| Case Report | ||
Open Vet. J.. 2024; 14(12): 3640-3648 Open Veterinary Journal, (2024), Vol. 14(12): 3640-3648 Case Report Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiologyLuigi Venco1*, Ștefan Adrian Geantă2, Mihaela Claudia Bolintineanu2, Alina Nechifor3 and Florin Leca21Ospedale Veterinario Città di Pavia, Pavia, Italy 2Doctor’s Veterinary University Veterinary Clinic, Bucharest, Romania 3Dr. Bercaru Veterinary Clinic, Bucharest, Romania *Corresponding Author: Luigi Venco. Ospedale veterinario Città di Pavia, Pavia, Italy. Email: luigivenco [at] libero.it Submitted: 16/11/2024 Accepted: 29/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
AbstractBackground: Cardiologists occlud most patent ductus arteriosus (PDA) defects in dogs using the Amplatz® canine duct occluder via a transarterial approach. However, this approach can be problematic in small dogs due to their small femoral artery diameters. In such cases, cardiologists have opted for lower profile devices or use coils or vascular plugs deployed using a more challenging transvenous approach. Case Description: The present report describes a 1-year-old, 6 kg male Cavalier King Charles Spaniel with a small PDA, which was successfully occluded using the Micro Plug Set (KA Medical, Minneapolis, MN) via a transarterial approach. Conclusion: This is the first case report in medicine of PDA closure with a KA Micro Plug Set via transarterial approach. This approach, which has been described for PDA closure in a few dozen premature human infants, could be considered as a minimally invasive PDA closure in small dogs by using a transarterial approach. Furthermore, in pediatric cardiology, the use of the device has been described only via a transvenous approach. The information from this case report could inspire a different and simpler approach to human medicine. Keywords: Case report, Micro Plug Set (KA Medical, Minneapolis, MN), Patent ductus arteriosus, Small dogs. IntroductionPatent ductus arteriosus (PDA), is the most common congenital heart disease in dogs (Hunt et al., 1995; McDonald, 2006). Some breeds appear to be particularly predisposed to this condition; however, defects can occur in any breed and are more often described in females than in males (Buchanan, 1994; Brambilla et al., 2020). Left-to-right PDA shunting can lead to pathological pulmonary overcirculation and left-sided volume overload, potentially resulting in congestive heart failure. A wide range of PDA sizes and morphologies has been described using multidimensional imaging (Doocy et al., 2018). Such variations in the morphology and size of the PDA, and in the size of the artery used for vascular access, influence closure recommendations and device selection when considering minimally invasive transcatheter closure (Doocy et al., 2018). A transcatheter closure device was developed specifically for use in dogs with PDA: the Amplatzer canine duct occluder (ACDO) (Infiniti Medical, Huddersfield, UK), which cardiologists currently consider as the treatment of choice for dogs with left-to-right shunting PDA (Nguyenba et al., 2007; Gordon et al., 2010). This device has a minimally invasive controlled catheter-based delivery system, is easily deployed, has a low complication rate, and is available in a broad range of sizes that allow the closure of a wide range of PDAs. However, the ACDO delivery system size and the need to use this device via a transarterial approach limits its use in small dogs and/or dogs with small-diameter femoral arteries. Consequently, veterinary investigators have examined lower profile plugs, such as the Amplatzer™ vascular plug 4 (AVP4, AGA Medical Corporation, Plymouth, MN) (Hogan et al., 2021, Hulsman et al., 2021), which has also been used in children (Mini et al., 2023). The AVP4 consists of two symmetrical self-expanding nitinol mesh lobes, and is available in the following sizes (diameter/length): 4/10, 5/10.5, 6/11, 7/12.5, and 8/13.5 mm). Its diameter is selected with 30%–50% oversizing. The maximal diameter of the ductal ampulla increases (constrained) its length, and is delivered directly through a 4-French diagnostic catheter, in a similar way to the procedure for ACDO implantation, without using an introducer with a hemostatic valve (Hogan et al., 2021). Other veterinary investigators have focused on a transvenous approach (femoral or jugular) when using Amplatzer™ vascular plug 2 devices (AGA Medical Corp), coils, or other devices, such as the Nit-Occlud PDA occlusion system (Pfm Medical GMBH, Cologne, Germany), for interventional closure of a PDA in dogs where routine ACDO closure is not feasible (Blossom et al., 2010; Bagardi et al., 2022; Belachsen et al., 2022; Hildebrandt et al., 2022; Morgan et al., 2022; Cala et al., 2024). However, transvenous access involving the cardiac chambers can be more challenging, and retrograde access to the PDA is time-consuming and sometimes impossible, particularly in cases with a small minimal ductal diameter (MDD). A new vascular occlusion device (Micro Plug Set, KA Medical, Minneapolis, MN) with a short length (2.5 mm, unconstrained) has become commercially available. It is microcatheter-deliverable and X-ray and ultrasound visible. It was developed for arterial embolization in peripheral vasculature, with early experiences described in a few dozen neonates and infants undergoing transcatheter PDA occlusion via transvenous access (Heyden et al., 2020, Guyon et al., 2022). The Micro Plug Set comprises a self-expanding braided nitinol cylindrical mesh with three equal-sized discs and radiopaque marker bands at each end (Fig. 1). The device is available in four sizes, with disc diameters ranging from 3 to 6 mm and an unconstrained length of 2.5 mm. According to the manufacturer, the device is prepared and advanced using a 2.9-French, 125-cm long delivery microcatheter and is released by using its delivery cable (Fig. 1). This case report aims to evaluate the first-time use of the Micro Plug Set for the closure of a PDA in a dog with transarterial access never described even in pediatric interventional cardiology. Case DetailsA 1-year-old, 6-kg male Cavalier King Charles Spaniel with subclinical PDA, diagnosed via transthoracic echocardiography, was referred for minimally invasive closure. On presentation, the dog was alert, and physical examination revealed pink, moist mucous membranes, a capillary refill time of less than 2 seconds, an IV/VI left basilar continuous murmur, and bounding femoral pulses. A transthoracic echocardiogram (TTE) (Siemens Acuson Juniper, Probe: 8V4, Siemens Healthcare, Erlangen, Germany) revealed mild left atrial and left ventricular dilation: Left atrium/aorta ratio: 1.8 ( reference value < 1.6), normalized to body weight), left ventricular internal diastolic diameter: 1.98 (reference value: ≤ 1.7), and left ventricle apical four-chamber view end-diastolic volume (Simpson biplane method): 23.6 mL (reference value < 20.1 mL), (Wess et al. 2021). It also revealed a small PDA, with approximate MDD and ampulla diameters of 1.2 mm and 3.7 × 7 mm, respectively, and a length of 7 mm (Fig. 2). Doppler echocardiography confirmed left-to-right flow across the PDA with pressure gradients of 113.30 mmHg in systole and 59.5 mmHg in diastole. Despite the modest overload of the left heart chambers, in agreement with the owners’ wishes, we decided to proceed with PDA closure with a minimally invasive technique, also considering the predisposition to mitral valve insufficiency development in middle-aged patients of this breed, exacerbating left volumetric overload.
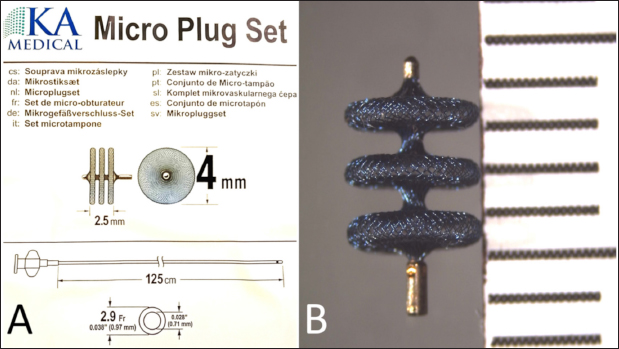
Fig. 1. Schematic drawing of the KA Micro Plug Set with the device and 2.9-French delivery catheter, along with the relevant sizes (A), and a photograph of the device (B) with a millimeter scale.
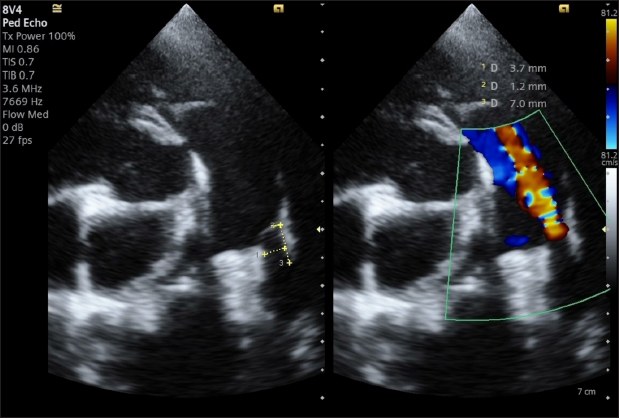
Fig. 2. Transthoracic echocardiography, cranial left lateral view, optimized for PDA visualization and its measurement (A) and and left-to-right flow on CFM Doppler (Dual-mode). We considered that the small MDD (1.2 mm) implied that it would be difficult or time-consuming to cross the PDA by transvenous access and that the use of even an ACDO of the smallest size (3-mm waist with a ratio to MDD: 2.5), inserted via the femoral artery, would have created excessive stretching at both the pulmonary ostium and the ampulla given the diameter of the proximal disc (8 mm, with a ratio of proximal disc diameter/ampulla diameter > 2.1). We also considered that a required delivery sheath thinner than ACDO would allow a faster and safer trans-arterial approach. Therefore, we proposed using the KA Micro Plug Set via femoral arterial access, applying the same indications that were used in a preliminary study on children (discs > 1 mm of the MDD and similar width to that of the mid-distal part of the ampulla, using a 4-mm device). The owner was informed of our suggestion and provided written consent for the off-label use of other devices if needed. Anesthesia was induced and maintained as previously described (Bagardi et al., 2022) and perioperative intravenous amoxicillin (22 mg/kg) was administered. The dog was placed in right lateral recumbency on a fluoroscopy table (C-arm: Siemens Cios Select Siemens Healthcare), and the area over the right femoral artery was clipped and prepared for surgery. The patient was monitored with transesophageal echocardiography (TEE; Probe: 5VT, Siemens Healthcare), which allowed optimal visualization of the ductus and its measurements (MDD 1.2 mm, ampulla diameter 3.8 mm, and length 4.4 mm), which confirmed the choice of the device (Fig. 3). The right femoral artery was accessed via surgical cut-down and the distal end of the femoral artery was ligated with a non-absorbable multifilament suture (3-0 PERMA-HAND™ Silk Suture, Ethiicon, Johnson & Johnson International, Diegem, Belgium). A 4-French, 10-cm long introducer vascular sheath (Pinnacle Precision Access System, Terumo Medical Corporation, Somerset, NJ) was inserted, after puncturing the artery with the appropriate 21-gauge needle, after which the 0.021” Nitinol Taper tip guidewire, also supplied with the set, was inserted. A 3.3-French MP angiographic catheter (Moongoose® Togo Medikit Co. Ltd., Oaza Hichiya Hyuga City, Japan) was placed into the femoral artery together with a 0.025” angled hydrophilic guidewire, 180-cm long (Terumo Radiofocus® Glidewire, Terumo Medical Corporation), through the 4-French vascular sheath. Once it had reached the ascending aorta, it was used to perform angiography (after removing the guide wire) with 3 mL iodinated contrast agent (350 mg/mL) diluted with 3 mL saline, to facilitate its manual injection through the 3.3-French vascular catheter. Angiography confirmed the earlier measurements of TEE.
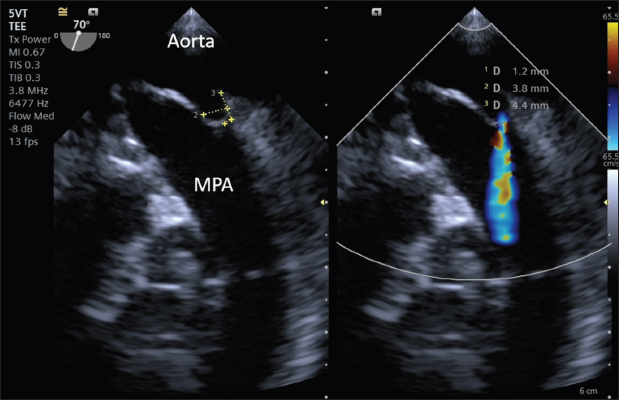
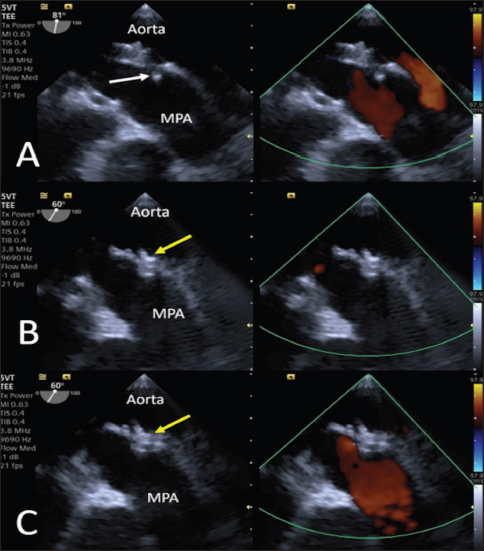
Fig. 3. Intraoperative TEE showing PDA and its measurements (MDD: 1.2 mm; ampulla diameter: 3.8 mm; length: 4.4 mm) and the flow from the aorta to the pulmonary artery (MPA) on CFM Doppler (Dual-mode).
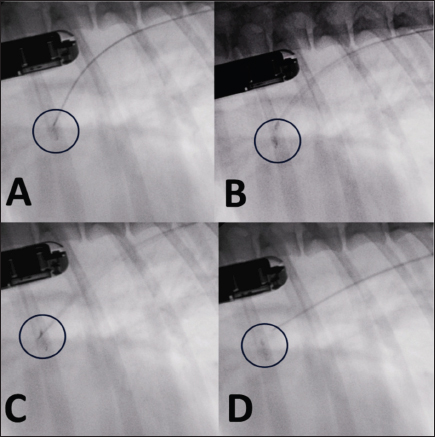
Fig. 4. Fluoroscopic sequence. (A) The first disc is deployed and the entire system is retracted until it engages the pulmonary ostium. (B) The second and third discs are released into the ampulla. (C) The device is released with anticlockwise rotation of the delivery cable. (D) The delivery catheter is removed along with the delivery cable.
Fig. 5. Intraoperative TEE. (A) The first disc is released into the pulmonary artery (yellow arrowhead). (B) The central and proximal discs are positioned inside the ampulla (yellow arrowhead). (C) No residual flow is visible on CFM (Dual-mode) examination, and no flow obstacles are present in the pulmonary artery. Subsequently, the guidewire (reinserted into the catheter) and catheter were passed under fluoroscopic and transthoracic echocardiography guidance through the PDA into the pulmonary trunk in an anterograde manner until the tip of the guidewire was clearly visible in the pulmonary artery. The catheter was then removed, leaving the guidewire, on which the dedicated 2.9-French KA manufacturer microcatheter was positioned such that its tip was clearly visible under both fluoroscopic and TEE guidance, in situ. The guidewire was subsequently removed. To facilitate the operations and avoid a mismatch with the length (180 cm) of the 0.0025” guidewire, given the length of the microcatheter (125 cm), its tip was shortened by 40 cm. The device, in its loader, was inserted into the delivery sheath through the appropriate Tuohy-Borst Sidearm Adapter provided by the manufacturer and was advanced carefully until the distal disc was expanded in the pulmonary artery. The delivery wire and catheter were gently withdrawn until resistance was experienced in the pulmonary ostium. The guiding sheath was then retracted, allowing expansion of the central and proximal discs of the device within the PDA ampulla. The stability of the device was subsequently tested with the push–pull “Minnesota wiggle” maneuver, and the cessation of transductal flow and the correct positioning of the device was verified using TEE (Figs. 4 and 5). The device was then deployed by rotating the delivery cable counterclockwise with the appropriate plastic vise. To verify the closure of the PDA and the absence of protrusions in the aorta further, a second angiography was performed as before (Fig. 6).
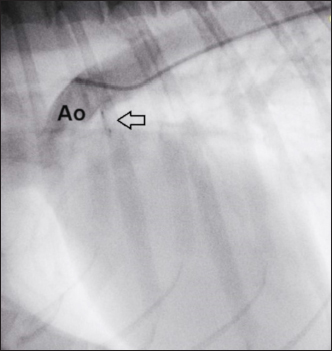
Fig. 6. Angiography conducted after device (arrow) release. The PDA is completely occluded and no residual shunt is visible. The delivery sheath and introducer sheath were then removed, the femoral artery was ligated, and the surgical wound was repaired in a routine manner using a 3-0 monofilament polydioxanone absorbable suture (PDS* II Ethicon, Raritan, NJ). The duration of the entire surgery was 40 minutes, with fluoroscopic exposure times of 3 minutes 40 seconds. Recovery from anesthesia was uneventful, and the dog was able to walk and eat 3 hours later. Thoracic radiographs after 24 hours showed correct positioning of the device (Fig. 7). The dog was discharged, and a check-up was scheduled for 30 days. The 1-month TTE echocardiographic examination confirmed complete occlusion of the PDA, without residual shunting on Color Flow Mapping (CFM), correct positioning of the device, with complete reverse remodeling of the cardiac chambers (Fig. 8). Ethical approvalEthics review and approval of this study were waived because this was not an experimental study, but a clinical report of a spontaneously occurring congenital disease, and detailed informed consent was obtained from the owner for the publication of the images and clinical case.
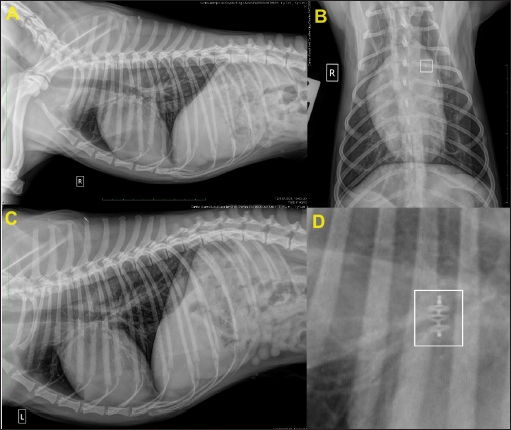
Fig. 7. Thoracic radiographs obtained 24 hours after surgery. (A) Right lateral, (B) dorso-ventral, (C) left lateral views, and (D) close-up view of the device in the right lateral view (white square). In (B), note the thickness of the device (white square) compared to that of the identification microchip. Post-operative films show that the device is positioned correctly.
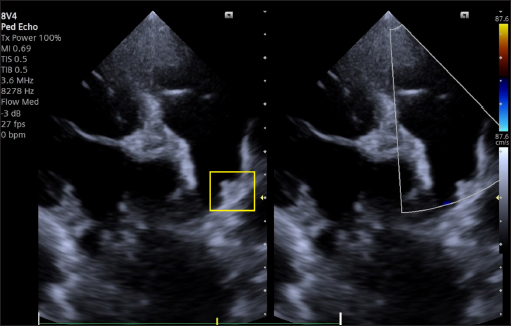
Fig. 8. Dual-mode transthoracic echocardiography (left cranial parasternal view optimized for duct visualization) at 30 days after surgery. The device (yellow square) is correctly positioned, and no evidence of residual shunt or pulmonary arterial obstruction is present on CFM Doppler. DiscussionOur case report details the first use of a KA Micro Plug Set via the transarterial approach for occluding a PDA in a small young dog. In human medicine, this device has been used in a few dozen neonates and infants undergoing transcatheter PDA occlusion via transvenous access only (Heyden et al., 2020, Guyon et al., 2022). Veterinary and human cardiologists have previously reported successful closure of PDAs in small dogs and children with the AVP4 and transarterial (Hogan, et al., 2021, Hulsman et al., 2021) or tranvenous access (Mini et al., 2023). However, the available lengths of the AVP4 (10–13.5-mm, unconstrained) make it potentially suitable only for long ducts. In the case of short ducts, the plug may protrude into the aorta or into the pulmonary artery, leading to stenosis (Mini et al., 2023). In veterinary medicine, the AVP4 is delivered through a 4-French angiographic catheter through the femoral artery, without the use of an introducer with a hemostatic valve, which poses a potential risk of bleeding, which can have a marked clinical impact in small dogs. The Micro Plug Set addresses the limitations of contemporary devices used for transcatheter PDA closure in small dogs, even those with a small-diameter femoral artery. First, this device is shorter (2.5 mm), which allows the closure of ducts much shorter than 10 mm, without any risk of protrusion and stenosis, and the shape is well suited for placing the distal disc in the pulmonary artery and the central and proximal discs of the device within the PDA ampulla, potentially reducing the risk of embolization. Second, the device is delivered through a soft, low-profile (2.9-French) microcatheter. It does not require catheter exchange, a stiff delivery catheter, a sheath, or a guide wire, and can easily be introduced through a 4-French introducer with a hemostatic valve. The device is available with disc diameters of 3, 4, 5, and 6 mm, and based on the experience in human medicine and in our canine clinical case, the diameter of the selected disc must be >2 mm larger than the MDD and should be similar to that of the mediodistal part of the ampulla closest to the pulmonary side, allowing occlusion of PDAs even of significant dimensions. Therefore, the Micro Plug Set appears to be a promising transcatheter device for use in small dogs that addresses the limitations of the currently available devices. Our first experience has shown that the device can be safely implanted, with no significant procedural complications or adverse events. The only critical issue noted in our study was the poor radiopacity and lower visibility of the device by fluoroscopy, due to its small size, relative to that of other devices. Intraprocedural monitoring with TEE provides useful support, in addition to shortening the exposure time to X-rays for operators and patients. In very small patients in whom the size of the probe does not allow TEE examination, we believe that TTE monitoring coupled with fluoroscopy could be very useful, as is used in very small, premature human infants (Heyden et al., 2020, Catalán et al., 2024). Our success, albeit in a single dog, demonstrated that the Micro Plug Set is a promising transcatheter device option for transarterial PDA occlusion in small dogs. More clinical cases are required, and its use via transvenous access in dogs, such as those used in children, is desirable. Finally, our report provides cardiologists, both veterinary and human, with a novel method of transvascular PDA occlusion in small dogs and potentially in children and demonstrates how the mutual exchange of information in the field of veterinary and human interventional cardiology can contribute to significant progress in addressing challenging clinical cases. AcknowledgementsThe authors thank Dr Altin Cala for his helpful suggestions in drafting the manuscript. Conflict of interestThe authors have no conflicts of interest to declare. No funding was received for the authors of this case report. FundingThe authors declare that there is no conflict of interest. No funding was received by the authors for this case report. Authors’ contributionsVenco L, Leca F Conceptualization and surgical management. S.A.Geanta Diagnosis, TEE management, and follow up. M.C. Bolintineanu surgical management A. Nechifor Anesthesia management. Venco L.: Manuscript supervision. All the authors manuscript drafting and approval of the final manuscript Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesBagardi, M., Domenech, O., Vezzosi, T., Marchesotti, F., Bini, M., Patata, V., Croce, M., Valenti, V. and Venco, L. 2022. Transjugular patent ductus arteriosus occlusion in seven dogs using the Amplatzer vascular plug II. Vet. Sci. 9(8), 431. Belachsen, O., Sargent, J., Koffas, H., Schneider, M. and Wagner, T. 2022. The use of Amplatzer vascular plug II in 32 consecutive dogs for transvenous occlusion of patent ductus arteriosus. J. Vet. Cardiol. 41, 88–98. Blossom, J.E., Bright, J.M. and Griffiths, L.G. 2010. Transvenous occlusion of patent ductus arteriosus in 56 consecutive dogs. J. Vet. Cardiol. 12(2), 75–84. Brambilla, P.G., Polli, M., Pradelli, D., Papa, M., Rizzi, R., Bagardi, M. and Bussadori, C. 2020. Epidemiological study of congenital heart diseases in dogs: prevalence, popularity, and volatility throughout twenty years of clinical practice. PLoS One. 15(7), e0230160. Buchanan, J.W. 1994. Patent ductus arteriosus. Semin. Vet. Med. Surg. 9, 168–176. Cala, A., Ferasin, L., Ferasin, H., Domenech, O., Bini, M., Valenti, V. and Venco, L. 2024. Transvenous closure of patent ductus arteriosus with Nit-Occlud® PDA occlusion system in 13 dogs weighing less than 3 kg. J. Vet. Cardiol. 56, 23–34. Catalán, A.I., Condori, K., Medina, M., Lucena, S., Montoya, D. and Gálvez-Arévalo, R. 2024. Use of echocardiography in percutaneous closure of patent ductus arteriosus at the Instituto Nacional de Salud del Niño, San Borja, Lima - Peru. Arch. Peru. Cardiol. Cir. Cardiovasc. 5(2), e350. Doocy, K.R., Saunders, A.B., Gordon, S.G. and Jeffery, N. 2018. Comparative, multidimensional imaging of patent ductus arteriosus and a proposed update to the morphology classification system for dogs. J. Vet. Intern. Med. 32(2), 648–657. Gordon, S.G., Saunders, A.B., Achen, S.E., Roland, R.M., Drourr, L.T., Hariu, C. and Miller, M.W. 2010. Transarterial ductal occlusion using the Amplatz Canine Duct Occluder in 40 dogs. J. Vet. Cardiol. 12(2), 85–92. Guyon, P., Duster, N., Katheria, A., Heyden, C., Griffin, D., Steinbergs, R., Moreno Rojas, A., Ratnayaka, K. and El-Said, H.G. 2022. Institutional trend in device selection for transcatheter PDA closure in premature infants. Pediatr. Cardiol. 43(8), 1716–1722. Heyden, C.M., El-Said, H.G., Moore, J.W. Guyon, P.W. Jr, Katheria A.C. and Ratnayaka, K. 2020. Early experience with the Micro Plug Set for preterm patent ductus arteriosus closure. Catheter Cardiovasc. Interv. 96(7), 1439–1444. Hildebrandt, N., Stosic, A., Henrich, E., Wiedemann, N., Wurtinger, G. and Schneider, M. 2022. Transvenous embolization of moderate to large patent ductus arteriosus in dogs using the Amplatzer vascular plug II. J. Vet. Intern. Med. 36(1), 20–28. Hogan, D.F. and Goldfeder, G.T. 2021. Transarterial correction of patent ductus arteriosus in small dogs with the Amplatz™ vascular plug 4: a pilot study. J. Vet. Cardiol. 35, 48–54. Hulsman, A.H., Breur, J.M.P.J. and Szatmári, V. 2021. Low profile vascular plug for transarterial occlusion of patent ductus arteriosus in small dogs. J. Vet. Intern. Med. 35(1), 98–106. Hunt, G.B., Church, D.B., Malik, R. and Bellenger, C.R. 1995. A retrospective analysis of congenital cardiac anomalies (1977–1989). Aust. Vet. Pract. 20, 70–75. MacDonald, K.A. 2006. Congenital heart diseases of puppies and kittens. Vet. Clin. North Am. Small Anim. Pract. 36(3), 503–531. Mini, N., Schneider, M.B.E. and Schneider K. 2023. Transcatheter closure of tubular PDA with amplatzer plug 4 in preterm infants weighing between 900 and 3,400 g: the pros and cons. Front. Cardiovasc. Med. 10, 1283992. Morgan, K.R.S., Stauthammer, C.D., Barncord, K., Pinkos, A., Fundingsland, S. and Rishniw, M. 2022. Transvenous detachable coiling of patent ductus arteriosus in small dogs. J. Vet. Cardiol. 42, 65–73. Nguyenba, T.P. and Tobias, A.H. 2007. The Amplatz canine duct occluder: a novel device for patent ductus arteriosus occlusion. J. Vet. Cardiol. 9, 109–117. Wess, G., Bauer, A. and Kopp, A. 2021. Echocardiographic reference intervals for volumetric measurements of the left ventricle using the Simpson’s method of discs in 1331 dogs. J. Vet. Intern. Med. 35(2), 724–738. | ||
| How to Cite this Article |
| Pubmed Style Venco L, Geantă �A, Bolintineanu MC, Nechifor A, Leca F. Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology. Open Vet. J.. 2024; 14(12): 3640-3648. doi:10.5455/OVJ.2024.v14.i12.45 Web Style Venco L, Geantă �A, Bolintineanu MC, Nechifor A, Leca F. Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology. https://www.openveterinaryjournal.com/?mno=228984 [Access: August 23, 2025]. doi:10.5455/OVJ.2024.v14.i12.45 AMA (American Medical Association) Style Venco L, Geantă �A, Bolintineanu MC, Nechifor A, Leca F. Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology. Open Vet. J.. 2024; 14(12): 3640-3648. doi:10.5455/OVJ.2024.v14.i12.45 Vancouver/ICMJE Style Venco L, Geantă �A, Bolintineanu MC, Nechifor A, Leca F. Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology. Open Vet. J.. (2024), [cited August 23, 2025]; 14(12): 3640-3648. doi:10.5455/OVJ.2024.v14.i12.45 Harvard Style Venco, L., Geantă, . �. A., Bolintineanu, . M. C., Nechifor, . A. & Leca, . F. (2024) Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology. Open Vet. J., 14 (12), 3640-3648. doi:10.5455/OVJ.2024.v14.i12.45 Turabian Style Venco, Luigi, Ștefan Adrian Geantă, Mihaela Claudia Bolintineanu, Alina Nechifor, and Florin Leca. 2024. Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology. Open Veterinary Journal, 14 (12), 3640-3648. doi:10.5455/OVJ.2024.v14.i12.45 Chicago Style Venco, Luigi, Ștefan Adrian Geantă, Mihaela Claudia Bolintineanu, Alina Nechifor, and Florin Leca. "Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology." Open Veterinary Journal 14 (2024), 3640-3648. doi:10.5455/OVJ.2024.v14.i12.45 MLA (The Modern Language Association) Style Venco, Luigi, Ștefan Adrian Geantă, Mihaela Claudia Bolintineanu, Alina Nechifor, and Florin Leca. "Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology." Open Veterinary Journal 14.12 (2024), 3640-3648. Print. doi:10.5455/OVJ.2024.v14.i12.45 APA (American Psychological Association) Style Venco, L., Geantă, . �. A., Bolintineanu, . M. C., Nechifor, . A. & Leca, . F. (2024) Low-profile KA microplug set for transarterial occlusion of patent ductus arteriosus in a small dog: First experience in interventional cardiology. Open Veterinary Journal, 14 (12), 3640-3648. doi:10.5455/OVJ.2024.v14.i12.45 |