| Research Article | ||
Open Vet. J.. 2025; 15(3): 1217-1225 Open Veterinary Journal, (2025), Vol. 15(3): 1217-1225 Research Article Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbitsAdil Jabbar Atiyah1*, Shireen Jamal Mahmood2 and Adil Jabbar Atiyah11Department of Veterinary Medicine Public Health, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq 2Ministry of Agriculture, Baghdad, Iraq *Corresponding Author: Mohammed Munis Dakheel. College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: adil.jabar [at] covm.uobaghdad.edu.iq Submitted: 11/11/2024 Accepted: 01/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
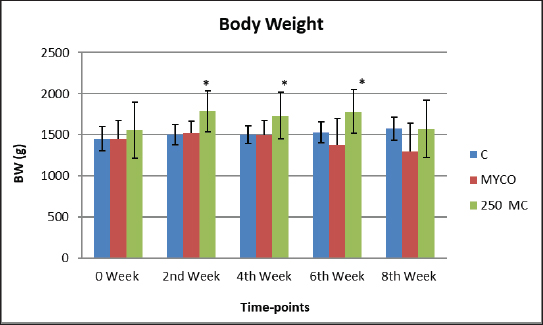
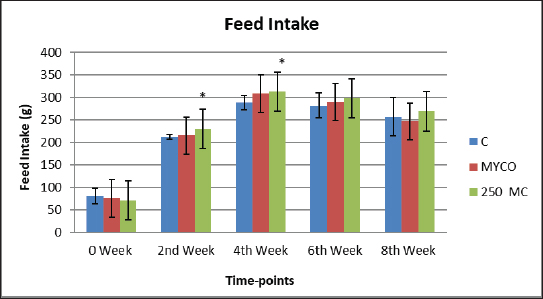
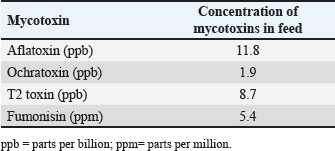
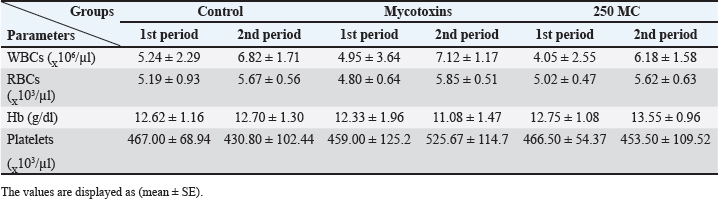
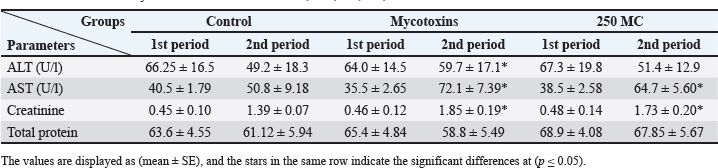
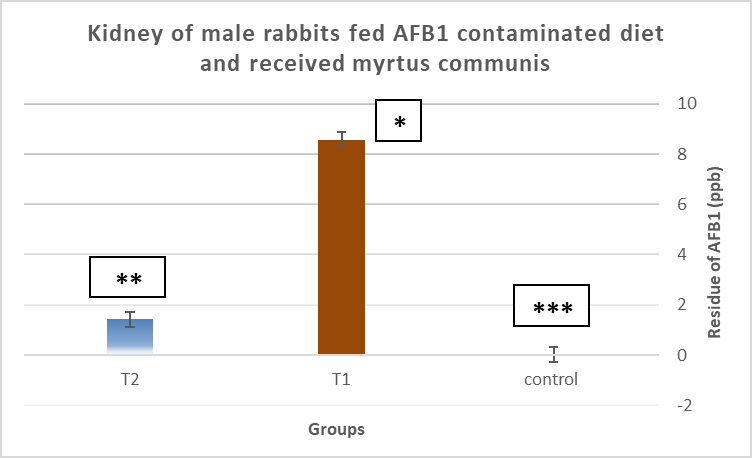
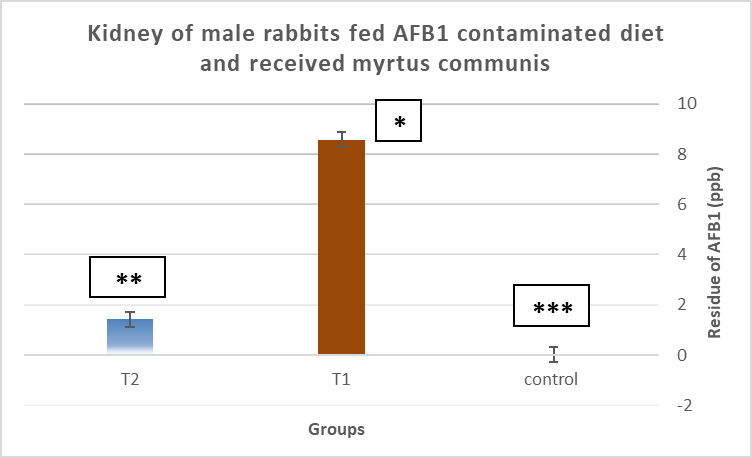
AbstractBackground: Mycotoxins, secondary metabolic compounds released by bacteria, negatively impact the environment, animals, and people. Extraction from Myrtus communis may be used instead of antibiotics to treat microbial infections. Aim: This study investigated the ability of a medicinal plant extract (M. communis) to reduce the harmful effect of mycotoxin on the productivity and health status of male rabbits. In addition, it improved the productivity and health status of male rabbits and detected Aflatoxin-B1 residues in kidney and liver tissues using the High-performance liquid chromatography (HPLC) technique. Methods: Twenty-four local male rabbits, aged 5–6 months with a mean body weight of 1393 ± 20 g, were uncontaminated in three groups based on body weight, each consisting of eight rabbits. The initial control group was provided with a basal diet uncontaminated (C); the second group was given a diet contaminated with state the concentration of mycotoxins in diet mycotoxins (T1); the third group feeds on the same diet as the second group received a diet contaminated with mycotoxins and was treated with M. communis at a dosage of 250 mg/head orally (T2). Results: A significant increase in body weight was observed in the T2 that was treated with M. communis after 2, 4, and 6 weeks; the feed intake showed that in the first 2 and 4 weeks, there was a significant increase in T2 compared with C and T1 in all groups of the experiment with no significant change in 6 and 8 weeks. Regarding the blood parameters white blood cells (WBCs), red blood cells (RBCs), Hemoglobin (Hb), and platelets, there was no change among groups; creatinine increased significantly in the T1 and T2 groups, whereas the total protein was unchanged. The liver enzymes AST enzyme increased in the T1 compared with the T2 group, representing improved liver functions. However, alanine aminotransferase was within the average level for the three groups; after detection of AFB1 in the kidney and liver by HPLC, the concentration of AFB1 in T1, T2 was 8.6,1.4 in the kidney tissue, 10.5, 1.6 in also liver T1, T2, respectively, while the control group was under detectable level. Conclusion: In conclusion, giving M. communis extract orally to rabbits can play an important role in improving production performance and reducing the toxic effects of mycotoxins. It was suggested that the activity of this organ to eliminate the mycotoxins by the M. communis action. In conclusion, giving M. communis orally to rabbits improves their productive performance and minimizes the toxic effects of mycotoxins. Keywords: Myrtus communis, Mycotoxins, Liver enzyme, Rabbits, HPLC. IntroductionMycotoxins are secondary metabolic substances secreted by fungi and have many harmful effects on humans, animals, and the environment (Zhou et al., 2019; Herman et al., 2021). The most dangerous is transmission in animal secretions such as milk, urine, and feces (Kemboi et al., 2020; Lippolis et al., 2020; Cha et al., 2021; Al-Rubaye et al., 2023) as well as in eggs and meat (Mahdi and Atiyah, 2021) as a residue that is only detected by sensitive devices for their presence in tiny proportions for consumption (De Santis et al., 2017; Wang et al., 2019), which are the permissible ranges according to USA and EU regulations. Mycotoxins have apparent effects on body weight, feed consumption, milk production, immunosuppression, and antioxidant activity (Huang et al., 2018; Hung et al., 2018; Cuciureanu et al., 2021). Studies confirm that 25%–50% of cereals are exposed to mycotoxins on a large scale worldwide. The problem facing the agricultural sector comes from exposure to these toxins, which are transmitted to animals through food and thus transfer to humans through production, and the cycle continues. To get rid of these toxins, several methods have been used to reduce the damage of mycotoxins (Horky et al., 2018; Hussein and Atiyah, 2020; Mahmood and Atiyah, 2021). Medicinal herbs have become common in recent years to support the health situation, treat many diseases, increase animal production, and benefit from the remnants of these plants (Sheikh-Zeinoddin et al., 2018; Li et al., 2021). There is global pressure to use potent herbal plants as a food ingredient because plants are widely relevant for treating disease (Damiano et al., 2020) and detoxification of mycotoxins (Al-Owaisi et al., 2022). The primary components of myrtle oil (up to 0.8% in the leaves) are myrtenol, myrtenol acetate, limonene (23%), linalool (20%), pinene (14%), cineol (11%), as well as p-cymene, geraniol, nerol, phenylpropanoid, and methyleugenol. The anti-fungal efficacy of Myrtus communis against Malassezia sp. isolated from the skin of individuals with pityriasis versicolor (Gultepe et al., 2020). The findings indicate that M. communis extraction might serve as an alternative to antifungal medications for treating fungal infections of the skin and mucous membranes, as well as combating dandruff. Another study measured the antioxidant activity of methanol, ethanol, water, and ethyl acetate extract of the leaves and berries. All of the extracts showed significant antioxidant capacity and higher antioxidant content in leaves (Amensour et al., 2010). Flavonoids and anthocyanins in berry extract were examined for their activity against free radicals. The myrtle extract demonstrated interesting free radical scavenging activity (Montoro et al., 2006). The M. communis extract was used to reduce the concentration of Aflatoxin B1 residue in rabbits’ livers and kidneys after mixing it with feed at a rate of 500–1,000 mg /kg diet (Mahmood and Atiyah, 2021). This study was designed to observe the effect of the medicinal plant M. communis on reducing the damage of mycotoxins. Materials and MethodsTwenty-four healthy local male rabbits were brought at the age of about 4–5 months, with a mean body weight (1,393 ± 20) were kept in cages at the animal house of the veterinary college, Baghdad University. All animals were fed on the same concentrated diet and water, and feed was offered for 3 weeks as a preliminary period. The animals were divided into three equal groups of eight animals each, as follows: The first group was fed a concentrated diet without contamination with the mycotoxin AFB1 or the addition of M. communis and kept as a control group (C). The second group was fed a concentrated diet naturally contaminated with mycotoxin (T1) The third group was fed on the concentrated diet contaminated with AFB1 and received M. communis at 250 mg/orally (T2). Preparation of mycotoxinMycotoxin was prepared naturally using contaminated corn, which was taken from where the corn was moldy; the corn was mixed with the concentrated diet according to the standard percentage of the control diet after mixing it well and estimating the concentration of mycotoxin three times at the beginning of the study, middle, and end of the study using the ELISA test; as illustrated in Table 1. Collection of the plant and preparation of the extractFresh M. communis leaves harvested from local trees in Baghdad were rinsed with tap water, air-dried, and ground into a fine powder using an electric grinder. The aqueous extraction involved placing 50 g of produced powder into 200 ml of distilled water. The solvent from the extracted substance was eliminated using a Soxhlet extractor at 2°C for 4 hours, followed by a rotary evaporator at 60°C for 2 hours. The extracted residue was obtained and stored in the freezer at −20°C for the duration of the investigation, following Dakheel et al. (2021). The samples and parameters of this research involve the following: Body weight (g) was calculated for each group biweekly at weeks (0, 2, 4, 6, and 8) to assess weight changes. Feed intake (g)The feed intake was measured daily by subtracting the offered feed (100 g/rabbit) from the remaining quantity the next day. Body weight was also measured to assess overall gain during the experiment. Blood samplesThe samples were collected on weeks 4 and 8 of the experiment to evaluate the abovementioned parameters. Blood samples were (5 ml) from the heart after sterilization of the site of blood drawn by using a disposable syringe. The samples were divided into two parts. The first part of the blood samples was kept in sterile tubes (capacity 10 ml) containing the anticoagulant ethyl diamine tetra acetic acid. The other part of the blood samples was kept in the sterilized tube containing gel and clot activator, and then separated by centrifuge (3,000 rpm) for 5 minutes. Estimation of AFB1 residue in liver and kidney by HPLCTwelve randomly chosen rabbits from each diet group, four per cage, were slaughtered, and their livers and kidneys were removed at the conclusion of the study. Pooling each group’s liver and kidneys yielded four tissue samples for each dietary treatment. Refrigerated or frozen samples were placed on a disposable counter. Tissue aflatoxins were eliminated. Sample preparationAfter 25 g were set up in 100 ml methanol/water (70:30 v/v) for 40 minutes and centrifuged for 5 minutes, 5 ml of the supernatant was extracted, diluted with 20 ml of water, and run through the immunoaffinity column at a rate of no more than 3 ml per minute (the column had been conditioned with 20 ml of distilled water beforehand). After removing the matrix components with 10 ml D.W., the column was allowed to air dry to eliminate any last traces of water. Following the quantitative elution, 1.4 ml of methanol was added to the column and flushed with air. The eluate was then diluted with 2 ml of water and filtered through a 0.45 mm filter before being injected into the HPLC apparatus. The samples were analyzed via HPLC (model SYKAMN/Germany) according to the methodology outlined by Dan et al. (2012): The mobile phase comprised acetonitrile and distilled water in a ratio of 60:40. A C18-ODS column (25 cm × 4.6 mm) was used, with a flow rate of 0.7 ml/min. The detection was performed using a fluorescent detector with an excitation wavelength of 365 nm and an emission wavelength of 445 nm. Statistical analysisThe Minitab (2022) software was used to detect the effects of different factors on the study. The least significant difference test (T-test) was used to compare means significantly (p ≤ 0.005). Results Figure 1 shows that in the first 2, 4, and 6 weeks, body weight significantly increased in T2 G3 compared with C and T1 in all groups of the experiment, with no significant change in 8 weeks. However, Figure 2 showed that in the first 2 and 4 weeks, feed intake was significantly increased in T2 compared with C and T1 in all groups of the experiment, with no significant change in 6 and 8 weeks. On the other hand, there were no significant changes in WBCs, RBCs, Hb, and platelets among all groups during the study, as shown in (Table 2). CreatinineThe results of serum creatinine at 4 and 8 weeks are presented in Table 2, showing that there was no significant change in 4 weeks and a significant increase (p ≤ 0.05) in T1 compared to c and T2 in 8 weeks. Total proteinTable 2 also shows the serum total protein levels during the 4 and 8 weeks, and there were no significant changes among the three groups during these periods. Serum alanine aminotransferase (ALT)Table 2 displays the serum ALT values at 4 and 8 weeks. The ALT enzyme results indicated a substantial rise (p ≤ 0.05) and no significant change at 4 weeks. T1 in contrast to c and T2. Serum aspartate aminotransferaseThe ALT enzyme results revealed that there was no significant change in 4 weeks and a significant rise (p ≤ 0.05) in T1 compared to c and T2, whereas the results of serum AST at 4 and 8 weeks are shown in Table 3. The concentrations of AFB1 in the kidney and liverThe results showed that the concentration of AFB1 in the kidney was 8 and 6 ppb compared with the group treated with M.C.E, where it was 1.4 ppb. In this instance, the level of AFB1 in the liver was measured at 10.5 ppb, which dropped to 1.8 ppb following daily oral treatment with M.C.E. This is illustrated in Figures 3 and 4, confirming a highly significant increase in AFB1 concentration in the kidney and liver when compared to the group that obtained M.O.E orally after a diet contaminated with AFB1.
Fig. 1. The body weight of treated groups at 0, 2nd, 4th, 6th, and 8th weeks (mean ± SE), and stars indicate the significant differences at (p ≤ 0.05).
Fig. 2. The feed intake of treated groups at 0, 2nd, 4th, 6th, and 8th weeks (mean ± SE), and stars indicate the significant differences at (p ≤ 0.05). Table 1. Concentration of mycotoxin in contaminated dietary feed according to the ELIZA test.
DiscussionAfter 2 weeks in T2, this trial showed a noticeable shift in body weight. Mycotoxin is the cause of the drop in body weight. According to several studies, different breeds of developing or adult rabbits treated with AF showed decreased body weight (Fayed, 1999; Ibrahim, 2000; Shehata, 2002). In general, Hafez et al. (1983) found that aflatoxin-B1 combined with aflatoxin-G caused a reduction in body weight in both male and female rabbits. Abd El-Hamid (1990) also produced a closely comparable work. The diet of Baladi rabbits consisted of AFs B1, B2, G1, and G2. Lower feed conversion caused by AFB1 handling could be attributed to aflatoxicosis or maybe its impact on the regulation of the hypothalamic feed intake core, in addition to the dangerous influence of AFB1 on the digestion and absorption of various nutrients. The changes in hormonal balance among animals cared for with AF may lead to a slight reduction in body weight and other functional requirements. Moreover, reduced feed intake and weight gain among animals fed AF-contaminated diets have been documented in commercial ducks (Han et al., 2008) and mice (Kocabas et al., 2003). In broiler chickens, feed consumption was reduced to 2.5 ppm/ diet AF (Shareef et al., 2019). The current results also agreed with previous results mentioned that there was a diminishment in body weight in various breeds of growing or adult rabbits handled by AF (Shehta et al., 2002; Hussein and Atiyah, 2020; Mahdi and Atiyah, 2021). Further, Hafez et al. (1983) reported a decrease in body weight for male and female rabbits through AFB with AFG. The decreased body weight with increasing aflatoxin concentrations in the diet was mainly related to the reduced feed intake. With increasing dietary aflatoxin concentrations, feed intake was linearly decreased. The current study did not agree with the previous research, which showed no effect change in body weight (Hussein and Atiyah, 2020; Mahdi and Atiyah, 2021; Abdulhussein and Dakheel, 2022). Previous research indicates comparable beneficial outcomes, including enhanced feed intake and egg yield in laying hens when myrtle plant extract is incorporated into drinking water at various levels (Goudarzi et al., 2016). Mahmoodi-Bardzardi et al. (2014) concurred with these findings, noting an increase in feed consumption and live weight in chickens with the addition of myrtle extract to the feed, whereas Biricik et al. (2012) reported a decrease in live weight. Bülbül et al. (2014) observed enhanced egg production in chickens administered myrtle plant extract at a dosage of 1000 mg/kg. Saei et al. (2013) reported that myrtle plant extract did not produce a significant effect on live weight gain in broilers when compared to the control group. It nonetheless decreased feed consumption and enhanced the feed conversion ratio. Taee et al. (2017) demonstrated that the inclusion of 1% myrtle powder in the diet of rainbow trout resulted in increased live weight gain and enhanced feed conversion ratios. This finding underscores the significance of incorporating M. communis into feed affected by mycotoxins. The beneficial effects observed on growth and feed intake in rabbits exposed to mycotoxins in the current study may be linked to the antioxidant, antibacterial, antifungal, and anti-inflammatory properties of myrtle plant extracts. Table 2. The complete blood count values of treated rabbits at 0, 2nd, 4th, 6th, and 8th weeks.
Table 3. The biochemistry values of treated rabbits at 0, 2nd, 4th, 6th, and 8th weeks.
Fig. 3. Concentration of AFB1 residue in the kidney after receiving Myrtus communis extract and/ or AFB1 in the kidney of male rabbits fed AFB1-contaminated diet. The stars indicate significantly different (p ≤ 0.01); means (n=5) ± SEM.
Fig. 4. Concentration of AFB1 residue in the liver after receiving Myrtus communis extract and/ or AFB1 in the kidney of male rabbits fed AFB1-contaminated diet. The stars indicate significantly different (p ≤ 0.01); means (n=5) ± SEM. Moreover, this result agrees with studies showing decreased total protein after administrating AFB1 (Sun et al., 2015). Also, some studies show increased total protein after administration (Zhou et al., 2019). The secondary metabolite of herbal plants may cause ameliorative effects on the productive traits and biochemical properties after contaminated feeding a diet with mycotoxins to rabbits (Mahmood and Atiyah, 2021; Abdulhussein and Dakheel, 2022). Increased creatinine was an important indicator of the action of mycotoxins in the damage of tissue in the kidney; this was observed in our study, where creatinine increased significantly in the group that was fed a diet contaminated with mycotoxins; this agreed with (Vaziriyan et al., 2018), the decrease of creatinine concentration in the blood after administration of M. communis confirms the positive action of this material, as it contains antioxidants such as flavonoids, which reduce the effect of free radicals that harmful to cell (Montoro et al., 2006; Amensour et al., 2010). In the current study, there is an increase in AFB1 compared to the control group, and that result agrees with the authors showing a higher increase in ALT in broiler chick after administrated AFB1 (Hussain et al., 2016). T1 shows a slight rise in ALT compared to the control group; this result agrees with a previous study using M. communis in water, which shows an increase in ALT (Gultepe et al., 2020). Another study shows a decrease in ALT after using M. communis (Bagcilar and Gezer, 2020). The result of AST shows no significant change in 4 weeks among the group; in the 8 weeks, there was a significant increase in T2 compared to the other group. The result agrees with another study (Qian et al., 2016), which shows an increase in AST after the administration of AFB1. The phenolic compounds in MCE likely protected the liver against toxic agents; thus, the AST decreased in C and T2 compared with T1. Saei et al. (2013) concluded that the use of M. communis as the treatment reduces the harmful effects of aflatoxin on various aspects, including glucose, creatinine, cholesterol (ALT), (AST), and (ALP) concentration. Flavonoid compounds in the Myrtle leaves, such as rosmarinic acid, caffeic acid, thymol, and carvacrol, are free radical absorbents. Free radicals cause lipid peroxidation, and these compounds are effective against lipid peroxidation (Yazdani et al., 2014). The levels of AFB1 in the kidney and liverThe presence of mycotoxins in the kidney and liver indicates that the body metabolizes mycotoxins. Biochemical analyses indicate a boost in the liver enzyme creatinine, alterations in certain blood parameters, and a decrease in body weight and feed intake in male rabbits. The findings match with those reported by (Hussein and Atiyah, 2020; Mahdi and Atiyah, 2021; Mahmood and Atiyah 2021), indicating that the concentration of AFB1 in the kidney and liver was 10.5 ppb, exceeding the acceptable limit of 8.6 ppb in the USA and EU. The concentration in the group administered MCE orally remained within acceptable limits. Rahmati-Joneidabad (2021), Mahmood and Atiyah (2021), Belmimoun et al. (2020), and Muhanna Al-Abdali et al. (2019) indicated that M.C.E. may inhibit fungal activity, thereby reducing mycotoxin production, suggesting beneficial treatment effects. This is consistent with the findings of Gumus et al. (2010). Bioactive compounds derived from medicinal plants, such as phenolic and flavonoid chemicals, are active therapeutic tools for treating many health problems. The active ingredients in M. communis are myrtenol, myrtenol acetate, limonene, linalool, pinene, and cineol. Moreover, p-cymene, geraniol, nerol, phenylpropanoid, and methyl eugenol can act against fungi immediately or indirectly as antioxidant materials, supportive immunity, and affirmed reactions (Mohammadi et al., 2020; Shaapan et al., 2021). ConclusionThe study concluded that the M. communis extract has a positive effect on the health status of male rabbits fed a contaminated diet with mycotoxins, which improved the productive trait and ameliorative effect on liver and kidney function, in addition to decreasing the concentration of aflatoxin-B1 in the kidney and liver. AcknowledgmentsThe authors acknowledge the University of Baghdad and the Veterinary Medicine College. They are also grateful to the Department of Ministry of Agriculture/ Iraq/ Baghdad. Conflict of interestThe authors did not declare any conflicts of interest. FundingNo grant was received for this study, and it has been supported by self-funding. Authors’ contributionsDr Mohammed Munis Dakheel was the corresponding author for this article. Dr Adil Jabbar Atiyah was responsible for study observations and research management. Shireen Jamal Mahmood was responsible for animal care, clinical parameters, and obtaining blood samples. Article writing was done equally by the authors. Data availabilityAll data were provided in the manuscript. ReferencesAbd El-Hamid, A.M. 1990. Occurrence of some mycotoxins (aflatoxins, ochratoxin A, citrinin, zearaltenone and vomitoxin) in various Egyptian feeds. Arch. Anim. Nutr. 40(1), 647–664. Abdulhussein, A.A. and Dakheel, M.M. 2022. Influence of tannin extracts on hematological and production properties of male rabbits fed mycotoxin diets. Indian J. Forensic Med. Toxicol. 16(1), 1437. Al-Owaisi, A., Al-Sadi, A.M., Al-Sabahi, J.N., Sathish Babu, S.P., Al-Harrasi, M.M.A., Hashil Al-Mahmooli, I., Abdel-Jalil, R. and Velazhahan, R. 2022. In vitro detoxification of aflatoxin B1 by aqueous extracts of medicinal herbs. All Life 15(1), 314–324. Al-Rubaye, H., Atiyah, A.J. and Huang, S. 2023. Comparative study of aflatoxin M1 carry-over from feed to raw milk in cow, buffalo, sheep, and goats in different areas of Baghdad Province. Iraqi J. Vet. Med. 47(2), 88–93. Amensour, M., Bouhdid, S., Fernández-López, J., Idaomar, M., Senhaji, N.S. and Abrini, J. 2010. Antibacterial activity of extracts of Myrtus communis against food-borne pathogenic and spoilage bacteria. Int. J. Food Properties 13(6), 1215–1224. Bagcilar, S. and Gezer, C. 2020. Myrtle (Myrtus communis L.) and potential health effects. EMU J. Pharm. Sci. 3(3), 205–214. Belmimoun, A., Meddah, B., Meddah, A.T.T., Gabaldon, J. and Sonnet, P. 2020. Antifungal activity of Myrtus communis and Zygophyllum album extracts against human pathogenic fungi. Europ. J. Biol. Res. 10(2), 45–56. Biricik, H., Yesilbag, D., Gezen, S.S. and Bulbul, T. 2012. Effects of dietary myrtle oil (Myrtus communis L.) supplementation on growth performance, meat oxidative stability, meat quality and erythrocyte parameters in quails. Revue. Méd. Vét. 163, 131–138. Bülbül, T., Yesilbag, D., Ulutas, E., Biricik, H., Gezen, S.S. and Bülbül, A. 2014. Effect of myrtle (Myrtus communis L.) oil on performance, egg quality, some biochemical values and hatchability in laying quails. Rev. Méd. Vét. 165, 280–288. Cha, M., Wang, E., Hao, Y., Ji, S., Huang, S., Zhao, L., Wang, W., Shao, W., Wang, Y. and Li, S. 2021. Adsorbents Reduce Aflatoxin M1 Residue in Milk of Healthy Dairy Cow Exposed to Moderate Level Aflatoxin B1 in Diet and Its Exposure Risk for Humans. Toxins, 13, 665. Cuciureanu, M., Tuchiluș, C., Vartolomei, A., Tamba, B.I. and Filip, L. 2021. An immunoenzymatic method for the determination of ochratoxin A in biological liquids (colostrum and cow’s Milk). Toxins 13(10), 673. Dakheel, M.M., Atshan, O.F. and Abdulwahed, A.A. 2021. Nutritional comparison of different levels of pomegranate peel extracts on production values and performance in female rabbits. Ann. Rom. Soc. Cell. Biol. 25(6), 4094–4110. Damiano, S., Andretta, E., Longobardi, C., Prisco, F., Paciello, O., Squillacioti, C., Mirabella, N., Florio, S. and Ciarcia, R. 2020. Effects of curcumin on the renal toxicity induced by ochratoxin a in rats. Antioxidants 9(4), 332. Dan, M., Lina, G., Yongping, C. and Chunqing, L. 2012. Determination of 17a-methyltestosterone in fish feed by HPLC method. Feed Ind. 4, 65–45. De Santis, B., Debegnach, F., Gregori, E., Russo, S., Marchegiani, F., Moracci, G. and Brera, C. 2017. Development of a LC-MS/MS method for the multi-mycotoxin determination in composite cereal-based samples. Toxins 9(5), 169. Fayed, A.M.A. 1999. Amononiation of the contaminated crop residues with aflatoxins and its effect on rabbits. M.Sc. Thesis, Faculty of Science, Cairo University, Egypt, 7(6), 770–772. Goudarzi, M., Samiei, I., Nanekarani, S. and Nasrolahi, F. 2016. The effect of Myrtus communis oil extract on growth performance and immune responses in ross and cobb strain broilers. J. Adv. Agric. Technol. 3, 10–14. Gultepe, E.E., Iqbal, A., Cetingul, I.S., Uyarlar, C., Ozcinar, U. and Bayram, I. 2020. Effect of Myrtus communis L. plant extract as a drinking water supplement on performance, some blood parameters, egg quality and immune response of older laying hens. Kafkas Üniv. Vet. FakDerg. 26(1), 9–16. Gumus, T., Demirci, A.S., Sagdic, O. and Arici, M. 2010. Inhibition of heat resistant molds: Aspergillus fumigatus and Paecilomycesvariotii by some plant essential oils. Food Sci. Bio. 19(5), 1241–1244. Hafez, A.H., Gomma, A., Mousa, S.A. and Megalla, S.E. 1983. Aflatxoins and aflatoxicosis .4. Effect of dietary aflatoxins on adult fertile male and female rabbits at various reproductive conditions. Mycopathologia 83, 183–186. Han, X., Huang, Q., Li, W., Jiang, J. and Xu, Z. 2008. Changes in growth performance digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livest. Sci. 119, 216–220. Herman, D. and Mantle, P. 2021. Rat tumour histopathology associated with experimental chronic dietary exposure to ochratoxin A in prediction of the mycotoxin’s risk for human cancers. Toxins 13(3), 205. Horky, P., Sylvie, S., Daria, B. and Jiri, S. 2018. Nanoparticles as a solution for eliminating the risk of mycotoxins. Nanomaterials 8(9), 727. Huang, S., Zheng, N., Fan, C., Cheng, M., Wang, S., Jabar, A., Wang, J. and Cheng, J. 2018. Effects of aflatoxin B1 combined with ochratoxin A and/or zearalenone on metabolism, immune function, and antioxidant status in lactating dairy goats. Astralas J. Anim. Sci. 31(4), 505–513. Hussain, Z., Khan, M.Z., Saleemi, M.K., Khan, A. and Rafique, S. 2016. Clinicopathological effects of prolonged intoxication of aflatoxin B1 in broiler chicken. Pak. Vet. J. 36(4), 477–481. Hussein, N.A.A.S. and Atiyah, A.J. 2020. The effect of N-carbamylglutamate supplement on carryover of aflatoxin B1 in liver and muscle tissues of male rabbits fed with contaminated diet by AFB1. Plant Arch. 20(2), 4653–4659. Ibrahim, K.I.K. 2000. Effect of aflatoxins and ascorbic acid on some productive and reproductive parameters in male rabbits. M.Sc. Thesis, Faculty of Agriculture, Alexandria University, Egypt, 6(1), 694–701. Kemboi, D.C., Ochieng, P.E., Antonissen, G., Croubels, S., Scippo, M.L., Okoth, S., Kangethe, E.K. and Gathumbi, J.K. 2020. Multi-mycotoxin occurrence in dairy cattle and poultry feeds and feed ingredients from Machakos Town, Kenya. Toxins 12(12), 762. Kocabas, C.N., Coskun, T., Yurdakok, M. and Haziroglu, R. 2003. The effects of aflatoxin B1 on the development of kwashiorkor in mice. Hum. Exp. Toxicol. 22, 155–158. Li, S., Liu, R., Wei, G., Guo, G., Yu, H., Zhang, Y., Ishfaq, M., Fazilani, S.A. and Zhang, X. 2021. Curcumin protects against Aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 208, 111725. Lippolis, V., Asif, S., Pascale, M., Cervellieri, S., Mancini, E., Peli, A., De Amicis, I., Robbe, D. and Minervini, F. 2020. Natural occurrence of ochratoxin A in blood and milk samples from jennies and their foals after delivery. Toxins 12(12), 758. Mahdi, M.R. and Atiyah, A.J. 2021. Biodegradation of lycopene on AFB1 naturally contaminated feed and their residue in liver and muscles tissues of local male rabbits. Ann. Rom. Soc. Cell. Biol. 25, 385–393. Mahmood, S.J. and Atiyah, A.J. 2021. Ameliorative effect of aqueous Myrtus communis extract on AFB1 residue in liver and kidney and some blood biochemical parameter of local male rabbits feeding naturally contaminated by mycotoxins. Biochem. Cell. Arch. 21(2), 4163–4169. Mahmoodi-Bardzardi, M., Ghazanfari, S. and Sharifi, S.D. 2014. Growth performance, carcass characteristics, antibody titer and blood parameters in broiler chickens fed dietary myrtle (Myrtus communis) essential oil as an alternative to antibiotic growth promoter. Poult. Sci. J. 2, 37–49. Mohammadi Moghaddam, M., Rezaee, S., Mohammadi, A.H., Zamanizadeh, H.R. and Moradi, M. 2020. Relationship between Aspergillus flavus growth and aflatoxin B1 and B2 production with phenolic and flavonoid compounds in green hull and kernels of pistachio cultivars. Appl. Entomol. Phytopathol. 87(2), 13–23. Montoro, P., Tuberoso, C.I., Piacente, S., Perrone, A., De Feo, V., Cabras, P. and Pizza, C. 2006. Stability and antioxidant activity of polyphenols in extracts of Myrtuscommunis L. berries used for the preparation of myrtle liqueur. J. Pharm. Biomed. Anal. 41(5), 1614–1619. Muhanna Al-Abdali, M.A., Al-Mahmooli, I.H., Al-Sadi, A.M., Al-Sabahi, J.N., Al-Ruqaishi, H.K. and Velazhahan, R. 2019. In vitro antifungal activity of leaf extract of myrtle (Myrtus communis L.) against phytopathogenic fungi. Biochem. Cell. Arch. 19(2), 4663–4668. Qian, G., Tang, L., Lin, S., Xue, K.S., Mitchell, N.J., Su, J., Gelderblom, W.C., Riley, R.T., Phillips, T.D. and Wang, J.S. 2016. Sequential dietary exposure to aflatoxin B1 and fumonisin B1 in F344 rats increases liver preneoplastic changes indicative of a synergistic interaction. Food Chem. Toxicol. 95, 188–195. Rahmati-Joneidabad, M. 2021. Investigation of the antifungal effect of Myrtus communis essential oil on Penicillium digitatum and Penicillium italicum (the green and blue fungi of orange). Food Sci. Tech. 17(109), 1–8. Saei, M.M., Sadeghi, A.A. and Ahmadvand, H. 2013. The effect of Myrtus communis oil extract on growth performance, serum biochemistry and humoral immune responses in broiler chicks fed diet containing aflatoxin B1. Arch. Anim. Breed. 56(1), 842–850. Shaapan, R.M., Al-Abodi, H.R., Alanazi, A.D., Abdel-Shafy, S., Rashidipour, M., Shater, A.F. and Mahmoudvand, H. 2021. Myrtus communis essential oil; anti-parasitic effects and induction of the innate immune system in mice with Toxoplasma gondii infection. Mol. 26(4), 819. Shareef, A.M. and Sito, E.O. 2019. Effect of (mycofix® plus) and aflatoxin on health and performance of broiler chickens. Bas. J. Vet. Res. 18(1), 283–287. Shehata, S.A. 2002. Detoxification of mycotoxin contaminated animal feedstuffs. Ph.D. Thesis, Faculty of Agriculture, Zagazig University, Egypt. Sheikh-Zeinoddin, M. and Khalesi, M. 2019. Biological detoxification of ochratoxin A in plants and plant products. Toxin Rev. 38(3), 187–199. Sun, L.H., Lei, M.Y., Zhang, N.Y., Gao, X., Li, C., Krumm, C.S. and Qi, D.S. 2015. Individual and combined cytotoxic effects of aflatoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on BRL 3A rat liver cells. Toxicon. 95, 6–12. Taee, H.M., Hajimoradloo1, A., Hoseinifar, S.H. and Ahmadvand, H. 2017. The effects of dietary Myrtle (Myrtus communis L.) supplementations on growth performance and some innate immune responses in rainbow trout (Oncorhynchus mykiss). Int. J. Aquat. Biol. 5, 252–259. Vaziriyan, M., Banaee, M., Nemadoost Haghi, B. and Mohiseni, M. 2018. Effects of dietary exposure to aflatoxins on some plasma biochemical indices of common carp (Cyprinus carpio). Iran J. Fish. Sci. 17(3), 487–502. Wang, Q., Zhang, Y., Zheng, N., Guo, L., Song, X., Zhao, S. and Wang, J. 2019. Biological system responses of dairy cows to aflatoxin B1 exposure revealed with metabolomic changes in multiple biofluids. Toxins 11(2), 77. Yazdani, M., Zamani, Z., Moghtari, M., Nozari, M. and Johari, H. 2014. The effect of Myrtus communis extract on liver enzymes and blood biochemical factors in diabetic adult male rats. Zahedan J. Res. Med. Sci. 16(10), 12–17. Zhou, R., Liu, M., Liang, X., Su, M. and Li, R. 2019. Clinical features of aflatoxin B1-exposed patients with liver cancer and the molecular mechanism of aflatoxin B1 on liver cancer cells. Environ. Toxicol. Pharmacol. 71, 103225. | ||
| How to Cite this Article |
| Pubmed Style Atiyah AJ, Mahmood SJ, Dakheel MM. Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits. Open Vet. J.. 2025; 15(3): 1217-1225. doi:10.5455/OVJ.2025.v15.i3.13 Web Style Atiyah AJ, Mahmood SJ, Dakheel MM. Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits. https://www.openveterinaryjournal.com/?mno=228261 [Access: January 22, 2026]. doi:10.5455/OVJ.2025.v15.i3.13 AMA (American Medical Association) Style Atiyah AJ, Mahmood SJ, Dakheel MM. Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits. Open Vet. J.. 2025; 15(3): 1217-1225. doi:10.5455/OVJ.2025.v15.i3.13 Vancouver/ICMJE Style Atiyah AJ, Mahmood SJ, Dakheel MM. Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits. Open Vet. J.. (2025), [cited January 22, 2026]; 15(3): 1217-1225. doi:10.5455/OVJ.2025.v15.i3.13 Harvard Style Atiyah, A. J., Mahmood, . S. J. & Dakheel, . M. M. (2025) Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits. Open Vet. J., 15 (3), 1217-1225. doi:10.5455/OVJ.2025.v15.i3.13 Turabian Style Atiyah, Adil Jabbar, Shireen Jamal Mahmood, and Mohammed Munis Dakheel. 2025. Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits. Open Veterinary Journal, 15 (3), 1217-1225. doi:10.5455/OVJ.2025.v15.i3.13 Chicago Style Atiyah, Adil Jabbar, Shireen Jamal Mahmood, and Mohammed Munis Dakheel. "Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits." Open Veterinary Journal 15 (2025), 1217-1225. doi:10.5455/OVJ.2025.v15.i3.13 MLA (The Modern Language Association) Style Atiyah, Adil Jabbar, Shireen Jamal Mahmood, and Mohammed Munis Dakheel. "Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits." Open Veterinary Journal 15.3 (2025), 1217-1225. Print. doi:10.5455/OVJ.2025.v15.i3.13 APA (American Psychological Association) Style Atiyah, A. J., Mahmood, . S. J. & Dakheel, . M. M. (2025) Effect of oral administration of Myrtus communis extract on reducing the negative impacts of feed contaminated with mycotoxins on productive performance and some blood characteristics in local male rabbits. Open Veterinary Journal, 15 (3), 1217-1225. doi:10.5455/OVJ.2025.v15.i3.13 |