Mancozeb. J. Grape Res. 21, 100870. Atashfaraz, E., Farokhi, F. and Najafi, G. 2013. Protective effect of ethyl pyruvate on epididymal sperm characteristics, oxidative stress and testosterone level in methotrexate treated mice. J. Reprod. Infertil. 14(4), 190. Atta, M.S., Farrag, F.A., Almadaly, E.A., Ghoneim, H.A., Hafez, A.S., Al Jaouni, S.K., Mousa, S.A. and Ali, H. 2020. Transcriptomic and biochemical effects of pycnogenol in ameliorating heat stress-related oxidative alterations in rats. J. Therm. Biol. 93, 102683; doi:10.1016/j.jtherbio.2020.102683. Bayer, J. and Högger, P. 2024. Review of the pharmacokinetics of French maritime pine bark extract (Pycnogenol®) in humans. Front. Nutr. 11, 1389422; doi:10.3389/fnut.2024.1389422. Bhaskar, R., Mishra, A.K. and Mohanty, B. 2014. Effects of mancozeb and imidacloprid pesticides on activities of steroid biosynthetic enzymes cytochromes P450. J. Kalash Sci. 2, 1–6. Björndahl, L., Söderlund, I. and Kvist, U. 2003. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 18(4), 813–816. Cao, Y., Wang, X., Li, S., Wang, H., Yu, L. and Wang, P. 2017. The effects of L-carnitine against cyclophosphamide-induced injuries in mouse testis. Basic Clin. Pharmacol. Toxicol. 120(2), 152–158; doi:10.1111/bcpt.12679. Chang, D., Kong, F., Jiang, W., Li, F., Zhang, C., Ding, H., Kang, Y., Li, W., Huang, C. and Zhou, X. 2023. Effects of L-carnitine administration on sperm and sex hormone levels in a male Wistar rat reproductive system injury model in a high-altitude hypobaric hypoxic environment. Reprod. Sci. 30(7), 2231–2247; doi:10.1007/s43032-022-00948-5. Dall’Agnol, J., Pezzini, M.F., Uribe, N.S. and Joveleviths, D. 2021. Systemic effects of the pesticide mancozeb: a literature review. Eur. Rev. Med. Pharmacol. Sci. 25(11), 4113–4120; doi:10.26355/eurrev_202106_26054. Dehghani, A., Pourjafari, F., Koohkan, F., Haghpanh, T., Pourjafari, F., Sheibani, V. and Afarinesh, M. R. 2023. L-carnitine attenuates acoustic startle reflex dysfunction in adult male rats exposed to mancozeb. Toxicol. Ind. Health 39(2), 115–126; doi:10.1177/07482337231151739. Elsharkawy, E.E., Abd El-Nasser, M. and Bakheet, A.A. 2019. Mancozeb impaired male fertility in rabbits with trials of glutathione detoxification. Regul. Toxicol. Pharmacol. 105, 86–98; doi:10.1016/j.yrtph.2019.04.012. Fan, B., Dun, S.H., Gu, J.Q., Guo, Y. and Ikuyama, S. 2015. Pycnogenol attenuates the release of proinflammatory cytokines and expression of perilipin 2 in lipopolysaccharide-stimulated microglia in part via inhibition of NF-κB and AP-1 activation. PLoS One 10(9), e0137837; doi:10.1371/journal.pone.0137837. Fernandes, I.A.A., Maciel, G.M., Bortolini, D.G., Pedro, A.C., Rubio, F.T.V., de Carvalho, K.Q. and Haminiuk, C.W.I. 2023. The bitter side of teas: pesticide residues and their impact on human health. Toxicol. Sci. 179, 113955. Ferreira-Santos, P., Genisheva, Z., Botelho, C., Santos, J., Ramos, C., Teixeira, J.A. and Rocha, C.M. 2020a. Unraveling the biological potential of Pinus pinaster bark extracts. Antioxidants 9(4), 334; doi:10.3390/antiox9040334. Ferreira-Santos, P., Genisheva, Z., Botelho, C., Santos, J., Ramos, C., Teixeira, J.A. and Rocha, C.M.J.A. 2020b. Unraveling the biological potential of Pinus pinaster bark extracts. Antioxidants 9(4), 334. Ferreira-Santos, P., Ibarz, R., Fernandes, J.M., Pinheiro, A.C., Botelho, C., Rocha, C.M., Teixeira, J.A. and Martín-Belloso, O.J.F. 2021. Encapsulated pine bark polyphenolic extract during gastrointestinal digestion: bioaccessibility, bioactivity and oxidative stress prevention. Food Funct. 10(2), 328. Gök, E. and Deveci, E. 2022. Histopathological, immunohistochemical and biochemical alterations in liver tissue after fungicide-mancozeb exposures in Wistar albino rats. Acta Cir. Bras. 37(4), e370404; doi:10.1590/acb370404. Hafezi, H., Vahdati, A., Forouzanfar, M. and Shariatic, M. 2022. Ameliorate effects of resveratrol and L-carnitine on the testicular tissue and sex hormones level in busulfan induced azoospermia rats. Theriogenology 191, 47–53; doi:10.1016/j.theriogenology.2022.06.006. Hashem, M.A., Mohamed, W.A., Attia, E.S. and Research, P. 2018. Assessment of protective potential of Nigella sativa oil against carbendazim-and/or mancozeb-induced hematotoxicity, hepatotoxicity, and genotoxicity. J. Environ. Sci. Res. 25, 1270–1282. Kei, S. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 90(1), 37–43. Khafaji, S.S. 2023. Antioxidant, anti-inflammatory, and anti-reprotoxic effects of kaempferol and vitamin E on lead acetate-induced testicular toxicity in male rats. Open Vet. J. 13(12), 1683. Khan, B.A., Nadeem, M.A., Nawaz, H., Amin, M.M., Abbasi, G.H., Nadeem, M., Ali, M., Ameen, M., Javaid, M.M. and Maqbool, R. 2023. Pesticides: impacts on agriculture productivity, environment, and management strategies. In Emerging contaminants and plants: interactions, adaptations and remediation technologies. Springer, pp: 109–134. Kim, S.H., Lee, I.C., Baek, H.S., Moon, C., Kang, S.S., Bae, C.S., Kim, S.H., Shin, D.H. and Kim, J.C. 2012. Pycnogenol® prevents hexavalent chromium-induced spermatotoxicity in rats. Mol. Cell. Toxicol. 8, 249–256; doi:10.1007/s13273-012-0030-8. Kistinger, B.R. and Hardej, D. 2022. The ethylene bisdithiocarbamate fungicides mancozeb and nabam alter essential metal levels in liver and kidney and glutathione enzyme activity in liver of Sprague-Dawley rats. Environ. Toxicol. Pharmacol. 92, 103849; doi:10.1016/j.etap.2022.103849. Ko, J.W., Park, S.W., Shin, N.R., Kim, W.I., Kim, J.C., Shin, I.S. and Shin, D.H. 2018. Inhibitory effects of Pycnogenol®, a pine bark extract, in a rat model of testosterone propionate-induced benign prostatic hyperplasia. Lab. Anim. Res. 34, 111–117. Koohpeyma, F., Gholizadeh, F., Hafezi, H., Hajiaghayi, M., Siri, M., Allahyari, S., Maleki, M. H., Asmarian, N., Bayat, E. and Dastghaib, S. 2022. The protective effect of L-carnitine on testosterone synthesis pathway, and spermatogenesis in monosodium glutamate-induced rats. BMC Complement. Med. Ther. 22(1), 269; doi:10.1186/s12906-022-03749-0. Kooshesh, L., Nateghian, Z. and Aliabadi, E. 2023. Evaluation of L-carnitine potential in improvement of male fertility. J. Reprod. Infertil. 24(2), 69; doi:10.18502/jri.v24i2.12491. Kumawat, S., Dumka, V., Kaur, R., Dattaray, D. and Lonare, M. 2024. Effect of subacute oral mancozeb exposure and its detrimental impact on male reproductive health in Wistar rats. Int. J. Adv. Biochem. Res. 8(1), 294–299; doi:10.33545/26174693.2024.v8.i1Se.399. Lee, G.H. and Choi, K.C. 2020. Adverse effects of pesticides on the functions of immune system. Toxicol. 235, 108789. Liu, H., Shi, J., Liu, F. and Zhang, L. 2024. Integrating network pharmacology and experimental verification to reveal the anti-inflammatory ingredients and molecular mechanism of pycnogenol. Front. Pharmacol. 15, 1408304; doi:10.3389/fphar.2024.1408304. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25(4):402–8. doi:10.1006/meth.2001.1262 Mardanshahi, T., Rezaei, N., Zare, Z., Shafaroudi, M.M. and Mohammadi, H. 2019. Effects of L-Carnitine on the sperm parameters disorders, apoptosis of spermatogenic cells and testis histopathology in diabetic Rats. Int. J. Reprod. Biomed. 17(5), 325; doi:10.18502/ijrm.v17i5.4600. Mesallam, D.I.A., Elkhishin, I.A.R., Abdelwahab, M., El-Fatah, A., Salah, S. and Atef, M. 2023. Cymbopogon Citratus Alleviates Mancozeb-mediated renal toxicity in a rat model through combating inflammation, oxidative stress, and apoptosis. Egypt. Soc. Clin. Toxicol. J. 11(2), 63–78; doi:10.21608/esctj.2023.242827.1043. Mescher, A.L. 2018. Junqueira’s basic histology: text and atlas: New York, NY: McGraw Hill. Mohammadi-Sardoo, M., Mandegary, A., Nabiuni, M., Nematollahi-Mahani, S.N. and Amirheidari, B. 2018. Mancozeb induces testicular dysfunction through oxidative stress and apoptosis: protective role of N-acetylcysteine antioxidant. Toxicol. Ind. Health 34(11), 798–811; doi:10.1177/0748233718778397. Mohammadi-Sardoo, M., Mandegary, A., Nematollahi-Mahani, S.N., Moballegh Nasery, M., Nabiuni, M. and Amirheidari, B. 2021. Cytotoxicity of mancozeb on Sertoli–germ cell co-culture system: role of MAPK signaling pathway. Toxicol. Ind. Health 37(11), 674–684; doi:10.1177/0748233721104402. Mohandas, J., Marshall, J.J., Duggin, G.G., Horvath, J.S. and Tiller, D.J. 1984. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem. Pharmacol. 33(11), 1801–1807. Narayana, K., Prashanthi, N., Nayanatara, A., Kumar, H.H.C., Abhilash, K. and Bairy, K. 2005. Effects of methyl parathion (o, o-dimethyl o-4-nitrophenyl phosphorothioate) on rat sperm morphology and sperm count, but not fertility, are associated with decreased ascorbic acid level in the testis. Mutation Res. Genet. Toxicol. Environ. Mutagen. 588(1), 28–34. Ng, C.M., Blackman, M.R., Wang, C. and Swerdloff, R.S. 2004. The role of carnitine in the male reproductive system. J. Androl. Soc. N. Y. Acad. Sci. 1033(1), 177–188. Nishikimi, M., Rao, N.A. and Yagi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46(2), 849–854. Raji, Y., Oloyo, A.K. and Morakinyo, A.O. 2006. Effect of methanol extract of Ricinus communis seed on reproduction of male rats. Asian J. Androl. 8(1), 115–121. Ramaswamy, S. and Weinbauer, G.F. 2014. Endocrine control of spermatogenesis: role of FSH and LH/testosterone. Spermatogenesis, 4(2), e996025; doi:10.1080/21565562.2014.996025.50. Ritchie, C. and Ko, E.Y. 2021. Oxidative stress in the pathophysiology of male infertility. Andrologia, 53(1), e13581; doi:10.1111/and.13581. Saddein, E., Haghpanah, T., Nematollahi-Mahani, S.N., Seyedi, F. and Ezzatabadipour, M. 2019a. Preventative effects of vitamin E on testicular damage and sperm parameters in the first-generation mice pups due to pre-and postnatal mancozeb exposure. J. Toxicol. 2019(1), 4763684; doi:10.1155/2019/4763684. Saddein, E., Haghpanah, T., Nematollahi-Mahani, S.N., Seyedi, F. and Ezzatabadipour, M. 2019b. Preventative effects of vitamin E on testicular damage and sperm parameters in the first-generation mice pups due to pre-and postnatal mancozeb exposure. J. Toxicol. 2019(1), 4763684. Sakr, S.A., Okdah, Y.A. and El-Adly, E.K. 2009. Effect of ginger (Zingiber officinale) on mancozeb fungicide-induced testicular damage in albino rats. Aust. J. Basic Appl. Sci. 3(2), 1328–1333. Salem, M.A., Ismail, R.S., Zaki, H.F., Arafa, H.M. and El-Khatib, A.S. 2021. L-carnitine extenuates endocrine disruption, inflammatory burst and oxidative stress in carbendazim-challenged male rats via upregulation of testicular StAR and FABP9, and downregulation of P38-MAPK pathways. Toxicol. 457, 152808; doi:10.1016/j.tox.2021.152808. Schefler, W.C. 1980. Statistics for the biological sciences. Reading, MA: Addison-Wesley Pub. Co. Seshoka, M., Van Zijl, M.C., Aneck-Hahn, N.H. and Barnhoorn, I. 2021. Endocrine-disrupting activity of the fungicide mancozeb used in the Vhembe District of South Africa. Afr. J. Aquat. Sci. 46(1), 100–109; doi:10.2989/16085914.2020.1803041. Singh, P., Arif, Y., Bajguz, A. and Hayat, S. 2021. The role of quercetin in plants. Plant Physiol. Biochem. 166, 10–19; doi:10.1016/j.plaphy.2021.05.023. Skalny, A., Aschner, M., Paoliello, M., Santamaria, A., Nikitina, N., Rejniuk, V., Jiang, Y., Roche, J. and Tinkov, A. 2021. Endocrine-disrupting activity of mancozeb. Arhiv za Farm. 71(6), 491; doi:10.5937/arhfarm71-34359. Sudjarwo, S.A., Anwar, C., Wardani, G. and Eraiko, K. 2019. Antioxidant and anti-caspase 3 effect of chitosan-Pinus merkusii extract nanoparticle against lead acetate-induced testicular toxicity in rat. Asian Pac. J. Reprod. 8(1), 13–19; doi:10.4103/2305-0500.250418. Surai, P.F. 2015. Antioxidant action of carnitine: molecular mechanisms and practical applications. EC Vet. Sci. 2(1), 66–84. Walke, G., Gaurkar, S.S., Prasad, R., Lohakare, T. and Wanjari, M.J.C. 2023. The impact of oxidative stress on male reproductive function: exploring the role of antioxidant supplementation. Clin. J. Reprod., 15(7), e42583. Yousuf, R., Verma, P.K., Sharma, P., Sood, S., Pankaj, N. and Agarwal, S. 2023. Testicular toxicity following subacute exposure of arsenic and mancozeb alone and in combination: ameliorative efficacy of Quercetin and Catechin. Toxicol. Int. 30(3), 255–267; doi:10.18311/ti/2023/v30i3/32276. | ||
| How to Cite this Article |
| Pubmed Style Alrubaie MFK, Khafaji SS. Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats. Open Vet. J.. 2025; 15(3): 1166-1177. doi:10.5455/OVJ.2025.v15.i3.9 Web Style Alrubaie MFK, Khafaji SS. Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats. https://www.openveterinaryjournal.com/?mno=228047 [Access: January 16, 2026]. doi:10.5455/OVJ.2025.v15.i3.9 AMA (American Medical Association) Style Alrubaie MFK, Khafaji SS. Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats. Open Vet. J.. 2025; 15(3): 1166-1177. doi:10.5455/OVJ.2025.v15.i3.9 Vancouver/ICMJE Style Alrubaie MFK, Khafaji SS. Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats. Open Vet. J.. (2025), [cited January 16, 2026]; 15(3): 1166-1177. doi:10.5455/OVJ.2025.v15.i3.9 Harvard Style Alrubaie, M. F. K. & Khafaji, . S. S. (2025) Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats. Open Vet. J., 15 (3), 1166-1177. doi:10.5455/OVJ.2025.v15.i3.9 Turabian Style Alrubaie, Mohammed Fadhil K., and Sura Safi Khafaji. 2025. Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats. Open Veterinary Journal, 15 (3), 1166-1177. doi:10.5455/OVJ.2025.v15.i3.9 Chicago Style Alrubaie, Mohammed Fadhil K., and Sura Safi Khafaji. "Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats." Open Veterinary Journal 15 (2025), 1166-1177. doi:10.5455/OVJ.2025.v15.i3.9 MLA (The Modern Language Association) Style Alrubaie, Mohammed Fadhil K., and Sura Safi Khafaji. "Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats." Open Veterinary Journal 15.3 (2025), 1166-1177. Print. doi:10.5455/OVJ.2025.v15.i3.9 APA (American Psychological Association) Style Alrubaie, M. F. K. & Khafaji, . S. S. (2025) Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozebinduced testicular toxicity in male rats. Open Veterinary Journal, 15 (3), 1166-1177. doi:10.5455/OVJ.2025.v15.i3.9 |