| Research Article | ||
Open Vet. J.. 2025; 15(3): 1150-1156 Open Veterinary Journal, (2025), Vol. 15(3): 1150-1156 Research Article Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairmentHuda S. Darusman1,2,3*, Lis Rosmanah1,3, Sela S. Mariya4, Uus Saepuloh1, Yuliana Yuliana1 and Jann Hau51Primate Research Center, Institute of Research and Community Service, Bogor Agricultural University (IPB University), Bogor, Indonesia 2School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 3Primatology Graduate Study Program, IPB University Graduate School, Bogor, Indonesia 4Center for Biomedical Research, National Research and Innovation Agency (BRIN), Cibinong, Indonesia 5Department of Experimental Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark *Corresponding Author: Huda S. Darusman. School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, West Java, Indonesia. Email: hudada [at] apps.ipb.ac.id Submitted: 12/11/2024 Accepted: 06/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
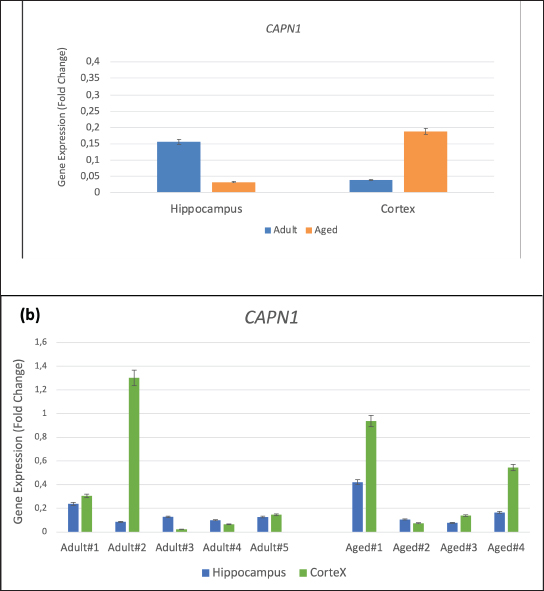
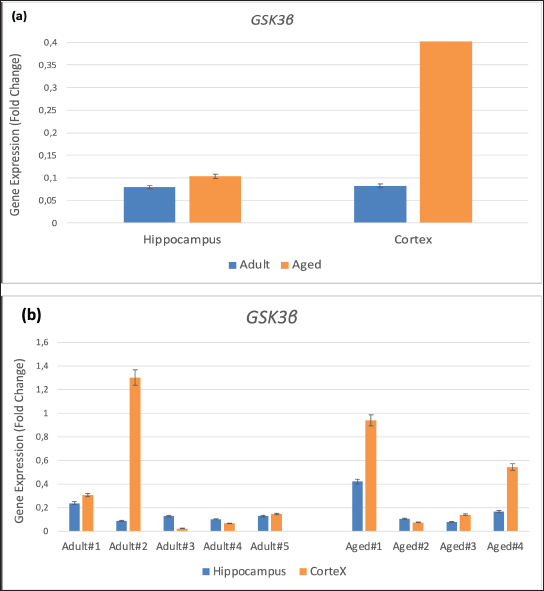
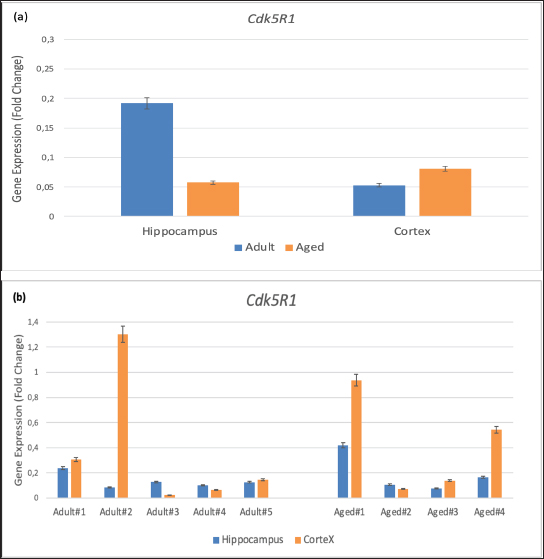
AbstractBackground: Alzheimer’s disease (AD) is characterized by the buildup and aggregation of misfolded proteins in the brain, including amyloid-β (Aβ) and hyperphosphorylated tau. The hyperphosphorylation state of Tau protein plays an important role in the development of AD. Our previous studies developed and characterized the cynomolgus monkey as a spontaneous animal model of AD. Aim: We demonstrated the validity of the model through experimental investigations of the relationship between cognitive decline and AD neuropathy. There is, however, little information about the expression of hyperphosphorylated tau-related genes in various brain areas in the cynomolgus monkey spontaneous AD model. Methods: In the present study, total RNA was extracted from archived cortex and hippocampus tissues from the brains of two groups of cynomolgus monkeys, adult (10–12 years old, n=5) and aged (> 20 years old, n=4). The expression of the tau-protein-associated genes kinase 3 beta, calpain 1, and cyclin-dependent kinase 5 regulatory subunit 1 was evaluated using RT-qPCR. Results: The expression of all three genes increased by up to fivefold in the cortical brain area of aged subjects compared with adults. Conclusion: Our results add weight to the utility of cynomolgus macaques as a valid spontaneous model in translational preclinical research involving studies of the effect of aging on the formation of hyperphosphorylated tau protein, which causes AD-related lesions in the brain. Keywords: Cynomolgus monkey, Hyperphosphorylated tau, Gene expression, Aging, Alzheimer. IntroductionAlzheimer’s disease (AD) is a neurodegenerative disease characterized by the buildup and aggregation of misfolded proteins in the brain, including amyloid-β (Aβ) and hyperphosphorylated tau (Jellinger, 1998). More than 55 million people worldwide are living with AD, and this number is expected to nearly double every 20 years. The Centers for Disease Control and Prevention reported that the number of people living with AD doubles every 5 years beyond the age of 65 (Alzheimer’s Disease Association, 2024). Animal models are important tools for the study of AD and in the preclinical testing of potential drug candidates (Chen and Zhang, 2022). Non-human primates (NHPs) are particularly relevant as reliable animal models of complex diseases like AD with respect to the etiology and treatment of the disease (Darusman et al., 2021; Jiang et al., 2024). We found that a proportion of aged cynomolgus monkeys (Macaca fascicularis, long-tailed macaques) spontaneously develop dementia resembling human AD with respect to memory loss and lesions in the brain, including the build-up of amyloid beta1–42 and phosphorylated tau threonine 231 (Darusman et al., 2014a). Cynomolgus monkeys have a similar amyloid beta 42 (Aβ42) as humans, and cerebral amyloid angiopathy was observed in aged monkeys. In contrast to humans, amyloids were found to deposit in the small veins and capillaries. Phosphorylated tau was found intracellularly in the neurons, indicating a possibility of an early stage of the formation of tangles (Darusman et al., 2014b). Other research has revealed similar qualitative disease sequences and patterns in aged Rhesus monkeys (Macaca mulatta) that are similar to those in humans (Paspalas et al., 2018). Hyperphosphorylation of tau protein results in the formation of neurofibrillary tangles. Tau proteins are hyperphosphorylated as a result of changes in kinase and phosphate activity, which can result in neurofibrillary tangles in brain cells and ultimately cell death (Holper et al., 2022). Hyperphosphorylated tau protein is a result of defective cellular signaling, and tau becomes abnormally hyperphosphorylated, which eventually leads to microtubule disassembling and the combination of free tau molecules into paired helical filaments (Yang et al., 2024). Natural tauopathy in monkeys older than 30 years of age resembles neurodegeneration or the advanced stages of human brain aging (Stonebarger et al., 2021). We recently (Rosmanah et al., 2024) analyzed the mRNA expression of the genes Presenilin2 (PSEN2) and A Disintegrin and Metalloproteinase 10 (ADAM10) in the aged cynomolgus monkey brain (Rosmanah et al., 2024). These genes are responsible for the amyloid precursor protein sequence of proteolytic cleavage and the subsequent formation of amyloid β peptides. PSEN2 expression was higher in the adult control group and ADAM10 expression was lower in the aged group, similar to that found in human AD patients (Kabir et al., 2020; Lipper et al., 2023). In the present study, we examined the expression of three genes that play a role in the processing of tau protein in the brain using RT-qPCR to evaluate the quantitative expression of glycogen synthase kinase 3 beta (GSK3β), calpain 1 (CAPN1), and cyclin-dependent kinase 5 regulatory subunit 1 (p35) (CDK5R1) with the aim to further consolidate the cynomolgus monkey as a high-validity animal model of human AD. Material and MethodsSubject and sample collectionFrom previous studies, we selected brain tissue from our archives. Four female cynomolgus monkeys aged >20 years met the requirements of low levels of Aβ42(<5000 pg/ml) and low Delayed Response Task (DRT) performance (<40%) for the memory-affected group (Darusman et al., 2014a, b). Five adult female subjects, 10–12 years of age, characterized by high-circulating Aβ42 and high DRT performance were selected as the control group. Subject age was determined from birth certificates for animals born in captivity and from dental scaling (Swindler, 2002) and the estimated year of birth for animals born in the wild. All subjects were housed at the AAALAC-accredited Primate Research Center, Bogor Agricultural University (PRC, IPB) in pairs or social groups of various sizes. Sample collection and project licenseThis study examined brain tissue from our archives from nine female cynomolgus macaques, which were separated into two groups: four aged (≥ 20 years) and five adults (ages 10–12 years). Brain tissue samples from the cortex and hippocampus were analyzed. Project authorization was granted by KPKPHP4 (Commission for Supervision of Animal Welfare in Research, Testing, Education, and Captivity) after an ethical cost-benefit analysis of the use of animals for this project (IPB number PRC-19-A012). RNA extraction and synthesis of cDNAThe brain cortex and hippocampus, 2–3 mm sections, were extracted for total RNA using RNeasy Mini Kits (Qiagen, Hilden, Germany) following the manufacturer’s guidelines. A Nanodrop 1000 spectrophotometer was used to measure the amount of RNA (Thermofisher Scientific, USA). Reverse transcriptase was used for cDNA synthesis following standard operating procedures (Sensifast cDNA Synthesis Kit, Bioline, Meridian Bioscience, USA). RT-qPCR amplificationA CFX Opus 96 instrument was used to perform the PCR amplification process (Biorad, USA). The following formulation was used for each reaction: 2 µl of cDNA as a template, 18 µl of a mixture containing 6 µl of nucleotide-free water, 10 µl of Sensifast Sybr mix (Bioline, Meridian Bioscience, USA), and 1 µl of forward and reverse primers for CAPN1, CDK5R1, GSK3, and GAPDH as reference genes. The nucleotide sequences of these primers are presented in Table 1 (Park et al., 2015). The following steps were performed in the qPCR amplification program: pre-denaturation at 95°C for 2 minutes, denaturation at 95°C for 10 seconds, annealing at 55°C for 20 seconds, and extension at 65°C for 10 seconds. This cycle was repeated 40 times. Data analysisSPSS version 26 and Microsoft Excel were used to analyze the data. The Shapiro–Wilk test and the Levene test were used to determine whether the data were normal or homogeneous. Then, an independent t-test was performed to check for variations across areas and age groups. Cycle threshold (Ct) data from qPCR is processed with the 2-Ct formula to obtain the relative quantification (RQ) result. mRNA expression levels in fold-change were determined using the RQ value after normalization to the GAPDH housekeeping gene. P-levels < 0.05 were considered significant. ResultsThe qPCR results demonstrated differences in the mRNA expression of CAPN1, CDK5R1, and GSK3 between adult and aged cynomolgus monkeys (Figs 1–3). There was a significantly higher expression of CAPN1, CDK5R1, and GSK3 mRNA in the cortex tissue of aged animals compared with the cortex tissue of the adults. The expression of CAPN1 was higher in older adult’s cortical region than in adult’s cortex region. In addition, expression in the hippocampus region was 4.9-fold higher in adults than in aged monkeys. The GSK3β gene expression in older adults’ cortex and hippocampus was significantly higher than in the animals in the adult group. The aged cortex expression was 5.9-fold higher and the hippocampus expression was 1.3-fold higher in the aged group than in the adult group. The CDK5 expression of CDK5R1 older adults’ cortex was 1.5-fold higher than that in the adult monkeys. Meanwhile, the adult hippocampus had a 3.3-fold higher expression compared to monkeys in the older adults group. Table 1. The primers used in this study were modified from those used by Park et al. (2015).
Fig. 1. Gene expression of CAPN1 in the hippocampus and cortex of adult and aged cynomolgus monkeys (a) Group gene expression (b) Individual gene expression. Values are presented as means error bars=SEM.
Fig. 2. Gene expression of GSK3β in brain hippocampus and cortex of adult monkeys compared with aged monkeys. (a) Group gene expression. (b) Individual gene expression. Values are presented as means error bars=SEM. DiscussionThe accumulation of phosphorylated tau proteins in neurofibrillary tangles in the brain is closely linked to neuronal loss and cognitive function in AD. Tau phosphorylation is regulated by genes including CAPN1, CDK5R1, and GSK3β (Park et al., 2015; Spreafico et al., 2018; Lauretti and Dincer, 2021), and in the present study, the cortical regions of older monkeys (aged) exhibited higher levels of gene expression compared with the cortical regions of younger monkeys (adult). The aging process involves a complex interplay of genetic, epigenetic, and environmental factors that lead to changes in gene expression. The increased expression of CAPN1, CDK5R1, and GSK3β may be part of a broader pattern of altered gene regulation in aging neurons. These changes could be driven by factors such as increased inflammatory signals, altered growth factor levels, and changes in cellular metabolism. Neurons become more susceptible to damage and degeneration as they age. The increased expression of these genes may be associated with early neurodegenerative changes, even in the absence of overt disease. This upregulation may reflect an attempt to cope with accumulating damage, but it may also contribute to a pathological cycle of tau phosphorylation and neuronal dysfunction.
Fig. 3. Gene expression of Cdk5R1 in the hippocampus and cortex of adult cynomolgus monkeys compared with aged monkeys. (a) Group gene expression. (b) Individual gene expression. Values are presented as means error bars=SEM. In addition to playing a physiological role in neuroprotection and synaptic plasticity in the mammalian brain, CAPN1 is involved in the proteolysis of tau kinases like GSK3β and CDK5R1 (Kurbatskaya et al., 2016; Mahaman et al., 2019; Su et al., 2020). The regulation of the activation of calpains, which are involved in numerous cell life processes, is impaired with advancing age, elevated oxidative stress, and other excitotoxicity. The increased activation of these proteases implicates their involvement in the pathogenesis of several illnesses, including neurodegenerative diseases like AD (Mahaman et al., 2019). According to the findings of this study, older cynomolgus monkeys had greater CAPN1 gene expression levels of CAPN1 in the cortex than adults did. This is due to the possibility that numerous physiological processes, such as increased oxidative stress, have taken in older adults cynomolgus monkeys, which may have an impact on the activation increase. Aging is associated with increased oxidative stress and calcium dysregulation, which can activate the synthesis of CAPN1. Higher expression of CAPN1 can lead to increased cleavage of various substrates, including the CDK5 activators p35 to p25, which can then abnormally activate CDK5. This response may be a response to increased cellular damage or an attempt to manage protein aggregates common in aging brains. The GSK3 kinase that eliminates Ser21/9, GSK3β, can be activated by calpain at its N-terminal portion (Hoffmeister et al., 2020). According to the findings indicating an increase in calpain and GSK3β activity in the cortex region of aged animals, there is a positive link between CAPN1 and GSK3β in the brains of elderly cynomolgus monkeys and calpain-mediated proteolysis. Numerous players participate in neurogenerative processes, such as GSK3β’s cleavage and tau phosphorylation. Calpain 1 may be responsible for the up-regulation of GSK3β, abnormal hyperphosphorylation of tau, and neurofibrillary degeneration in AD (Jin et al., 2015). Therefore, it seems likely that in aged cynomolgus monkeys, tau phosphorylation occurs as a result of gradually increasing GSK3β expression, which confirms the findings of Lauretti and co-workers that GSK3β expression level increases with advancing age (Lauretti et al., 2020). The key component of the helical filament pairs is the phosphorylated tau protein, which can possibly lead to the development of paired helix filaments if GSK3β levels rise (Rankin et al., 2007). The GSK3β gene is involved in a variety of activities, including hyperphosphorylation, memory loss, increased amyloid-beta formation, inflammation, and apoptosis mediators, directly causing the loss of neurons in AD (Rankin et al., 2007). CDK5R1 is a CDK5/p25 activator that may play a role in AD-related nerve degeneration. The protease calpain converts p35/CDK5R1 to CDK5/p25, which may have a detrimental effect on AD by activating CDK5 and tau phosphorylation (Rankin et al., 2007). Older cynomolgus monkeys have higher CAPN1 and CDK5R1 gene expression in the cortex than do younger monkeys. Accumulation of p25/CDK5, which may have a role in the etiology of cytoskeletal abnormalities and neuronal death in neurodegenerative illnesses, has been reported after increased expression of the CDK5R1/p35 gen (Cooper-Patrick et al., 2024). It has been suggested that as long as NHPs are allowed to age naturally, they are somewhat resistant to the development of tau pathology (Jester et al., 2022). However, the findings of the present study demonstrate that gene expression in aged cynomolgus monkeys (>20 years) is increased in the cortex region for the three genes we examined. Research utilizing different gene markers and cynomolgus monkeys older than 30 years is required to determine whether other regulatory genes are involved in the creation of neurofibrillary tangles. Although this study successfully utilized qRT-PCR methods to analyze the expression of CAPN1, CDK5R1, and GSK3β in the cortex of aged and adult monkeys, it was limited by the lack of protein-level validation and functional assays to directly correlate gene expression changes with tau phosphorylation and neuronal health. The protein expression analysis and functional experiments is needed for the future study to provide a comprehensive understanding of the molecular mechanisms driving these changes in the aging brain. ConclusionIn summary, the higher expression of CAPN1, CDK5R1, and GSK3β in the cortex of aged monkeys suggests a complex interplay between aging, cellular stress responses, and potential early neurodegenerative changes. These gene expression changes may contribute to altered tau phosphorylation and other protein modifications that influence neuronal health and function in the aging brain. Further studies are needed to elucidate the precise mechanisms and consequences of these changes, which could provide new insights into the molecular understanding of brain aging and neurodegenerative diseases. AcknowledgmentsThe authors thank Yumni K Ghasani M.Si, DVM, Dyah Setyawati M.Si, DVM, Dr (med) Irma H. Soeparto MS, and Prof Steven J Scaphiro for their technical assistance in research preparation. This study was supported by the Research and Productive Innovation (RISPRO) LPDP program (Contract Id. PRJ/29/LPDP/2019). Conflict of interestThe authors declare no conflict of interest. FundingThis study was supported by the Research and Productive Innovation (RISPRO) LPDP program (Contract Id. PRJ/29/LPDP/2019). Author’s contributionsStudy design and supervised research: HSD, SSM, US, and LR. Sample collection and facility: HSD, US, and LR. Laboratory work and manuscript writing: HSD, LR, SSM, US, and JH. Manuscript revised by J. H. All authors have read and approved the final manuscript. Data availabilityAll data were provided in the manuscript. ReferencesAlzheimer’s Disease Association. 2024. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 20(5), 3708–3821. Chen, Z.Y. and Zhang, Y. 2022. Animal models of Alzheimer’s disease: applications, evaluation, and perspectives. Zool Res. 43(6), 1026–1040. Cooper-Patrick, L., Gallo, J.J., Gonzales, J.J., Vu, H.T., Powe, N.R., Nelson, C. and Ford, D.E. 1999. Race, gender, and partnership in the patient–physician relationship. JAMA J. Amr. Med. Ass. 282(6), 583–589. Darusman, H.S., Gjedde, A., Sajuthi, D., Schapiro, S.J., Kalliokoski, O., Kristianingrum, Y.P., Handaryani, E. and Hau, J. 2014a. Amyloid beta1-42 and the phoshorylated tau threonine 231 in brains of aged cynomolgus monkeys (Macaca fascicularis). Front. Aging Neurosci. 6, 313. Darusman, H.S., Pandelaki, J., Mulyadi, R., Sajuthi, D., Putri, I.A., Kalliokoski, O.H., Call, J., Abelson, K.S., Schapiro, S.J., Gjedde, A. and Hau, J. 2014b. Poor memory performance in aged Cynomolgus monkeys with hippocampal atrophy, depletion of amyloid beta 1-42 and accumulation of Tau proteins in cerebrospinal fluid. In Vivo 28(2), 173–184. Darusman, H.S., Saepuloh, U., Mariya, S.S., Sajuthi, D., Schapiro, S.J., and Hau, J. 2021. Increased expression of GAPDH in Cynomolgus monkeys with spontaneous cognitive decline and amyloidopathy reminiscent of an Alzheimer’s-type disease is reflected in the circulation. Am. J. Primatol. 83(11), e23296. Hoffmeister, L., Diekmann, M., Brand, K. and Huber, R. 2020. GSK3: a kinase balancing promotion and resolution of inflammation. Cells 9(4), 820. Holper, S., Watson, R. and Yassi, N. 2022. Tau as a biomarker of neurodegeneration. Int. J. Mol. Sci. 23, 7307. Jellinger, K.A. 1998. The neuropathological diagnosis of Alzheimer disease. In: Jellinger, K., Fazekas, F., Windisch, M. (eds) Ageing and dementia. J. Neur. Trans. 53, 97–118. Jester, H.M., Gosrani, S.P., Ding, H., Zhou, X., Ko, M.C. and Ma, T. 2022. Characterization of early Alzheimer’s disease-like pathological alterations in non-human primates with aging: a pilot study. J. Alzheimers Dis. 88(3), 957–970. Jiang, Z., Wang, J., Qin, Y., Liu, S., Luo, B., Bai, F., Wei, H., Zhang, S., Wei, J., Ding, G., Ma, L., He, S., Chen, R., Sun, Y., Chen, Y., Wang, L., Xu, H., Wang, X., Chen, G. and Lei, W. 2024. A nonhuman primate model with Alzheimer’s disease-like pathology induced by hippocampal overexpression of human tau. Alzheimers Res. Ther. 16(1), 22. Jin, N., Yin, X., Gu, J., Zhang, X., Shi, J., Qian, W., Ji, Y., Cao, M., Gu, X., Ding, F., Iqbal, K., Gong, C.X. and Liu, F. 2015. Truncation and activation of dual specificity tyrosine phosphorylation-regulated kinase 1A by Calpain I: a molecular mechanism linked to tau pathology in Alzheimer disease. J. Biol. Chem. 290(24), 15219–15237. Kabir, M.T., Uddin, M.S., Setu, J.R., Ashraf, G.M., Bin-Jumah, M.N. and Abdel-Daim, M.M. 2020. Exploring the role of PSEN mutations in the pathogenesis of Alzheimer’s disease. Neurotox Res. 38(4), 833–849. Kurbatskaya, K., Phillips, E.C., Croft, C.L., Dentoni, G., Hughes, M.M., Wade, M.A., Al-Sarraj, S., Troakes, C., O’Neill, M.J., Perez-Nievas, B.G., Hanger, D.P. and Noble, W. 2016. Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer’s disease brain. Acta Neuropathol. Commun. 4, 34 Lauretti, E., Dincer, O. and Praticò, D. 2020. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 1867(5), 118664. Lipper, C.H., Egan, E.D., Gabriel, K.H. and Blacklow, S.C. 2023. Structural basis for membrane-proximal proteolysis of substrates by ADAM10. Cell 186(17), 3632–3641.e10. Mahaman, Y.A.R., Huang, F., Kessete, A.H., Maibouge, T.M.S., Ghose, B. and Wang, X. 2019. Involvement of calpain in the neuropathogenesis of Alzheimer’s disease. Med. Res. Rev. 39(2), 608–630. Park, S.J., Kim, Y.H., Nam, G.H., Choe, S.H., Lee, S.R., Kim, S.U., Kim, J.S., Sim, B.W., Song, B.S., Jeong, K.J., Lee, Y., Park, Y.I., Lee, K.M., Huh, J.W. and Chang, K.T. 2015. Quantitative expression analysis of APP pathway and tau phosphorylation-related genes in the ICV STZ-induced non-human primate model of sporadic Alzheimer’s disease. Int. J. Mol. Sci. 16(2), 2386–2402. Paspalas, C.D., Carlyle, B.C., Leslie, S., Preuss, T.M., Crimins, J.L., Huttner, A.J., van Dyck, C.H., Rosene, D.L., Nairn, A.C. and Arnsten, A.F.T. 2018. The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement. 14(5), 680–691. Rankin, C.A., Sun, Q. and Gamblin, T.C. 2007. Tau phosphorylation by GSK-3beta promotes tangle-like filament morphology. Mol. Neurodegener. 2, 12. Rosmanah, L., Saepuloh, U., Mariya, S.S, Suparto, I.H., Manalu, W., Winarto, A., and Darusman, H.S. 2024. Expression of APP, CDK5, and AKT1 gene related to Alzheimer disease in brain of long-tailed macaques. HAYATI J. Biosci. 31(1), 145–152. Spreafico, M., Grillo, B., Rusconi, F., Battaglioli, E. and Venturin, M. 2018. Multiple layers of CDK5R1 regulation in Alzheimer’s disease implicate long non-coding RNAs. Int. J. Mol. Sci. 19(7), 2022. Stonebarger, G.A., Bimonte-Nelson, H.A., and Urbanski, H.F. 2021. The rhesus macaque as a translational model for neurodegeneration and Alzheimer’s disease. Front. Aging Neurosci. 13, 734173. Su, Y., Fu, J., Yu, J., Zhao, Q., Guan, Y., Zuo, C., Li, M., Tan, H. and Cheng, X. 2020. Tau PET imaging with [18F]PM-PBB3 in frontotemporal dementia with MAPT mutation. J. Alzheimers. 76(1), 149–157. Swindler, D.R. 2002. Primate dentition: an introduction to the teeth of non-human primates. Cambridge, MA: Cambridge University Press. Yang, J., Zhi, W. and Wang, L. 2024. Role of tau protein in neurodegenerative diseases and development of its targeted drugs: a literature review. Molecules 29(12), 2812. | ||
| How to Cite this Article |
| Pubmed Style Darusman HS, Rosmanah L, Mariya SS, Saepuloh U, Yuliana Y, Hau J. Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment. Open Vet. J.. 2025; 15(3): 1150-1156. doi:10.5455/OVJ.2025.v15.i3.7 Web Style Darusman HS, Rosmanah L, Mariya SS, Saepuloh U, Yuliana Y, Hau J. Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment. https://www.openveterinaryjournal.com/?mno=228287 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.7 AMA (American Medical Association) Style Darusman HS, Rosmanah L, Mariya SS, Saepuloh U, Yuliana Y, Hau J. Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment. Open Vet. J.. 2025; 15(3): 1150-1156. doi:10.5455/OVJ.2025.v15.i3.7 Vancouver/ICMJE Style Darusman HS, Rosmanah L, Mariya SS, Saepuloh U, Yuliana Y, Hau J. Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1150-1156. doi:10.5455/OVJ.2025.v15.i3.7 Harvard Style Darusman, H. S., Rosmanah, . L., Mariya, . S. S., Saepuloh, . U., Yuliana, . Y. & Hau, . J. (2025) Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment. Open Vet. J., 15 (3), 1150-1156. doi:10.5455/OVJ.2025.v15.i3.7 Turabian Style Darusman, Huda S., Lis Rosmanah, Sela S. Mariya, Uus Saepuloh, Yuliana Yuliana, and Jann Hau. 2025. Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment. Open Veterinary Journal, 15 (3), 1150-1156. doi:10.5455/OVJ.2025.v15.i3.7 Chicago Style Darusman, Huda S., Lis Rosmanah, Sela S. Mariya, Uus Saepuloh, Yuliana Yuliana, and Jann Hau. "Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment." Open Veterinary Journal 15 (2025), 1150-1156. doi:10.5455/OVJ.2025.v15.i3.7 MLA (The Modern Language Association) Style Darusman, Huda S., Lis Rosmanah, Sela S. Mariya, Uus Saepuloh, Yuliana Yuliana, and Jann Hau. "Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment." Open Veterinary Journal 15.3 (2025), 1150-1156. Print. doi:10.5455/OVJ.2025.v15.i3.7 APA (American Psychological Association) Style Darusman, H. S., Rosmanah, . L., Mariya, . S. S., Saepuloh, . U., Yuliana, . Y. & Hau, . J. (2025) Expression profiles of the Tau-associated genes GSk3β, CAPN1, and CDK5R1 in the brain cortex of aged female cynomolgus monkeys with cognitive impairment. Open Veterinary Journal, 15 (3), 1150-1156. doi:10.5455/OVJ.2025.v15.i3.7 |