| Research Article | ||
Open Vet. J.. 2025; 15(3): 1206-1216 Open Veterinary Journal, (2025), Vol. 15(3): 1206-1216 Research Article Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting seasonKhalid M. Al Khodair1, Abdulkarem Al-Shabebi2,3, Thnaian A. Al-Thnaian1, Osamah I. M. Alturki1, Marwa-Babiker A.M.1,4, Saeed Y Al-Ramadan1, Abdelhay M. Ali1 and Abdelrahman M.A. Elseory1,5*1Department of Anatomy, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia 2Department of Animal Resources, Ministry of Municipality, Doha, Qatar 3Veterinary Department, Faculty of Agriculture and Veterinary Medicine, Thamar University, Dhamar, Yemen 4Department of Anatomy, College of Veterinary Medicine, University of Bahri, Khartoum North, Sudan 5Department of Anatomy, Faculty of Veterinary Medicine, University of Khartoum, Khartoum North, Sudan *Corresponding Author: Abdelrahman M.A. Elseory. Department of Anatomy, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia. Email: amamohamed [at] kfu.edu.sa Submitted: 17/11/2024 Accepted: 13/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
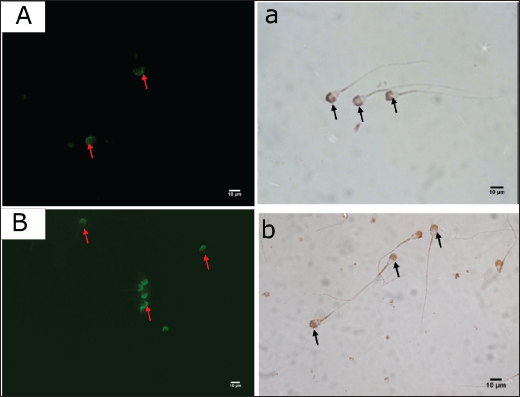
AbstractBackground: Numerous plant and animal species have β-defensins, antimicrobial peptides involved in immunity and reproduction. Beta-defensin 126 (DEFB126) belongs to the β-defensin family and is a vital component of sperm function. It regulates the capacitation and sperm-egg interaction. Aim: Clarify the expression of DEFB126 in the dromedary camel’s sperm and reproductive tract. Methods: The current work used immunohistochemical (IHC) and quantitative real-time polymerase chain reaction (qRT-PCR) methods to clarify the distribution and expression of DEFB126 in the sperm and male reproductive tract (MRT) of dromedary camels in the time of rutting. Samples of fresh and epididymal sperm, testicular, epididymis, ductus deferens, and male accessory gland tissues were obtained from 20 male camels. Results: The results of IHC showed that the fresh and epididymal sperms had a positive immunoreaction to DEFB126 antibodies. Also, all parts of the testicles, epididymis, vas deferens, prostate, and bulbourethral glands were positively stained to various degrees with a strong immunoreaction in the epididymal’s tail. qRT-PCR results showed that expression levels of DEFB126 mRNA varied in the fresh and epididymal sperms and throughout all parts of the MRT; the tail of the epididymis had the most significant expression levels (p < 0.05). Conclusion: This study’s results indicated that DEFB126 protein is expressed in the sperm and MRT of the dromedary camel, with the acrosomal cap of the sperm and the epididymis tail exhibiting the highest levels of expression. These findings imply that DEFB126 could be involved in the reproductive processes of sperm maturation, capacitation, and sperm-zona recognition. Keywords: Camel, DEFB126, Immunohistochemistry, Male reproductive tract, qRT-PCR, Sperm. IntroductionIn many desert places, dromedary camels (Camelus dromedarius) have been long valued for their capacity to withstand harsh environments and serve as a reliable source of food, transportation, beauty festivals, and racing sports (Ali et al., 2009; Wu et al., 2014; Khalafalla et al., 2021). The poor genetic potential of local camels, traditional livestock management, and unstable production circumstances all contribute to their low reproductive efficiency in arid environments (Gherissi et al., 2020). The morphology of the male reproductive system of the dromedary camel is made up of the testes, penis, epididymis, and accessory sex glands (prostate, ampulla, and bulbourethral glands), where seminal vesicle glands are absent (Jarrar and Faye, 2013; Bello and Umaru, 2014; Saini et al., 2022). Three parts make up the camel spermatozoon: the head, middle section, and tail. Its size is often less than other animals (Tingari et al., 1986). However, standard sperm parameters are deemed inadequate because they do not accurately indicate breeding potential and correlate poorly with fertility (Khatun et al., 2018). Understanding the proteins involved in male fertility is crucial for developing treatments for male infertility (Beeram et al., 2019). Therefore, molecular methods have been developed to accurately measure the sperm’s expression levels of particular proteins utilized as male fertility indicators (Ashrafzadeh et al., 2013). β-Defensins are small, cationic antimicrobial peptides first identified in the bovine trachea (Diamond et al., 1991). They consist of over 80 amino acids, with 5–12 positively charged residues, and play vital roles in immunity and reproduction (Ganz, 2004; Pazgier et al., 2006; Pujianto et al. 2020; Zupin et al. 2019; Wilson et al., 2013; Ribeiro et al., 2016; Fesahat et al., 2023). In adult animals, β-defensins are highly expressed in the testis and epididymis, where they support sperm glycocalyx formation and provide bacteriostatic and anti-inflammatory functions (Zhou et al., 2004; Dorin and Barratt, 2014; Avellar et al., 2019). Their expression is developmentally regulated, increasing in puberty (Sangeeta and Yenugu, 2019). Beta-defensin 126 (DEFB126) is a partner of the family β-defensin and is crucial to sperm functioning (Hall et al., 2017). It is a highly sialylated glycopeptide and is added to the surface of sperm after their passage through the epididymis. Also, this protein is required for sperm to migrate efficiently into the female reproductive system. Furthermore, it regulates the capacitation and sperm-egg interaction (Narciandi et al., 2016). Additionally, sperms that are migrating to the upper female reproductive tract are coated with an immunoprotective layer by DEFB126, which aids in sperm penetration through cervical mucus and sperm protection (Yudin et al., 2003; Yudin et al., 2005; Tollner et al., 2011; Tollner et al., 2012). Studies conducted in the first quarter of this century focused on the expression of DEFB126 in the spermatozoon and testicular and epididymal epithelium of several mammalian species, such as humans, bulls, and macaques, as well as its relationship to infertility mainly in humans (Yamaguchi et al., 2002; Yudin et al., 2003; Thimon et al., 2007; Narciandi et al, 2011; Tollner et al., 2012; Fernandez-Fuertes et al., 2016; Lyons, 2016; Narciandi et al., 2016; Lyons et al., 2018; Aram et al, 2020; He et al., 2022; Solanki et al., 2023). There is no information on DEFB126 expression in the reproductive system of the male dromedary camel. The current work investigates the localization pattern and expression of the DEFB126 protein in the camel sperm, testis, epididymis, vas deferens, prostate, and bulbourethral glands in the rutting season using immunohistochemistry (IHC) and quantitative polymerase chain reaction (qR-PCR) techniques. Materials and MethodsSampling and ethical approvalAll animal samples were collected in the middle of the rutting season (December and January). The sample procedures followed the animal protocol authorized by King Faisal University’s ethics committee (Ref. No. KFU-REC-2023-FEB-ETHICS614). Semen collection and sperm samplingFive healthy, adult dromedary camels were used to produce ejaculated semen samples following the established standards for ethical treatment of animals (Tingari et al., 1986; Al-Shabebi et al., 2021). The animals were provided by the Camel Research Centre, King Faisal University-Al-Ahsa, Saudi Arabia. After being cleaned with phosphate-buffered saline (PBS), the semen samples were centrifuged twice for 5 minutes at room temperature (RT) at 2,000 rpm. Sperm were then diluted to around 20 × 104 cell/ml in PBS and counted using a Makler chamber. Following smearing on Superfrost® slides, the sperms were air-dried and kept at −20°C until needed for further investigations. Camel epididymal semen was collected from Al Omran abattoir, Al-Ahsa, Saudi Arabia. Samples were collected within 30 minutes of slaughtering from the epididymal tails of five adult camels. In each instance, a small incision was made in the epididymis wall, and the semen was carefully extracted and transferred into sterile, collection tubes. 20 μl of semen and 200 μl of PBS were combined. The mixture was then applied directly onto Superfrost® slides, air-dried, and stored at −20°C until further use. Tissues samplesTissue samples of various organs from 10 clinically healthy adult dromedary camels (aged ≥ 5 years) were obtained from the previous abattoir. These animals had not been used before in this study. These included the testicles (seminiferous tubules and rete testis), epididymis (head, body, and tail), vas deferens (initial, middle, and ampullary parts), prostate gland (corpus and disseminated parts), and bulbourethral gland. Immediately after collection, tissue samples were fixed in 10% buffered formalin for 36 hours to preserve their integrity for subsequent IHC analysis. Within 10 minutes of evisceration, additional samples of the same organs were frozen in liquid nitrogen at −80°C and stored for the qR-PCR procedure. IHC techniqueImmunostaining of sperm For sperm immunostaining, sperm-smeared slides from fresh and epididymal sperm samples were encircled with the PAP pen (Cloud-Clone Corp., USA, cat. no. PAQ483Hu01). After 5 minutes of PBS incubation, 70 μl of normal goat serum (NGS) (Abcam, Inc., cat. no. ab7481) at a 1:20 dilution was applied, and the slides were incubated for 10 minutes at RT. Then, each slide received 100 μl of the primary antibody (diluted 1:100) and was incubated for an hour at RT. For 5 minutes, PBS washing was done on each slide three times. The slides were then treated with a 1:100 dilution of goat anti-rabbit fluorescent secondary antibody (Abcam, Inc., cat. no. ab6717) and incubated for 2 hours at RT in a dark environment. The slides were cleaned in between each reagent application. A cover slip made of an aqueous, non-fluorescent mounting solution was placed over the plates. Immediately, a fluorescent microscope (Fully Automated Upright Microscope, Leica DM6000 B, Germany) was used to visualize and analyze the slides. Immunostaining of tissues Samples fixed in formalin were dried using a series of ethanol, cleared in xylene, and inserted into paraffin wax for tissue immunostaining. Using a microtome (Leica, Germany), sections 4 µm thick were cut and then put on Superfrost slides (VWR, cat. no. 631-0108). Following that, sections were stained using the avidin-biotin-peroxidase complex method (Hsu et al., 1981; Adeghate et al., 2001) using rabbit polyclonal anti-human BEFD126 antibody (1:100) (cat. no. PAQ483Hu01, Cloud-Clone Corp. USA) (Table 1). Antigen-retrieval buffer (sodium citrate 50 mM, pH 6.0) was made and heated to 100°C for 20 minutes to enhance the epitope access immunostaining signal. The tissue slides were treated for 10 minutes with 90 μl of 3% hydrogen peroxide, 10 minutes with NGS (Cloud-Clone Corp. USA, Inc., cat. no. PAQ483Hu01) at a 1:100 dilution, and 1 hour at RT with diluted primary antibody (dilution 1:100). Next, a secondary goat anti-rabbit biotinylated antibody (ab64261, Abcam, Cambridge, UK) was applied for 1 hour at RT. After that, the slides received an incubation for 30 minutes at RT. For 20 minutes, each slide was treated with streptavidin HRP (Abcam, Inc., cat. no. ab64269). An appropriate 3,3’-diaminobenzidine tetrahydrochloride chromogen substrate was added for 5 minutes to create the color. Among each reagent, PBS was utilized for the wash operations. The slides were then counterstained with hematoxylin, dehydrated, cleared, and then mounted with DPX. Samples were then inspected under a light microscope (Microscope Leica DM6000 B, Germany) connected to a digital camera (Leica DFC420, Germany). Immunoreactive staining positivity was assessed as (++++) strong, (+++) moderate, (++) weak, and (+) very weak. Quantitative real-time polymerase chain reaction (qRT-PCR) technique50 mg of the tissue samples were collected using the qRT-PCR method on a Bead Ruptor Homogenizer (OMNI International, NW Ken Nesaw, GA, USA). To extract the whole RNA, TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) was done as directed by the manufacturer. Next, the RNA was suspended in ultrapure diethylpyrocarbonate (DEPC)-treated RNase-free water (Invitrogen, USA) using chloroform and isopropanol. After that, the purity and concentration of the total RNA were assessed using the BioTek Synergy HTX reader (BioTek, USA). Subsequently, the purity RNA was reverse-transcribed to cDNA with the iScript cDNA Synthesis Kit (BioRad, Hercules, CA, USA) in a 20 μl total volume mixture that included 4 μl iScript® Reaction Mix, 1 μl iScript® Reverse Transcriptase, and nuclease-free distilled water. The mixture was then incubated at 25°C for 5 minutes, 46°C for 20 minutes, and 95°C for 60 seconds to deactivate the reverse transcriptase. The CFX96® Touch Real-time PCR (BioRad, USA) and SsoAdvanced SYBR Green Supermix dye (BioRad, USA) were used to conduct the qPCR experiment. The camels DEFB126 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes were examined using the gene-specific primers in Table 2. 10 µl of the master mix, 2 µl of each forward and reverse primer pm/µl, 2 µl of the specimens’ cDNA, and 4 µl of nuclease-free water comprised the 20 µl reaction mixture. The thermal cycling program as follows: 30 seconds at 95°C followed by 40 cycles at 95°C for 15 seconds; after that, 30 seconds at 60°C, and 10 seconds at 72°C. To demonstrate fluorescence emission, every cDNA template was used in duplicate. The CFX Mana gerTM software V3.1 (BioRad, Hercules, CA, USA) was used to automatically calculate the corresponding levels of expression of the DEFB126 gene by fold change using the 2-ΔΔCt (delta-delta cycle threshold) method, applying the housekeeping GAPDH gene as a reference. Statistical analysisThe data for the DEFB126 gene were analyzed by applying SPSS® 16 software. One-way analysis of variance (one-way) via post hoc analysis was used to compare the variance expressions’ mean ± SE of the different organs (10 variables for 11 parameters). ResultsDetection of DEFB126 in the spermThe fresh and epididymal sperms showed a positive immunoreaction to DEFB126 antibodies (Table 3). The expression of this protein showed identical spatial patterns in both types of sperm and no differences in localization. However, the fresh sperm exhibited better response resolution than the epididymal sperm. The acrosomal cap was stained more strongly than the midpiece. The sperm’s equatorial band, post-acrosomal cap, neck, annulus, and tail showed no apparent staining (Fig. 1). Table 1. Showing primary antibodies used to determine protein distribution in the camel sperm and tissues.
Table 2. Showing the real-time qPCR oligonucleotide primers that used on qRT-PCR.
Table 3. Showing summary for the distribution and localization of DEFB126 on the camel sperm.
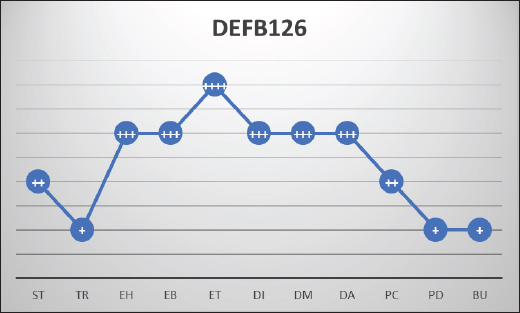
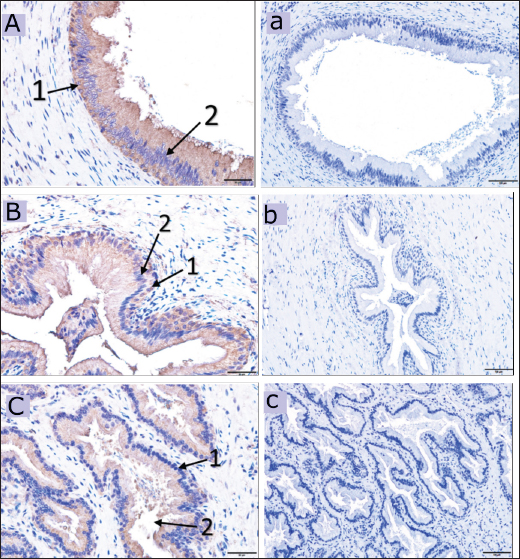
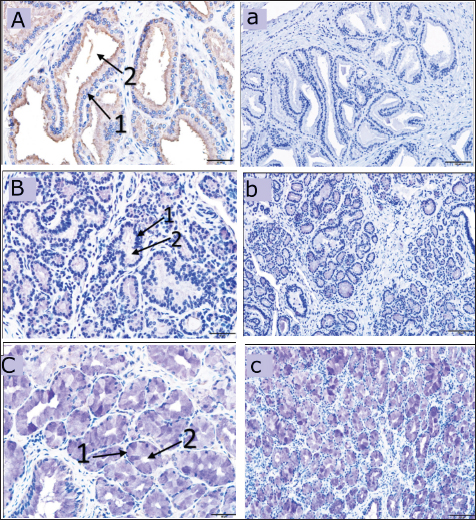
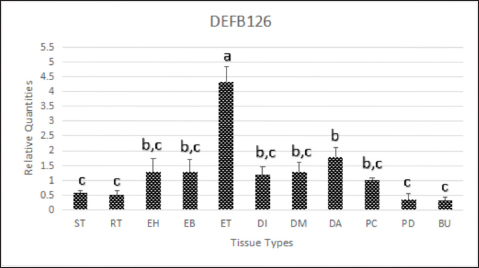
Fig. 1. DEFB126 protein immunostaining for fresh (A, a) and epididymal (B, b) sperm of dromedary camels with fluorescent and chromogenic stains. Arrows indicate the immunostaining of DEFB126 protein on sperm. (60×, scale bar=10 μm). Immunohistochemistry of the male reproductive tract (MRT)DEFB126 antibodies showed positive staining in the MRT organs with different intensities (Fig. 2). Both round and elongated spermatids in the seminiferous tubules of the camel’s testis exhibited weak staining for DEFB126. Notably, other cells within the seminiferous tubules showed no signs of staining. Furthermore, a very mild stain was observed in the lining epithelium of the rete testis (Fig. 3). The pseudo-stratified epithelial cells in the head, body, and tail of the epididymis showed different DEFB126 staining patterns. The staining was fainter in the basal, principal, and stereocilia cells at the head and body but more intense in the tail (Fig. 4). A moderate amount of DEFB126 protein was found in the initial, middle, and ampullary parts of the vas deferens’ epithelial layer (Fig. 5). The glandular epithelium of the male camel accessory glands exhibited a negligible amount of DEFB126 protein. The compact prostate showed a modest response to the DEFB126 antibodies, but the disseminated prostate and bulbourethral gland had a weak response (Fig. 6). qRT-PCRThe expressed level of DEFB126 mRNA in the testis, epididymis, vas deferens, prostate, and bulbourethral glands of the dromedary camel is represented in Figure 7. The seminiferous tubules and rete testis sections of the camel’s testis exhibited a modest degree of DEFB126 expression. While the two portions of the epididymis had the same expression, the tail component exhibited more significant percentages of DEFB126 mRNA expression (p < 0.05) than the head and body parts. In the other parts of the MGT, the vas deferens and the male accessory glands revealed moderate to weak expression of DEFB126 mRNA.
Fig. 2. Showing the intensity of DEFB126 protein in the dromedary camel’s male reproductive system. The level of positivity for immunoreactive staining was rated as (++++) strong, (+++) moderate, (++) weak, (+) very weak. BU, bulbourethral gland; DA, ampulla; DI, vas deferens initial part; DM, vas deferens middle part; EB, epididymis body; EH, epididymis head; ET, epididymis tail; PC, prostate compact; PD, prostate disseminated; ST, seminiferous tubules; TR, rete testis.
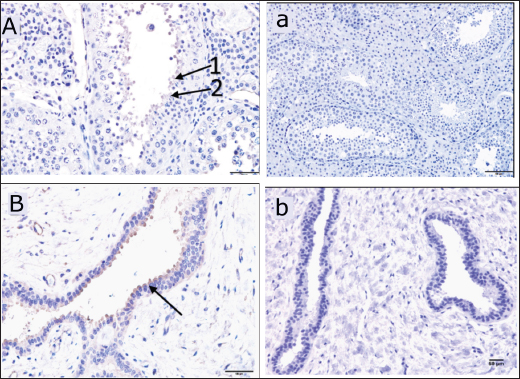
Fig. 3. Immunoreactive staining of the camel’s testis with DEFB126 antibody showing a weak reaction in the seminiferous tubules (spermatids) and the lining epithelium of the rete testis. (A) +ve, DEFB126 for seminiferous tubules, 40×; (B) +ve, DEFB126 rete testis, 40×; (a) and (b) negative control for seminiferous tubules and rete testis, respectively. 20×; (1) and (2) spermatids; (arrow) lining epithelial cells.
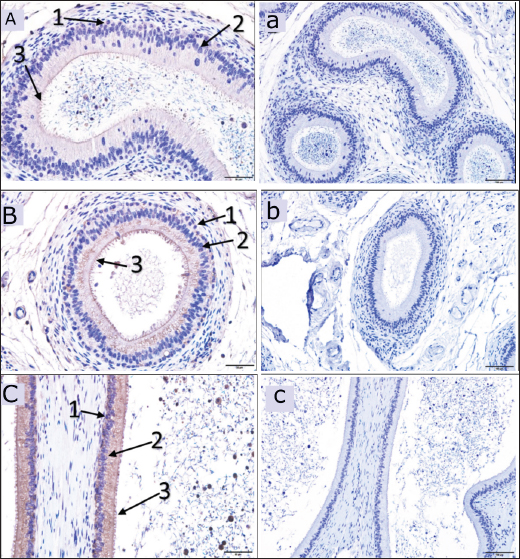
Fig. 4. Immunoreactive staining of the camel’s epididymis showing moderate reaction with DEFB126 antibody in the head (A) and body (B), whereas, the tail (C) has a strong immunoreaction. ×40; (a), (b) and (c) are negative control for epididymal head, body and tail in that order. ×20; (1) Basal cells; (2) Principal cells; (3) Stereocilia. DiscussionDEFB126 protein’s presence in camel sperm may influence the sperm’s transit into the female reproductive system. It can also control sperm-egg contact, capacitation, and sticking to the oviduct epithelium, as suggested earlier by Xin et al. (2016). Additionally, it backed up the theory that DEFB126 provides an immunoprotective layer to sperm traveling to the upper female reproductive system, which helps them pass through cervical mucus and be preserved (Yudin et al., 2003; Yudin et al., 2005; Tollner et al., 2011; Tollner et al., 2012). In this investigation, DEFB126 was found in the fresh and epididymal sperm of the dromedary camel. It displayed intense staining in the acrosomal cap and limited low staining in the midpiece. As known, the sperm acquire epididymal proteins through epididymosomes (Barrachina et al., 2022); the present result supports the findings of Yudin et al. (2003), who reported that the DEFB126 protein is produced by the principal cells of the epididymal epithelium. It is maximally expressed in the distal head, and the proximal tail is added to the entire surface of the ejaculated macaque sperm, which decreases on the head and midpiece after capacitation. Furthermore, Tollner et al. (2008) recorded that in the macaque monkey, this protein was coated on the entire surface of the sperm during passage through the epididymis tail. They also observed it on the sperms collected from the female reproductive tract of the same animal.
Fig. 5. Immunoreactive staining of the vas deferens of the dromedary camel showing a moderate reaction with DEFB126 antibody in the initial (A), middle (B) and ampullary parts (C). ×40; (a), (b) and (c) are negative control for initial, middle and ampullary parts, respectively. ×20; (1) Columnar epithelium, (2) Basal cell. Despite the DEFB126 protein localized in the acrosomal cap of the sperm of the camel, it was found over the whole mouse sperm surface, except in the equatorial region (Yudin et al., 2008). In contrast, it was reported only in the bovine epididymal sperm’s tail (Narciandi et al., 2016). At the same time, Fernandez-Fuertes et al. (2016) observed that it was found in the bull sperm’s tail and post-acrosomal region. The epididymis tail and vas deferens showed high expression of the DEFB126 protein in this study. In contrast, it was weak in the camel’s testes and moderate in the epididymis’s head and body. Nonetheless, DEFB126 protein was found in the epithelial cells of the epididymis tail and vas deferens of bulls but not in the epididymis head, testis, or body. This information was reported by (Narciandi et al., 2016). As is known, during their migration through the epididymis, immature sperm are submerged in region-specific epididymal fluid, which causes their surface proteins to be added, deleted, and modified sequentially. This process eventually gives the sperm the ability to move and fertilize (Dacheux et al., 2003; Gatti et al., 2004; Cornwall, 2014). In addition, proteins can be released in the tail of the epididymis to preserve sperm metabolic quiescence and avoid premature sperm activation and capacitation (Ni et al., 2009; Baskaran et al., 2020; Chen et al., 2020). Thimon and others have observed DEFB126 being generated in the human and macaque epididymis bodies and tails (Yudin et al., 2003; Thimon et al., 2007). The previously reported findings were consistent with the current investigation, which elucidated DEFB126 expression in the camel’s epididymis, particularly the tail. The expression of DEFB126 mRNA in the epididymis and vas deferens of humans, bovines, and buffalo as reported by (Thimon et al., 2007; Narciandi et al., 2011; Batra et al., 2019), respectively, aligns with our findings in camels. This expression agrees with existing research and reassures us of the validity of our findings and their relevance to the field of reproductive biology. Moreover, this study noticed a high expression of DEFB126 mRNA in the epididymis tail as sperm storage and less in other examined tissues of MRT. The same finding was mentioned in the different parts of the MRT of the ram; it showed the highest expression in the epididymis tail, a moderated in the head and body of the epididymis as well as in the testes (Hall et al., 2017). According to Batra et al. (2019), the epididymis tail has more significant levels of DEFB126 mRNA expression than the epididymis head. These statistics are similar to those discovered in the current study on the epididymis of camels.
Fig. 6. The dromedary camel’s accessory glands immunoreactive stain revealing a weak reaction with the DEFB126 antibodies in the compact prostate (A), weak immunoreaction in the disseminated prostate (B) and bulbourethral gland (C), ×40. (a) negative control for a compact prostate, (b) for a dispersed prostate and (c) for bulbourethral gland, ×20. (1) Secretory epithelium, (2) Lumen of the secretory units.
Fig. 7. Comparison of DEFB126 mRNA expression in the testis and epididymis of the dromedary camel. BU, bulbourethral gland; DA, ampulla; DI, initial part of vas deferens; DM, middle part of vas deferens; EB, epididymis body; EH, epididymis head; ET, epididymis tail; PC, prostate compact; PD, prostate disseminated; RT, rete testis; ST, seminiferous tubules. Narciandi et al. (2016) quantitative analysis showed notable staining of DEFB126 protein in bull seminal fluid, suggesting that this protein was produced from the male accessory glands. Our finding of DEFB126 protein in the male accessory glands of the camel may support this suggestion. ConclusionThe DEFB126 protein is a highly expressed gene and protein in the camel’s epididymis tail. Furthermore, it significantly concentrated on the acrosomal cap of the epididymal and fresh sperm. The investigation showed no discernible difference between camels and other animals or primates regarding BBD126 expression. These findings sported the previously suggested function of DEFB126 as linked to sperm maturation, capacitation, providing an immunoprotective layer for survival through cervical mucus and in the female reproductive canal, and sperm-zona recognition fertility. Thus, this protein’s presence, especially in sperm, suggested that the sperm were good quality and might help reduce camel infertility issues. AcknowledgmentsThe authors thank the staff of Camel Research Center, King Faisal University, Al-Ahsa, Kingdom of Saudi Arabia, and the director of Al Omran abattoir, Al-Ahsa, Kingdom of Saudi Arabia for providing the samples. Conflict of interestThe authors declare that they have no conflict of interest. FundingThe authors thank the Deanship of Scientific Research Deanship of King Faisal University in Saudi Arabia for their financial support (Grant #KFU242456). Authors’ contributionsAl Khodair K.: investigation, methodology, data curation, software, writing—original draft. Al-Shabebi A.: investigation, methodology. Al-Thnaian T.: investigation, data curation. Alturki O.: resources, methodology. Babiker M.: conceptualization, methodology. Al-Ramadan S.: conceptualization, methodology, investigation. Ali A.: review and editing, supervision, funding acquisition. Elseory A.: investigation, writing—original draft. All authors have read and agreed to the published version of the manuscript. Data availabilityThe data used to support this study’s findings are included in the article and will be made available on request. ReferencesAdeghate, E., Ponery, A., Pallot, D. and Singh, J. 2001. Distribution of vasoactive intestinal polypeptide, neuropeptide-Y and substance P and their effects on insulin secretion from the in vitro pancreas of normal and diabetic rats. Peptides 22(1), 99–107; doi:10.1016/s0196-9781(00)00361-2. Ali, I., Chaudhry, M.S. and Farooq, U. 2009. Camel rearing in Cholistan Desert of Pakistan. Pak. Vet. J. 29(2), 85–92. Al-Shabebi, A., Althnaian, T. and Alkhodair, K. 2021. Localization and expression of ADAM2 in the dromedary camel testis, epididymis and sperm during rutting season. Anim. Reprod. 18(1), e20200241; doi:10.1590/1984-3143-ar2020-0241. Aram, R., Chan, P.T. and Cyr, D.G. 2020. Beta-defensin126 is correlated with sperm motility in fertile and infertile men. Biol. Reprod. 102(1), 92–101; doi:10.1093/biolre/ioz171. Ashrafzadeh, A., Karsani, S.A. and Nathan, S. 2013. Mammalian sperm fertility related proteins. Int. J. Med. Sci. 10(12), 1649; doi:10.7150/ijms.6395. Avellar, M.C.W., Ribeiro, C.M., Dias-da-Silva, M. and Silva, E.J. 2019. In search of new paradigms for epididymal health and disease: innate immunity, inflammatory mediators, and steroid hormones. Andrology 7(5), 690–702; doi:10.1111/andr.12654. Barrachina, F., Battistone, M.A., Castillo, J., Mallofré, C., Jodar, M., Breton, S. and Oliva, R. 2022. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 37(4), 651–668. doi:10.1093/humrep/deac015. Baskaran, S., Selvam, M.K.P. and Agarwal, A. 2020. Exosomes of male reproduction. Adv. Clin. Chem. 95, 149–163; doi:10.1016/bs.acc.2019.08.004. Batra, V., Maheshwarappa, A., Dagar, K., Kumar, S., Soni, A., Kumaresan, A., Kumar, R. and Datta, T. 2019. Unusual interplay of contrasting selective pressures on β-defensin genes implicated in male fertility of the Buffalo (Bubalus bubalis). BMC Evol. Biol. 19, 1–19; doi:10.1186/s12862-019-1535-8. Beeram, E., Suman, B. and Divya, B. 2019. Proteins as the molecular markers of male fertility. J. Hum. Reprod. Sci. 12(1), 19; doi:10.4103/jhrs.JHRS_9_18. Bello, A., and Umaru. 2014. An over view on the anatomy and physiology of male one humped camel (Camelus Dromedarius) reproductive system. Scientific Journal of Review, 2, 340–347. Chen, Y., Wang, K., Zhang, D., Zhao, Z., Huang, J., Zhou, L., Feng, M., Shi, J., Wei, H., Li, L., Wu, Z., and Li, L. 2020. GPx6 is involved in the in vitro induced capacitation and acrosome reaction in porcine sperm. Theriogenology 156, 107–115; doi:10.1016/j.theriogenology.2020.06.020. Cornwall, G.A. 2014. Role of posttranslational protein modifications in epididymal sperm maturation and extracellular quality control. Adv. Exp. Med. Biol. 759, 159–180; doi:10.1007/978-1-4939-0817-2_8. Dacheux, J.L., Gatti, J.L. and Dacheux, F. 2003. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc. Res. Tech. 61(1), 7–17; doi:10.1002/jemt.10312. Diamond, G., Zasloff, M., Eck, H., Brasseur, M., Maloy, W.L. and Bevins, C.L. 1991. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. USA, 88(9), 3952–3956; doi:10.1073/pnas.88.9.3952. Dorin, J.R. and Barratt, C.L. 2014. Importance of β-defensins in sperm function. Mol. Hum. Reprod. 20(9), 821–826; doi:10.1093/molehr/gau050. Fernandez-Fuertes, B., Narciandi, F., O’Farrelly, C., Kelly, A.K., Fair, S., Meade, K.G. and Lonergan, P. 2016. Cauda epididymis-specific beta-defensin 126 promotes sperm motility but not fertilizing ability in cattle. Biol. Reprod. 95(6), 122, 121–112; doi:10.1095/biolreprod.116.138792. Fesahat, F., Firouzabadi, A.M., Zare-Zardini, H. and Imani, M. 2023. Roles of different b-defensins in the human reproductive system: a review study. Am. J. Mens Health. 17(3), 15579883231182673; doi:10.1177/15579883231182673. Ganz, T. 2004. Defensins: antimicrobial peptides of vertebrates. C R Biol. 327(6), 539–549; doi:10.1016/j.crvi.2003.12.007. Gatti, J.L., Castella, S., Dacheux, F., Ecroyd, H., Métayer, S., Thimon, V. and Dacheux, J.L. 2004. Post-testicular sperm environment and fertility. Anim. Reprod. Sci. 82–83, 321–339; doi:10.1016/j.anireprosci.2004.05.011. Gherissi, D.E., Monaco, D., Bouzebda, Z., Bouzebda, F.A., Gaouar, S.B.S. and Ciani, E. 2020. Camel herds’ reproductive performance in Algeria: objectives and thresholds in extreme arid conditions. J. Saudi Soc. Agric. Sci. 19(7), 482–491. Hall, T., McQuillan, C., Finlay, E.K., O’Farrelly, C., Fair, S. and Meade, K.G. 2017. Comparative genomic identification and validation of β-defensin genes in the Ovis aries genome. BMC Genomics 18(1), 1–11; doi:10.1186/s12864-017-3666-x. He, J.Y., Peng, J.Y., Li, Q.F., Lin, X.L., Cui, Y.R., Ma, S.Y., Fan, S.Y., Liu, Y.R, Song, Z.L., Deng, J.H., Wei, X. and Deng, J.H. 2022. DEFB126 polymorphisms and association with idiopathic asthenozoospermia in China. Asian J. Androl. 24(6), 607–614; doi:10.4103/aja2021115. Hsu, S.M., Raine, L. and Fanger, H. 1981. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am. J. Clin. Pathol. 75(6), 816–821; doi:10.1093/ajcp/75.6.816. Jarrar, B. and Faye, B. 2013. Normal pattern of camel histology. Saudi Arabia, FAO Publications. Khalafalla, A.I., Hussein, M.F. and Bornstein, S. 2021. Evolution, distribution, and economic importance of the camels. In Infectious diseases of dromedary camels: a concise guide, 1–19. Springer, Cham, Switzerland. 1–19. Khatun, A., Rahman, M.S. and Pang, M.G. 2018. Clinical assessment of the male fertility. Obstet. Gynecol. Sci. 61(2), 179–191; doi:10.5468/ogs.2018.61.2.179. Lyons, A. 2016. The role of β-defensin 126 on the ability of bull sperm to bind oviductal epithelium. Limerick, Ireland, University of Limerick. Lyons, A., Narciandi, F., Donnellan, E., Romero-Aguirregomezcorta, J., O’Farrelly, C., Lonergan, P., Meade KG, Fair, S. 2018. Recombinant β-defensin 126 promotes bull sperm binding to bovine oviductal epithelia. Reproduction, Reprod. Fertil. Dev. 30(11), 1472–1481; doi:10.1071/RD17415. Narciandi, F., Fernandez-Fuertes, B., Khairulzaman, I., Jahns, H., King, D., Finlay, E.K., Mok, K.H., Fair, S., Lonergan, P. and Farrelly, C.O. 2016. Sperm-coating beta-defensin 126 is a dissociation-resistant dimer produced by epididymal epithelium in the bovine reproductive tract. Biol. Reprod., 95(6), 121–129; doi:10.1095/biolreprod.116.138719. Narciandi, F., Lloyd, A.T., Chapwanya, A., O’Farrelly, C. and Meade, K.G. 2011. Reproductive tissue-specific expression profiling and genetic variation across a 19 gene bovine β-defensin cluster. Immunogenetics 63, 641–651; doi:10.1007/s00251-011-0551-7. Ni, Y., Zhou, Y., Chen, W.Y., Zheng, M., Yu, J., Li, C., Zhang, Y. and Shi, Q.X. 2009. HongrES1, a cauda epididymis-specific protein, is involved in capacitation of guinea pig sperm. Mol. Reprod. Dev. 76(10), 984–993; doi:10.1002/mrd.21063. Pazgier, M., Hoover, D., Yang, D., Lu, W. and Lubkowski, J. 2006. Human β-defensins. Cell Mol. Life Sci. 63, 1294–1313; doi:10.1007/s00018-005-5540-2. Pujianto, D.A., Muliawati, D., Rizki, M.D., Parisudha, A. and Hardiyanto, L. 2020. Mouse defensin beta 20 (Defb20) is expressed specifically in the caput region of the epididymis and regulated by androgen and testicular factors. Reprod. Biol. 20(4), 536–540. doi:10.1016/j.repbio.2020.09.003. Ribeiro, C.M., Silva, E.J., Hinton, B.T. and Avellar, M.C.W. 2016. β-defensins and the epididymis: contrasting influences of prenatal, postnatal, and adult scenarios. Asian J. Androl. 18(2), 323; doi:10.4103/1008-682X.168791. Saini, M.K., Joshi, S., Thanvi, P., Panwar, A., Singh, D. and Siyag, R. 2022. Gross anatomical study on the testes of camel (Camelus dromedarius). J. Pharm. Innov. 11(SP), 2673–2679. Sangeeta, K. and Yenugu, S. 2019. Male reproductive tract antimicrobial expression in the extremes of ages of rats. Gene 710, 218–232. Scholar, A. 2020. An overview of the anatomy and physiology of the male one-humped camel (Camelus dromedarius) reproductive system. 17(2), 141–152. Solanki, S., Kumar, V., Kashyap, P., Kumar, R., De, S. and Datta, T. K. 2023. Beta-defensins as marker for male fertility: a comprehensive review. Biol. Reprod. 108(1), 52–71; doi:10.1093/biolre/ioac197. Thimon, V., Koukoui, O., Calvo, E. and Sullivan, R. 2007. Region-specific gene expression profiling along the human epididymis. Mol. Hum. Reprod. 13(10), 691–704; doi:10.1093/molehr/gam051. Tingari, M., El-Manna, M., Rahim, A., Ahmed, A. and Hamad, M. 1986. Studies on camel semen. I. Electroejaculation and some aspects of semen characteristics. Animal Reprod. Sci. 12(3), 213–222. doi.org/10.1016/0378-4320(86)90042-4. Tollner, T.L., Bevins, C.L. and Cherr, G.N. 2012. Multifunctional glycoprotein DEFB126—a curious story of defensin-clad spermatozoa. Nat. Rev. Urol. 9(7), 365–375; doi:10.1038/nrurol.2012.109. Tollner, T.L., Venners, S.A., Hollox, E.J., Yudin, A.I., Liu, X., Tang, G., Xing, H., Kays, R.J., Lau, T., Overstreet, J.W. and Overstreet, J.W. 2011. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci. Transl. Med. 3(92), 92ra65; doi:10.1126/scitranslmed.3002289. Tollner, T.L., Yudin, A.I., Treece, C.A., Overstreet, J.W. and Cherr, G.N. 2008. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum. Reprod. 23(11), 2523–2534; doi:10.1093/humrep/den276. Wilson, S.S., Wiens, M.E. and Smith, J.G. 2013. Antiviral mechanisms of human defensins. J. Mol. Biol. 425(24), 4965–4980; doi:10.1016/j.jmb.2013.09.038. Wu, H., Guang, X., Al-Fageeh, M.B., Cao, J., Pan, S., Zhou, H., Abutarboush, M.H., Xing, Y., Xie, Z. and Alshanqeeti, A.S. 2014. Camelid genomes reveal evolution and adaptation to desert environments. Nat. Commun. 5(1), 5188; doi:10.1038/ncomms6188. Xin, A., Cheng, L., Diao, H., Wu, Y., Zhou, S., Shi, C., Sun, Y., Wang, P., Duan, S., Zheng, J., Wu, B., Yuan, Y., Gu, Y., Chen, G., Sun, X., Shi, H., Tao, S. and Zheng, J. 2016. Lectin binding of human sperm associates with DEFB126 mutation and serves as a potential biomarker for subfertility. Sci. Rep. 6(1), 20249; doi:10.1038/srep20249. Yamaguchi, Y., Nagase, T., Makita, R., Fukuhara, S., Tomita, T., Tominaga, T., Kurihara, H. and Ouchi, Y. 2002. Identification of multiple novel epididymis-specific β-defensin isoforms in humans and mice. J. Immunol. 169(5), 2516–2523; doi:10.4049/jimmunol.169.5.2516. Yudin, A.I., Generao, S.E., Tollner, T.L., Treece, C.A., Overstreet, J.W. and Cherr, G.N. 2005. Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biol. Reprod. 73(6), 1243–1252; doi:10.1095/biolreprod.105.042432. Yudin, A.I., Tollner, T.L., Li, M.-W., Treece, C.A., Overstreet, J.W. and Cherr, G.N. 2003. ESP13. 2, a member of the β-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol. Reprod. 69(4), 1118–1128; doi:10.1095/biolreprod.103.016105. Yudin, A.I., Tollner, T.L., Treece, C.A., Kays, R., Cherr, G.N., Overstreet, J.W. and Bevins, C. L. 2008. b-Defensin 22 is a major component of the mouse sperm glycocalyx. Reproduction 136, 753–765; doi:10.1530/REP-08-0164. Zhou, C.X., Zhang, Y.-L., Xiao, L., Zheng, M., Leung, K.M., Chan, M.Y., Lo, P.S., Tsang, L.L., Wong, H.Y., Ho, L,S,, Chung, Y.W. and Ho, L.S. 2004. An epididymis-specific β-defensin is important for the initiation of sperm maturation. Nat. Cell. Biol. 6(5), 458–464; doi:10.1038/ncb1127. Zupin, L., Polesello, V., Martinelli, M., Luppi, S., Giolo, E., Zito, G., Romano, F., Segat, L., Crovella, S, and Ricci, G. 2019. Human β-defensin 1 in follicular fluid and semen: impact on fertility. J. Assist. Reprod. Genet. 36, 787–797; doi:10.1007/s10815-019-01409-w. | ||
| How to Cite this Article |
| Pubmed Style Khodair KMA, Al-shabebi A, Al-thnaian TA, Alturki OIM, A.m. M, Al-ramadan SY, Ali AM, Elseory AM. Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season. Open Vet. J.. 2025; 15(3): 1206-1216. doi:10.5455/OVJ.2025.v15.i3.12 Web Style Khodair KMA, Al-shabebi A, Al-thnaian TA, Alturki OIM, A.m. M, Al-ramadan SY, Ali AM, Elseory AM. Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season. https://www.openveterinaryjournal.com/?mno=229123 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.12 AMA (American Medical Association) Style Khodair KMA, Al-shabebi A, Al-thnaian TA, Alturki OIM, A.m. M, Al-ramadan SY, Ali AM, Elseory AM. Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season. Open Vet. J.. 2025; 15(3): 1206-1216. doi:10.5455/OVJ.2025.v15.i3.12 Vancouver/ICMJE Style Khodair KMA, Al-shabebi A, Al-thnaian TA, Alturki OIM, A.m. M, Al-ramadan SY, Ali AM, Elseory AM. Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1206-1216. doi:10.5455/OVJ.2025.v15.i3.12 Harvard Style Khodair, K. M. A., Al-shabebi, . A., Al-thnaian, . T. A., Alturki, . O. I. M., A.m., . M., Al-ramadan, . S. Y., Ali, . A. M. & Elseory, . A. M. (2025) Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season. Open Vet. J., 15 (3), 1206-1216. doi:10.5455/OVJ.2025.v15.i3.12 Turabian Style Khodair, Khalid M. Al, Abdulkarem Al-shabebi, Thnaian A. Al-thnaian, Osamah I. M. Alturki, Marwa-babiker A.m., Saeed Y Al-ramadan, Abdelhay M. Ali, and Abdelrahman M.a. Elseory. 2025. Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season. Open Veterinary Journal, 15 (3), 1206-1216. doi:10.5455/OVJ.2025.v15.i3.12 Chicago Style Khodair, Khalid M. Al, Abdulkarem Al-shabebi, Thnaian A. Al-thnaian, Osamah I. M. Alturki, Marwa-babiker A.m., Saeed Y Al-ramadan, Abdelhay M. Ali, and Abdelrahman M.a. Elseory. "Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season." Open Veterinary Journal 15 (2025), 1206-1216. doi:10.5455/OVJ.2025.v15.i3.12 MLA (The Modern Language Association) Style Khodair, Khalid M. Al, Abdulkarem Al-shabebi, Thnaian A. Al-thnaian, Osamah I. M. Alturki, Marwa-babiker A.m., Saeed Y Al-ramadan, Abdelhay M. Ali, and Abdelrahman M.a. Elseory. "Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season." Open Veterinary Journal 15.3 (2025), 1206-1216. Print. doi:10.5455/OVJ.2025.v15.i3.12 APA (American Psychological Association) Style Khodair, K. M. A., Al-shabebi, . A., Al-thnaian, . T. A., Alturki, . O. I. M., A.m., . M., Al-ramadan, . S. Y., Ali, . A. M. & Elseory, . A. M. (2025) Beta-defensin 126 (DEFB126) localization and expression in the sperms and male reproductive tract of the dromedary camel (Camelus dromedarius) during the rutting season. Open Veterinary Journal, 15 (3), 1206-1216. doi:10.5455/OVJ.2025.v15.i3.12 |