| Research Article | ||
Open Vet. J.. 2025; 15(6): 2374-2385 Open Veterinary Journal, (2025), Vol. 15(6): 2374-2385 Research Article Therapeutic effects of single-clove garlic extract on nicotine-induced inflammation and histopathological changes in ratsMaya Nurwartanti Yunita1,2*, Hani Plumeriastuti3, Wiwiek Tyasningsih4, Bodhi Agustono1,2, Andhika Lutfhi Hardiono1, Gabrian Wahyu Prasetyanuri1, Shofia Shofia1, Reina Puspita Rahmaniar5 and Md. Aliar Rahman61Faculty of Health, Medicine and Life Sciences (FIKKIA), Universitas Airlangga, Surabaya, Indonesia 2Animal Biomedical and Conservation Research Group 3Department of Veterinary Science, Division of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Department of Microbiology, Faculty of Veterinary Medicine, Wijaya Kusuma University, Surabaya, Indonesia 6Department of Animal Nutrition, Faculty of Animal Husbandry, Bangladesh Agricultural University, Mymensingh, Bangladesh *Correspondence to: Maya Nurwartanti Yunita. Faculty of Health, Medicine and Life Sciences (FIKKIA), Universitas Airlangga, Surabaya, Indonesia. Email: mayanurwantanti [at] fkh.unair.ac.id Submitted: 16/11/2024 Revised: 07/05/2025 Accepted: 23/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
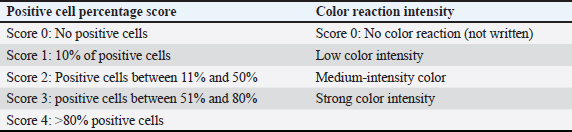
AbstractBackground: Nicotine exposure causes cellular damage by generating reactive oxygen species, leading to inflammation. DNA damage, and diseases such as atherosclerosis. Single-clove garlic extract (SCGE) contains antioxidants such as allicin and flavonoids that may counteract nicotine’s harmful effects by reducing pro-inflammatory cytokines, suggesting SCGE’s potential as a natural therapeutic intervention to support good health. Aim: This study aimed to investigate the effects of SCGE against nicotine-induced pneumotoxicity in rats by assessing its impact on TNF-α, interleukin 6 (IL-6), and structural integrity tracheal, pulmonary, and coronary artery tissues. Methods: The subjects of this study were 30 male rats which were divided into the following 5 treatment groups: T1 (negative control), T2 (3 mg/ml of nicotine), and T3 (3 mg/ml of nicotine and 3.6 mg/day vitamin C), T4; T5; T6 groups (3 mg/ml nicotine and 75; 100; 125 kg/mg body weight of SCGE). A nicotine exposure followed by oral SCGE was administered twice a day consecutively for 14 days. The TNF-α and IL-6 expression was evaluated using immunohistochemical staining. Histopathology of the tracheal and pulmonary organs and coronary arteries was performed using H&E staining. Results: The T4, T5, and T6 groups revealed that TNF-α and IL-6 levels were significantly lower than in T2 (2.90 and 4.72), reducing ciliary damage, epithelial height, and mucosal thickness. The T6 group exhibited significant differences in the coronary arteries (14.97 µm) compared with the T2 (121.36 µm). The mean lumen diameter of the coronary artery of the T4, T5, and T6 groups was increased compared with T2 (49.71 µm). Significant decreases (42,65%) were observed in hemorrhage, alveolar septal thickening, and inflammatory cell infiltration in the T6 compared to T2. Conclusion: In conclusion, SCGE treatment can potentially mitigate pneumotoxicity caused by nicotine, as evidenced by improvements in TNF-α, IL-6, and histopathological damage of the tracheal, pulmonary, and coronary arteries. These findings suggest that SCGE could be explored as a potential natural therapeutic intervention for preventing or treating nicotine-induced lung damage. These findings suggest that SCGE could be explored as a potential natural therapeutic intervention to prevent or treat nicotine-induced lung damage. Further studies could explore the mechanisms underlying SCGE’s anti-inflammatory and antioxidant properties. Keywords: Allium sativum var. solo garlic, Good health, IL-6, Nicotine, Pneumotoxicity, TNF-α. IntroductionThe pervasive and detrimental effects of nicotine exposure at both cellular and systemic levels have been well-documented in the scientific literature. Nicotine, a key component of tobacco products, is known to induce significant cellular damage via the generation of reactive oxygen species (ROS), which subsequently triggers inflammatory responses and DNA damage in animals and humans (Ebersole et al., 2020; Wang et al., 2022). Specifically, nicotine exerts direct harmful effects on pulmonary tissues resulting in respiratory diseases and structural lung damage (Layden et al., 2020). Numerous cases of severe lung disease as a consequence of nicotine toxicity (Arter et al., 2019; Shehata et al., 2023) and animal experiments suggested that nicotine may enhance the risk of lung cancer (Lee et al., 2018). In addition to the respiratory system, nicotine also adversely affects the cardiovascular system, primarily through the development of atherosclerosis. Nicotine exposure significantly elevates pro-inflammatory cytokines, such as TNF-α, interleukin 6 (IL-6), and IL-1, in the lungs, indicating a systemic inflammatory response (Glynos et al., 2018). This response is particularly harmful, as elevated TNF-α levels enhance prothrombotic functions by stimulating leukocyte adhesion molecules, activating endothelial cells, and regulating immune cell activity via macrophage and cytokine stimulation (Urschel and Cicha, 2015). IL-6 also plays a pivotal role in immune and inflammatory responses, regulating T-cell activation and differentiation of B cells while modulating the proliferation of various cells (Yang et al., 2020). Elevated IL-6 levels contribute to increased inflammatory cell (IC) infiltration and promote a cascade of respiratory issues. In the respiratory tract, nicotine exposure damages epithelial cells and promotes chronic respiratory conditions, such as chronic obstructive pulmonary disease (COPD), that further intensify oxidative stress and airway inflammation (Shraideh et al., 2013). Given nicotine’s deleterious effects, there is growing interest in exploring natural therapeutic agents that can mitigate these impacts. Single-clove garlic extract (SCGE) has considerable potential to alleviate the detrimental effects of nicotine, primarily due to its antioxidant and anti-inflammatory properties. The unique structure of single-clove garlic (SCG) includes a single, uniform bulb instead of the segmented cloves seen in multiple garlic (Brewster and Rabinowitch, 2020). SCG contains higher levels of allicin, phenolic compounds, and antioxidants compared to multiple types of garlic. Due to SCG’s unique composition, including a higher concentration of volatile oils and organosulfur compounds, it has a stronger bioactive profile, particularly in terms of antioxidant activity (Subramanian et al., 2020). Among different garlic types, SCG exhibits the highest antioxidant activity because of its enriched volatile compounds and flavonoids, which are essential for combating oxidative stress (Naji et al., 2017). The therapeutic benefits of SCGE are primarily attributable to such as allicin, alliin, saponins, and flavonoids, which have notable anti-inflammatory and antioxidant properties (Khanarmuei et al., 2019). Allicin, an organosulfur compound in SCGE, enhances blood circulation and reduces inflammation. Allicin is formed when non-protein amino acids such as alliin interact with the enzyme alliinase to generate a compound that improves antioxidant activity particularly superoxide dismutase (SOD). This enzyme is crucial in reducing oxidative stress neutralizing ROS and decreasing pro-inflammatory cytokines such as TNF-α (Shang et al., 2019). Research indicates that SCGE significantly reduces oxidative stress and inflammation cytokines, such as TNF-α and IL-6, which are elevated following nicotine exposure, protective effects against oxidative damage (Ziamajidi et al., 2016; Wlosinska et al., 2021). The bioactive compounds in garlic, particularly S-allyl cysteine, play crucial roles in promoting cellular health and reducing apoptosis in stressed cells, further enhancing its protective effects against nicotine (Gómez Sierra et al., 2014; Tsukioka et al., 2017). Moreover, previous studies have shown that garlic extract can modulate the expression of genes involved in detoxification processes, thereby facilitating the elimination of nicotine and its metabolites from the body. Additionally, the high flavonoid content in SCGE contributes to its antioxidant capabilities, which are essential for defending cells against ROS-induced damage, especially in nicotine-exposed tissues (Nasr and Saleh, 2014; Ziamajidi et al., 2016). This comprehensive antioxidant mechanism underscores the potential of garlic extract as a natural therapeutic agent to mitigate the adverse effects of nicotine exposure. The unique composition of SCG, which is rich in organosulfur compounds and allicin, underscores its bioactive profile, making it a potent agent for managing oxidative stress-related conditions (Tran et al., 2018; Gruhlke et al., 2019). As interest in herbal therapies increases, the effects of SCGE highlight the significance of plant-based antioxidants in combating environmental toxins and promoting health (Moodley et al., 2015; Metwally et al., 2018). This study is the first to explore the potential of SCGE in reducing the negative effects of nicotine exposure in animal models. The findings suggest that SCGE can mitigate pneumotoxicity and reduce tissue damage by lowering inflammatory markers, particularly TNF-α and IL-6. These markers are key mediators of nicotine-induced inflammation. SCGE indicates the potential to protect against oxidative stress. Herbal therapies have gained interest in both traditional and modern medicine, and the promising effects of SCG underscore the of plant-based antioxidants in combating environmental toxins. The high antioxidant and anti-inflammatory properties of SCGE can be used as a protective mechanism against nicotine-induced damage in pulmonary and cardiovascular tissues. By targeting pro-inflammatory cytokines and reducing oxidative stress, SCGE has the potential therapeutic approach to reduce the harmful effects of nicotine. The findings of this study support further exploration of SCGE as a natural remedy for managing inflammatory responses in oxidative stress, offering hope to individuals exposed to environmental toxins or those seeking herbal alternatives, and managing respiratory and cardiovascular health. Materials and MethodsEthical approvalThis study was approved by the Health Research Ethics Clearance Commission of Universitas, Airlangga, Indonesia (Approval No. 049/HRECC). (FODM/II/2022). Euthanasia was injected with a combination of Xylazine [1 mg/kg body weight (BW) and Ketamine HCl (8 mg/ kg BW)] intramuscularly. After the mice showed signs of adequate anesthesia, such as loss of response to stimuli and pedal reflexes, euthanasia was continued using the cervical dislocation method (American Veterinary Medical Association, 2020). Plant identificationAllium sativum var. solo garlic plants were identified at the UPT Herbal Materia Medica Batu in Malang, East Java. The results were recorded in the letter of identification Number 074/027/102.20-C/2022. Characteristics of A. sativum var solo garlicAllium sativum var. solo garlic contains only one clove per bulb, unlike other garlic varieties. Several aspects: The outer skin of the SCG bulb is white, like paper, and has a bulb diameter of ˜ 3.3 – 3.8 cm. Compared with the multiclove ordinary garlic, the SCG is distinguished by a richer flavor and fragrant taste, as well as greater nutritional and therapeutic benefits. Preparation of A. sativum var. solo garlic extractApproximately 3,000 g of SCG was used in this study. The garlic bulbs were peeled off, cleaned, and then chopped to a thickness as thin as possible to speed up the drying process. The thin slices were then pulverized in a blender and sieved through a sieve no. 40 to obtain garlic powder; 150 g of the SCG powder was added to 450 ml of 96% ethanol, covered, and left for 3 days with occasional stirring. Next, the garlic dregs were separated, collected, and further macerated in 450 ml of 96% ethanol solvent for 2 days and then filtered. A rotary evaporator was used to concentrate the immersion extract at 50□ and 150 rpm for 20 minutes. The SCG extract was then re-evaporated in a water bath until thickened. Experimental animalsThe experimental animals used were healthy male Rattus norvegicus Sprague–Dawley (WT) white rats aged 3–4 months and weighing 150–200 g. In this study, we used 30 rats. The rats were kept in cages for an adaptation period of 1 week and were given water ad libitum. After completing the adaptation period, we divided 30 rats into treatment groups as follows: T1 consisted of rats provided feed only and without exposure to SCGE or nicotine; T2 group included rats exposed to nicotine at a concentration of 3 mg/ml; T3 group comprised rats exposed to nicotine at 3 mg/ml and supplemented with vitamin C at a dose of 3.6 mg/ day; and T4, T5, and T6 group rats exposed to nicotine at 3 mg/ml and treated with SCGE at doses of 75, 100, and 125 mg/kg BW, respectively. The e-liquid used in this study contained nicotine at the concentration of 3 mg/ml (Goniewicz et al., 2015). The Chemnovatic liquid nicotine (1 ml) was measured using a syringe to determine the amount of treatment needed to be homogeneous. An e-cigarette that had been dripped with 1 ml of e-liquid and was lit and automatically stopped after 10 seconds rats exposed to nicotine was left in the treatment cage for 4 minutes for maximal e-cigarette vapor extraction. The nicotine-induced and SCGE treatment groups in rats were administered twice a day for 14 days. After the completion of the treatment period, the animals were euthanized. The euthanized rats were subjected to necropsy to determine the tracheal, pulmonary, and coronary organs. The organs were fixed in 10% formalin buffer solution for further preparation of histopathological specimens and immunohistochemical observations of TNF-α and IL-6 expression. Immunohistochemical observationImmunohistochemical analysis of lung and heart tissues was performed to evaluate the expression of TNF-α and IL-6. IHC is highly specific, as it allows the detection of target proteins (e.g., TNF-α and IL-6 at the cellular level within tissue sections). In this study, TNF-α and IL-6 were critical markers of inflammation and immune response, particularly in lung tissue affected by nicotine exposure. By using specific antibodies against these cytokines, IHC enables precise localization, ensuring that researchers can determine the exact cells or regions in which these proteins are expressed in response to nicotine-induced inflammation (Strzelak et al., 2018). Observations were conducted using a Nikon Eclipse-type Ei light microscope at 400× magnification, capturing images for analysis. The expression levels of TNF-α and IL-6 were semi-quantitatively assessed using a modified Remmele Scale Index (IRS) as can be observed in Table 1, calculated by multiplying the percentage of positive cells (A) by the intensity of the color and the reaction (B) (Eshraghian et al., 2013). Additionally, P-glycoprotein expression was rated at 9.5 across five fields of view, providing insights into the inflammatory response in these organs (Fasinu et al., 2012; Arifah et al., 2020). Tracheal observationsHistopathological examination of the tracheal organs was conducted using a trinocular microscope. Capturing images at 400× magnification across five fields of view. The key variables assessed were deciliation, epithelial height, and tracheal mucosa thickness (TMT). The deciliation was scored on a scale from 0 to 3 based on the percentage of the visual field that was deciliate, with 0 indicating no deciliation and 3 indicating over 60% deciliation (Wlosinska et al., 2021). The epithelial height and mucosal thickness were measured using the microscope imaging software Nikon Instrument NIS-Elements (RRID: SCR_014329) and a trinocular microscope (Nikon Eclipse type Ei). The deciliation score can be linked as follows: to various functional or biochemical metrics, such as mucociliary clearance efficiency and inflammatory markers to evaluate the overall effects of damage (Perea et al., 2024). Pulmonary observationsThe pulmonary organs were examined using a trinocular microscope at 400× magnification to capture histopathological images across five fields of view. The assessment focused on three key parameters: hemorrhage, IC infiltration, and interalveolar thickening septa. Each parameter was scored based on the extent of damage observed established by Hansel and Barnes as can be observed in Table 3. (Eshraghian et al., 2013). Histopathological analysis of lung tissue focuses on assessing the degree and severity of organ damage by examining specific structural and cellular alterations (Table 2). Increasing hemorrhagic scores indicate greater vascular injury, indicating more severe mechanical or pathological disruption of the pulmonary capillaries (Krause et al., 2012). The degree of inflammatory infiltration indicates immune response activation, with elevated scores being linked to increased inflammation and potential tissue damage. Thickening signifies structural alteration of the alveolar walls is frequently linked to inflammatory and fibrotic activity (Savin et al., 2022). Coronary arteryThe method used to observe coronary artery histopathological preparations quantitative measurements of wall thickness and lumen diameter (LD). In a Nikon Eclipse Trinocular microscope at 400× magnification, wall thickness was assessed at eight distinct positions, and the LD was measured at four positions, with the results averaged for accuracy (Arifah et al., 2020). The measurement using the microscope imaging software Nikon Instrument NIS-Elements (RRID: SCR_014329) and a trinocular microscope to provide representative values for the observed artery. Variations in the thickness around the circumference may indicate abnormalities. A narrowed LD may indicate stenosis due to plaque, atherosclerosis, thrombus, or arterial constriction. The comparison of LD with wall thickness provides a complete assessment of vascular pathology (Wentzel et al., 2003). Table 1. Semi-quantitative scale of the IRS.
Table 2. Score for assessing the degree of damage to pulmonary organs.
Table 3. Score for assessing damage to the histopathology of the pulmonary organs of experimental animals (Hansel and Barnes, 2003).
Statistical analysisResults were expressed using means and standard deviations. The Shapiro–Wilk test data were analyzed using SPSS (version 22.0; IBM Corp., NY). If the data were not distributed normally, then a one-way parametric analysis of variance was performed, and if the data were not normally distributed, a non-parametric analysis of variance was used. Duncan’s parametric test was performed if a significant difference (p < 0.05) to determine the variation in TNF- and IL-6 levels. The non-parametric Kruskal–Wallis test was used to determine differences between treatment groups histopathological parameters of the tracheal, pulmonary, and coronary arteries. Any significant differences between treatment groups as determined by the Kruskal–Wallis test and the Mann–Whitney U test. To assess the correlation between non-normally distributed variables, Spearman’s rank correlation coefficient was also calculated. This nonparametric method measures the monotonic relationship between two variables, regardless of their distribution. A significant Spearman’s correlation (p < 0.05) indicates a monotonic association between variables. ResultsTNF-α and IL-6 expression in pulmonary and cardiac tissueThe expression of TNF-α in pulmonary tissue was measured using the post hoc Duncan test, as presented in Table 4. At T2, IL-6 levels increased by 100% compared to T1 and represented the highest value observed, whereas at T6, the treatment successfully reduced it by 87%, approaching normal levels. Similarly, TNF-α levels at T2 increased by 100% from T1, and the T6 treatment reduced it by 70%, nearly returning to control levels, as shown in Figure 3. Tracheal histopathologyThe administration of SCG extracts notably improved the tracheal histopathology of nicotine-exposed groups (T4, T5, and T6) as can be observed from Figure 1. T1 had the lowest score (0.10 ± 0.308), whereas T2 exhibited the highest (2.85 ± 0.366), indicating a dramatic increase in deciliation damage as can be observed in Table 5. SCG also reduces the tracheal deciliation and epithelial thinning, T2 exhibited a severe reduction in tracheal epithelial height (TEH) (41% lower than T1) and a significant thickening of the TMT (148% than T1), whereas T6 most effectively recovered with epithelial height and mucosal thickness nearly returning to control levels. Table 4. Observations and scoring of immunohistochemical changes in IL-6 and TNF-α expression in heart and lung tissues.
Pulmonary histopathologyPulmonary parameters (alveolar septa thickness, hemorrhage, IC infiltration) were evaluated in Table 5. T2 showed the most severe damage (96.53), which was approximately 213% higher than that of control T1 (30.88). T6 and T3 had the best therapeutic effects, reducing damage to 42.65 and 43.48, respectively. These patients had significantly lower alveolar septal thickness, hemorrhage, and inflammation (p < 0.05) as can be observed in Figure 2. Coronary artery histopathologyCoronary artery wall thickness and LD are presented in Table 5. T2 exhibited the highest coronary artery wall thickening (59% higher than T1) and severe lumen narrowing (52% reduction compared with T1), whereas T6 effectively normalized the wall thickness and restored the LD to control levels. Spearman correlationThe Spearman correlation analysis revealed several significant relationships among the measured parameters. Strong positive correlations were observed between IC presence and pulmonary damage (PD) (0.97) and between septal thickening and PD (0.91) as can be observed in Table 6, indicating that these factors are closely linked to the progression of pulmonary conditions. Additionally, TMT and tracheal deciliation scoring (TDS) exhibit a strong positive correlation (0.88) suggesting that mucosal changes were associated with deciliation severity. Conversely, TEH showed strong negative correlations with TMT (−0.89) and TDS (−0.88), highlighting the inverse relationship between epithelial integrity and tracheal damage. Increased mucosal thickness and severe cilia damage are associated with a reduction in TEH as can be observed in Figure 4.
Fig. 1. Histopathological description of the tracheal organ, deciliation parameters in each treatment. Sign (→) deciliation. (1,000× magnification) T1 (rats were only given feed without an SCGE and nicotine), T2 (exposure with 3 mg/ml nicotine), and T3 (exposure with 3 mg/ml nicotine and vitamin C of 3.6 mg/day). T4 (exposure to 3 mg/ml nicotine and given a SCGE of 75 mg/kg BW), T5 (exposure with 3 mg/ml nicotine and an SCGE of 100 mg/kg BW), and T6 (exposure with 3 mg/ml nicotine and an SCGE of 125 mg/kg BW). Table 5. Observation and scoring of histopathological preparations of tracheal, pulmonary, and coronary arteries.
Wall thickness (WT) and LD display weaker correlations with other parameters indicating their relatively independent roles. These findings underscore the interconnected nature of pulmonary and tracheal parameters, providing insights into the pathological mechanisms at play. DiscussionNicotine exposure has long been associated with significant pulmonary and cardiovascular systems. PD results from nicotine’s direct impact on respiratory organs impaired lung function and structural integrity, thereby compromising overall respiratory health (Krishnasamy et al., 2020). One major mechanism through which nicotine harms the lungs is the promotion of oxidative stress and inflammation. These processes damage the ciliated epithelial cells in the airways, leading to resulting in reduced mucociliary clearance and increased vulnerability to COPD and pulmonary hypertension (Shang et al., 2019). This study aims to investigate the potential therapeutic effects of SCGE, in bioactive compounds to mitigate the negative impacts of nicotine. Notably, SCG treatment to a dose-dependent reduction in inflammatory markers TNF-α and IL-6 (observed in groups T4, T5, and T6). This decrease was significant because these cytokines play central roles in mediating inflammatory responses. By attenuating the levels of TNF-α and IL-6, SCG demonstrates a protective effect that curtails oxidative stress and inflammation, which are key contributors to the structural and functional decline of respiratory tissues.
Fig. 2. Histopathology of the pulmonary organs of white rats after being exposed to nicotine, H: Hemorraghi; PS: The thickening of the alveolar septa is indicated by arrows (↔); SR: IC infiltration; Staining: HE (400× magnification). Pulmonary organ damage in rats exposed to nicotine was measured by observing pulmonary parameters (thickening of the alveolar septa, hemorrhage, and IC infiltration). These measurements revealed that the SCG Extract treatment groups T4 (D), T5 (E), and T6 (F) showed significant decreases compared to group T2 (B). The impact of nicotine extends to cardiovascular health, with atherosclerosis being one of the most serious nicotine-induced conditions. Atherosclerosis is characterized by arterial wall thickening the accumulation of lipids, fibrous tissue, and calcium caused by chronic oxidative stress and inflammation (Leyva-López et al., 2016). This study found that SCG extract administration promoted improved cardiovascular parameters, specifically, increased coronary artery LD treated with SCG (groups T4, T5, and T6). Allicin, a key sulfur-containing compound in garlic, is believed to be the main driver of these effects. Allicin’s potent antioxidant properties facilitate vasodilation by reducing ROS levels and increasing glutathione production promoting vascular health and function (Nadeem et al., 2021). The findings suggest that SCG’s ability to counteract oxidative damage and enhance endothelial function may help prevent or mitigate the occurrence of the development of atherosclerosis and associated cardiovascular conditions. At the cellular level, SCG’s protective effects of SCG were evident in the trachea, and lung tissue exposure disrupts the structural integrity of ciliated epithelial cells, leading to cilia loss and impaired mucociliary clearance. This damage can trigger a cascade of events leading to chronic inflammation increased susceptibility to infections and eventual development of COPD and pulmonary hypertension (Hirayama and Nakase, 2017). The current study revealed that rats treated with SCG extract showed that histopathological improvement was observed in the tracheal and lung tissue compared with the untreated controls. The antioxidant effects of SCG are attributed to its high alliin levels, which enzymatic conversion, form allicin. Allicin stimulates antioxidant enzyme activity, including SOD, glutathione peroxidase, catalase, glutathione S-transferase (Kano et al., 2019). These enzymes play crucial roles in neutralizing oxidative damage, thereby protecting delicate respiratory structures from nicotine-induced degradation. The therapeutic properties of SCGE are substantiated by its anti-inflammatory effects, which are consistent with the initial hypothesis that SCGE can mitigate nicotine-induced inflammatory responses. Nicotine induces oxidative stress, leading to the release of pro-inflammatory cytokines, such as IL-8, activate immune cells, including macrophages and neutrophils (Alexy et al., 2022). This inflammatory cascade results in the accumulation of immune cells in the lung tissue and contributes to pathological changes, such as alveolar thickening (Lobo et al., 2010). Organosulfur compounds, particularly allicin, play critical roles in countering these effects down regulating the cyclooxygenase-2 and inducible nitric oxide synthase pathways, thereby reducing the production of inflammatory mediators, such as prostaglandin E-2 and nitric oxide (Park et al., 2014). Furthermore, the presence of polysaccharides in garlic enhances its immunomodulatory effects, effectively limiting the production of pro-inflammatory cytokines such as IL-6 and TNF-α, which are crucial for managing systemic inflammation (Recinella et al., 2021). Similar studies have shown that aged red garlic extract suppresses lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages and alleviates acute pulmonary inflammation by promoting heme oxygenase-1 induction (Park et al., 2012). Shang et al. (2019) reported that quercetin has dose-dependent effects on reducing lung inflammation and oxidative stress. Their research indicated that quercetin mitigates oxidative stress and inflammation caused by ROS by inhibiting the production of NOX2 (Sul and Ra, 2021). These findings underscore SCGE’s potential as a therapeutic agent against nicotine-induced damage attenuating oxidative stress and inflammation. Furthermore, SCGE’s bioactive components inhibit NFκB signaling, which upregulates nicotine and leads to decreased levels of key cytokines α and IL-6. The polysaccharides in SCGE also modulate cytokine production, thereby limiting pro-inflammatory cytokines and thereby controlling systemic inflammation (Arreola et al., 2015). In addition, by downregulating NFκB signaling, SCGE effectively reduced the expression of inflammatory cytokines, supporting the hypothesis that SCGE can reduce chronic inflammation and related diseases (Zamani Taghizadeh Rabe et al., 2015). The study findings demonstrate a reduction in leukocyte accumulation and inflammatory responses supporting the hypothesis that SCGE protects against nicotine-induced damage. By mitigating oxidative stress and inflammation, SCGE is a promising natural intervention for respiratory and cardiovascular health.
Fig. 3. Immunohistochemistry (A) TNF-α expression of pulmo in each treatment; (B) IL-6 expression of heart in each treatment (400× magnification). Dark blue arrow has strong color reaction; Light blue arrow has moderate color reaction; and Yellow arrow has no color reaction. T1 (rats were only given feed without an SCGE and nicotine), T2 (exposure with 3 mg/ml nicotine), and T3 (exposure with 3 mg/ml nicotine and vitamin C of 3.6 mg/day). T4 (exposure with 3 mg/ml nicotine and an SCGE of 75 mg/kg BW), T5 (exposure with 3 mg/ml nicotine and an SCGE of 100 mg/kg BW), and T6 (exposure with 3 mg/ml nicotine and an SCGE of 125 mg/kg BW). Table 6. Spearman correlation of histopathological preparations of tracheal, pulmonary, and coronary arteries.
Fig. 4. Heatmap visualization of the Spearman correlations. Note: Wall Thickness (WT); Lumen Diameter (LD); Hemorrhages Pulmo (HP); Inflammatory Cell on Pulmo (ICP); Septa Thickening Pulmo (STP); Pulmonary Damage (PD); Tracheal Epithelial Height (TEH); Tracheal Mucosa Thickness (TMT); Tracheal Deciliation (TD). However, several limitations of this study must be acknowledged. The relatively small sample size may limit the statistical power and generalizability of the findings. Additionally, the 14-day treatment duration cannot capture the long-term effects of SCGE on nicotine-induced tissue damage and inflammatory responses. There is also the possibility of observer bias in histopathological examinations. Scoring systems include subjective evaluations despite the need for standardization. Overall, the results emphasize the potential clinical applications of SCGE as a therapeutic agent for managing nicotine induced by oxidative stress and inflammation. Despite these limitations, this study provides compelling evidence of SCGE’s potential as a natural intervention for managing oxidative stress and inflammation. Future research should focus on long-term studies to evaluate the sustained efficacy and safety of SCGE in chronic nicotine exposure models. Investigation of the modulation of molecular pathways by SCGE bioactive compounds could further elucidate its therapeutic mechanisms. Moreover, exploring SCGE’s synergistic effects with other natural antioxidants may enhance its therapeutic potential, paving the way for its application in human clinical settings. Practical developments, such as SCGE-based supplements or dietary interventions, have promise for public health, especially populations at risk of nicotine or toxin exposure. SCGE demonstrates significant potential as a natural therapeutic agent that mitigates oxidative stress and inflammation induced by nicotine exposure, particularly in the respiratory and cardiovascular systems. Further research is required to explore its long-term efficacy and the underlying molecular mechanisms, paving the way for practical applications in public health. Conflict of interestThe authors declare that they have no competing interests. FundingThe authors did not receive any funds for this study. Authors’ contributionThe authors are grateful to the Faculty of Health, Medicine, and Life Sciences for providing animal enclosures for this study. M.N.Y.: Conceptualization, methodology, data analysis, and writing of the original draft. H.P., B.A., A.L.H., G.W.P, S., M.A.R., and R.P.R.: Contributed to the conceptualization and methodology, conducted the study, and revised the manuscript. All authors have read and approved the manuscript, the final version of the manuscript. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesAlexy, T., Detterich, J., Connes, P., Toth, K., Nader, E., Kenyeres, P., Arriola-Montenegro, J., Ulker, P. and Simmonds, M.J. 2022. Physical properties of blood and their relationship to clinical conditions. Front. Physiol. 13, 906768. American Veterinary Medical Association. 2020. AVMA Guidelines for the Euthanasia of animals, 2020 ed. Schaumburg (IL): American Veterinary Medical Association. Arifah, S.N., Atho’illah, M.F., Lukiati, B. and Lestari, S.R. 2020. Herbal medicine using single-cleve garlic oil extracts ameliorate hepatic steatosis and oxidative status in high fat diet mice. Malaysian J. Med. Sci. 27(1), 46–56. Arreola, R., Quintero-Fabián, S., Ivette López-Roa, R., Flores-Gutiérrez, E.O., Reyes-Grajeda, J.P., Carrera Quintanar, L. and Ortuño-Sahagún, D. 2015. Immunomodulatory and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 401630. Arter, Z.L., Wiggins, A., Hudspath, C., Kisling, A., Hostler, D.C. and Hostler, J.M. 2019. Acute eosinophilic pneumonia following electronic cigarette use. Respir. Med. Case. Rep. 27, 100825; doi:10.1016/j.rmcr.2019.100825 Brewster, J.L. and Rabinowitch, H.D. 2020. Garlic agronomy. In Onions allied Crop. Boca Raton, FL: CRC Press, vol. 11, pp: 9780429355752. Ebersole, J., Samburova, V., Son, Y., Cappelli, D., Demopoulos, C., Capurro, A., Pinto, A., Chrzan, B., Kingsley, K., Howard, K. and Clark, N. 2020. Harmful chemicals emitted from electronic cigarettes and potential deleterious effects in the oral cavity. Tob. Induced. Dis. 18, 41; doi:10.18332/ TID/116988 Eshraghian, A., Kamyab, A.A. and Yoon, S.K. 2013. Pharmacological treatment for hepatopulmonary syndrome. Biomed. Res. Int. 2013, 670139; doi:10.1155/2013/670139 Fasinu, P.S., Bouic, P. and Rosenkranz, B. 2012. An overview of the evidence and mechanisms of herb drug use interactions. Front. Pharmacol. 3, 69; doi:10.3389/fphar.2012.00069 Glynos, C., Bibli, S.I., Katsaounou, P., Magkou, C., Karavana, V. and Topouzis, S. 2018. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. J. Physiol. Lung. Cell. Mol. Physiol. 315(5), L662–L672. Gómez-Sierra,T.,Molina-Jijón,E.,Tapia,E.,Hernández-Pando, R., García-Niño, W.R., Maldonado, P.D., Reyes, J.L., Barrera-Oviedo, D., Torres, I. and Pedraza-Chaverri, J. 2014. S-allylcysteine prevents cisplatin-induced nephrotoxicity and oxidative stress. J. Pharm. Pharmacol. 66(9), 1271–1281; doi:10.1111/jphp.12263 Goniewicz, M.L., Gupta, R., Lee, Y.H., Reinhardt, S., Kim, S., Kim, B., Kosmider, L. and Sobczak, A. 2015. Nicotine levels in electronic cigarette refill solutions: a comparative analysis of products from the US, Korea, and Poland. Int. J. Drug. Policy. 26, 583–588; doi:10.1016/j.drugpo.2015.01.020 Gruhlke, M.C., Antelmann, H., Bernhardt, J., Kloubert, V., Rink, L. and Slusarenko, A. 2019. The human allicin proteome: S-Thioallylation of proteins by the garlic defense substance allicin and its biological effects. Free Radic. Biol. Med. 131, 144–153; doi:10.1016/j.freeradbiomed.2018.11.022 Hansel, T.T. and Barnes, P.J. 2003. Novel drugs for treating asthma. Curr. Allergy Asthma Rep. 1(2), 164–173; doi: 10.1007/s11882-001-0084-5 Hirayama, D. and Nakase, H. 2017. Molecular sciences the phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 19(1), 92; doi:10.3390/ijms19010092 Kano, S.I., Choi, E.Y., Dohi, E., Agarwal, S., Chang, D.J., Wilson, A.M., Lo, B.D., Rose, I.V., Gonzalez, S., Imai, T. and Sawa, A. 2019. Glutathione S-transferases promote proinflammatory astrocyte-microglia communication during brain inflammation. Sci. Signal 12(569), 2124; doi:10.1126/scisignal. aar2124 Khanarmuei, M., Rahimzadeh, H. and Sarkardeh, H. 2019. Effect of dual intake direction on critical function submergence and vortex strength. J. Hydraul. Res. 57, 272–279; doi:10.1080/00221686.2018.1459896 Krause, M.L., Cartin-Ceba, R., Specks, U. and Peikert, T. 2012. Update on diffuse alveolar hemorrhage and pulmonary vasculitis. Immunol. Allergy. Clin. North. Am. 32, 587–600; doi:10.1016/j. iac.2012.08.001 Krishnasamy, V.P., Hallowell, B.D., Ko, J.Y., Board, A., Hartnett, K.P., Salvatore, P.P., Danielson, M., Kite-Powell, A., Twentyman, E., Kim, L., Cyrus, A., Wallace, M., Melstrom, P., Haag, B., King, B.A., Briss, P., Jones, C.M., Pollack, L.A., Ellington, S. and Lung Injury Response Epidemiology/Surveillance Task Force. 2020. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping product use-associated lung injury-United States. Morb. Mortal. Wkly. Rep. 69(3), 90–94; doi: 10.15585/mmwr.mm6903e2 Layden, J.E., Ghinai, I., Pray, I., Kimball, A., Layer, M., Tenforde, M.W., Navon, L., Hoots, B., Salvatore, P.P., Elderbrook, M. and Haupt, T. 2020. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin. N. Engl. J. Med. 382, 903–916; doi:10.1056/nejmoa1911614 Lee, H.W., Park, S.H., Weng, M.W., Wang, H.T., Huang, W.C., Lepor, H., Wu, X.R., Chen, L.C. and Tang, M.S. 2018. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. 115, E1560–E1569; doi:10.1073/pnas.1718185115 Leyva-López, N., Gutierrez-Grijalva, E.P., Ambriz-Perez, D.L. and Heredia, J.B. 2016. Molecular sciences flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int. J. Mol. Sci. 17(6), 921; doi:10.3390/ ijms17060921 Lobo, V., Patil, A., Phatak, A. and Chandra, N. 2010. Free radicals, antioxidants and functional foods: impact human health. Pharmacogn. Rev. 4(8), 118; doi:10.4103/0973-7847.70902 Metwally, D.M., Al-Olayan, E.M., Alanazi, M., Alzahrany, S.B. and Semlali A. 2018. Antischistosomal and anti-inflammatory activity of garlic and allicin compared with that of praziquantel in civo. BMC Complement. Altern. Med. 18(1), 135. doi:10.1186/s12906-018-2191-z Moodley, K., Joseph, K., Naidoo, Y., Islam, S. and Mackraj, I. 2015. Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (Wild Garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement. Altern. Med. 15, 408; doi:10.1186/s12906-015-0932-9 Nadeem, M.S., Kazmi, I., Ullah, I., Muhammad, K. and Anwar, F. 2021. Allicin, an antioxidant and neuroprotective agent, ameliorates cognitive impairment. Antioxidants 11(1), 87; doi:10.3390/ antiox11010087 Naji, K.M., Al-Shaibani, E.S., Alhadi, F.A., Al-Soudi, S.A.A. and D’souza, M.R. 2017. Hepatoprotective and anti-oxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits. BMC. Complement. Altern. Med. 17(1), 411; doi:10.1186/s12906-017-1916-8 Nasr, A.Y. and Saleh, H.A. 2014. Aged garlic extract protects against oxidative stress and renal changes in cisplatin-treated adult male rats. Cancer Cell Int. 14(1), 92; doi:10.1186/s12935-014-0092-x Park, H.J., Jeon, B.T., Kim, H.C., Roh, G.S., Shin, J.H., Sung, N.J., Han, J. and Kang, D. 2012. Aged red garlic extract reduces lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages and acute pulmonary inflammation through haeme oxygenase-1 induction. Acta. Physiol. (Oxf). 205, 61–70; doi:10.1111/j.1748-1716.2012.02425.x Park, S.Y., Seetharaman, R., Ko, M.J., Kim, D.Y., Kim, T.H., Yoon, M.K., Kwak, J.H., Lee, S.J., Bae, Y.S. and Choi, Y.W. 2014. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced proinflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int. Immunopharmacol. 19, 253–261; doi: 10.1016/j. intimp.2014.01.017 Perea, L., Bottier, M., Cant, E., Richardson, H., Dicker, A.J., Shuttleworth, M., Giam, Y.H., Abo-Leyah, H., Finch, S., Huang, J.T. and Shteinberg, M. 2024. Airway IL-1β is related to disease severity and mucociliary function in bronchiectasis. Eur. Respir. J. 200, 401–407; doi:10.1183/13993003.01966-2023 Recinella, L., Chiavaroli, A., Masciulli, F., Fraschetti, C., Filippi, A., Cesa, S., Cairone, F., Gorica, E., De Leo, M., Braca, A. and Martelli, A. 2021. Protective effects induced by a hydroalcoholic Allium sativum extract in isolated mouse heart. Nutrients 13(7), 2332; doi:10.3390/nu13072332 Savin, I.A., Zenkova, M.A. and Sen’kova, A.V. 2022. Pulmonary fibrosis as a result of acute lung inflammation: molecular mechanisms, relevant in vivo models, prognostic and therapeutic approaches. Int. J. Mol. Sci. 23(23), 14959; doi:10.3390/ijms232314959 Shang, A., Cao, S.Y., Xu, X.Y., Gan, R.Y., Tang, G.Y. and Corke, H. 2019. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 23(23), 14959; doi:10.3390/foods8070246 Shehata, S.A., Toraih, E.A., Ismail, E.A., Hagras, A.M., Elmorsy, E. and Fawzy, M.S. 2023. Vaping, environmental toxicants exposure, and lung cancer risk. Cancers 15(18), 4525; doi:10.3390/ cancers15184525 Shraideh, Z., Awaida, W. and Badran, D.H. 2013. Effects of cigarette smoking on the histological findings of the trachea and lungs albino rat effects of medicinal plants on smooth muscle cells: a medical education project view project. Res. Op. Ani. Vet. Sci. 3(10), 356–362. Strzelak, A., Ratajczak, A., Adamiec, A. and Feleszko, W. 2018. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int. J. Environ. Res. Public. Health. 15(5), 1033; doi:10.3390/ijerph15051033 Subramanian, M.S., Nandagopal, G.M.S., Nordin, S.A., Thilakavathy, K. and Joseph, N. 2020. Prevailing knowledge of the bioavailability and biological activities of sulfur compounds from Alliums: a potential drug candidate. Molecules 25(18), 4111; doi:10.3390/molecules25184111 Sul, O.J. and Ra, S.W. 2021. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules 26(22), 6949; doi:10.3390/ molecules26226949. Tran, G.B., Dam, S.M. and Le, N.T.T. 2018. Amelioration of single clove black garlic aqueous extract on dyslipidemia and hepatitis in chronic carbon tetrachloride intoxicated Swiss Albino mice. Int. J. Hepatol. 2018, 9383950; doi:10.1155/2018/9383950 Tsukioka, T., Takemura, S., Minamiyama, Y., Mizuguchi, S., Toda, M. and Okada, S. 2017. Attenuation of bleomycin-induced pulmonary fibrosis in rats with S-allyl cysteine. Molecules 22(4), 543; doi:10.3390/molecules22040543 Urschel, K. and Cicha, I. 2015. TNF-α in the cardiovascular system: from physiology to therapy. Int. J. Interf. Cytokine. Mediat. Res. 7, 9–25; doi:10.2147/IJICMR.S64894 Wang, L., Wang, Y., Chen, J., Liu, P. and Li, M. 2022. A review of toxicity mechanism studies of electronic cigarettes on respiratory system. Int. J. Mol. Sci. 23(9), 5030; doi:10.3390/ijms23095030 Wentzel, J.J., Janssen, E., Vos, J., Schuurbiers, J.C., Krams, R., Serruys, P.W., de Feyter, P.J. and Slager, C.J. 2003. Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation 108, 17–23; doi:10.1161/01. CIR.0000078637.21322.D3 Wlosinska, M., Nilsson, A., Hlebowicz, J., Fakhro, M., Malmsjö, M. and Lindstedt, S. 2021. Aged garlic extract reduces IL-6: a double-blind placeboZcontrolled trial in females with a low risk of cardiovascular disease. Evidence-Based Complement. Altern. Med. 2021, 6636875; doi:10.1155/2021/6636875 Yang, Z., Du, J., Zhu, J., Rong, Y., Chen, S., Yu, L., Deng, X., Zhang, X., Sheng, H., Yang, L. and Lu, X. 2020. Allicin inhibits proliferation by decreasing IL-6 and IFN-β in HCMV-infected glioma cells. Cancer Manag. Res. 12, 7305–7317. Zamani Taghizadeh Rabe, S., Ghazanfari, T., Siadat, Z., Rastin, M., Zamani Taghizadeh Rabe, S. and Mahmoudi, M. 2015. Anti-inflammatory effect of garlic 14-kDa protein on LPS-stimulated-J774A. 1 macrophages. Immunopharmacol. Immunotoxicol. 37(2), 158–64; doi:10.3109/08923973.2015.1005229 Ziamajidi, N., Nasiri, A., Abbasalipourkabir, R. and Sadeghi Moheb, S. 2016. Effects of garlic extract on TNF-α expression and oxidative stress status in the kidneys of rats with STZ+ nicotinamide-induced diabetes. Pharm. Biol. 55(1), 526–531; doi:10.1080/13880209.2016.1255978 | ||
| How to Cite this Article |
| Pubmed Style Yunita MN, Plumeriastuti H, Tyasningsih W, Agustono B, Hardiono AL, Prasetyanuri GW, Shofia S, Rahmaniar RP, Rahman MA. Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats. Open Vet. J.. 2025; 15(6): 2374-2385. doi:10.5455/OVJ.2025.v15.i6.10 Web Style Yunita MN, Plumeriastuti H, Tyasningsih W, Agustono B, Hardiono AL, Prasetyanuri GW, Shofia S, Rahmaniar RP, Rahman MA. Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats. https://www.openveterinaryjournal.com/?mno=228943 [Access: December 10, 2025]. doi:10.5455/OVJ.2025.v15.i6.10 AMA (American Medical Association) Style Yunita MN, Plumeriastuti H, Tyasningsih W, Agustono B, Hardiono AL, Prasetyanuri GW, Shofia S, Rahmaniar RP, Rahman MA. Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats. Open Vet. J.. 2025; 15(6): 2374-2385. doi:10.5455/OVJ.2025.v15.i6.10 Vancouver/ICMJE Style Yunita MN, Plumeriastuti H, Tyasningsih W, Agustono B, Hardiono AL, Prasetyanuri GW, Shofia S, Rahmaniar RP, Rahman MA. Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats. Open Vet. J.. (2025), [cited December 10, 2025]; 15(6): 2374-2385. doi:10.5455/OVJ.2025.v15.i6.10 Harvard Style Yunita, M. N., Plumeriastuti, . H., Tyasningsih, . W., Agustono, . B., Hardiono, . A. L., Prasetyanuri, . G. W., Shofia, . S., Rahmaniar, . R. P. & Rahman, . M. A. (2025) Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats. Open Vet. J., 15 (6), 2374-2385. doi:10.5455/OVJ.2025.v15.i6.10 Turabian Style Yunita, Maya Nurwartanti, Hani Plumeriastuti, Wiwiek Tyasningsih, Bodhi Agustono, Andhika Lutfhi Hardiono, Gabrian Wahyu Prasetyanuri, Shofia Shofia, Reina Puspita Rahmaniar, and Md. Aliar Rahman. 2025. Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats. Open Veterinary Journal, 15 (6), 2374-2385. doi:10.5455/OVJ.2025.v15.i6.10 Chicago Style Yunita, Maya Nurwartanti, Hani Plumeriastuti, Wiwiek Tyasningsih, Bodhi Agustono, Andhika Lutfhi Hardiono, Gabrian Wahyu Prasetyanuri, Shofia Shofia, Reina Puspita Rahmaniar, and Md. Aliar Rahman. "Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats." Open Veterinary Journal 15 (2025), 2374-2385. doi:10.5455/OVJ.2025.v15.i6.10 MLA (The Modern Language Association) Style Yunita, Maya Nurwartanti, Hani Plumeriastuti, Wiwiek Tyasningsih, Bodhi Agustono, Andhika Lutfhi Hardiono, Gabrian Wahyu Prasetyanuri, Shofia Shofia, Reina Puspita Rahmaniar, and Md. Aliar Rahman. "Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats." Open Veterinary Journal 15.6 (2025), 2374-2385. Print. doi:10.5455/OVJ.2025.v15.i6.10 APA (American Psychological Association) Style Yunita, M. N., Plumeriastuti, . H., Tyasningsih, . W., Agustono, . B., Hardiono, . A. L., Prasetyanuri, . G. W., Shofia, . S., Rahmaniar, . R. P. & Rahman, . M. A. (2025) Therapeutic effects of single-clove garlic extract on nicotineinduced inflammation and histopathological changes in rats. Open Veterinary Journal, 15 (6), 2374-2385. doi:10.5455/OVJ.2025.v15.i6.10 |