| Review Article | ||
Open Vet. J.. 2025; 15(3): 1066-1077 Open Veterinary Journal, (2025), Vol. 15(3): 1066-1077 Review Article B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A reviewIago Martins Oliveira1*, Wanessa Patrícia Rodrigues da Silva2 and Naida Cristina Borges31Pontifícia Universidade Católica de Goiás, Escola de Ciências Médicas e da Vida, Goiânia, Goiás, Brazil 2Programa de Pós-graduação em Ciência Animal, Escola de Veterinária e Zootecnia, Universidade Federal de Goiás, Goiânia, Brazil 3Departamento de Medicina Veterinária, Escola de Veterinária e Zootecnia, Universidade Federal de Goiás, Goiânia, Brazil *Corresponding Author: Iago Martins Oliveira. Escola de Ciências Médicas e da Vida, Pontifícia Universidade Católica de Goiás, Goiânia, Brazil. Email: iago.vetufg [at] gmail.com Submitted: 17/11/2024 Accepted: 08/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
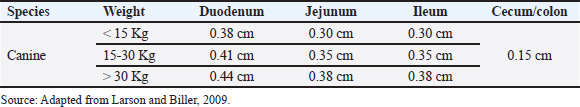
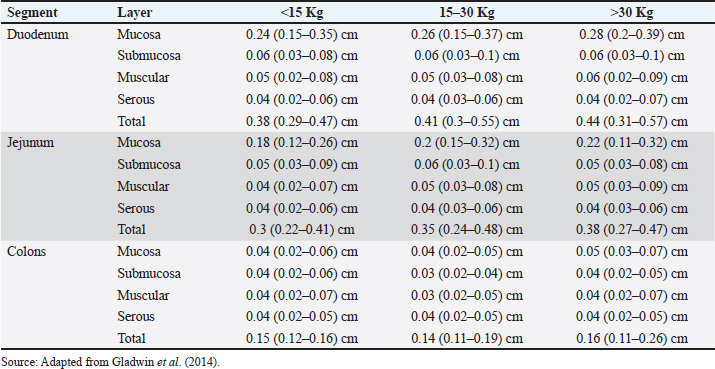
AbstractUltrasound (USG) is a valuable diagnostic tool for evaluating the gastrointestinal tract of small animals, providing noninvasive and dynamic information. This review discusses intestinal evaluation in dogs using B-mode ultrasound and advanced contrast-enhanced ultrasound (CEUS) with microbubbles. B-mode USG allows the examination of the thickness and stratification of the intestinal wall, motility, and adjacent structures, such as lymph nodes and peritoneum to be examined. Reference values for intestinal wall thickness in dogs are based on body weight. The duodenum, jejunum, and ileum can be differentiated sonographically by location wall stratification, and relationship to other intestinal segments. Intestinal motility is assessed by counting peristaltic waves, and abnormalities indicate various diseases. CEUS uses microbubble-based contrast agents, such as sulfur hexafluoride, which remain stable in the vascular system and are eliminated by the lungs. This technique improves the perception of ultrasound echoes, making it possible to quantify tissue perfusion using parameters such as entry time, peak enhancement, and exit time. In human medicine, CEUS is used to assess intestinal perfusion in conditions such as Crohn’s disease and ischemia. In veterinary medicine, this technique has been used to assess duodenal perfusion in healthy dogs and those with chronic enteropathies or alimentary lymphoma. Although CEUS shows potential in differentiating dogs with chronic enteropathies from healthy controls, more research is needed to standardize the methodology, establish reference values, and define clinical applications in various canine intestinal diseases. The combination of B-mode USG and CEUS provides a noninvasive assessment of intestinal morphology and function, contributing to the understanding of the pathophysiology of enteropathies in dogs. Keywords: Canine, Contrast, Ultrasound, Intestinal wall thickness, Microbubbles. IntroductionIntestinal ultrasound evaluation provides relevant information on topography, wall thickness, layer stratification, echogenicity, motility, luminal content, and characteristics of adjacent structures such as lymph nodes, peritoneum, and other organs attached to the gastrointestinal tract (GIT) (Larson and Biller, 2009). USG can be helpful in cases where patients present symptoms of gastrointestinal disease, but clinical confrontation of the findings is always required (Larson and Biller, 2009; Penninck and D’anjou, 2015). However, there is a description of the limitations of USG in terms of its potential to define the diagnosis in some situations, especially when the examination is carried out based exclusively on clinical signs such as vomiting and diarrhea (Leib et al., 2010; Leib et al., 2012; Mapletoft et al., 2018). Diffuse or focal thickening, loss of parietal stratification, and increased or decreased echogenicity are findings on USG in dogs with GIT diseases (Penninck and D’anjou, 2015). Contrast-enhanced ultrasound (CEUS) is a sensitive method for assessing the vascular perfusion of organs (Nyman et al., 2005). This innovative and noninvasive test can be useful for the intestinal evaluation of dogs (Cordella et al., 2021). In medicine, CEUS is used to assess intestinal vascularization in inflammatory (Lassau et al., 2006) and neoplastic diseases (Serra et al., 2007). In Crohn’s disease, CEUS revealed an increase in the enhancement pattern of the intestinal vessels, which was correlated with the clinical activity of the disease. Thus, the test is a tool for monitoring patients and has prognostic potential (Robotti et al., 2004). In veterinary medicine, only a few studies have assessed CEUS for intestinal assessment. More recent research has been based on evaluating intestinal perfusion in dogs (Nisa et al., 2018) and cats (Diana et al., 2011), studying intestinal ischemia in cats (Linta et al., 2020), canine inflammatory bowel disease, differentiating canine chronic enteropathy from alimentary lymphoma (Linta et al., 2021), and a case report of histiocytic colitis in a dog (Cordella et al., 2021). Ultrasound modalities integrate and provide important information about the intestine (Larson and Biller, 2009; Heilmann, 2015). Given the scarcity of studies, the aim of this literature review was to address the intestinal evaluation of dogs using B-mode USG and the advanced CEUS technique. B-mode ultrasound for the intestinal assessment of dogs USG is an important diagnostic imaging modality for assessing the gastrointestinal tract of small animals, as it is a noninvasive test that provides dynamic information and can be recommended for acute and chronic enteropathies (Haber et al., 2002; Bhavani et al., 2021). It is recommended for dogs showing clinical signs of gastrointestinal disease (Delaney et al., 2003; Seiler et al., 2022). However, Leib et al. (2010) found that in dogs with chronic vomiting, the diagnostic usefulness of USG was greater in older animals, in cases where there was a greater number of vomiting episodes and when there was weight loss. In dogs with chronic diarrhea, the usefulness of this imaging test was greater in cases of weight loss, alterations in abdominal palpation, and suspected GIT neoplasia (Leib et al., 2012). In another study, ultrasound was considered counterproductive in some situations where only clinical signs justified its indication (Mapletoft et al., 2018). We believe that USG has some limitations and that its use should be based on not only clinical manifestation but also anamnesis, the evolution of the presenting symptom, and the results of laboratory tests. In the intestinal evaluation of dogs, USG has been used in cases of gastrointestinal foreign bodies (Dandrieux, 2016; Bhavani et al., 2021) and to suggest a differential between inflammation and neoplastic processes (Simeoni et al., 2020). The evaluation focused on the intestine of dogs provides information on the thickness of the organ wall layer, motility, parietal stratification, and peripheral structures, such as lymph nodes and peritoneum (Larson and Biller, 2009). Gladwin et al. (2014) determined reference values for the thickness of the layers of the duodenum, jejunum, and colon in dogs with the aim of providing support in the management of disorders that alter the thickness and stratification of these intestinal layers. This evaluation was the subject of a study that correlated the ultrasound intestinal layer of canine cadavers with histological aspects, except for the serous layer. There was no correlation between USG and histology; however, there was a hyperechoic line in the muscle, referring to the interface of the circular and longitudinal fibers (Le Roux et al., 2016). Abdominal USG provides dynamic information that reflects the physiology and anatomy of the structures assessed (Malancus and Malancus, 2017). The method was performed in a systematic evaluation pattern with an organized analysis of the components (Nyland et al., 2015). Knowledge of normal and altered ultrasound features is advantageous in the diagnosis of GIT diseases (Larson and Biller, 2009). Basic principles of bowel ultrasoundTo perform the examination, the patient must be properly positioned in relation to the operator, USG equipment, and transducer (Carvalho, 2004). These considerations are related to obtaining consistent images and the comfort of the animal and examiner. The projected image is classified in the sagittal plane into ventral, cranial dorsal, and caudal, while in the transverse plane, the images formed are divided into medial and lateral (Nautrup, 2000). For evaluating the GIT, high-frequency transducers (7.5–10 MHz) improve image resolution and allow for greater detail, as well as the measurement of the layers (Penninck and D’anjou, 2015). In addition to the frequency mentioned above, linear transducers are the most recommended. For a more detailed analysis, the scans should be in the longitudinal and transverse planes (Penninck and D’anjou, 2015). Preparing the animal for the examination is of fundamental importance and reflects on the quality of the images generated. For ultrasound examinations focused on intestinal assessment, it is necessary to perform a wide trichotomy of the ventral region of the abdomen, from the seventh intercostal space to the caudal inguinal region. Because the ultrasound beam does not penetrate the air trapped between the hairs, it generates reverberation artifacts that impair the interpretation of the findings. The close contact between the skin and the transducer facilitates image formation without interference (King, 2006). To remove air and fat from the surface of the skin, which could potentially meet the transducer, in addition to the trichotomy technique, acoustic gel, and 70% isopropyl alcohol should be used before using any other material (Carvalho, 2004). The acoustic gel should be applied abundantly to the skin to favor contact and prevent the formation of reverberation artifacts (Nyland et al. 2015). Ideally, an 8-hour food fast is recommended in dogs without water restriction to help reduce gas inside the GIT, as this gas in the GIT promotes the formation of elements that hinder the assessment of abdominal structures (Penninck and D’anjou, 2015). The order of assessment varies, but in general, for intestinal assessment, the duodenum is assessed halfway through the procedure, while the general assessment of other intestinal segments (jejunum, ileum, cecum, ileoceocolic valve, ascending, descending, and transverse colon) along with lymph nodes is carried out at the end of the USG (Nautrup, 2000). The sequence is individual to the practitioner, but all structures should be categorically assessed (Nyland et al., 2015). The thickness of the intestinal segments in dogs can be measured using cursors in the USG software at the outer edge of the serous layer and the inner portion of the mucosal layer (Riedesel, 2015). However, it is known that there is a positive correlation between the measurement of the intestinal wall and the dog’s body weight, so reference values are divided based on weight ranges (Gladwin et al., 2014). The reference values for the total measurements of the intestinal walls of dogs are presented in Table 1 and stratified in Table 2. Ultrasound bowel anatomyThe normal topography of the duodenum is in the right cranial abdominal quadrant starting between the last ribs and distal along the right abdominal wall, and the ultrasound evaluation of the intestinal segments is done by moving the transducer from right to left and the other way around, then cranial and caudal (Agut, 2009). These maneuvers were performed to obtain images of the intestines in all sections, including sagittal transverse, and oblique images (Penninck and D’anjou, 2015). The duodenum (Fig. 1B) of a healthy dog can be differentiated from the jejunum (Fig. 1C) by USG because of its more superficial topography in the right epigastric region and because it is cranially close to the stomach (Fig. 1A). In addition, it is possible to observe the duodenal papilla as an anatomical reference if the image resolution is of high quality (Riedesel, 2015). The ileum (Fig. 1D) was anatomically located in the craniomedial abdomen to the right of the ascending colon and cecum (Goggin et al., 2000). This intestinal segment can be visualized by USG in the right abdominal quadrant and is the most robust and bright submucosal layer in the image (Prestes et al., 2019). The jejunum can be found and evaluated by the US in practically every abdominal cavity (Goggin et al., 2000). Although the jejunal loops can be found and form an image during the evaluation of the other structures during the complete examination, systematic and detailed scanning of the jejunal segments is recommended (Larson and Biller, 2009). Table 1. Reference values for the wall thickness of intestinal segments in dogs divided by region and body weight.
Table 2. Reference values for the thickness of the intestinal wall layers (in cm) of healthy dogs according to body weight with segmentation of values with variations in measurements according to each layer (mucosa, submucosa, muscle and serosa).
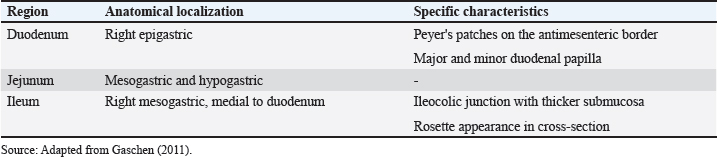
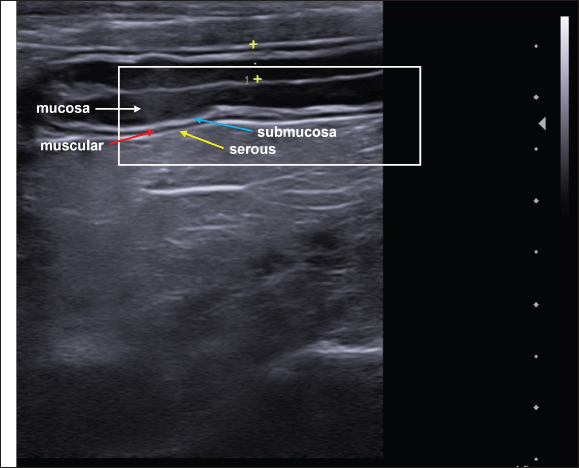
There is difficulty in scanning the colon due to the large amount of gas that is naturally present, plus the fecal material that is stored is an additive factor in the difficulty of evaluation (Larson and Biller, 2009). The transverse colon lies immediately caudally to the stomach and duodenum, while the descending branch can be found caudally and dorsally to the urinary bladder (Goggin et al., 2000). The segments of the colon are shown in Figure 2. When the colon is seen to be distended on ultrasound, it can be mistakenly characterized as a full uterus. However, the differentiation between the abdominal organs and the intestines on ultrasound, in addition to differential ultrasound diagnosis of diseases, can be obtained by checking intestinal peristalsis, the pattern of stratification of layers, and the topography of the intestine and its adjacencies (Drost, 2015). In dogs, the segments of the colon are often visualized on USG with gas or fecal distension and have a thinner wall than the other parts of the intestine in dogs (Penninck and D’anjou, 2015). The duodenum, jejunum, and ileum can be characterized on USG and differentiated based on some image findings, topography, mural stratification, and syntony with other intestinal segments (Gaschen, 2011). This information is detailed in Table 3. On USG, the five parietal layers can be assessed (Fig. 3), with an interface between the intestinal lumen and the mucosa that is hyperechoic and forms a bright, central line on the image. The mucosal layer is associated with this and is hypoechogenic, followed by the hyperechogenic submucosa. The thin muscular layer is hypoechogenic, and the outermost layer is the serosa, which is hyperechogenic (Goggin et al., 2000; Agut, 2009; Larson and Biller, 2009).
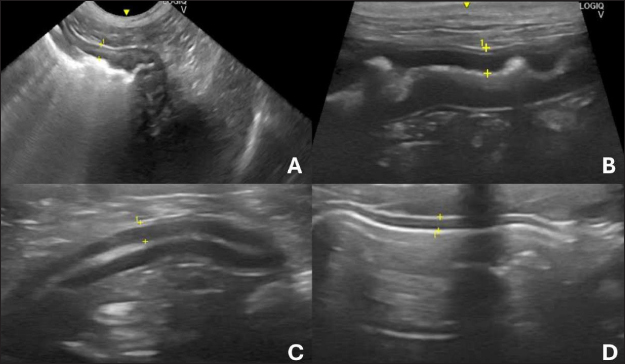
Fig. 1. Ultrasound images of the gastrointestinal tract of healthy dogs. A: gastric body, B: duodenum, C: jejunum, D: ileum. All segments with wall thickness were measured using a cursor in the ultrasound machine software. 8 MHz microconvex transducer.
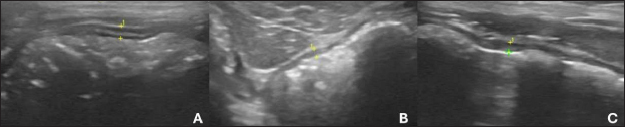
Fig. 2. Ultrasound images of the colon in healthy dogs. A, ascending colon; B, transverse colon; C, descending colon. All segments with wall thickness were measured using the ultrasound machine’s software cursor. An 8-MHz microconvex transducer was used, and longitudinal section images. Table 3. Intestinal ultrasound properties of dogs separated by region, anatomical location and specific characteristics.
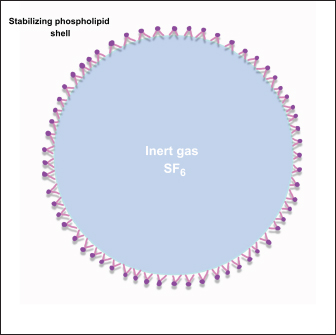
Fig. 3. Ultrasound image of the intestinal segments of a dog. The image shows transverse and longitudinal sections. The stratified layers are the mucosal layer (white arrow), submucosal layer (blue arrow), muscular layer (red arrow), and serosal layer (yellow arrow). The content in the intestines, as well as its quantity, is directly related to the ultrasound appearance, and when there is little intestinal filling, it is possible to observe a mucous pattern with a hyperechogenic nucleus surrounded by a hypoechogenic halo of the intestinal wall (Penninck and Nyland, 1990; Agut, 2009). The nucleus formed generally represents the mucoid content and air bubbles that are trapped at the interface between the lumen and mucosa. However, when the intestine is filled with fluid, an anechoic area can be observed between the intestinal walls (Agut, 2009). Gaseous filling of the intestinal loops leads to the formation of a highly echogenic interface with posterior acoustic shadowing (Penninck and Nyland, 1990). Ultrasound evaluation of intestinal motilityIntestinal motility can be assessed by counting peristaltic waves, and the expected contractions for the intestines are 3–5 movements per minute (Penninck and Nyland, 1990). This assessment can be performed using USG in different segments of the GIT (Riedesel, 2015). Inflammatory, infectious, and neoplastic intestinal diseases can cause functional alterations with compromised motility, and the ileum is the segment most frequently affected. The dysmotility that occurs in the adynamic ileus must be differentiated from mechanical obstructions, and USG is essential in the differential diagnosis, since the sonographic characteristics of the functional condition are mild to moderate, generalized dilation, predominantly liquid filling, reduced or absent motility with movement of the intraluminal intestinal contents (Penninck and D’anjou, 2015). In the case of altered intestinal motility due to mechanical causes, USG can show dilatation of varying proportions in the intestinal portions, but most commonly, there is dilatation cranial to the obstructive point or stenosis, with increased peristalsis in the region, moderate to severe distension of the intestine, reduced diameter, and motility in the section caudal to the point of obstruction. In these cases, the foreign body can usually be visualized by a hyperechoic surface that promotes posterior acoustic shadowing (Gaschen, 2011). Intestinal hypermotility can be detected using ultrasound and occurs secondary to the presence of an obstructive or non-obstructive foreign body, dietary changes, intestinal parasitism, enteritis, and previous intestinal surgery. This increased mobility can lead to intussusception (Oliveir , 2019). Intestinal motility was assessed using USG with the influence of fasting in healthy dogs, and it was found in this study that the test is effective in assessing the motility of the GIT and that food restriction of 12–24 hours led to a reduction in the contractility of the stomach, duodenum, jejunum, and ileum. However, there was a need for further studies with longer fasting times postoperatively and in dogs with gastrointestinal diseases (Sanderson et al., 2017). In addition to B-mode USG, the use of pulsed Doppler ultrasound makes it possible to graphically observe the movements of the small intestine in dogs, and these values can be analyzed for qualitative and quantitative data, which provides a noninvasive way of assessing intestinal kinetics (An et al., 2001). Basic principles and physical properties of contrast-enhanced ultrasonographyThe CEUS is a diagnostic imaging technique based on the use of contrast-enhanced images associated with ultrasound examination. It is a current tool applied to routine veterinary medicine and has led to the modernization and optimization of the diagnosis of various conditions in animals, especially in diseases that compromise the vascular perfusion of the organ (Nyman et al., 2005). CEUS uses microbubbles (Fig. 4) as a contrast medium to intensify the perception of ultrasound echoes and quantify tissue perfusion. Microbubbles have a high reflective capacity and are therefore able to increase the Doppler signal, allowing the perception of flows that cannot be verified by other techniques. The presence of these microbubbles in each region visualized by USG generates a contrast effect in the structure analyzed, which favors the observation of microcirculation (Nyman et al., 2005). Microbubbles are gases with a high molecular weight and a diameter of between 1 and 6 μm, which favors and allows them to pass through the bed of the blood capillaries into the intravascular space without diffusing into the interstitium and renal excretion, making them safe to use with no hemodynamic effects (Kalantarinia and Okusa, 2007). The use of microbubble contrast was made unfeasible for a time because there was no commercially available product that could remain in the intravascular space after passing through the pulmonary circulation. Subsequently, more resistant media capable of remaining in the systemic circulation were created (Ignee et al., 2016). Currently, most contrast mediums are stable because they contain microbubbles that are covered by a capsule, usually a thin membrane of phospholipids arranged in a monolayer with hydrophilic poles on the outside toward the circulating blood and lipophilic chains arranged toward the encapsulated gas (Baun, 2017; Takahashi et al., 2021). These contracts are currently classified as first-generation, when the gas used has a lower diffusion coefficient and is, therefore, less stable, and second-generation, which is more modern because the gas has a higher diffusion coefficient and is, therefore, more stable (Takahashi et al., 2021).
Fig. 4. Representative image of a microbubble used as an ultrasound contrast agent. The material consists of a lipidcoated membrane filled with gaseous content. The lumen is filled with sulfur hexafluoride (SF6). The figure was created using the Penup Samsung® application (Windows 11, Ldta, Samsung Electronics, Suwon, South Korea) and Canva Pro® (Canva Pty Ltd, Sydney, Australia). The product most commonly used in this technique is sulfur hexafluoride, a contrast element capable of remaining stable inside the vessels without diffusing through the vascular bed. The medium also has the potential to remain in the bloodstream for prolonged periods due to its low solubility in water and the high molecular weight of its gaseous molecules, which makes it resistant to external pressure (Volta et al., 2014) and is excreted by the lungs (Nyman et al., 2005). It can be administered mainly by two routes: intravenous and intracavitary (Takahashi et al., 2021). Contrasts have a low capacity to cause adverse reactions. In veterinary medicine, syncope and emesis are described after 24 hours of use (Seiler et al., 2013). In medicine, there are few reports of headaches, nausea, and increased body temperature after CEUS (Haers et al., 2010). The product is contraindicated for individuals with hypersensitivity reactions to any component of the contrast medium (Takahashi et al., 2021). The safety profile of the material used to perform CEUS has been extensively studied in children and has been shown to be highly safe with discrete, self-limiting, or easily reversible reactions (Rosado and Riccabona, 2016; Mao et al., 2019). The intravenous administration of CEUS is indicated for assessing intestinal perfusion. After application, the microbubbles are restricted to the intravascular space without entering the organ parenchyma (Fig. 5). Contrast media classified as second generation can remain in the vascular bed for approximately 10 minutes, and as the bubbles break, they are gradually eliminated through respiration (Takahashi et al., 2021). The physical properties of using microbubbles are due to the nonlinear detection that bubbles of different sizes produce when they are diluted in the patient’s blood and meet ultrasound echoes and, consequently, produce harmonic frequencies. The oscillation of this material in the evaluation region generates an intense contrast enhancement on the surface of the organ, as shown in the USG image, allowing microcirculation to be observed (Nyman et al., 2005). The image parameters used to evaluate this imaging method are related according to the degree of homogeneous or heterogeneous filling of the organ’s microcirculation by the microbubbles, mainly in specific circular regions within the tissues being examined. This defines the intensity patterns as “hyperintense” in tissues with greater intensity of visualization of the contrasted region, “isointense” in which it is not possible to distinguish the tissue adjacent to the evaluated region even with the use of contrast and “hypointense”, which determines a pattern of low intensity (Volta et al., 2014).
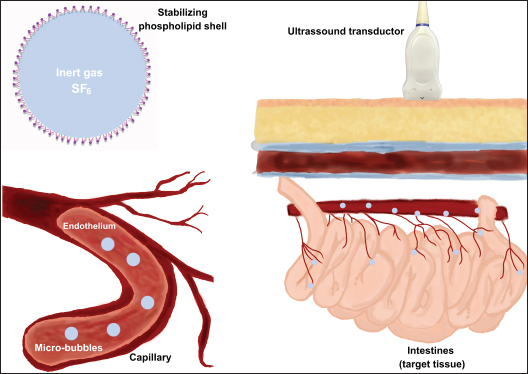
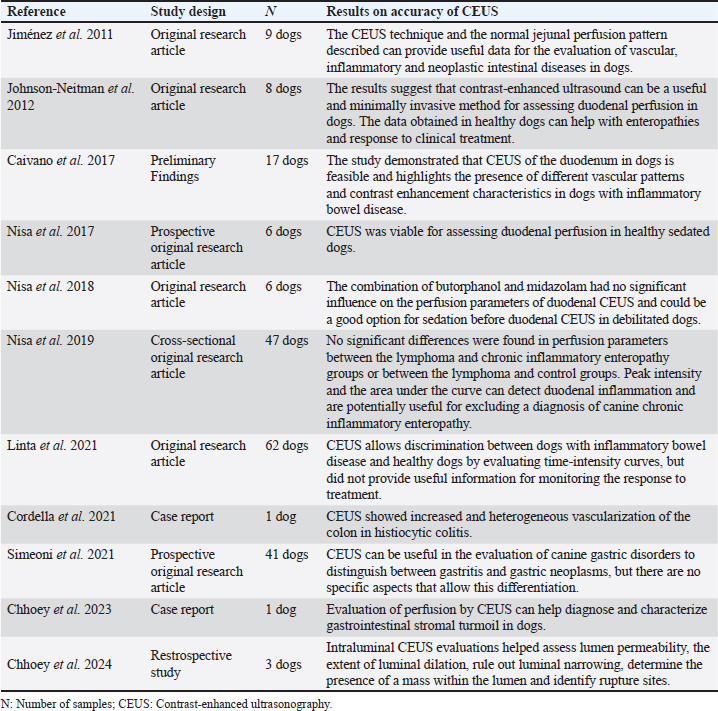
Fig. 5. Representative images of the basic principles of ultrasound with microbubble contraction. When exposed to ultrasound echoes, the microbubbles containing gas and coated with lipid membrane oscillate in expansion and generate vascular markings in the tissue. The migration of the contrast into the intestinal vessels can be observed. The figure was created using the Penup Samsung® application (Windows 11, Ldta, Samsung Electronics, Suwon, South Korea) and Canva Pro® (Canva Pty Ltd, Sydney, Australia). In addition, CEUS can be used to assess vessel filling time from the intravenous application of contrast to the start of perfusion of the organ under analysis, known as wash-in. The peak of contrast enhancement corresponds to the moment of greatest perfusion after wash-in and the time of total contrast elimination, known as wash-out (Lock et al., 2011). Contrast-enhanced ultrasound for intestinal evaluation in dogsIn medicine, among the various organs evaluated using this technique, there have been studies on intestinal ischemia (Kalantarinia and Okusa, 2007). Furthermore, the intestinal perfusion of people with Crohn’s disease was assessed using CEUS, and changes were observed in the contrast enhancement pattern and intestinal perfusion parameters when compared with healthy control subjects (Quaia, 2013). In addition, CEUS has been used for intestinal assessment in medicine to monitor the activity and response to treatment of Crohn’s disease (Saevik et al., 2014). Some CEUS studies used in the intestinal evaluation of dogs are presented in Table 4. The evaluation of intestinal perfusion in dogs provides important information for the diagnosis and monitoring of chronic intestinal diseases (Nisa et al., 2019). Jiménez et al. (2011) published quantitative and qualitative data that allowed the intestinal vascularization of dogs without enteropathy to be characterized, but clinical applicability had not been established at the time of the study. Table 4. Contrast-enhanced ultrasonography studies for intestinal evaluation in dogs organized by author, study design, number of samples and main results.
One study evaluated the repeatability and reproducibility of perfusion parameters using CEUS of the duodenum of healthy dogs under sedation. The results showed that in this experimental unit profile and under sedation, the parameters were stable, namely the time taken for the contrast to reach the duodenum until maximum enhancement, the degree of intensity of this enhancement, and the entry and exit rates of the microbubbles. Despite these results, a further study in dogs with intestinal disorders was suggested to determine the clinical applicability of the test (Nisa et al., 2019). Subsequently, the duodenum of dogs with CE and alimentary lymphoma was evaluated using CEUS. The CEUS-derived data showed that the regional blood volume in the duodenal mucosa was increased in dogs with CE compared with the control group. These results also suggest that perfusion parameters can be used as predictive values for duodenal inflammation in symptomatic animals. However, the modality was not sufficient to differentiate CE from alimentary lymphoma given the similarity of CEUS findings in both conditions (Nisa et al., 2019). In a more recent study, CEUS was effective in distinguishing dogs with CE from those without intestinal disease using the results of the microbubble contraction time and intensity curve. However, qualitative evaluation could not determine this differentiation. Furthermore, the method is not capable of evaluating the monitoring of response to treatment independently if qualitative and quantitative metrics are considered (Linta et al., 2021). In a case report of granulomatous colitis in a French Bulldog multiple diagnostic imaging techniques were used, such as B-mode USG, elastography, and CEUS. Contrast enhancement showed greater demarcation in the serosa and muscular region and, to a lesser extent, in the mucosa and submucosa, as well as an accelerated wash-in and wash-out pattern, which determined increased and heterogeneous circulation in the colon (Cordella et al., 2021). In general, in the veterinary medical literature, the application and use of CEUS are more restricted to intestinal perfusion studies, specifically in the duodenum. In some cases, the technique has been described in the use of dogs with CE and alimentary lymphoma (Nisa et al., 2019). Considering the scarcity of publications on the subject, a recent study aimed to prospectively evaluate the feasibility of CEUS in the duodenal perfusion of dogs, to observe the enhancement pattern and, in addition, to follow up dogs treated in a standardized way for intestinal affection using this imaging exam (Linta et al., 2021). We emphasize that although USG can provide important information about the GIT in dogs, it is important to consider that its indication should not be based solely on isolated clinical signs. It is necessary to associate clinical information, physical examination findings, and correlations with laboratory results. In some situations, ultrasound examination may be inconclusive because of the lack of specificity of clinical gastrointestinal signs in dogs. Therefore, we believe that USG is an important diagnostic tool, but it must be used based on well-defined clinical criteria. ConclusionThe anatomical and functional complexity of GIT in dogs, together with the frequent diseases that affect the intestines of these animals and the nonspecific clinical signs, highlight the importance of auxiliary diagnostic methods. This underscores the relevance of basic and advanced CEUS. These dynamic procedures help assess both functionality and morphology. We emphasize that USG results can be nonspecific if the underlying cause of the disease is not related to the gastrointestinal tract. Due to the nonspecificity of gastrointestinal clinical signs, ultrasound examination should be indicated based on the entire clinical context, history, and other complementary tests. B-mode ultrasound has limitations for gastrointestinal assessment, such as the experience of the examiner, the presence of gas in the GIT, the depth of the organs assessed, and anatomical variations between species and breeds. CEUS for intestinal assessment has limitations, such as being restricted to use in animals with heart disease and respiratory disorders and the lack of studies with reference parameters for different enteropathies. An analysis of the descriptions of the sources used in this study shows that B-mode and CEUS are valuable approaches for noninvasive intestinal assessment. These findings emphasize the growing importance of combining different complementary tests to understand the pathophysiology of enteropathies. It is worth noting that studies are still needed to fill the gaps in the literature on the methodology of applying these imaging tests, to standardize reference values and measurements, to systematize execution, and to provide practical clinical applications for the different diseases affecting the intestines of dogs. AcknowledgmentsThe Veterinary and Zootechnical School of the Federal University of Goiás, Brazil. Conflicts of interestThe authors declare no conflicts of interest. FundingThis research did not receive any specific grant. Data availabilityAll data supporting the findings of this study are available in the manuscript. Authors’ contributionConceptualization I. M. O., N. C. B., W. P. R. S., methodology I. M. O. and W. P. R. S., formal analysis N. C. B., data curation; I. M. O., W. P. R. S., drafted the manuscript; N. C. B., revised the manuscript; I. M. O., and N. C. B., translated the manuscript. All authors have read and agreed with the published version of the manuscript. ReferencesAgut, A. 2009. Ultrasonography of the small intestine in small animals. Vet. Focus 19, 20–28. An, Y.J., Lee, H., Chang, D., Lee, Y., Sung, J.K., Choi, M. and Yoon, J. 2001. Application of pulsed Doppler ultrasound for the evaluation of small intestinal motility in dogs. J. Vet. Sci. 2, 71–74. Baun, J. 2017. Contrast-enhanced ultrasound: a technology primer. J. Diagn. Med. Sonogr. 33, 446–452. Bhavani, M.S., Kavitha, S., Sumathi, D. and Bhat, A.A. 2021. Abdominal ultrasonography in canine inflammatory bowel disease: a short study. J. Entomol. Zool. Stud. 9, 1977–1981. Caivano, D., Marchesi, M.C., Rishniw, M., Timpano, C., Giorgi, M.E., Antognoni, M.T., Conti, M.B., Miglio, A., Lepri, E. and Birettoni, F. 2017. Contrast-enhanced ultrasonography of the duodenum in dogs with inflammatory bowel disease: preliminary findings. Proceedings of the 27th ECVIM-CA Annual Conference, St. Julian’s, Malta. Carvalho, C.F. 2004. Bases físicas da formação da imagem ultrassonográfica. Ultrassonografia em Pequenos Animais, São Paulo, Brazil: Roca, pp: 1–7. Chhoey, S., Kim, S., Kang, K., Keo, S. and Choi, J. 2023. Contrast enhanced ultrasonography and CT features of gastrointestinal stromal tumor in a dog. J. Vet. Clin. 40, 375–381. Chhoey, S., Kim, S., Kim, E., Lee, D., Kang, K., Keo, S., Acorda, J.A., Yoon, J. and Choi, J. 2024. Intraluminal contrast-enhanced ultrasonography application in dogs and cats. Vet. Sci. 11, 443–450. Cordella, A., Stock, E., Van de Maele, I., Willems, A. and Saunders, J. 2021. Use of contrast-enhanced ultrasonography and shear-wave elastography in the diagnosis of granulomatous colitis in a French Bulldog. Vet. Sci. 8, 133–140. Dandrieux, J.R.S. 2016. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J. Small Anim. Pract. 57, 589–599. Delaney, F., O’Brien, R.T. and Waller, K. 2003. Ultrasound evaluation of small bowel thickness compared to weight in normal dogs. Vet. Radiol. Ultrasound 44, 577–580. Diana, A., Specchi, S., Baron Toaldo, M., Chiocchetti, R., Laghi, A. and Cipone, M. 2011. Contrast-enhanced ultrasonography of the small bowel in healthy cats. Vet. Radiol. Ultrasound 52, 555–559. Drost, W.T. 2015. Física do ultrassom. In: Thrall, D. E. Diagnóstico de Radiologia Veterinária, 6th ed. Rio de Janeiro, Brazil: Elsevier. Gaschen, L. 2011. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 41, 329–344. Gladwin, N.E., Penninck, D.G. and Webster, C.R. 2014. Ultrasonographic evaluation of the thickness of the wall layers in the intestinal tract of dogs. Am. J. Vet. Res. 75, 349–353. Goggin, J., Biller, D.S., Debey, B.M., Pickar, J.G. and Mason, D. 2000. Ultrasonographic measurement of gastrointestinal wall thickness and the ultrasonographic appearance of the ileocolic region in healthy cats. J. Am. Anim. Hosp. Assoc. 36, 224–228. Haber, H.P., Busch, A., Ziebach, R., Dette, S., Ruck, P. and Stern, M. 2002. Ultrasonographic findings correspond to clinical, endoscopic, and histologic findings in inflammatory bowel disease and other enterocolitides. J. Ultrasound. Med. 21, 375–382. Haers, H., Vignoli, M., Paes, G., Rossi, F., Taeymans, O., Daminet, S. and Saunders, J.H. 2010. Contrast harmonic ultrasonographic appearance of focal space-occupying renal lesions. Vet. Radiol. Ultrasound 51, 516–522. Heilmann, R.M. 2015. Evaluation of canine S100A12 and sRAGE as novel disease markers in dogs with inflammatory bowel disease. College Station, TX: Texas A&M University. Ignee, A., Atkinson, N.S., Schuessler, G. and Dietrich, C.F. 2016. Ultrasound contrast agents. Endosc. Ultrasound 5, 355–362. Jiménez, D.A., O’Brien, R.T., Wallace, J.D. and Klocke, E. 2011. Intraoperative contrast-enhanced ultrasonography of normal canine jejunum. Vet. Radiol. Ultrasound 52, 196–200. Johnson-Neitman, J.L., O’Brein, R.T. and Wallace, J.D. 2012. Quantitative perfusion analysis of the pancreas and duodenum in healthy dogs by use of contrast-enhanced ultrasonography. Am. J. Vet. Res. 73, 385–392. Kalantarinia, K. and Okusa, M.D. 2007. Ultrasound contrast agents in the study of kidney function in health and disease. Drug Discov. Today Dis. Mech. 4, 153–158. King, A.M. 2006. Development, advances and applications of diagnostic ultrasound in animals. Vet. J. 171, 408–420. Larson, M.M. and Biller, D.S. 2009. Ultrasound of the gastrointestinal tract. Vet. Clin. North Am. Small Anim. Pract. 39, 747–759. Lassau, N., Lamuraglia, M., Chami, L., Leclère, J., Bonvalot, S., Terrier, P., Roche, A. and Le Cesne A. 2006. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am. J. Roentgenol. 187, 1267–1273. Le Roux, A.B., Granger, L.A., Wakamatsu, N., Kearney, M.T. and Gaschen, L. 2016. Ex vivo correlation of ultrasonographic small intestinal wall layering with histology in dogs. Vet. Radiol. Ultrasound 57, 534–545. Leib, M.S., Larson, M.M., Grant, D.C., Monroe, W.E., Troy, G.C., Panciera, D.L., Rossmeisl, J.H. and Werre, S.R. 2012. Diagnostic utility of abdominal ultrasonography in dogs with chronic diarrhea. J. Vet. Intern. Med. 26, 1288–1294. Leib, M.S., Larson, M.M., Panciera, D.L., Troy, G.C., Monroe, W.E., Rossmeisl, J.H., Forrester, S.D. and Herring, E.S. 2010. Diagnostic utility of abdominal ultrasonography in dogs with chronic vomiting. J. Vet. Intern. Med. 24, 803–808. Linta, N., Baron Toaldo, M., Del Magno, S., Pey, P., Quinci, M. and Diana A. 2020. Two-dimensional and contrast-enhanced ultrasound of intestinal ischemia in four cats: four cases. J. Feline Med. Surg. 22, 384–390. Linta, N., Pey, P., Baron Toaldo, M., Pietra, M., Felici, M., Bettini, G., Cipone, M. and Diana, A. 2021. Contrast-enhanced ultrasonography in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 35, 2167–2176. Lock, G., Schmidt, C., Helmich, F., Stolle, E. and Dieckmann, K.P. 2011. Early experience with contrast-enhanced ultrasound in the diagnosis of testicular masses: a feasibility study. Urology 77, 1049–1053. Malancus, R.N. and Malancus, C.M. 2017. Assessment of ultrasonographic and endoscopic changes in dogs with gastrointestinal disorders. Arq. Bras. Med. Vet. 69, 1451–1455. Mao, M., Xia, B., Chen, W., Gao, X., Yang, J., Li, S., Wang, B., Mai, H., Liu, S., Wen, F., Gan, Y., Song, J., Wei, H., Yang, W., Wu, Y., Yang, S., Yu, W., Yu, H., Fan, S., Tao, H., Feng, X., Lin, Z. and Liu, L. 2019. The safety and effectiveness of intravenous contrast-enhanced sonography in Chinese children—a single center and prospective study in China. Front. Pharmacol. 10, 1447–1455. Mapletoft, E.K., Allenspach, K. and Lamb, C.R. 2018. How useful is abdominal ultrasonography in dogs with diarrhoea? J. Small Anim. Pract. 59, 32–37. Nautrup, C.P. and Tobias, R. 2000. Atlas and textbook of diagnostic ultrasonography of the dog and cat. CRC Press, Boca Raton, Estados Unidos, p: 400. Nisa, K., Lim, S.Y., Osuga, T., Yokoyama, N., Tamura, M., Nagata, N., Sasaoka, K., Dermlim, A., Leela-Arporn, R., Morita, T., Sasaki, N., Morishita, N., Nakamura, K., Ohta, H. and Takiguchi, M. 2018. The effect of sedation with a combination of butorphanol and midazolam on quantitative contrast-enhanced ultrasonography of the duodenum in healthy dogs. J. Vet. Med. Sci. 80, 453–459. Nisa, K., Lim, S.Y., Shinohara, M., Nagata, N., Sasaoka, K., Dermlim, A., Leela-Arporn, R., Morita, T., Yokoyama, N., Osuga, T., Sasaki, N., Morishita, K., Nakamura, K., Ohta, H. and Takiguchi, M. 2017. Repeatability and reproducibility of quantitative contrast-enhanced ultrasonography for assessing duodenal perfusion in healthy dogs. J. Vet. Med. Sci. 79, 1585–1590. Nisa, K., Lim, S.Y., Shinohara, M., Osuga, T., Yokoyama, N., Tamura, M., Nagata, N., Sasaoka, K., Dermlim, A., Leela-Arporn, R., Morita, T., Sasaki, N., Morishita, K., Nakamura, K., Ohta, H. and Takiguchi, M. 2019. Evaluation of duodenal perfusion by contrast-enhanced ultrasonography in dogs with chronic inflammatory enteropathy and intestinal lymphoma. J. Vet. Intern. Med. 33, 559–568. Nyland, T.G., Neels, D.A. and Mattoon, J.S. 2015. Gastrointestinal tract. In: Mattoon, J.S., and Nyland, T.G. Small Animal Diagnostic Ultrasound, 3rd ed. Elsevier Saunders, St. Louis, Estados Unidos, pp: 468–500. Nyman, H.T., Kristensen, A.T., Kjelgaard-Hansen, M. and McEvoy, F.J. 2005. Contrast-enhanced ultrasonography in normal canine liver. Evaluation of imaging and safety parameters. Vet. Radiol. Ultrasound 46, 243–250. Oliveira, M.N.B. 2019. Intussuscepção intestinal secundária a parasitose por Ancylostoma spp. em um cão. Trabalho de Conclusão de Curso, Centro Universitário do Planalto Central Apparecido dos Santos, Gama, Brasil. Penninck, D.G., Nyland, T.G., Kerr, L.Y. and Fisher, P.E. 1990. Ultrasonographic evaluation of gastrointestinal diseases in small animals. Vet. Radiol. Ultrasound 31, 134–141. Penninck, D. and D’Anjou, M.A. 2015. Gastrointestinal tract. In: Atlas of Small Animal Ultrasonography. Wiley-Blackwell, Ames, Estados Unidos, pp: 259–308. Prestes, R.S., Coelho, N.G.D., Pinto, P.C.O., Santos, A.B., Gomes, P.P.R., Souza, I.P., Paula, T., Souza, A.C.F., Torres, R.C.S. and Nepomuceno, A.C. 2019. Exames radiográficos e ultrassonográficos em pequenos animais: riscos de interpretação. Ars Vet. 35, 127–137. Quaia, E. 2013. Contrast-enhanced ultrasound of the small bowel in Crohn’s disease. Abdom. Imaging. 38, 1005–1013. Riedesel, E.A. 2015. Intestino delgado. In: Thrall, D.E. Diagnóstico de Radiologia Veterinária, 6th ed. Elsevier, Rio de Janeiro, Brasil, pp: 789–811. Robotti, D., Cammarota, T., Debani, P., Sarno, A. and Astegiano, M. 2004. Activity of Crohn’s disease: value of color-power-Doppler and contrast-enhanced ultrasonography. Abdom. Imaging. 29, 648–652. Rosado, E. and Riccabona, M. 2016. Off-label use of ultrasound contrast agents for intravenous applications in children: analysis of the existing literature. J. Ultrasound. Med. 35, 487–496. Saevik, F., Nylund, K., Hausken, T., Ødegaard, S. and Gilja, O.H. 2014. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with Crohn’s disease. Inflamm. Bowel Dis. 20, 2029–2037. Sanderson, J.J., Boysen, S.R., McMurray, J.M., Lee, A. and Stillion, J.R. 2017. The effect of fasting on gastrointestinal motility in healthy dogs as assessed by sonography. J. Vet. Emerg. Crit. Care 27, 645–650. Seiler, G.S., Brown, J.C., Reetz, J.A., Taeymans, O., Bucknoff, M., Rossi, F., Ohlerth, S., Alder, D., Rademacher, N., Drost, W.T., Pollard, R.E., Travetti, O., Pey, P., Saunders, J.H., Shanaman, M.M., Oliveira, C.R., O’Brien, R.T. and Gaschen, L. 2013. Safety of contrast-enhanced ultrasonography in dogs and cats: 488 cases (2002-2011). J. Am. Vet. Med. Assoc. 242, 1255–1259. Seiler, G.S., Cohen, E.B., D’Anjou, M.A., French, J., Gaschen, L., Knapp, S., Salwei, R.M. and Saunders, H.M. 2022. ACVR and ECVDI consensus statement for the standardization of the abdominal ultrasound examination. Vet. Radiol. Ultrasound 63, 661–674. Serra, C., Menozzi, G., Morselli Labate, A.M., Giangregorio, F., Gionchetti, P., Beltrami, M., Robotti, D., Fornari, F. and Cammarota, T. 2007. Ultrasound assessment of vascularization of the thickened terminal ileum wall in Crohn’s disease patients using a low-mechanical index real-time scanning technique with a second-generation ultrasound contrast agent. Eur. J. Radiol. 62, 114–121. Simeoni, F., Del Signore, F., Terragni, R., Tamburro, R., Aste, G. and Vignoli, M. 2020. Diagnostic imaging of gastrointestinal tumours in dogs and cats: a review. Am. J. Anim. Vet. Sci. 15, 89–101. Takahashi, M.S., Yamanari, M.G.I., Suzuki, L., Pedrosa, É.F.N.C., Lopes, R.I. and Chammas, M.C. 2021. Utilização da ultrassonografia com contraste de microbolhas na pediatria. Radiologia Brasileira 54, 321–328. Volta, A., Manfredi, S., Vignoli, M., Russo, M., England, G.C.W., Rossi, F., Bigliardi, E., Di Ianni, F., Parmigiani, E., Bresciani, C. and Gnudi, G. 2014. Use of contrast-enhanced ultrasonography in chronic pathologic canine testes. Reprod. Domest. Anim. 49, 202–209. | ||
| How to Cite this Article |
| Pubmed Style Oliveira IM, Silva WPRD, Borges NC. B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review. Open Vet. J.. 2025; 15(3): 1066-1077. doi:10.5455/OVJ.2025.v15.i3.1 Web Style Oliveira IM, Silva WPRD, Borges NC. B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review. https://www.openveterinaryjournal.com/?mno=229127 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.1 AMA (American Medical Association) Style Oliveira IM, Silva WPRD, Borges NC. B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review. Open Vet. J.. 2025; 15(3): 1066-1077. doi:10.5455/OVJ.2025.v15.i3.1 Vancouver/ICMJE Style Oliveira IM, Silva WPRD, Borges NC. B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1066-1077. doi:10.5455/OVJ.2025.v15.i3.1 Harvard Style Oliveira, I. M., Silva, . W. P. R. D. & Borges, . N. C. (2025) B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review. Open Vet. J., 15 (3), 1066-1077. doi:10.5455/OVJ.2025.v15.i3.1 Turabian Style Oliveira, Iago Martins, Wanessa Patrícia Rodrigues Da Silva, and Naida Cristina Borges. 2025. B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review. Open Veterinary Journal, 15 (3), 1066-1077. doi:10.5455/OVJ.2025.v15.i3.1 Chicago Style Oliveira, Iago Martins, Wanessa Patrícia Rodrigues Da Silva, and Naida Cristina Borges. " B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review." Open Veterinary Journal 15 (2025), 1066-1077. doi:10.5455/OVJ.2025.v15.i3.1 MLA (The Modern Language Association) Style Oliveira, Iago Martins, Wanessa Patrícia Rodrigues Da Silva, and Naida Cristina Borges. " B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review." Open Veterinary Journal 15.3 (2025), 1066-1077. Print. doi:10.5455/OVJ.2025.v15.i3.1 APA (American Psychological Association) Style Oliveira, I. M., Silva, . W. P. R. D. & Borges, . N. C. (2025) B-mode and contrast-enhanced ultrasonography for intestinal assessment in dogs: A review. Open Veterinary Journal, 15 (3), 1066-1077. doi:10.5455/OVJ.2025.v15.i3.1 |