| Research Article | ||
Open Vet. J.. 2025; 15(3): 1187-1205 Open Veterinary Journal, (2025), Vol. 15(3): 1187-1205 Research Article Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public healthPalash Bose1, Kazi Abdus Sobur1, Md. Bakhtiar Lijon1,2, Md. Zaminur Rahman1, Tanvir Ahamed1, Papia Sultana1, Muhammad Muktaruzzaman1, Mst. Minara Khatun1, Md. Ariful Islam1*1Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh, Bangladesh 2Graduate School of Environmental and Life Science, Okayama University, Okayama 7008530, Japan *Corresponding Author: Md. Ariful Islam. Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh, Bangladesh. Email: islamma [at] bau.edu.bd Submitted: 16/11/2024 Accepted: 13/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
AbstractBackground: Staphylococcus aureus is a significant pathogen in both clinical and food safety contexts, capable of contaminating table eggs, which are a common dietary staple worldwide. Aim: This study aimed to assess the prevalence, molecular characteristics, and antibiotic resistance profiles of S. aureus isolated from table eggs. The focus was on identifying methicillin-resistant S. aureus (MRSA) strains that produce enterotoxin (seb), resistance to β-lactam antibiotics (blaTEM), tetracycline (tetA), and vancomycin-resistant S. aureus. Methods: A total of 200 egg samples were collected from various retail sources in Mymensingh City Corporation, Bangladesh. Swab samples (n=100) were collected from eggshells, and another 100 samples were collected from the inner membrane, egg white, and yolk. Samples were enriched in trypticase soy broth and cultured on mannitol salt agar. Staphylococcus aureus was isolated through conventional culture techniques, confirmed by polymerase chain reaction targeting the nuc, and further screened for the mecA, seb, blaTEM, tetA, vanA, and vanC genes. Antibiotic susceptibility testing was conducted using the disc diffusion method against 13 antibiotics. Bivariate analysis is used to assess the strong and significant correlations between virulence genes and the pairs of any of two antibiotic-resistant S. aureus. Results: Staphylococcus spp. and S. aureus were detected in 53% and 21% of eggshell samples, respectively, and 41% and 13% of egg content samples. Among 34 coagulase-positive isolates, 12 (57.14%) from eggshells and 4 (30.78%) from egg contents were positive for the nuc gene. Resistance was observed in eggshell isolates for mecA (33.33%), blaTEM (85.71%), tetA (33.33%), vanA (19.04%), vanC (33.33%), and seb (20.50%), whereas egg content isolates showed resistance to blaTEM (46.15%) and vanC (7.80%). All coagulase-positive isolates exhibited significant resistance to β-lactam antibiotics, cephalosporins, and glycopeptides, especially vancomycin. Notably, 19 (90.47%) and 12 (92.30%) eggshell and egg content isolates, respectively, were multidrug-resistant, with multiple antibiotic resistance indices ranging from 0.23 to 0.76. Conclusion: The study revealed a high prevalence of multidrug-resistant S. aureus in table eggs, indicating a significant public health risk. The presence of MRSA and strains with enterotoxins and resistance genes underscores the need for enhanced monitoring, stricter biosecurity measures, and robust control strategies for egg production and distribution to ensure food safety. Keywords: Antibiogram, Enterotoxin, MDR, MRSA, Table eggs, VRSA. IntroductionTable eggs are a widely consumed and affordable food source that are rich in protein, fats, vitamins, and minerals, making them a popular choice for a healthy diet worldwide (Puglisi and Fernandez, 2022; Myers and Ruxton, 2023). Although eggs are shielded by a hard shell and semipermeable membrane, they remain susceptible to microbial contamination, posing a potential risk of transmitting infectious agents that can induce illness in humans (Verma et al., 2023). Even apparently clean, unbroken, and freshly laid shell eggs may contain harmful bacteria, potentially resulting in egg-borne diseases (Damena et al., 2022). Consuming raw or undercooked eggs carries a notable risk of contamination due to pathogens naturally found in hens (Solís et al., 2023). These pathogens can migrate from the eggshell surface to its inner structures through pores, a process influenced by factors, such as humidity, temperature, and storage duration (Kulshreshtha et al., 2021; Rachtanapun et al., 2022). Inadequate handling and storage of eggs in unhygienic conditions at poultry farms or retail settings can negatively impact egg quality and present a potential health hazard to consumers (Jones et al., 2018; Damena et al., 2022). Previous studies have identified various bacterial species known to cause foodborne illnesses, such as Listeria monocytogenes, Escherichia coli, Salmonella enterica, and Campylobacter jejuni, in table eggs (Fonseca et al., 2014; Kubo et al., 2020; Ricke et al., 2023; Sharan et al., 2023). Staphylococcus spp. have been implicated in causing several diseases in poultry, with approximately 50% of Staphylococcus aureus strains producing enterotoxins capable of causing food poisoning in consumers (Pondit et al., 2018). Staphylococcal food poisoning, ranking third among all foodborne diseases worldwide, is caused by S. aureus, a Gram-positive, facultative anaerobic, nonmotile, nonspore-forming bacterium with a round shape, renowned for its toxic properties (Kadariya et al., 2014; Şanlıbaba, 2022). Staphylococcus aureus is typically found on the skin and in the nasal cavities of humans and animals as a commensal resident, which can spread from its natural habitats to other parts of the body, causing a range of clinical infections and foodborne intoxication (Achek et al., 2021; Raineri et al., 2022). Staphylococcus aureus is a Gram-positive bacterium known for causing a wide range of infections, from minor skin conditions to life-threatening systemic diseases (Lang et al., 2024). Staphylococcus aureus, a medically significant bacterium, causes various infections, ranging from skin and soft tissue issues to more severe conditions such as pneumonia, fasciitis, otitis media, and urinary tract infections (David and Daum, 2010; Tong et al., 2015). Staphylococcus aureus is a leading cause of food poisoning due to its production of heat-stable enterotoxins, which can persist in the food environment and cause illness (Grispoldi et al., 2021; Liang et al., 2023). Enterotoxin-producing S. aureus requires a temperature range of 10°C to 46°C for toxin secretion, with optimal growth and toxin production observed between 30°C and 40°C; conditions outside this range significantly reduce or inhibit toxin production (Argudín et al., 2010; Hennekinne et al., 2012). The rise of multidrug-resistant S. aureus, including MRSA, has challenged healthcare globally, resisting most β-lactam antibiotics such as penicillin and cephalosporins (Alghamdi et al., 2023). Table eggs may contain medically significant bacterial species, including antibiotic-resistant strains, raising concerns (Kapena et al., 2020). Evidence suggests that antimicrobial resistance can be transferred from food-producing animals to humans through the food chain, direct handling, or contact with infected animals and contaminated animal products (Samtiya et al., 2022). The World Organization for Animal Health categorizes antibiotics based on their necessity for animal treatment, impacting both animal and human health settings (Scott et al., 2019; Van et al., 2020). Staphylococcus aureus is a prevalent pathogen causing a range of human infections, from skin issues to severe conditions such as bacteremia and endocarditis (Tong et al., 2015). Resistant strains, such as MRSA and vancomycin-resistant S. aureus (VRSA), present significant treatment challenges (Cong et al., 2020). Enterotoxin-producing S. aureus (SESA) can contaminate food, including eggs, leading to food poisoning (Grispoldi et al., 2021). Staphylococcus aureus, commonly found in food, produces heat-resistant enterotoxins causing food poisoning, while antibiotic-resistant strains pose significant health concerns, with methicillin and vancomycin vital for treatment (Naeim et al., 2023). Table eggs contaminated with antibiotic-resistant Staphylococcus strains pose a risk of foodborne infections in humans, especially MRSA, a significant bacterium that can develop multidrug resistance through horizontal gene transfer (Parvin et al., 2021). The rise of multidrug-resistant (MDR) microorganisms is a global public health concern, making it essential to study the prevalence of S. aureus, its antimicrobial resistance, and clonal lineages across human, animal, and food chain contexts. This research is particularly important given the diverse lifestyles influenced by diverse ethnicities, cultures, and food habits (Lozano et al., 2016; Rao et al., 2022). The proliferation of retail shops, super shops, farm outlets, and wholesale marketplaces selling table eggs in Bangladesh has led to an elevated risk of MRSA transmission to humans through egg intake. However, no investigation has been conducted on the prevalence of MRSA-resistant genes in table eggs within the country. This research hypothesizes that table eggs sold in retail markets within Mymensingh City Corporation, Bangladesh, may harbor MDR-SA, including MRSA, enterotoxin-producing strains (seb), and VRSA. Therefore, the purpose of this study is to isolate and identify S. aureus from eggshells and egg contents, assess their phenotypic and genotypic antibiotic resistance patterns, and detect the presence of MRSA, VRSA, and enterotoxin-producing S. aureus (seb) in both the eggshells and egg contents. Materials and MethodsSample collectionA total of 200 random eggs were collected from different places in Mymensingh City Corporation, including retail shops, super shops, farm outlets, and wholesale markets. Each egg sample was placed in a separate, sterile plastic bag and brought to the Bacteriology Laboratory, Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh, Bangladesh, for testing (Fig. 1).
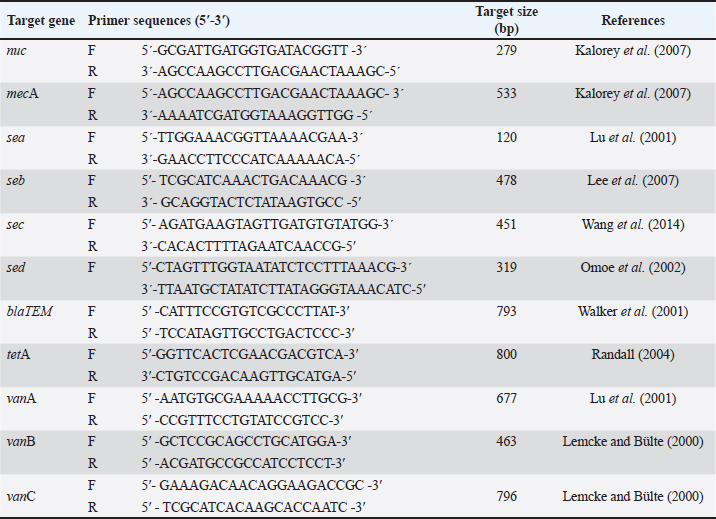
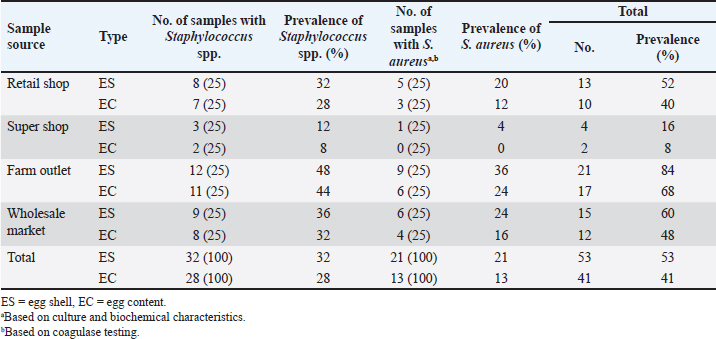
Fig. 1. Map of the site, where the study was conducted. Bacteriological analysisSample preparation and isolation of S. aureus The eggs were swabbed with sterile cotton buds and placed in trypticase soy broth for enrichment. The broth was then incubated in a bacteriological incubator overnight at 37°C. Following enrichment, samples were streaked onto mannitol salt agar (MSA) to isolate S. aureus. Pure colonies were obtained by subculturing all isolates on MSA plates, which were incubated for 24 hours at 37°C. The surfaces of the eggs were disinfected by dipping them in 70% ethanol for 3–5 seconds, followed by air drying in a safety cabinet. Each egg was then cracked using a sterile rod, and the egg contents (the inner membrane, egg white, and yolk) were transferred into a sterile petri dish. Microbial analysis was performed on the mixer comprising three components of the egg: the inner membrane, egg white, and yolk. To isolate S. aureus, 1 mm of the egg mixture contents was diluted tenfold with sterile phosphate-buffered saline (1X PBS; HI Media, India) and homogenized for 1–2 minutes. Then, a 100-µl aliquot of the resulting mixture was spread onto an MSA plate (HI Media) and, after solidification, incubated for 24 hours at 37°C. Identification of S. aureus The identification of S. aureus was conducted by observing the cultural characteristics and colony morphology of MSA. Gram staining method and biochemical tests (catalase test and coagulase test) were performed to identify bacteria. Molecular analysisDNA extraction and polymerase chain reaction (PCR) detection of enterotoxin and antibiotic resistance genes The boiling method was used to extract genomic DNA from isolated organisms (Ahmed and Dablool, 2017). Briefly, a 1 ml sample of the enriched culture was initially centrifuged at 5,000 rpm for 5 minutes. After discarding the supernatant, 200 μl of phosphate buffer solution was added to the pellet to create a suspension. The suspension was then boiled and allowed to cool for 10 minutes, followed by centrifugation at 10,000 rpm for 10 minutes. The resulting supernatant, which contained genomic DNA, was collected and stored at –20°C for future study. A final reaction mixture of 20 μl was used for all PCR experiments, comprising 3 μl of nuclease-free water, 10 μl of master mix (Promega, Madison, WI), 1 μl each of forward and reverse primers, and 5 μl of DNA template. After amplification, the PCR results were analyzed by electrophoresis on a 1.5% agarose gel. The gel was then stained with ethidium bromide and documented using a Biometra UV trans illuminator (Göttingen, Germany). A 100-bp DNA ladder (Promega, Madison, WI) was used as a reference marker to confirm the anticipated size of the amplified PCR products. A gene-specific PCR assay was performed to detect the specific gene associated with the detection of S. aureus. The assay successfully amplified the nuc, mecA, seb, blaTEM, tetA, vanA, and vanC genes using the PCR technique. The oligonucleotide primers used in this study for detecting these genes in S. aureus are listed in Table 1. Antibiotic susceptibility testing The antibiogram of S. aureus was performed using 13 commonly prescribed antibiotics: azithromycin (AZM, 15 μg), erythromycin (E, 5 μg), oxacillin (OX, 1 μg), penicillin (P, 10 μg), cefoxitin (CX, 30 μg), ciprofloxacin (CIP, 5 μg), levofloxacin (LEV, 5 μg), norfloxacin (NX, 10 μg), doxycycline (DO, 30 μg), tetracycline (TE, 30 μg), clindamycin (CD, 2 μg), gentamicin (GEN, 5 μg), and vancomycin (VAN, 30 μg). Prior to testing, each bacterial isolate was standardized to a McFarland 0.5 standard (Nix et al., 2020; Haile et al., 2022). The antibiotic disks were purchased from HI Media, India. The antibiotic sensitivity test was conducted using the disk diffusion method described by Bauer et al. (1966). The zones of inhibition produced by S. aureus were interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2021). Statistical analysisThe Statistical Package for Social Science (SPSS.v.25, IBM, Chicago, IL) and GraphPad Prism (Prism.v.8.4.2, San Diego, CA) were used to conduct analyses after the data from this study were integrated using Excel 365 (Microsoft/Office 365, Redmond, WA). The frequencies of the different variables were determined by descriptive analysis. By applying a prior technique (Brown, 2001) in GraphPad Prism, a binomial 95% confidence interval (CI) was computed to estimate the prevalence. To evaluate the possible relationship between the virulence genes of S. aureus isolates with a p-value of less than 0.05, a bivariate analysis was also carried out using SPSS. Additionally, SPSS bivariate analysis was used to determine any link between the two antibiotics that were shown to be resistant to the isolates. A p-value of less than 0.05 (p < 0.05) denoted statistical significance. Ethical approvalNo ethics approval was required for this study. ResultsPrevalence of Staphylococcus spp. and S. aureusBased on cultural and biochemical assessments, the prevalence of Staphylococcus spp. was 53% (n=100) in eggshell samples and 41% (n=100) in egg content samples. Among these isolates, 21% of the eggshell samples and 13% of the egg content samples were confirmed to be S. aureus via coagulase test. The prevalence of Staphylococcus spp. and S. aureus is summarized in Table 2. Table 1. Oligonucleotide primers used in the PCR assays.
Table 2. The overall prevalence of Staphylococcus spp. and Staphylococcus aureus in table eggshell and content samples.
Table 3. Molecular detection of nuc gene of Staphylococcus aureus from eggshells and contents.
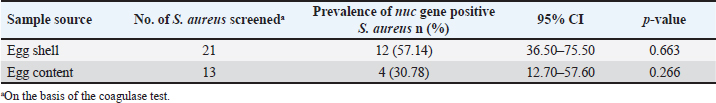
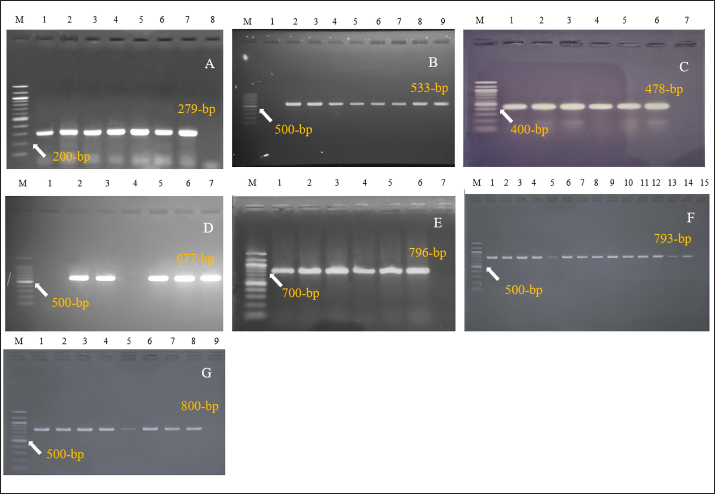
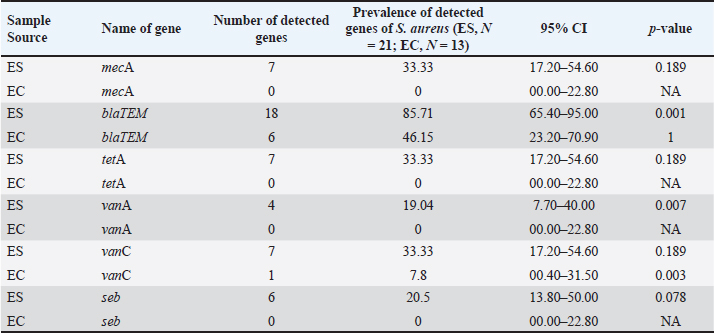
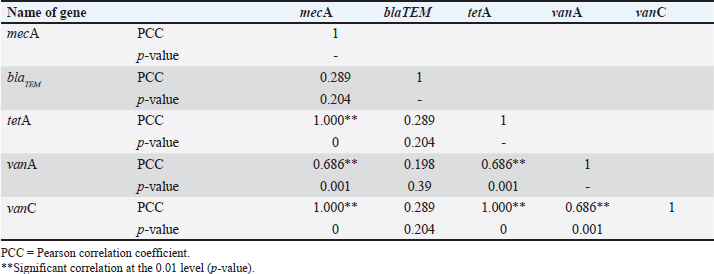
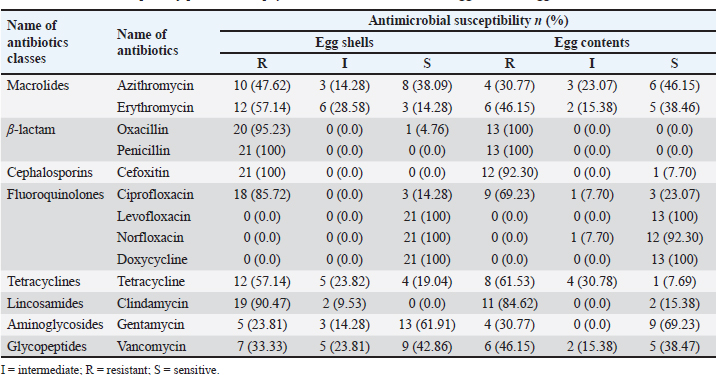
Molecular detection of enterotoxin and antibiotic resistance genesAll 34 coagulase-positive S. aureus isolates were analyzed using PCR to detect the nuc gene. The results showed that 12 out of 21 eggshell isolates (57.14%, CI: 36.50–75.50) and 4 out of 13 egg content isolates (30.78%, CI: 12.70–57.60) were positive for the nuc gene (Table 3 and Fig. 3A). These isolates were also tested for several antibiotic resistance genes (mecA, blaTEM, tetA, vanA, and vanC) and the enterotoxigenic gene (seb). The results indicated that among the eggshell isolates, 7 (33.33%, CI: 17.20–54.60) carried the mecA gene, 18 (85.71%, CI: 65.40–95.00) carried blaTEM, 7 (33.33%, CI: 17.20–54.60) carried tetA, 4 (19.04%, CI: 7.70–40.00) carried vanA, 7 (33.33%, CI: 17.20–54.60) carried vanC, and 6 (20.5%, CI: 13.80–50.00) carried the seb gene (Table 4 and Fig. 3B–G). For the egg content isolates, 6 (46.15%, CI: 23.20–70.90) were positive for the blaTEM gene, and 1 (7.8%, CI: 00.40–31.50) was positive for the vanC gene, respectively (Table 4 and Fig. 3E and F). However, none of the egg content isolates were positive for the mecA, tetA, vanA antibiotic resistance genes and the seb enterotoxin gene. Bivariate analysis revealed strong and significant positive correlations between several virulence genes. Specifically, strong correlations were observed between mecA and tetA (Pearson correlation coefficient, p=1.000), mecA and vanA (p =0.686), mecA and vanC (p=1.000), tetA and vanA (p=0.686), and vanA and vanC (p=0.686). A summary of the correlation results between the antibiotic resistance genes of the S. aureus isolates is provided in Table 5 and Figure 2. Antibiotic susceptibility testing of S. aureus isolated from eggshellsAll 34 coagulase-positive S. aureus isolates were tested for antibiotic susceptibility using eight groups of 13 commonly prescribed antibiotics. Among the 21 isolates from table eggshell samples, high resistance levels were observed to cephalosporins, specifically CX (100%), β-lactam antibiotics such as P (100%) and OX (95%), lincosamides such as CD (90%), and fluoroquinolones, particularly CIP (85%). Resistance to macrolides (E) and tetracyclines (TE) was observed in 57% of all eggshell isolates. The lowest resistance levels were noted for aminoglycosides, such as GEN (23%), and glycopeptides, such as VAN (33%). Conversely, fluoroquinolones, including LEV, NX, and DO, demonstrated the highest sensitivity (100%) across all isolates. Moderate sensitivity was observed for aminoglycosides (GEN [61%]) and glycopeptides (VAN [42%]). Lower sensitivity rates were observed for macrolides (E [14%]) and fluoroquinolones (CIP [14%]). Additionally, 19% of the isolates were sensitive to tetracyclines (TE) (Table 6 and Fig. 4).
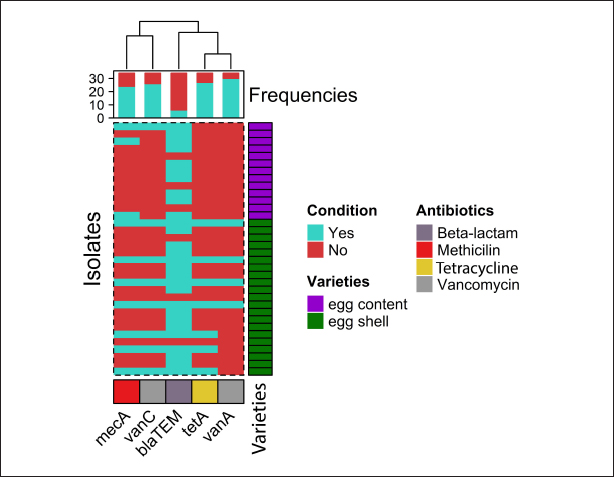
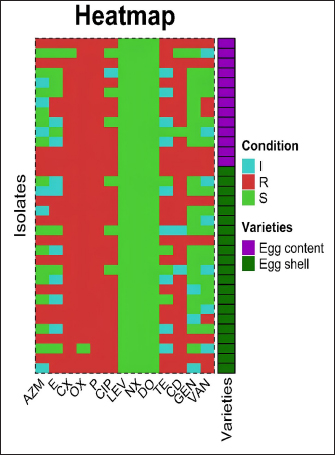
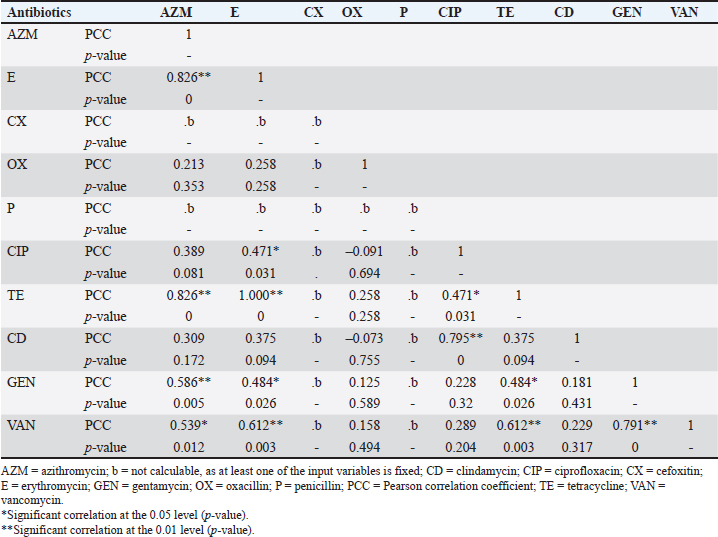
Fig. 2. The heatmap represents the distribution of the different types of antibiotic-resistant genes of Staphylococcus aureus isolated from eggshells and contents. Here, the top of the dendrogram represents the frequency of present genes and the neighborhood relationship among the genes. These resistance and sensitivity patterns were further supported by strong and statistically significant positive associations observed between several antibiotics in the correlation analysis. Notably, AZM showed strong correlations with E (Pearson correlation coefficient, p=0.826), TE (p=0.826), GEN (p =0.586), and VAN (p=0.539). This finding aligns with the observed resistance trends, in which E and TE displayed notable resistance levels, whereas GEN and VAN showed lower resistance. Similarly, E was strongly correlated with CIP (p=0.471), a fluoroquinolone that exhibited both high resistance and low sensitivity, as well as CD (p=0.795), reflecting the resistance trends in lincosamide. Further strong associations were noted between E and TE (p=1.000), CIP and TE (p=0.471), E and GEN (p=0.484), TE and GEN (p=0.484), E and VAN (p=0.612), TE and VAN (p=0.612), and GEN and VAN (p=0.791). These correlations underscore the complex relationship between resistance patterns and antibiotic effectiveness, particularly among macrolides, aminoglycosides, and glycopeptides (Table 7). Antibiotic susceptibility testing of S. aureus isolated from egg contentsThirteen coagulase-positive S. aureus isolates from table egg contents were tested for antibiotic susceptibility. The higher resistance levels were observed to β-lactam antibiotics, including P (100%) and OX (100%), cephalosporins such as CX (92%), lincosamides such as CD (84%), fluoroquinolones such as CIP (69%), and tetracyclines, specifically TE (61%). Additionally, 30% of the isolates showed resistance to macrolides (E) and aminoglycosides (GEN), whereas 46% were resistant to VAN. Among the egg content isolates, all (100%) were sensitive to LEV and DO, with NX and GEN showing sensitivity in 92% and 69% of the isolates, respectively. Sensitivity to E and VAN was observed in 38% of the isolates. The lowest sensitivity rates were observed for CX and TE, with only 7% of the isolates responding to these antibiotics (Table 6 and Fig. 4). These resistance and sensitivity patterns align with the statistical findings of the bivariate analysis, in which strong and positive significant correlations were observed between several antibiotics. In this study, AZM was strongly correlated with E (p=0.033, coefficient=0.592), GEN (p=0.001, coefficient=0.822), and VAN (p=0.033, coefficient=0.592). These correlations reflect the observed resistance trends, particularly in the case of E, GEN, and VAN, in which resistance was noted in 30% and 46% of the isolates, respectively.
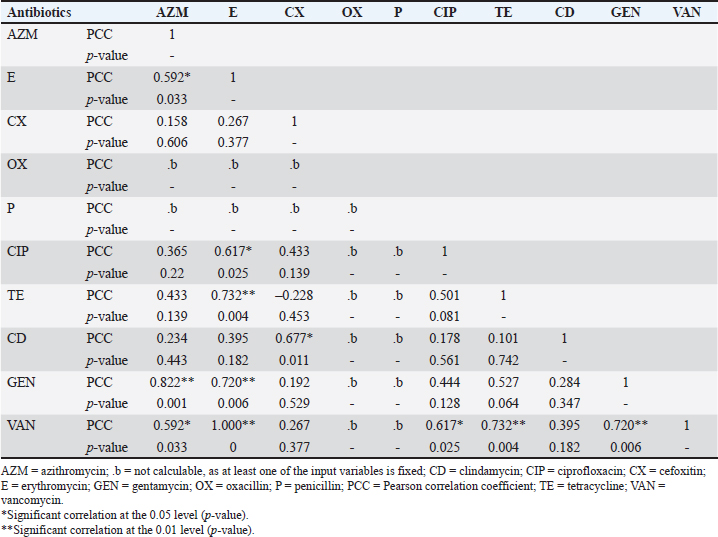
Fig. 3. PCR detection of Staphylococcus aureus. (A) Amplification of 279-bp fragment of nuc gene (Lane 1–6: DNA samples, Lane M: 100-bp size DNA marker, Lane 7: positive control, and Lane 8: negative control) of S. aureus. (B) Amplification of 533-bp fragment of mecA gene (Lane 2–8: DNA samples, Lane M: 100-bp size DNA marker, Lane 9: positive control, and Lane 1: negative control) of S. aureus. (C) Amplification of 478-bp fragment of seb gene. (Lane 1–5: DNA samples, Lane M: 100-bp size DNA marker, Lane 6: positive control, and Lane 7: negative control) of S. aureus (D) Amplification of 677-bp fragment of vanA gene (Lane 2, 3, 5, and 6: DNA samples, Lane M: 100-bp size DNA marker, Lane 7: positive control, and Lane 4: negative control) of S. aureus. (E) Amplification of 796-bp fragment of vanC gene (Lane 1–5: DNA samples, Lane M: 100-bp size DNA marker, Lane 6: positive control, and Lane 7: negative control) of S. aureus. (F) Amplification of 793-bp fragment of blaTEM gene (Lane 1–13: DNA samples, Lane M: 100-bp size DNA marker, Lane 14: positive control, and Lane 15: negative control) of S. aureus. (G) Amplification of 800-bp fragment of tetA gene (Lane 1–7: DNA samples, Lane M: 100-bp size DNA marker, Lane 8: positive control, and Lane 9: negative control) of S. aureus. Additionally, E exhibited strong correlations with CIP (p=0.025, coefficient=0.617), TE (p=0.004, coefficient=0.732), and VAN (p=1.000, coefficient=1.000), supporting the resistance trends observed for these antibiotics. The correlation between E and CIP, for instance, is consistent with the moderate resistance levels observed for CIP (69%) and E (30%). Moreover, significant correlations between CIP and VAN (p=0.617, coefficient=0.617), TE and VAN (p=0.732, coefficient=0.732), and GEN and VAN (p=0.720, coefficient=0.720) underscore the observed patterns of resistance and sensitivity. The resistance observed in VAN (46%) is particularly notable in light of these correlations, which highlight the complex interplay between these antibiotics in egg content isolates (Table 8). Table 4. Molecular detection of enterotoxin and antibiotic resistance genes of Staphylococcus aureus isolated from eggshells and contents.
Table 5. Pearson correlation with different types of antibiotic resistance genes of eggshell isolates.
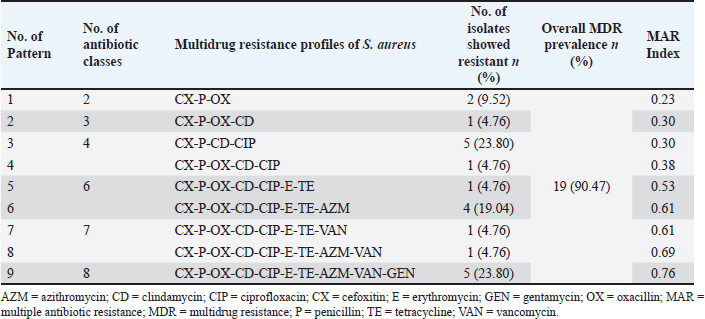
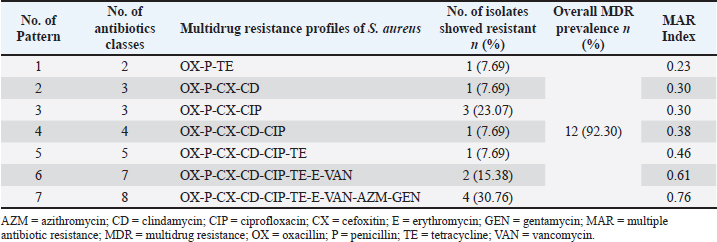
Phenotypic MDR of S. aureus isolated from eggshells and egg contentsOut of 21 eggshell coagulase-positive S. aureus isolates, 19 (95% CI: 71.1%–98.3%) were identified as MDR based on their phenotype. A total of nine resistance patterns were observed, of which eight were classified as MDR patterns. The overall prevalence of MDR was 90.47%. Among the MDR patterns, pattern number 3 (CX-P-CD-CIP) and pattern number 9 (CX-P-OX-CD-CIP-E-TE-AZM-VAN-GEN) were the most frequently observed, each found in 5 out of 21 isolates (23.80%, 95% CI: 10.6%–45.1%). Another notable MDR pattern, number 6 (CX-P-OX-CD-CIP-E-TE-AZM), was found in 4 out of 21 isolates (19.04%, 95% CI: 0.77%–40.0%). In contrast, resistance pattern number 1 (CX-P-OX) was not considered MDR in terms of phenotype, although it was present in two isolates (95% CI: 0.17%–28.9%). Additionally, five isolates exhibited resistance to four different classes of antibiotics, corresponding to patterns 2, 4, 5, 7, and 8 (Table 9). Similarly, of 13 coagulase-positive S. aureus isolates from the egg content, 12 (95% CI: 66.7%–99.6%) were identified as MDR based on their phenotype. Eight resistance patterns were observed, of which seven were classified as MDR patterns, resulting in an overall MDR prevalence of 92.30%. The most frequently observed MDR patterns were pattern number 2 (OX-P-CX-CIP) and pattern number 7 (OX-P-CX-CD-CIP-TE-E-VAN-AZM-GEN), which were present in 3 out of 13 isolates (23.07%, 95% CI: 0.82%–50.3%) and 4 out of 13 isolates (30.76%, 95% CI: 12.7%–57.6%), respectively. Resistance pattern number 1 (OX-P-TE) was detected in 2 isolates (95% CI: 0.17%–28.9%) but was not classified as MDR phenotypically. Antibiotic resistance was detected in seven isolates against four different classes of antibiotics, corresponding to patterns 2, 4, 5, 7, and 8 (Table 10). The antibiotic resistance profiling of each S. aureus isolates varied, with multiple antibiotic resistance indices ranging from 0.23 to 0.76 (Tables 9 and 10). Table 6. Antibiotic susceptibility profiles of Staphylococcus aureus isolated from eggshells and egg contents.
Fig. 4. Antibiotic susceptibility profiles of S. aureus isolated from eggshells and contents isolate. I=Intermediate; R=Resistant; S=Sensitive; AZM=Azithromycin; E=Erythromycin; CX=Cefoxitin; OX=Oxacillin; P=Penicillin; CIP=Ciprofloxacin; TE=Tetracycline; CD=Clindamycin; GEN=Gentamycin; VAN=Vancomycin. DiscussionThe issue of antibiotic-resistant bacteria, particularly those caused by resistant strains of S. aureus, poses a significant challenge to public health. This challenge is especially pronounced in individuals undergoing prolonged antibiotic treatment, where controlling infections becomes increasingly difficult (Nwobodo et al., 2022; Tălăpan et al., 2023). The spread of resistant bacteria to animals, the environment, and food is a growing concern, especially because the antimicrobial resistance profiles of S. aureus strains found in egg samples are alarmingly similar to those identified in humans. This resemblance, particularly in certain regions of the world, is a cause for concern and underscores the need for vigilant monitoring and control measures (Manyi-Loh et al., 2018; Samreen et al., 2021). When food contaminated with S. aureus is ingested, it can lead to a severe health condition known as staphylococcal food poisoning (Romano et al., 2023). Eggs, which are easily contaminated, can harbor S. aureus, and even cooking may not completely eliminate the heat-stable enterotoxins produced by this bacterium (Grispoldi et al., 2021; Khoothiam et al., 2023). Limited studies on S. aureus in eggs have demonstrated that the bacteria can be present on the eggshell, yolk, and egg white, with some isolates also exhibiting resistance to antimicrobials (Zhang et al., 2023). Table 7. Pearson correlation coefficient to assess the pairs of any of the two antibiotic-resistant Staphylococcus aureus isolated from table eggshell samples.
Previous studies have shown that bacteria can penetrate eggshells (Gole et al., 2014; Kulshreshtha et al., 2021). The widespread use of antibiotics in treating farm animals and in poultry feed may contribute to the presence of MRSA in eggs, facilitating the infection of eggs by resistant bacteria (Abreu et al., 2023). The current study aimed to detect S. aureus in egg samples collected from various retail sources to understand its prevalence, molecular characteristics, antimicrobial sensitivity, and MDR profile in Bangladesh. This research sheds light on how bacteria enter eggs, potentially through human or animal sources. The study found that Staphylococcus spp. were present in 53% of eggshell samples and 41% of egg contents. Among these, 21% of the eggshell isolates and 13% of the egg content isolates were confirmed as S. aureus. These findings emphasize the presence of potentially harmful bacteria in both the outer and inner parts of table eggs, underscoring the importance of good hygiene practices in egg production and consumption to ensure food safety and protect public health. Previous studies have indicated that bacteria, especially Staphylococcus spp., can be found on the outer shell of eggs (Verma et al., 2023). Punom et al. (2020) investigated unhatched leftover duck eggs from selected mini hatcheries in Kishoreganj, Bangladesh, and found a high prevalence of Salmonella spp., E. coli, Staphylococcus spp., and Clostridium spp. in unhatched leftover duck eggs. These bacterial species potentially contribute to decreased hatchability and increased embryo mortality. Pondit et al. (2018) also reported a high prevalence rate of S. aureus in chicken and quail eggshells in Mymensingh, Bangladesh. Table 8. Pearson correlation coefficient to assess the pairs of any of the two antibiotic-resistant Staphylococcus aureus isolated from table egg content samples.
Table 9. Multidrug resistance profiles of Staphylococcus aureus isolated from table eggshells.
Table 10. Multidrug resistance profiles of Staphylococcus aureus isolated from table egg contents.
The MDR profile analysis of coagulase-positive S. aureus isolates from egg content samples revealed a high prevalence of MDR, with 92.30% of isolates exhibiting resistance to multiple antibiotic classes. Previous studies have reported similarly high levels of MDR, with 95.10% of isolates from Shanghai and 12.5%–100% of isolates from S. aureus from Bangladesh showing similar resistance patterns (Ou et al., 2020; Ain et al., 2022). The most common resistance patterns observed in this study were OX, P, CX, CD, CIP, TE, E, VAN, AZM, and gentamycin (GEN). Similar resistance patterns have been reported in studies conducted in South Africa, northeast Ethiopia, and Panama (Shittu and Lin, 2006; Kibret and Abera, 2011; Mejía et al., 2021). Notably, certain resistance patterns were frequently observed, indicating the presence of potentially dominant strains with broad antibiotic resistance profiles (Russo et al., 2020; Bashir et al., 2023). The present study also revealed significant differences in the prevalence of virulence and antibiotic resistance genes between coagulase-positive S. aureus isolates from eggshell and egg content samples. The eggshell, which is the primary barrier between the external environment and the egg interior, is more susceptible to contamination from various sources, such as handling, storage conditions, and exposure to contaminated surfaces (De Reu et al., 2006; Kulshreshtha et al., 2021). This external exposure increases the likelihood of acquiring diverse antibiotic resistance genes (Kunhikannan et al., 2021). In contrast, the egg content is relatively protected and is less exposed to environmental contaminants, which may explain the lower prevalence and diversity of resistance genes. Differences in the microenvironments of the eggshell and egg content could also influence bacterial adaptation and gene expression, contributing to the observed variations in gene prevalence (Grizard et al., 2015; Chen et al., 2019). Specifically, 57.14% of eggshell isolates were positive for the nuc gene, compared with 30.78% of egg content isolates. Antibiotic resistance genes were more prevalent in eggshell isolates, with blaTEM detected in 85.71% and mecA detected in 33.33% of these isolates. In egg content isolates, blaTEM was found in 46.15% of isolates and vanC in 7.8%, while none tested positive for mecA, tetA, vanA, or the enterotoxigenic gene seb. Similar patterns of antibiotic resistance and distribution of resistance genes have been detected in various bacteria, including Salmonella and S. aureus, across different bird species, such as chickens and quails (Pondit et al., 2018; Ganjeer et al., 2023). These findings underscore the higher prevalence and diversity of antibiotic resistance genes in eggshell isolates than in egg content isolates, highlighting potential differences in contamination sources or bacterial adaptation mechanisms. The strong and statistically significant positive correlations observed between certain antibiotic resistance genes in the S. aureus isolates suggest a consistent co-occurrence of these genes, likely due to co-selection or co-inheritance mechanisms (Tasneem et al., 2022; Murray et al., 2024). The high correlation between mecA and tetA, mecA and vanC, and vanA and vanC (with Pearson correlation coefficients of 1.000) indicates that these genes often appear together within the same bacterial isolates (Ballah et al., 2022; Nepal et al., 2023). This co-occurrence may result from genetic linkage, where resistance genes are located close to each other on mobile genetic elements such as plasmids or transposons, facilitating their simultaneous transfer during bacterial reproduction or horizontal gene transfer (Stalder et al., 2012; Partridge et al., 2018). Additionally, exposure to multiple antibiotics can create selective pressure, encouraging the survival of bacteria harboring multiple resistance genes and further promoting the co-selection of these genes (Davies and Davies, 2010). The notable patterns of antibiotic resistance and sensitivity observed among coagulase-positive S. aureus isolates from eggshell and egg content samples can be attributed to several factors. The high levels of resistance to cephalosporins (CX), β-lactam antibiotics (P and OX), lincosamides (CD), and fluoroquinolones (CIP) suggest widespread and possibly prolonged exposure to these antibiotics, either in veterinary medicine or through environmental contamination, leading to selective pressure favoring resistant strains (Ali et al., 2022; Urban-Chmiel et al., 2022). Moderate resistance to macrolides (E) and tetracyclines (TE) indicates some level of exposure, but not as intense or frequent as with previously mentioned antibiotics (Chopra and Roberts, 2001; LaPlante et al., 2022). The lower levels of resistance to aminoglycosides (GEN) and glycopeptides (VAN) could be due to their less frequent use or effective regulatory measures limiting their application in agriculture and veterinary practices (Jain et al., 2011; Miller et al., 2020). On the other hand, the highest sensitivity observed with fluoroquinolones (LEV, NX, and DO) suggests that these antibiotics may not be commonly used or that they remain effective against these S. aureus isolates, maintaining their efficacy (Clay et al., 2021). The moderate sensitivity to aminoglycosides (GEN) and glycopeptides (VAN) further supports this, indicating that although resistance exists, these antibiotics retain substantial activity against the isolates (Adhikari, 2010). The lower sensitivity to macrolides (E) and fluoroquinolones (CIP) points to emerging resistance, but not yet at a critical level, while the moderate sensitivity to tetracyclines (TE) suggests they remain effective to a certain extent but may be under increasing pressure (Wieczorek and Osek, 2013; Urban-Chmiel et al., 2022). The high levels of antibiotic resistance found in S. aureus isolates from table eggs pose serious concerns about public health and the food industry. These findings underscore the crucial need for stronger regulatory mechanisms to monitor and limit antibiotic usage in chicken agriculture. Antibiotic stewardship initiatives combined with stricter limits on the use of important antibiotics may help reduce the emergence and spread of resistance strains. By aligning with established guidelines, such as those set by the World Health Organization and regulatory bodies such as the FDA, the food industry can contribute significantly to the global fight against antimicrobial resistance. The bivariate analysis demonstrated strong and statistically significant positive correlations between certain pairs of antibiotics used against eggshell isolates, suggesting potential mechanisms of co-resistance or co-selection (Pouwels et al., 2019; Robas et al., 2021). The strong correlations between AZM and E, AZM and TE, AZM, and GEN indicate that resistance to these antibiotics may be linked, possibly due to the presence of multi-resistance plasmids or genes that confer resistance to multiple drugs simultaneously (Chen et al., 2013; Abotsi et al., 2022; Heidary et al., 2022). Moderate correlations observed between AZM and VAN, E and CIP, and CIP and CD suggest a less direct but still significant association between resistance mechanisms for these antibiotics (Arabestani et al., 2017; Yamanaka et al., 2019). These relationships may be influenced by similar selective pressures or genetic elements that carry resistance determinants for multiple antibiotics (Munita and Arias, 2016). Strong correlations between E and TE, as well as between GEN and VAN, further support the idea of shared resistance pathways or genetic linkages (Chopra and Roberts, 2001; Conwell et al., 2017). Additionally, moderate correlations between E and GEN, TE and GEN, E and VAN, TE and VAN, and GEN and VAN highlight a network of interconnected resistance traits, reinforcing the complexity of antibiotic resistance patterns (Baquero et al., 2021). The bivariate analysis of egg content isolates also revealed strong and statistically significant positive correlations between several pairs of antibiotics, indicating potential patterns of co-resistance or co-selection. Specifically, AZM exhibited strong correlations with E, GEN, and VAN, suggesting that resistance mechanisms affecting AZM may also confer resistance to E, GEN, and VAN, possibly due to shared resistance genes or the presence of multi-resistance plasmids that carry these genes together (Vrancianu et al., 2020; Nusrin et al., 2022). Additionally, E showed strong correlations with CIP and TE, indicating potential cross-resistance or co-selection mechanisms (Shariati et al., 2022). This suggests that the use of E might be selected for bacteria that are also resistant to CIP and TE, likely because of linked genetic elements or overlapping resistance pathways (Shariati et al., 2022; Hasan et al., 2023). The strong correlation between CX and CD suggests a similar association, where resistance to one could imply resistance to the other, potentially due to co-located resistance genes (Duche et al., 2023). Furthermore, the strong correlations between CIP, TE, GEN, and VAN with each other emphasize a network of interconnected resistance traits, indicating that resistance to one of these antibiotics often coincides with resistance to others. This interconnected resistance complicates treatment options and highlights the necessity for careful antibiotic use and comprehensive resistance management strategies (Gajdács et al., 2021; Muteeb et al., 2023). The high prevalence of MDR among coagulase-positive S. aureus isolates from both eggshell and egg content samples, with prevalence of 90.47% and 92.30%, respectively, can be attributed to several factors. The frequent observation of resistance patterns involving CX, P, OX, CD, CIP, E, TE, AZM, VAN, and GEN suggests the widespread use of these antibiotics in agricultural settings (Parvin et al., 2021; Ballah et al., 2022). This widespread use likely exerts selective pressure on bacterial populations, favoring the survival and proliferation of resistant strains (Tello et al., 2012). The frequent occurrence of certain resistance patterns indicates the presence of dominant strains with broad antibiotic resistance profiles (Russo et al., 2020; Sultan et al., 2020). These strains could have acquired multiple resistance genes through horizontal gene transfer mechanisms, such as conjugation, transformation, or transduction, often facilitated by mobile genetic elements such as plasmids, transposons, and integrons (Bello-López et al., 2019; Michaelis and Grohmann, 2023). Additionally, the shared resistance patterns between eggshell and egg content isolates suggest that these dominant strains are capable of colonizing both the external and internal environments of the eggs, possibly due to contamination during handling and processing (De Reu et al., 2006; Gantois et al., 2009). The study’s limitations include geographic bias and a narrow sampling of egg sources, potentially restricting its broad applicability. Future research should expand regionally, include diverse egg types and supply chain stages, and explore contamination trends through longitudinal studies. These findings underscore the importance of stringent antibiotic stewardship practices and improving biosecurity measures in poultry farming to reduce the selection pressure for resistant strains and prevent the spread of MDR bacteria. ConclusionThe findings of this study indicate the significant presence of MDR S. aureus in eggs collected from the market of Mymensingh City Corporation, Bangladesh. This high prevalence poses a public health risk for consumers purchasing eggs from retail shops, supermarkets, local markets, and farm outlets. The presence of MDR S. aureus in eggs also highlights the potential for the spread of resistant strains to humans through the food chain. Implementing proper sanitation and biosafety measures is essential to mitigate this public health threat. The antibiotic resistance profiles identified in S. aureus strains from table egg samples in this study provide a valuable reference for future research. Monitoring antibiotic use in livestock and farm animals is crucial for reducing the risk of MDR S. aureus infection. Expanding similar studies to other regions of Bangladesh and other countries would help determine the prevalence and distribution of MDR strains on a broader scale. AcknowledgmentsThe authors would like to acknowledge the Ministry of Education (MoE) for funding this research. Conflict of interestThe authors declare no conflict of interest. FundingThe Ministry of Education (MoE) funded this research (Project No. LS 2018784/MoE). Author’s contributionsPB designed and conducted the experiment, collected data, analyzed data, and wrote the manuscript. KAS, MBL, and MZR wrote the manuscript, collected samples, conducted the experiment, helped in various molecular research and analysis, and interpreted the results. TA, PS, and MM conducted laboratory work, gathered data, and performed a review. MMK participated in the methodology, wrote the first draft, reviewed it, revised the final version, and edited the manuscript. MAI conceptualized and designed the experiment, developed the draft, arranged funds, critically analyzed the data, and wrote and revised the final version of the manuscript. All authors contributed to the review of the manuscript and approved the final manuscript. Data availabilityThe data generated from this study might be available on a valid request from the corresponding author. ReferencesAbotsi, R.E., Nicol, M.P., McHugh, G., Simms, V., Rehman, A.M., Barthus, C., Ngwira, L.G., Kwambana-Adams, B., Heyderman, R.S., Odland, J.Ø., Ferrand, R.A. and Dube, F.S. 2022. The impact of long-term azithromycin on antibiotic resistance in HIV-associated chronic lung disease. ERJ Open Res. 8, 00491–2021. Abreu, R., Semedo-Lemsaddek, T., Cunha, E., Tavares, L. and Oliveira, M. 2023. Antimicrobial drug resistance in poultry production: current status and innovative strategies for bacterial control. Microorganisms 11, 953. Achek, R., El-Adawy, H., Hotzel, H., Hendam, A., Tomaso, H., Ehricht, R., Neubauer, H., Nabi, I., Hamdi, T.M. and Monecke, S. 2021. Molecular characterization of Staphylococcus aureus isolated from human and food samples in Northern Algeria. Pathogens 10, 1276. Adhikari, L. 2010. High-level aminoglycoside resistance and reduced susceptibility to vancomycin in nosocomial enterococci. J. Glob. Infect. Dis. 2, 231. Ahmed, O.B. and Dablool, A.S. 2017. Quality improvement of the DNA extracted by boiling method in Gram negative bacteria. Int. J. Bioassays. 6, 5347. Ain, Q., Arif, M., Ema, F., Shathi, M., Islam, M. and Khatun, M. 2022. Multidrug-resistant Staphylococcus aureus isolated from chicken nuggets sold at superstores in Mymensingh, Bangladesh. J. Adv. Vet. Anim. 9, 601. Alghamdi, B.A., Al-Johani, I., Al-Shamrani, J.M., Alshamrani, H.M., Al-Otaibi, B.G., Almazmomi, K. and Yusof, N.Y. 2023. Antimicrobial resistance in methicillin-resistant staphylococcus aureus. Saudi J. Biol. Sci. 30, 103604. Ali, S.A., Taj, M.K., Ali, S.H., Farooqi, S., Hussain, A., Azam, S. and Hanif, Z.U.N. 2022. Emerging resistance of antibiotics among various bacterial infections. Int. J. Biosci. 21, 121–168. Arabestani, M.R., Nasaj, M. and Mousavi, S.M. 2017. Correlation between infective factors and antibiotic resistance in Enterococci clinical isolates in West of Iran. Chonnam Med. J. 53, 56. Argudín, M.Á., Mendoza, M.C. and Rodicio, M.R. 2010. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2, 1751–1773. Ballah, F.M., Islam, M.S., Rana, M.L., Ullah, M.A., Ferdous, F.B., Neloy, F.H., Ievy, S., Sobur, M.A., Rahman, A.T., Khatun, M.M., Rahman, M. and Rahman, M.T. 2022. Virulence determinants and methicillin resistance in biofilm-forming Staphylococcus aureus from various food sources in Bangladesh. Antibiotics (Basel) 11, 1666. Baquero, F., Martínez, J.L., Lanza, F.V., Rodríguez-Beltrán, J., Galán, J.C., San Millán, A., Cantón, R. and Coque, T.M. 2021. Evolutionary pathways and trajectories in antibiotic resistance. Clin. Microbiol. Rev. 34, e0005019. Bashir, N., Dablool, A.S., Khan, M.I., Almalki, M.G., Ahmed, A., Mir, M.A., Hamdoon, A.A.E., Elawad, M.A., Mosa, O.F., Niyazov, L.N., Elkhalifa, M.E.M., Alghamdi, M.A., Anwar, A. and Ayaz, M. 2023. Antibiotics resistance as a major public health concern: a pharmaco-epidemiological study to evaluate prevalence and antibiotics susceptibility-resistance pattern of bacterial isolates from multiple teaching hospitals. J. Infect. Public Health 16, 61–68. Bauer, A.W., Kirby, W.M.M., Sherris, J.C. and Turck, M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin Pathol. 45(4_ts), 493–496. Bello-López, J.M., Cabrero-Martínez, O.A., Ibáñez-Cervantes, G., Hernández-Cortez, C., Pelcastre-Rodríguez, L.I., Gonzalez-Avila, L.U. and Castro-Escarpulli, G. 2019. Horizontal gene transfer and its association with antibiotic resistance in the genus Aeromonas spp. Microorganisms 7, 363. Brown, A.M. 2001. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput. Methods Programs Biomed. 65, 191–200. Chen, L., Song, Y., Wei, Z., He, H., Zhang, A. and Jin, M. 2013. Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J. Vet. Med. Sci. 75, 583–587. Chen, X., Li, X., He, Z., Hou, Z., Xu, G., Yang, N. and Zheng, J. 2019. Comparative study of eggshell antibacterial effectivity in precocial and altricial birds using Escherichia coli. PLoS One 14, e0220054. Chopra, I. and Roberts, M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. 65, 232–260. Clay, K.A., Hartley, M.G., Armstrong, S., Bewley, K.R., Godwin, K., Rayner, E., Vipond, J., Bailey, M., Atkins, T.P. and Norville, I.H. 2021. Evaluation of the efficacy of doxycycline, ciprofloxacin, levofloxacin, and co-trimoxazole using in vitro and in vivo models of Q fever. Antimicrob. Agents. Chemother. 65, e0067321. CLSI. 2021. Performance standards for antimicrobial susceptibility testing; document M100, 31st ed. Berwyn, PA: Clinical and Laboratory Standards Institute. Cong, Y., Yang, S. and Rao, X. 2020. Vancomycin resistant Staphylococcus aureus infections: a review of case updating and clinical features. J. Adv. Res. 21, 169–176. Conwell, M., Daniels, V., Naughton, P.J. and Dooley, J.S.G. 2017. Interspecies transfer of vancomycin, erythromycin and tetracycline resistance among Enterococcus species recovered from agrarian sources. BMC Microbiol. 17, 19. Damena, A., Mikru, A., Adane, M. and Dobo, B. 2022. Microbial profile and safety of chicken eggs from a poultry farm and small-scale vendors in Hawassa, Southern Ethiopia. J. Food Qual. 2022, 1–16. David, M.Z. and Daum, R.S. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23, 616–687. Davies, J. and Davies, D. 2010. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433. De Reu, K., Grijspeerdt, K., Messens, W., Heyndrickx, M., Uyttendaele, M., Debevere, J. and Herman, L. 2006. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 112, 253–260. Duche, R.T., Singh, A., Wandhare, A.G., Sangwan, V., Sihag, M.K., Nwagu, T.N.T., Panwar, H. and Ezeogu, L.I. 2023. Antibiotic resistance in potential probiotic lactic acid bacteria of fermented foods and human origin from Nigeria. BMC Microbiol. 23, 142. Fonseca, B.B., Beletti, M.E., de Melo, R.T., Mendonça, E.P., Coelho, L.R., Nalevaiko, P.C. and Rossi, D.A. 2014. Campylobacter jejuni in commercial eggs. Braz. J. Microbiol. 45, 76–79. Gajdács, M., Bátori, Z. and Burián, K. 2021. Interplay between phenotypic resistance to relevant antibiotics in gram-negative urinary pathogens: a data-driven analysis of 10 years’ worth of antibiogram data. Life 11, 1059. Ganjeer, T., Patyal, A., Shakya, S., Chandrakar, C., Verma, S.K., Gade, N.E., Sannat, C., Naik, V.K. and Parkar, S. 2023. Antibiotic resistance pattern and distribution of resistance genes in Salmonella isolated from chicken and duck eggs in Chhattisgarh, India. Asian J. Dairy Food Res. 2033, 1–8. Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., Gast, R., Humphrey, T.J. and Van Immerseel, F. 2009. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 33, 718–738. Gole, V.C., Chousalkar, K.K., Roberts, J.R., Sexton, M., May, D., Tan, J. and Kiermeier, A. 2014. Effect of egg washing and correlation between eggshell characteristics and egg penetration by various Salmonella Typhimurium strains. PLoS One 9, e90987. Grispoldi, L., Karama, M., Armani, A., Hadjicharalambous, C. and Cenci-Goga, B.T. 2021. Staphylococcus aureus enterotoxin in food of animal origin and staphylococcal food poisoning risk assessment from farm to table. Ital. J. Anim. Sci. 20, 677–690. Grizard, S., Versteegh, M.A., Ndithia, H.K., Salles, J.F. and Tieleman, B.I. 2015. Shifts in bacterial communities of eggshells and antimicrobial activities in eggs during incubation in a ground-nesting passerine. PLoS One 10, e0121716. Haile, Z., Mengist, H.M. and Dilnessa, T. 2022. Bacterial isolates, their antimicrobial susceptibility pattern, and associated factors of external ocular infections among patients attending eye clinic at Debre Markos Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS One 17, e0277230. Hasan, M., Wang, J. and Ahn, J. 2023. Ciprofloxacin and tetracycline resistance cause collateral sensitivity to aminoglycosides in Salmonella typhimurium. Antibiotics 12, 1335. Heidary, M., Ebrahimi Samangani, A., Kargari, A., Kiani Nejad, A., Yashmi, I., Motahar, M., Taki, E. and Khoshnood, S. 2022. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 6, e24427. Hennekinne, J.A., De Buyser, M.L. and Dragacci, S. 2012. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol. Rev. 36, 815–836. Jain, S., Kumar, A., Kashyap, B. and Kaur, I. 2011. Clinico-epidemiological profile and high-level aminoglycoside resistance in enterococcal septicemia from a tertiary care hospital in east Delhi. Int. J. Appl. Basic Med. Res. 1, 80. Jones, D.R., Ward, G.E., Regmi, P. and Karcher, D.M. 2018. Impact of egg handling and conditions during extended storage on egg quality. Poult. Sci. 97, 716–723. Kadariya, J., Smith, T.C. and Thapaliya, D. 2014. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. Biomed Res. Int. 2014, 1–9. Kalorey, D.R., Shanmugam, Y., Kurkure, N.V., Chousalkar, K.K. and Barbuddhe, S.B. 2007. PCR-based detection of genes encoding virulence determinants in Staphylococcus aureus from bovine subclinical mastitis cases. J. Vet. Sci. 8, 151. Kapena, M.S., Muma, J.B., Mubita, C.M. and Munyeme, M. 2020. Antimicrobial resistance of Escherichia coli and Salmonella in raw retail table eggs in Lusaka, Zambia. Vet. World 13, 2528–2533. Khoothiam, K., Prapasawat, W., Yosboonruang, A., Rawangkan, A., Phuangsri, C., Rupprom, K., Kraivuttinun, P., Tanomsridachchai, W., Suthienkul, O. and Siriphap, A. 2023. Prevalence, antimicrobial resistance, and enterotoxin gene profiles of Staphylococcus aureus isolated from mobile phones of the food vendors in Phayao province, Thailand. Ann. Clin. Microbiol. Antimicrob. 22, 68. Kibret, M. and Abera, B. 2011. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr. Health Sci. 11, 40–45. Kubo, I., Kajiya, M., Aramaki, N. and Furutani, S. 2020. Detection of Salmonella enterica in egg yolk by PCR on a microfluidic disc device using immunomagnetic beads. Sensors 20, 1060. Kulshreshtha, G., Benavides-Reyes, C., Rodriguez-Navarro, A.B., Diep, T. and Hincke, M.T. 2021. Impact of different layer housing systems on eggshell cuticle quality and Salmonella adherence in table eggs. Foods 10, 2559. Kunhikannan, S., Thomas, C.J., Franks, A.E., Mahadevaiah, S., Kumar, S. and Petrovski, S. 2021. Environmental hotspots for antibiotic resistance genes. MicrobiologyOpen 10, e1197. Lang, J.C., Shahata, M. and Melican, K. 2024. Towards sustainable antimicrobial therapies for Staphylococcus aureus skin infections. Sustainable Microbiol. 1, qvae023. LaPlante, K.L., Dhand, A., Wright, K. and Lauterio, M. 2022. Re-establishing the utility of tetracycline-class antibiotics for current challenges with antibiotic resistance. Ann. Med. 54, 1686–1700. Lee, T.C., Carrick, M.M., Scott, B.G., Hodges, J.C. and Pham, H.Q. 2007. Incidence and clinical characteristics of methicillin-resistant Staphylococcus aureus necrotizing fasciitis in a large urban hospital. Am. J. Surg. 194, 809–813. Lemcke, R. and Bülte, M. 2000. Occurrence of the vancomycin-resistant genes vanA, vanB, vanC1, vanC2 and vanC3 in Enterococcus strains isolated from poultry and pork. Int. J. Food Microbiol. 60, 185–194. Liang, T., Liang, Z., Wu, S., Ding, Y., Wu, Q. and Gu, B. 2023. Global prevalence of Staphylococcus aureus in food products and its relationship with the occurrence and development of diabetes mellitus. Med. Adv. 1, 53–78. Lozano, C., Gharsa, H., Ben Slama, K., Zarazaga, M. and Torres, C. 2016. Staphylococcus aureus in animals and food: methicillin resistance, prevalence and population structure. a review in the African continent. Microorganisms 4, 12. Lu, J.J., Perng, C.L., Chiueh, T.S., Lee, S.Y., Chen, C.H., Chang, F.Y., Wang, C.C. and Chi, W.M. 2001. Detection and typing of vancomycin-resistance genes of enterococci from clinical and nosocomial surveillance specimens by multiplex PCR. Epidemiol. Infect. 126, 357–363. Manyi-Loh, C., Mamphweli, S., Meyer, E. and Okoh, A. 2018. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23, 795. Mejía, F., Castro-del Campo, N., García, A., Rodríguez, K., Cornejo, H., Ahumada-Ruiz, S., Soto-Beltrán, M., Castillo, M., Querol-Audi, J., Chaidez-Quiroz, C. and Martínez-Torres, A.O. 2021. Prevalence and characterization of antibiotic-resistant Staphylococcus aureus recovered from pasteurized cheese commercialized in Panama city markets. J. Food Qual. 2021, 9923855. Michaelis, C. and Grohmann, E. 2023. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 12, 328. Miller, W.R., Murray, B.E., Rice, L.B. and Arias, C.A. 2020. Resistance in vancomycin-resistant Enterococci. Infect. Dis. Clin. North. Am. 34, 751–771. Munita, J.M. and Arias, C.A. 2016. Mechanisms of antibiotic resistance. Microbiol. Spectr. 4, VMBF-0016-2015. pp: 481–511. Murray, L.M., Hayes, A., Snape, J., Kasprzyk-Hordern, B., Gaze, W.H. and Murray, A.K. 2024. Co-selection for antibiotic resistance by environmental contaminants. NPJ Antimicrob. Resist. 2, 9. Muteeb, G., Rehman, M.T., Shahwan, M. and Aatif, M. 2023. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: a narrative review. Pharmaceuticals (Basel) 16, 1615. Myers, M. and Ruxton, C.H.S. 2023. Eggs: healthy or risky? A review of evidence from high quality studies on hen’s eggs. Nutrients 15, 2657. Naeim, D.E., Eldesoukey, I.E., Moawad, A.A. and Ahmed, A.M. 2023. Molecular detection of methicillin-resistant Staphylococcus aureus isolated from different foodstuffs in Egypt. Vet. Res. Forum. 14, 243–248. Nepal, N., Mahara, P., Subedi, S., Rijal, K.R., Ghimire, P., Banjara, M.R. and Shrestha, U.T. 2023. Genotypically confirmed Vancomycin-resistant Staphylococcus aureus with vanB gene among clinical isolates in Kathmandu. Microbiol. Insight. 16, 11786361231183676. Nix, I.D., Idelevich, E.A., Storck, L.M., Sparbier, K., Drews, O., Kostrzewa, M. and Becker, K. 2020. Detection of methicillin resistance in Staphylococcus aureus from agar cultures and directly from positive blood cultures using MALDI-TOF mass spectrometry-based direct-on-target microdroplet growth assay. Front. Microbiol. 1, 232. Nusrin, S., Asad, A., Hayat, S., Islam, B., Begum, R., Nabila, F.H. and Islam, Z. 2022. Multiple mechanisms confer resistance to azithromycin in Shigella in Bangladesh: a comprehensive whole genome-based approach. Microbiol. Spectr. 10, e0074122. Nwobodo, D.C., Ugwu, M.C., Anie, C.O., Al-Ouqaili, M.T.S., Ikem, J.C., Chigozie, U.V. and Saki, M. 2022. Antibiotic resistance: the challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 36, e24655. Omoe, K., Ishikawa, M., Shimoda, Y., Hu, D.-L., Ueda, S. and Shinagawa, K. 2002. Detection of seg , seh , and sei genes in Staphylococcus aureus Isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, seh, or sei genes. J. Clin. Microbiol. 40, 857–862. Ou, C., Shang, D., Yang, J., Chen, B., Chang, J., Jin, F. and Shi, C. 2020. Prevalence of multidrug-resistant Staphylococcus aureus isolates with strong biofilm formation ability among animal-based food in Shanghai. Food Control 112, 107106. Partridge, S.R., Kwong, S.M., Firth, N. and Jensen, S.O. 2018. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 31, e00088-17. Parvin, M.S., Ali, M.Y., Talukder, S., Nahar, A., Chowdhury, E.H., Rahman, M.T. and Islam, M.T. 2021. Prevalence and multidrug resistance pattern of methicillin resistant S. aureus isolated from frozen chicken meat in Bangladesh. Microorganisms 9, 636. Pondit, A., Haque, Z., Sabuj, A., Khan, M. and Saha, S. 2018. Characterization of Staphylococcus aureus isolated from chicken and quail eggshell. J. Adv. Vet. Anim. Res. 5, 466. Pouwels, K.B., Muller-Pebody, B., Smieszek, T., Hopkins, S. and Robotham, J.V. 2019. Selection and co-selection of antibiotic resistances among Escherichia coli by antibiotic use in primary care: an ecological analysis. PLoS One 14, e0218134. Puglisi, M.J. and Fernandez, M.L. 2022. The health benefits of egg protein. Nutrients 14, 2904. Punom, S., Khan, M., Pritha, S., Hassan, J., Rahman, S., Mahmud, M. and Islam, M. 2020. Isolation and molecular-based identification of bacteria from unhatched leftover eggs of ducks in selected mini-hatcheries of Kishoreganj, Bangladesh. J. Adv. Vet. Anim. Res. 7, 164. Rachtanapun, P., Homsaard, N., Kodsangma, A., Phongthai, S., Leksawasdi, N., Phimolsiripol, Y., Seesuriyachan, P., Chaiyaso, T., Chotinan, S., Jantrawut, P., Ruksiriwanich, W., Wangtueai, S., Sommano, S.R., Tongdeesoontorn, W., Sringarm, K. and Jantanasakulwong, K. 2022. Effects of storage temperature on the quality of eggs coated by cassava starch blended with carboxymethyl cellulose and paraffin wax. Poult. Sci. 101, 101509. Raineri, E.J.M., Altulea, D. and van Dijl, J.M. 2022. Staphylococcal trafficking and infection—from ‘nose to gut’ and back. FEMS Microbiol. Rev. 46, fuab041. Randall, L.P. 2004. Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J. Antimicrob. Chemother. 53, 208–216. Rao, S., Linke, L., Magnuson, R., Jauch, L. and Hyatt, D.R. 2022. Antimicrobial resistance and genetic diversity of Staphylococcus aureus collected from livestock, poultry and humans. One Health 15, 100407. Ricke, S.C., O’Bryan, C.A. and Rothrock, M.J. 2023. Listeria occurrence in conventional and alternative egg production systems. Microorganisms 11, 2164. Robas, M., Probanza, A., González, D. and Jiménez, P.A. 2021. Mercury and antibiotic resistance co-selection in Bacillus spp. isolates from the Almadén mining district. Int. J. Environ. Res. Public Health 18, 8304. Romano, A., Carrella, S., Rezza, S., Nia, Y., Hennekinne, J.A., Bianchi, D.M., Martucci, F., Zuccon, F., Gulino, M., Di Mari, C., Zaccaria, T. and Decastelli, L. 2023. First report of food poisoning due to Staphylococcal enterotoxin type B in Döner Kebab (Italy). Pathogens 12, 1139. Russo, N., Stamilla, A., Cascone, G., Randazzo, C.L., Messina, A., Lanza, M., Pino, A., Caggia, C. and Antoci, F. 2020. The wide range of antibiotic resistance and variability of genotypic profiles in Escherichia coli from domestic animals in Eastern Sicily. Antibiotics (Basel) 10, 28. Samreen, Ahmad, I., Malak, H.A. and Abulreesh, H.H. 2021. Environmental antimicrobial resistance and its drivers: a potential threat to public health. J. Glob. Antimicrob. Resist. 27, 101–111. Samtiya, M., Matthews, K.R., Dhewa, T. and Puniya, A.K. 2022. Antimicrobial resistance in the food chain: trends, mechanisms, pathways, and possible regulation strategies. Foods 11, 2966. Şanlıbaba, P. 2022. Prevalence, antibiotic resistance, and enterotoxin production of Staphylococcus aureus isolated from retail raw beef, sheep, and lamb meat in Turkey. Int. J. Food Microbiol. 361, 109461. Scott, H.M., Acuff, G., Bergeron, G., Bourassa, M.W., Gill, J., Graham, D.W., Kahn, L.H., Morley, P.S., Salois, M.J., Simjee, S., Singer, R.S., Smith, T.C., Storrs, C. and Wittum, T.E. 2019. Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 1441, 8–16. Sharan, M., Dhaka, P., Bedi, J.S., Singh, R. and Mehta, N. 2023. Characterization of chicken eggs associated Escherichia coli and Staphylococcus aureus for biofilm production and antimicrobial resistance traits. Anim. Biotechnol. 34, 3533–3544. Shariati, A., Arshadi, M., Khosrojerdi, M.A., Abedinzadeh, M., Ganjalishahi, M., Maleki, A., Heidary, M. and Khoshnood, S. 2022. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 10, 1025633. Shittu, A.O. and Lin, J. 2006. Antimicrobial susceptibility patterns and characterization of clinical isolates of Staphylococcus aureus in KwaZulu-Natal province, South Africa. BMC Infect. Dis. 6, 125. Solís, D., Cordero, N., Quezada-Reyes, M., Escobar-Astete, C., Toro, M., Navarrete, P. and Reyes-Jara, A. 2023. Prevalence of Salmonella in eggs from conventional and cage-free egg production systems and the role of consumers in reducing household contamination. Foods 12, 4300. Stalder, T., Barraud, O., Casellas, M., Dagot, C. and Ploy, M.C. 2012. Integron involvement in environmental spread of antibiotic resistance. Front. Microbiol. 3, 119. Sultan, I., Ali, A., Gogry, F.A., Rather, I.A., Sabir, J.S.M. and Haq, Q.M.R. 2020. Bacterial isolates harboring antibiotics and heavy-metal resistance genes co-existing with mobile genetic elements in natural aquatic water bodies. Saudi J. Biol. Sci. 27, 2660–2668. Tălăpan, D., Sandu, A.M. and Rafila, A. 2023. Antimicrobial resistance of Staphylococcus aureus isolated between 2017 and 2022 from infections at a tertiary care hospital in Romania. Antibiot. 12, 974. Tasneem, U., Majid, M., Mehmood, K., Redaina, Ur Rehman, F., Andleeb, S. and Jamal, M. 2022. Co-occurrence of antibiotic resistance and virulence Genes in Methicillin Resistant Staphylococcus aureus (MRSA) Isolates from Pakistan. Afr. Health Sci. 22, 486–495. Tello, A., Austin, B. and Telfer, T.C. 2012. Selective pressure of antibiotic pollution on bacteria of importance to public health. Environ. Health Perspect. 120, 1100–1106. Tong, S.Y.C., Davis, J.S., Eichenberger, E., Holland, T.L. and Fowler, V.G. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. Urban-Chmiel, R., Marek, A., Stępień-Pyśniak, D., Wieczorek, K., Dec, M., Nowaczek, A. and Osek, J. 2022. Antibiotic resistance in bacteria—a review. Antibiotics (Basel) 11, 1079. Van, T.T.H., Yidana, Z., Smooker, P.M. and Coloe, P.J. 2020. Antibiotic use in food animals worldwide, with a focus on Africa: pluses and minuses. J. Glob. Antimicrob. Resist. 20, 170–177. Verma, S., Wadhwa, N.K., Bajaj, D., Sharma, R., Rajni, E. and Priyadarshani1, A. 2023. A microbiological analysis of egg shell bacteria. Vantage J. Thematic Analysis 4, 34–46. Vrancianu, C.O., Popa, L.I., Bleotu, C. and Chifiriuc, M.C. 2020. Targeting plasmids to limit acquisition and transmission of antimicrobial resistance. Front. Microbiol. 11, 761. Walker, R.A., Lindsay, E., Woodward, M.J., Ward, L.R. and Threlfall, E.J. 2001. Variation in clonality and antibiotic-resistance genes among multiresistant Salmonella enterica Serotype typhimurium phage-type U302 (MR U302) from humans, animals, and foods. Microb. Drug. Resist. 7, 13–21. Wang, X., Wang, X., Wang, Y., Guo, G., Usman, T., Hao, D., Tang, X., Zhang, Y. and Yu, Y. 2014. Antimicrobial resistance and toxin gene profiles of Staphylococcus aureus strains from Holstein milk. Lett. Appl. Microbiol. 58, 527–534. Wieczorek, K. and Osek, J. 2013. Antimicrobial resistance mechanisms among Campylobacter. Biomed. Res. Int. 2013, 1–12. Yamanaka, H., Kadomatsu, R., Takagi, T., Ohsawa, M., Yamamoto, N., Kubo, N., Takemoto, T. and Ohsawa, K. 2019. Antimicrobial resistance profiles of vancomycin-resistant Enterococcus species isolated from laboratory mice. J. Vet. Sci. 20, e13. Zhang, P., Wang, P., Fu, X., Xu, X., Ruan, F., Wang, T., Chang, G., Wan, Y., Zhang, Y. and Wang, X. 2023. Prevalence and characterization of Staphylococcus aureus in raw eggs and it’s growth and enterotoxin a production in egg contents. LWT 174, 114379. | ||
| How to Cite this Article |
| Pubmed Style Bose P, Sobur KA, Lijon MB, Rahman MZ, Ahamed T, Sultana P, Muktaruzzaman M, Khatun MM, Islam MA. Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health. Open Vet. J.. 2025; 15(3): 1187-1205. doi:10.5455/OVJ.2025.v15.i3.11 Web Style Bose P, Sobur KA, Lijon MB, Rahman MZ, Ahamed T, Sultana P, Muktaruzzaman M, Khatun MM, Islam MA. Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health. https://www.openveterinaryjournal.com/?mno=228992 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i3.11 AMA (American Medical Association) Style Bose P, Sobur KA, Lijon MB, Rahman MZ, Ahamed T, Sultana P, Muktaruzzaman M, Khatun MM, Islam MA. Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health. Open Vet. J.. 2025; 15(3): 1187-1205. doi:10.5455/OVJ.2025.v15.i3.11 Vancouver/ICMJE Style Bose P, Sobur KA, Lijon MB, Rahman MZ, Ahamed T, Sultana P, Muktaruzzaman M, Khatun MM, Islam MA. Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health. Open Vet. J.. (2025), [cited January 24, 2026]; 15(3): 1187-1205. doi:10.5455/OVJ.2025.v15.i3.11 Harvard Style Bose, P., Sobur, . K. A., Lijon, . M. B., Rahman, . M. Z., Ahamed, . T., Sultana, . P., Muktaruzzaman, . M., Khatun, . M. M. & Islam, . M. A. (2025) Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health. Open Vet. J., 15 (3), 1187-1205. doi:10.5455/OVJ.2025.v15.i3.11 Turabian Style Bose, Palash, Kazi Abdus Sobur, Md. Bakhtiar Lijon, Md. Zaminur Rahman, Tanvir Ahamed, Papia Sultana, Muhammad Muktaruzzaman, Mst. Minara Khatun, and Md. Ariful Islam. 2025. Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health. Open Veterinary Journal, 15 (3), 1187-1205. doi:10.5455/OVJ.2025.v15.i3.11 Chicago Style Bose, Palash, Kazi Abdus Sobur, Md. Bakhtiar Lijon, Md. Zaminur Rahman, Tanvir Ahamed, Papia Sultana, Muhammad Muktaruzzaman, Mst. Minara Khatun, and Md. Ariful Islam. "Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health." Open Veterinary Journal 15 (2025), 1187-1205. doi:10.5455/OVJ.2025.v15.i3.11 MLA (The Modern Language Association) Style Bose, Palash, Kazi Abdus Sobur, Md. Bakhtiar Lijon, Md. Zaminur Rahman, Tanvir Ahamed, Papia Sultana, Muhammad Muktaruzzaman, Mst. Minara Khatun, and Md. Ariful Islam. "Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health." Open Veterinary Journal 15.3 (2025), 1187-1205. Print. doi:10.5455/OVJ.2025.v15.i3.11 APA (American Psychological Association) Style Bose, P., Sobur, . K. A., Lijon, . M. B., Rahman, . M. Z., Ahamed, . T., Sultana, . P., Muktaruzzaman, . M., Khatun, . M. M. & Islam, . M. A. (2025) Characterization of enterotoxin, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: Implications for food safety and public health. Open Veterinary Journal, 15 (3), 1187-1205. doi:10.5455/OVJ.2025.v15.i3.11 |