| Review Article | ||
Open Vet. J.. 2025; 15(3): 1078-1090 Open Veterinary Journal, (2025), Vol. 15(3): 1078-1090 Review Article Review of neosporosis: Disease insights and control approachesRimayanti Rimayanti1*, Aswin Rafif Khairullah2, Suzanita Utama1, Riza Zainuddin Ahmad2, Sri Mulyati1, Ratna Damayanti3, Tita Damayanti Lestari1, Imam Mustofa1, Tatik Hernawati1, Wasito Wasito2, Ikechukwu Benjamin Moses4, Bantari Wisynu Kusuma Wardhani5, Dea Anita Ariani Kurniasih6, Shelly Kusumarini7, Syahputra Wibowo8, Sheila Marty Yanestria9, Muhammad Khaliim Jati Kusala2, Ertika Fitri Lisnanti10 and Ima Fauziah21Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 5Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 7Department of Veterinary Parasitology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 8Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 9Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 10Program of Animal Husbandry, Faculty of Agriculture, Universitas Islam Kadiri, Kediri, Indonesia *Corresponding Author: Rimayanti Rimayanti. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: rimayanti [at] fkh.unair.ac.id Submitted: 22/11/2024 Accepted: 13/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
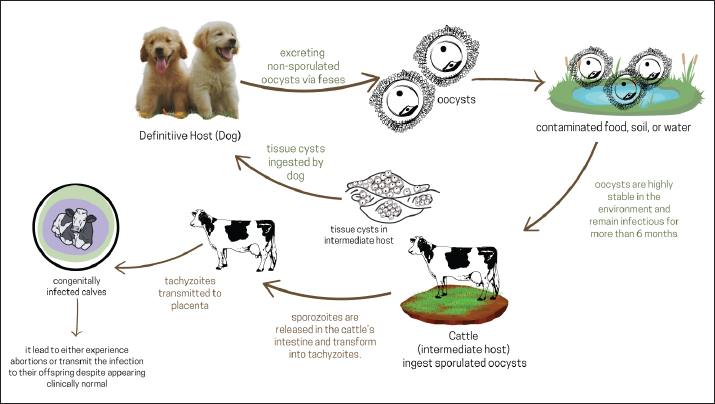
AbstractThe protozoan parasite Neospora caninum is a cause of infectious disease neosporosis. Neospora caninum is a major parasite affecting dogs and livestock worldwide. Neosporosis is a major cause of abortion in cattle, particularly in cattle raised in intensive agriculture. For diagnosis, the indirect enzyme-linked immunosorbent assay and immunofluorescence antibody test are employed. Neospora caninum goes through three different stages in its life cycle: sporozoites, tachyzoite, and bradyzoite. The primary method of N. caninum transmission in cattle is believed to be transplacental. Dogs are the definitive hosts of N. caninum, and the organisms in dogs and cattle are indistinguishable from one another. A high prevalence of N. caninum infection in animals was linked to the presence of dogs that tested positive for the parasite. Although exact statistics on the financial losses resulting from neosporosis in the global livestock sector are unavailable, losses are estimated to be millions of dollars. A number of medications have been investigated against N. caninum. In infected cell cultures, piritrexim, monensin, pyrimethamine, and trimethoprim stop N. caninum from growing intracellularly. Taking action to stop vertical transmission is the most practical way to control neosporosis in cattle herds, considering the current state of knowledge. Keywords: Abortion, Cattle, Dog, Infectious disease, N. caninum. IntroductionNeosporosis is an infectious disease caused by the protozoan parasite Neospora caninum (Donahoe et al., 2015). This parasite species and its close relative Toxoplasma gondii share many physical and biochemical traits (Athanasiou et al., 2021). Neospora caninum is now a major parasite that affects dogs and livestock all over the world. However, animals such as sheep, goats, rabbits, buffalo, horses, camels, and chickens can also contract N. caninum (Dubey, 2003). Neosporosis is one of the causes of abortion in cattle, especially in cattle raised on intensive farming (Dubey et al., 2007; Kasap et al., 2020). Sheep, goats, buffalo, and camels can also have miscarriages due to neosporosis, although they might not be as vulnerable as cattle (Shaapan, 2016; Wang et al., 2018; Ciuca et al., 2020). The disease was first identified in Norway in 1984 in dogs, and in 1988, the causative agent was identified as N. caninum (Klevar et al., 2010). According to recent reports, dogs are the definitive hosts of N. caninum, and the organisms in dogs and cattle are indistinguishable from one another (Reichel et al., 2020). Neosporosis has become a major problem in dogs and livestock. The N. caninum parasite has been linked to bovine miscarriages in several countries, including the United States, Europe, Australia, New Zealand, Africa, and Japan (Reichel et al., 2013). Neospora caninum goes through three different stages in its life cycle: sporozoites, tachyzoite, and bradyzoite (Winzer et al., 2020). The ingestion of sporozoites, a dormant stage of the oocyst, can result in infection of an intermediate host. Two separate intracellular stages are discernible in the intermediate host: the quickly replicating tachyzoite, which is present in multiple organs during the acute phase of the disease, and the slowly dividing bradyzoite, which lie dormant in tissue cysts mainly found in the central nervous system until reactivation (Winzer et al., 2020). The biology and pathogenesis of this parasite have been better understood in the ten and a half years since its identification. The excretion of N. caninum oocysts can be a risk factor when they are shed in feces and eventually mix with the environment of the definitive host; this can cause stillbirths and abortions in cattle and other intermediate hosts (Lefkaditis et al., 2020). Congenitally infected young pups (less than 4 months old) account for most recorded cases of clinical neosporosis (Dubey et al., 2007). However, neosporosis can infect and kill dogs of any age. The most consistent sign of canine neosporosis is paresis of the limbs, especially the hind limbs (Fayisa, 2023). Cattle neosporosis has been linked to endemics, epidemics, and occasional miscarriages, resulting in significant financial losses globally (Wilson et al., 2016). Neosporosis is a leading cause of infectious abortions in major livestock-producing nations and is currently a global concern. This review aims to provide information on neosporosis. Information on neosporrosis serves as a guide for unbiased and successful strategies to prevent and manage N. caninum infections over an extended period. EtiologyNeospora caninum is a heteroxene cyst-forming apicomplexan and is a common cause of foetopathy and abortion in cattle worldwide (Rosa et al., 2021). This parasite shares many morphological and biological features with T. gondii. The main differences that distinguish the two parasites are their natural host range, antigenicity, virulence factors, and pathogenesis (Nazari et al., 2020). Because the oocysts of the closely related parasite Hammondia heydorni resemble those of N. caninum, it is important to distinguish between them when examining N. caninum in dog feces. Additionally, the number, appearance, and placement of the rhoptria allow the tachyzoites and bradyzoites to be differentiated under an electron microscope despite their comparable appearance under a light microscope (Dubey et al., 2007). Tachyzoites measure 4–8 × 2–4 μm. Tachyzoites are crescent- or banana-shaped with pointed and rounded ends. Bradyzoites are slender and measure about 6.5 × 1.5 μm, have a nucleus located at the end, and contain several amylopectin granules that are red in color with the periodic acid Schiff reaction (Dubey et al., 2004). Although there are few recorded cases of neosporosis in wild animals, the existence of antibodies to N. caninum in a variety of wild mammal species increases the probability that the parasite is common in wildlife (Almería, 2013). HistoryThe protozoan N. caninum has recently been found in dogs and other animals (Lindsay and Dubey, 1989). In retrospective studies, N. caninum was found in dogs that died in the United States in 1957 and 1958, demonstrating that neosporosis was not a novel illness (Dubey, 1989; Dubey, 1992). In the past, Bjerkås et al. (1984) were the first to report disorders that resembled neosporosis. They found that six Boxer dogs in Norway had protozoa that formed cysts. Neurological problems appeared in five of these canines between 2 and 6 months after birth. Lesions in the brain and muscles include parasites like T. gondii. Nevertheless, dog parasites are not contagious to mice, and dog serum does not contain T. gondii antibodies. The illness was mistakenly identified as T. gondii in 1988. Dubey et al. (1988a) identified the parasite as N. caninum after identifying a similar infection in 10 dogs in the US and differentiating it from T. gondii. Dubey et al. (1988b) induced neosporosis in dogs and identified N. caninum alive in cell cultures and mice implanted with tissue from naturally infected dogs. The parasite that was first identified in Norwegian dogs was N. caninum, or a similarly related parasite, according to Bjerkas and Dubey’s (1991) comparison of the structure and antigenicity of parasites in fixed tissues from dogs in Norway and the US. Despite not having been isolated and described from cattle, parasites like N. caninum are classified as Neospora. Life cycleDogs that consume tissue cysts act as definitive hosts, releasing unsporulated oocysts into the environment for 5–17 days. These oocysts are highly stable in the environment, have a robust outer shell, and, in mild conditions, can remain infectious for up to 6 months (Al-Qassab et al., 2010). Cattle, the intermediary host, consume oocysts from tainted food and water. Sporozoites are released into the intestinal tract where they penetrate cells and become tachyzoite (a rapidly dividing asexual phase) (Almería, 2013). Invading and frequently destroying other host cells, tachyzoites divide and spread quickly. Hepatocytes, vascular endothelial cells, nerve cells, macrophages, fibroblasts, and muscle cells, including the placenta and myocardium in pregnant cows, have all been shown to contain tachyzoite (Benavides et al., 2012). Figure 1 illustrates N. caninum’s life cycle. The tachyzoite tries to evade the immune system by hiding dormant inside tissue cysts, which can contain hundreds of tachyzoites (Fereig and Nishikawa, 2020). The term “bradyzoite” refers to this delayed reproductive stage of the parasite. Herbivores can contract the disease by consuming food or water tainted with sporulated N. caninum oocysts, whereas carnivores can contract the disease by consuming tissue cysts harboring bradyzoite (Dubey et al., 2007). However, the parasite has developed a backup strategy; during pregnancy, the parasite becomes reactivated, breaks out of the tissue cyst, and migrates to the placenta (Regidor-Cerrillo et al., 2014). Placental infection can cause abortion, providing a source of meat contaminated by bradyzoite, which is then consumed by the definitive host (the dog).
Fig. 1. The life cycle of Neospora caninum: transmission dynamics in definitive and intermediate host. In addition, the parasite can be transmitted to the fetus, resulting in the birth of congenitally and persistently infected calves (Staska et al., 2003; Şentürk et al., 2020). When they are adults, cows that have had abortions will transmit the parasite to their offspring so that the calves that are born become infected even though they are clinically normal (show no symptoms) (Dubey, 2003). Because the only clinical manifestation of N. caninum is occasional abortion, the infection may go unnoticed. The parasite can live in cattle herds for multiple generations due to congenital transmission from cattle to offspring (Nam et al., 2011). However, miscarriages in nonbreeding herds can occur when oocysts from the definitive host contaminate drinking water and animal feed (Lefkaditis et al., 2020). EpidemiologyHerds of cattle may have a high incidence of infection without any obvious abortion issues; however, endemic and epidemic abortion patterns are the symptoms of neosporosis. Most cows that are not initially infected with N. caninum become infected from their cows before giving birth (Benavides et al., 2012). The primary method of N. caninum transmission in cattle is believed to be transplacental (González-Warleta et al., 2018). The distribution of N. caninum is widespread worldwide, with the prevalence of infection in cattle and sheep approaching 100% (González-Warleta et al., 2018; Noori et al., 2019; Selim et al., 2023). Neospora caninum was identified in the United States where it caused significant rates of neonatal calf mortality and abortions in cows (Barr et al., 1993). Additionally, neosporosis has been linked to miscarriages and stillbirths in dairy cows in Brazil (Cerqueira-Cézar et al., 2017), Italy (Manca et al., 2022), and Costa Rica (Romero et al., 2005). Antibodies against T. gondii and N. caninum were discovered in Swiss South American camels for the first time (Basso et al., 2014). The high sheep herding production rate has made it popular in the Mediterranean region. Poor management and feeding methods, stressful environments, and weakened resistance to opportunistic illnesses like N. caninum have made this disease a problem in recent years (Tamponi et al., 2015). Even though N. caninum is found in sheep and goats throughout the world (Dahourou et al., 2019). The sheep and goat herds in Khuzestan province had positive N. caninum rates of 10.8% and 32.4%, respectively. The combined infection rate of T. gondii and N. caninum was 5.4% (Gharekhani et al., 2018). In a study by Nasir et al. (2011) in Pakistan, the results showed the presence of N. caninum in buffaloes. Because the majority of animals are pregnant, immune system changes and parasite transmission, particularly in the summer, can have an impact (Regidor-Cerrillo et al., 2014). In India, Neospora linked to buffalo abortions was first identified when tissue samples from two fetuses showed positive immunohistochemistry results (Mahajan et al., 2020). The overall seroprevalence of N. caninum in central China is 15% (172/1176) (Wang et al., 2016). PathogenesisThe ability of tachyzoite to enter and proliferate inside host cells and to prevent parasite growth is both necessary for the pathophysiology of neosporrosis (Imhof et al., 2024). The cell invasion process, which can last up to 5 minutes, consists of two distinct processes: adhesion to the host cell surface and penetration inside the cell. The adhesion first phase is mediated by low-affinity interactions that cause the release of proteins containing micronemes (Keller et al., 2002). In this manner, more specific ligation to the host cell surface occurs, ultimately leading to invasion. Thus, receptors on the host cell surface are required to provide sufficient signals for parasite invasion into the cytoplasm. The tachyzoite first advances the anterior end until it reaches the cytoplasm, which is surrounded by the parasitophorous vacuole (PV) and then shifts its orientation perpendicular to the host cell surface membrane to begin host cell invasion (Nolan et al., 2015). Since invasion is an active process, the parasite alone needs metabolic energy to proceed; the host cell does not. Neospora caninum tachyzoite uses a highly conserved apicomplexan method to engage with their host cells (Pollo-Oliveira et al., 2013). Neospora caninum, in contrast to other genera, is able to identify one or more host cell surface receptors that are in charge of initial adhesion and invasion (Silva and Machado, 2016). Neospora caninum tachyzoite differs significantly from T. gondii in terms of nutrition scavenging, host organelle interactions, and surface carbohydrate content (Naguleswaran et al., 2003). Once inside, the parasite alters the host’s metabolism to aid in its maintenance. The endoplasmic reticulum, lysosomes, mitochondria, multivesicular bodies (transient storage compartments loaded with sphingolipids and cholesterol at the intersection of endocytosis and exocytosis), and Golgi vesicles are drawn to the PVs of both parasites by reorganizing the host microtubular cytoskeleton. However, in N. caninum infection, the host endoplasmic reticulum aggregates around the PV rather than physically affixing it to the vacuole membrane (Nolan et al., 2015). The parasite surrounds its PV by drawing the host’s Golgi apparatus around it although it fragments into minitracks less frequently than T. gondii. As a result, they take cholesterol from organelles and store it in lipid bodies while recovering sphingolipids from host Golgi vesicles and storing them in their PVs. They also attract host mitochondria to their PVs, retain these organelles, and use the host mitochondria to generate energy. Both parasites manipulate host cells and take advantage of mammalian resources (Paone and Olivieri, 2022). Both parasites exhibit a high degree of conservation in this phase. Immune responseTo evade the host’s immune reaction, parasites must infiltrate host cells. The CD8+ T cell response is one of these defense mechanisms. According to Jordan and Hunter (2010), the CD8+ T cell population is involved in the host’s defense mechanisms against apicomplexan protozoa infections. CD8+ T cells can secrete cytokines or act as cytotoxic T lymphocytes. The development of CD8+ T-cell responses is influenced by a variety of variables, including cytokines like IL-2 and IL-12, which support T-cell proliferation, survival, and effector function acquisition (Jordan et al., 2009). Correia et al. (2015) found that CD8+ T cells play a significant role in host protection during neosporosis. The humoral immune response and this response cooperate to keep infection under control. Typically, intestinal disease appears 5–8 days after consumption of tissue cysts, but it is difficult to know the location of the cysts in organs and tissues (Lindsay and Dubey, 2000). PathologyThe occurrence of abortion due to neosporosis is triggered by several factors, such as the release of bovine prostaglandins, which cause luteolysis resulting in abortion, proliferation of parasites, which damage important fetal or placental tissue, and changes in placental immunity associated with the release of bovine proinflammatory cytokines, which can cause fetal rejection (Rosbottom et al., 2011). Early pregnancy loss of fetuses frequently results in autolysis, and infected placentas often present with intact intercotyledonary areas and multifocal necrotic foci within the cotyledons (Dubey, 2003). Necrotic foci are commonly characterized by significant inflammatory infiltration by mononuclear cells and dystrophic calcifications. After infection in the bovine caruncle, the cellular infiltration spreads to the fetal cotyledons, where it manifests as areas of necrosis and bleeding (Cantón et al., 2014). Lesions most commonly observed in the brains of aborted fetuses and stillborn newborns include perivascular cuffs, gliosis, and small, multifocal regions of liquefactive necrosis encircled by gliosis (Benavides et al., 2022). Less frequently, the liver, kidneys, skeletal muscle, or lungs may also have comparable nonsuppurative inflammatory foci. Hydrocephalus can also occur, which explains the ataxia, reduced reflexes, and paralysis that may be seen in congenitally infected calves with clinical symptoms; inflammatory lesions in the spinal cord are more common in these calves than in the brain (Malaguti et al., 2012). Lesions in adult animals and calves without clinical symptoms are mainly limited to tissue cysts, which are mainly seen in the brain and less commonly in the heart, liver, and muscle (Dubey et al., 2007). Clinical symptomsBecause there are few parasites in the affected tissue and the early clinical signs and symptoms are unclear, neosporosis is challenging to diagnose. Neospora caninum causes abortion in dairy and beef cattle. Cows of all ages can abort as early as 3 months of gestation, with most abortions occurring due to neosporosis occurring between the fifth and sixth weeks of gestation (Rosbottom et al., 2011). There are several possible outcomes for the fetus: autolysis, mummification, resorption, death in utero, stillbirth, clinically normal birth, and persistent infection (Williams et al., 2000). Cows that have seropositive N. caninum antibodies (seropositive) are more likely to have abortions than cows that do not have seronegative N. caninum antibodies (seronegative). This applies to both dairy and beef cattle (Peregrine et al., 2004). Nonetheless, up to 95% of congenitally infected calves from seropositive cows maintain a clinically normal state (Dubey, 2003). The rate of congenital infection is normally unaffected by the dam’s age, number of lactations, or history of abortion (Stenlund et al., 2003). Clinical symptoms have only been documented in cattle aged 2 months. Calves infected with N. caninum may be born without clinical symptoms, have neurologic symptoms, be underweight, or be unable to stand (Uesaka et al., 2018). One or two of the cow’s four legs may be hyperextended or flexed. A neurological examination may show a loss of conscious proprioception, ataxia, and a diminished patellar reflex (Malaguti et al., 2012). Calves’ eyes may seem asymmetrical or exhibit exophthalmia (Aroch et al., 2008). Sometimes, N. caninum results in birth abnormalities, such as spinal cord stenosis and hydrocephalus (Kamali et al., 2014). Abortion can occur in an endemic state or an endemic state. It has been estimated that up to 33% of dairy cow fetuses in contaminated areas abort within a few months (Selim et al., 2023). Abortion is considered epidemic if more than 10% of cows are at-risk abortion within 6–8 weeks. A small percentage (<5%) of cows undergo recurrent abortions due to neosporosis (Basso et al., 2022). DiagnosisFurthermore, diagnosis of neosporosis is costly and challenging. A variety of potential causes should be the focus of diagnostic efforts because neosporosis is just one of several possible causes of abortion. Placental and serum samples from aborted cows should be sent to the veterinary diagnostic laboratory together with the aborted fetuses (Corbellini et al., 2006). A correct diagnosis is more likely to be obtained when several fetuses are examined. For diagnosis, the indirect enzyme-linked immunosorbent assay and immunofluorescence antibody test are employed (Packham et al., 1998). The features of nonsuppurative encephalitis on brain histological examination point to a Neospora infection, and the diagnosis is also characterized by heart abnormalities (Gaitero et al., 2006). Histological and polymerase chain reaction investigations, along with serological testing of maternal blood and fetal bodily fluids, can offer preliminary, but not conclusive, proof of abortion linked to N. caninum (Baszler et al., 1999). It is acceptable to presume that N. caninum caused the abortion if the pathologist determines that the fetal tissue shows lesions that are incompatible with life and are immunohistochemically connected to the infection (González-Warleta et al., 2018). The discovery of a statistically significant relationship between seropositivity and abortion in the group of dams at risk of abortion (at-risk dams) further supports the function of N. caninum in bovine abortion (Sánchez-Sánchez et al., 2021). TransmissionNo cow-to-cow transmission of N. caninum was observed. Anderson et al. (1997) conducted a study in which 25 seronegative heifers were raised alongside 25 seropositive heifers from birth, and the progeny were assessed for the presence of N. caninum infection. Cows that test negative for N. caninum remain seronegative and give birth to calves that are not infected. Seropositive cows remain clinically normal but give birth to congenitally infected calves. Four of these congenitally infected calves were in recumbency, and seven of them showed the histological identity of N. caninum infection upon autopsy. Although most N. caninum infections in cattle are disseminated through the placenta, postnatal rates vary depending on the country’s location, test type, and cutoff values (Reichel et al., 2014). In cattle, N. caninum is unlikely to be spread via sexual contact or embryo transfer, and in fact, it is recommended to use embryo transfer as a control measure to avoid vertical transmission (Dubey, 2003). Researchers used 87 recipient cows or heifers to conduct groundbreaking research on embryo transfer and N. caninum infection (Baillargeon et al., 2001). None of the 70 fetuses or calves delivered to seronegative cows that received embryos from seropositive donors exhibited evidence of N. caninum infection. However, five out of six calves that transferred embryos from seronegative donors to seropositive receivers had N. caninum infection. These results were supported by Landmann et al. (2002), who also demonstrated that commercially available embryo transfer techniques prevent N. caninum from being transferred from seropositive cattle to seronegative recipients. In addition, bovine embryos that had already been implanted showed resistance to N. caninum invasion. Although there is no confirmation that lactogenic transmission of N. caninum occurs spontaneously, it has been experimentally proven to occur in newborn calves fed colostrum mixed with tachyzoite (Lefkaditis et al., 2020). Dogs do not produce oocysts when they drink milk containing tachyzoite. Risk factorsGiven that dogs are Neospora’s definitive host, it is conceivable that livestock could contract the disease by coming into contact with dog oocysts. According to two recent epidemiological studies, having dogs on farms increases the likelihood of livestock miscarriage (Fávero et al., 2017; Semango et al., 2019). Furthermore, a high prevalence of N. caninum infection in animals was linked to the presence of dogs on farms that tested positive for the parasite (Gao and Wang, 2019). These results indicate a relationship between livestock and dog infections caused by N. caninum. Nonetheless, it is unlikely that exposure to the recently released oocysts from dogs directly causes abortion (Dubey et al., 2007). Since the majority of farmers have owned dogs for a long time, it is possible that the cattle in this herd have previously been infected (Reichel et al., 2020). The presence of domestic poultry on farms was also found to be a risk factor (Llano et al., 2018). It is believed that these animals could act as oocyst mechanical vectors. Other sources of postnatal infection in cattle are difficult to eliminate because tachyzoites are present in the fetal membranes and uterine fluid (Gondim and McAllister, 2022). Tachyzoites derived from cultures added to milk have been shown to be infectious to newborn calves when given orally (Uggla et al., 1998). Since cattle that are seropositive for N. caninum are more likely to experience an abortion, it seems that the majority of endemic and sporadic neosporosis-related abortions in cattle are caused by the reactivation of a chronic infection (Sánchez-Sánchez et al., 2021). Seropositive cows are 2–3 times more likely to have an abortion than seronegative cows (Špilovská et al., 2015). In fact, compared with seronegative heifers, congenitally infected heifers are up to 7.4 times more likely to have an abortion during their first pregnancy (Ståhl et al., 2006). Immune suppression-related conditions can lead to the reactivation of persistent Neospora infection. Eating moldy corn silage appeared to be a risk factor for abortions linked to Neospora (Vanleeuwen et al., 2010). Mycotoxins have been demonstrated to depress the immune system, and they may be present in moldy feed (Kraft et al., 2021). Economic impactAlthough exact statistics on the financial losses resulting from neosporosis in the global livestock sector are unavailable, losses are estimated to be millions of dollars (Reichel et al., 2013). The direct expenses and the value of the lost fetuses determine the economic impact of neosporosis, which can cause up to 42% of cows to abort (Ghanem et al., 2009). Professional help and expenses related to diagnosis, rebreeding, potential loss of milk output, and replacement costs in the event that aborted cows are put down are examples of indirect costs (Lefkaditis et al., 2020). Iranian dairy cattle incur significant financial losses due to culling, abortions, and reproductive issues caused by N. caninum infection (Gharekhani and Yakhchali, 2019). The reasons for abortion in beef cattle are generally less understood than in dairy cattle because of the difficulties in identifying small fetuses that are discharged during the first trimester (Dubey et al., 2007). Therefore, the losses caused by Neospora in beef cattle cannot be accurately estimated. There is no concrete proof that N. caninum causes morbidity in adult cattle because no clinical illness has been documented in calves older than 2 months. However, Barling et al. (2001) estimated a loss of $15.62 per calf and discovered a strong correlation between weight increase and N. caninum antibody status in calves in a seroepidemiological investigation. Many wild and domestic animals exhibit significant financial losses due to N. caninum (Fávero et al., 2017). Sheep with neosporosis are unable to reproduce, which has a large effect on the economy (Benavides et al., 2022). Fereig et al. (2016) stated that because neosporosis can result in reproductive losses and persistent infections, it can lead to abortion and high culling rates. Neosporosis, a significant cause of miscarriage in dairy cows makes it challenging to boost livestock productivity, a crucial source of revenue for low-income nations like Egypt (Selim et al., 2023). Given the higher number of services required for seropositive cows per conception, neosporosis may contribute to greater economic losses (Tagwireyi et al., 2024). Furthermore, compared with other seropositive cows, the study’s seropositive cows tended to have longer open days. The odds of a positive cow not becoming pregnant are 1.8 times higher than those of a negative cow (Muñoz-Zanzi et al., 2004). TreatmentA number of toxoplasmosis medications against N. caninum have been investigated. In infected cell cultures, piritrexim, monensin, lasalocid, pyrimethamine, and trimethoprim stop N. caninum from growing intracellularly (Qian et al., 2015). In experimentally infected mice, sulfadiazine reduced clinical neosporosis (Lindsay dan Dubey, 1990). However, the administration of sulfadiazine after the onset of clinical symptoms is ineffective. Several treatments have been shown to be effective against Neospora tachyzoite in vitro, although chemotherapy is thought to be ineffective against encysted bradyzoite in vivo (Ojo et al., 2014). Given the duration of milk withholding, treatment of dairy cows is not feasible. It is possible to medicate pregnant cows to avoid vertical infection transmission and abortion (Imhof et al., 2024). However, it has been noted that heifers are not entirely protected against Neospora-induced abortion during the first trimester by monensin treatment (40–120 mg/animal/day) (Sánchez-Sánchez et al., 2018). With an emphasis on medication toxicity and pharmacokinetics, in vivo experiments were performed in standard mouse models to determine the efficacy against acute infections and placentally transmitted infections, among other conditions. Drugs such as toltrazuril, ponazuril, thiazoles, and the bumped-BKI-1294 kinase inhibitor have demonstrated efficacy in the treatment of neosporosis (Hemphill et al., 2016). Chemotherapeutic methods for managing N. caninum infections in cattle have demonstrated the potential in lowering the parasite burden, vertical transmission, and abortion rates. Cuteri et al. (2005) used trimethoprim in conjunction with toltrazuril and sulphadiazine in a field trial, including 936 Friesian cattle spread across 18 herds in Italy (Cuteri et al., 2005). Within a year, the abortion rate dropped dramatically from 188 to 9 and the seroprevalence rate dropped from 68.7% to 0%. To support its action for sustained parasite suppression, Dirikolu et al. (2009) investigated the pharmacokinetics of toltrazuril sulfone (Ponazuril) in six calves and observed high absorption with a lengthy elimination half-life. Toltrazuril may help create parasite-free offspring in infected herds, as demonstrated by Haerdi et al. (2006), who investigated its use on newborn calves from seropositive moms and found lower levels of parasite infection and elimination. Kritzner et al. (2002) examined 19 experimentally infected calves that received Ponazuril treatment. The majority of parasites found in the brains and other organs of treated animals were eradicated after a 6-day treatment. In contrast, untreated calves displayed significant parasite loads along with associated clinical symptoms. These results highlight the value of chemotherapeutic treatments such as toltrazuril and ponazuril as practical instruments for treating cow neosporosis. The treatment of neosporosis is typically challenging and ineffective, either fully or partially. Patients can be required to receive treatment for 8 weeks. The prognosis for dogs with neurological symptoms is dismal, and treatment takes too long (Fisher et al., 2024). Early treatment is most successful when muscle contractures develop. In cases of cutaneous neosporosis, it appears to work better (Jiménez-Pelayo et al., 2019). The main medication used to treat canine neosporosis is clindamycin (Silva and Machado, 2016). It is the only lincosamide with extra antiprotozoal properties (Wang et al., 2022). Consequently, this medication works well against N. caninum tachyzoite. Sulfonamides and clindamycin work together to prevent neoplasia. Furthermore, pyrimethamine and sulfonamides work in concert to enhance antiprotozoal activity (McFarland et al., 2016). Clindamycin is believed to have little to no effect on bradyzoite but does affect the growth of N. caninum tachyzoite (Silva and Machado, 2016). Thus, tissue cysts may continue to exist for around 2 months after therapy, and bradyzoite exposure to an immune response as a treatment for persistent neosporosis should be taken into consideration and studied. Every parent group member should have their N. caninum antibody levels checked if one of them has neosporosis and those who test seropositive should receive treatment. Seropositive puppies should not receive immunosuppressive medications (Lyon, 2010). Because of the lack of preventative therapy, the parasite can spread from an infected bitch to her children several times. VaccinationThere is currently no cure or vaccine to stop the spread of neosporosis that causes abortion. The only licensed N. caninum vaccine is Bovilis Neoguard (Intervet International B.V., Boxmeer; The Netherlands), which consists of inactivated N. caninum tachyzoite (3 × 106 ml−1), 10% Havlogen adjuvant, 5% stabilizer, and 5% phosphate-buffered saline (Mazuz et al., 2021). However, this vaccine was withdrawn from the market due to its limited efficacy (20%) and increased transplacental transmission resulting in embryonic death (Weston et al., 2012; Mansilla et al., 2015). Recently, pregnant cows exhibited strong immunogenicity and activation of interferons (IFN)-c responses to a soluble fraction of tachyzoite’s lysate and a soy-based aqueous adjuvant (sNcAg/AVEC) (Mansilla et al., 2012). Abortion rates have significantly decreased as a result of live tachyzoite vaccines, which include naturally attenuated or less virulent isolates such as Nc-Nowra, Nc-Spain1H, and the Argentine isolate Nc-6 (Imhof et al., 2024). These vaccines also significantly increase N. caninum antibody responses. Inactivated or subunit vaccinations are more appealing choices since live vaccines have certain intrinsic disadvantages, such as the danger of pathogenicity reoccurring after inoculation and the bulk maintenance of live parasites (Hou et al., 2023). Unfortunately, recombinant NcGRA7 (50–200 µg) entrapped in oligo-mannose microsomes (M3-NcGRA7), as well as recombinant proteins expressed and purified by bacteria, including rNcSAG1, rNcHSP20, and rNcGRA7, showed little promise, as they failed to prevent infection in pregnant cows (Reichel et al., 2015). To guarantee long-term protection against Neospora, it is crucial to identify novel, more effective vaccine strategies, such as live attenuated strains of Neospora (containing tachyzoites lacking in Ca2+-dependent protein kinase 2) (Khan et al., 2020). ControlThe economic significance of neosporrosis and the absence of effective treatments and vaccines make appropriate prevention and control measures the most effective way to eliminate N. caninum. Serological investigations to gather seroprevalence data are crucial for the efficient management of neosporosis (Guido et al., 2016). Reducing the risk of vertical or horizontal transmission can help prevent and manage neosporosis. One of the main causes of Neospora infection persistence in herds has been identified as vertical transmission (Marugan-Hernandez, 2017). Therefore, action to stop vertical transmission is the most practical way to control neosporosis in cattle herds, considering the current state of knowledge. Only herds with a low incidence of disease can implement the most extreme measure, namely slaughtering all diseased animals and their affected offspring. Focusing on keeping calves contaminated at birth from replacement is more practical and cost-effective in herds with moderate to high infection prevalence (Haddad et al., 2005). This strategy is recommended in light of mounting data showing that heifers infected at birth are at significantly increased risk of both vertical transmission of the virus to their progeny and abortion, particularly during the first pregnancy. Seropositivity to Neospora can be used as a criterion for selective culling of dairy cows within general herd management limitations, in addition to allowing only seronegative heifers as replacement stock (Pabón et al., 2007). It is possible to transfer embryos from seropositive cattle to seronegative recipients to preserve valuable seropositive animals as breeding stock (Baillargeon et al., 2001). Since dogs play a significant role in the spread of the disease and its transmission, stopping the parasite’s life cycle through dog quarantine aids in its control by blocking horizontal transfer between the final and intermediate hosts (Silva and Machado, 2016). Furthermore, the host must not have access to aborted fetuses or placentas from cows and calves. To prevent oocyst infection, cows should only be fed closed foods and water. It is important to prevent dog excrement from contaminating feed. Avoid feeding moldy cattle feed because it may contain mycotoxins (Dubey et al., 2007). Stressors and nutritional imbalances are two more factors that might lead to a weakened immune response and are difficult to control (Monney et al., 2011). To stop the spread of disease, eradication measures should be implemented for all rodents, including mice, rats, and rabbits (Jenkins et al., 2007). Similar tactics should be used for poultry because pigeons and chickens can serve as intermediary hosts for the parasite (Furuta et al., 2007). An effective control program must include a calculation of the costs of testing and control measures against reduced economic losses due to N. caninum infection or abortion (Liu et al., 2020). ConclusionNeosporosis is a frequent disease of cattle caused by the protozoan parasite N. caninum. Infection in pregnant cows leads to miscarriage. Dogs are recognized to be definitive hosts N. caninum, which can excrete oocysts in feces. Other animals are affected after consuming Neospora oocysts. The economic significance of neosporosis and the absence of effective treatments and vaccines make appropriate prevention and control measures the most effective way to eliminate N. caninum. Future prospectsTo develop an effective vaccine and uncover the connection between domestic and wild disease cycles, research on the genotype and molecular diagnostics of N. caninum is required. To determine the actual incidence of neosporosis, research on other hosts is recommended, particularly wild animal species. Furthermore, farmers require instructional programs on the risk factors associated with neosporosis. AcknowledgmentsThe authors would like to acknowledge the Kementerian Pendidikan, Kebudayaan, Riset, and Teknologi who funded this research. Conflict of interestThe authors declare no conflict of interest. FundingThe authors thank Universitas Airlangga for their managerial support. Author’s contributionsTDL, ARK, SW, and BWKW drafted the manuscript. SK, IF, DAAK, IM, and IBM revised and edited the manuscripts. RZA, EFL, TH, RR, and RD participated in preparing and critical checking this manuscript. SU, WW, SM, SMY, and MKJK edit the references. All authors have read and approved the final manuscript. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAlmería, S. 2013. Neospora caninum and related insects. ISRN Parasitol. 2013(1), 947347. Al-Qassab, S.E., Reichel, M.P. and Ellis, J.T. 2010. On the biological and genetic diversity in Neospora caninum. Diversity 2(3), 411–438. Anderson, M.L., Reynolds, J.P., Rowe, J.D., Sverlow, K.W., Packham, A.E., Barr, B.C. and Conrad, PA. 1997. Evidence of vertical transmission of Neospora sp infection in dairy cattle. J. Am. Vet. Med. Assoc. 210(8), 1169–1172. Aroch, I., Ofri, R. and Sutton, G.A. 2008. Ocular manifestations of systemic diseases. Slatter Fundamentals Vet. Ophthalmol. 2008(1), 374–418. Athanasiou, L.V., Papatsiros, V.G., Spanou, V.M., Katsogiannou, E.G. and Dedousi, A. 2021. Neospora caninum and/or Toxoplasma gondii seroprevalence: vaccination against PCV2 and muscle enzyme activity in seropositive and seronegative pigs. Microorganisms 9(5), 1097. Baillargeon, P., Fecteau, G., Paré, J., Lamothe, P. and Sauvé, R. 2001. Evaluation of the embryo transfer procedure proposed by the International Embryo Transfer Society for controlling vertical transmission of Neospora caninum in cattle. J. Am. Vet. Med. Assoc. 218(11), 1803–1806. Barling, K.S., McNeill, J.W., Paschal, J.C., McCollum, F.T. 3rd, Craig, T.M., Adams, L.G. and Thompson, J.A. 2001. Ranch-management factors associated with antibody seropositivity for Neospora caninum in consignments of beef calves in Texas, USA. Prev. Vet. Med. 52(1), 53–61. Barr, B.C., Conrad, P.A., Breitmeyer, R., Sverlow, K., Anderson, M.L., Reynolds, J., Chauvet, A.E., Dubey, J.P. and Ardans, A.A. 1993. Congenital Neospora infection in calves born from cows that had previously aborted Neospora-infected fetuses: four cases (1990-1992). J. Am. Vet. Med. Assoc. 202(1), 113–117. Basso, W., Holenweger, F., Schares, G., Müller, N., Campero, L.M., Ardüser, F., Moore-Jones, G., Frey, C.F. and Zanolari, P. 2022. Toxoplasma gondii and Neospora caninum infection of sheep and goats in Switzerland: seroprevalence and occurrence in aborted fetuses. Food Waterborne Parasitol. 28(1), e00176. Basso, W., Moré, G., Quiroga, M.A., Balducchi, D., Schares, G. and Venturini, M.C. 2014. Neospora caninum is a cause of perinatal mortality in axis deer (Axis axis). Vet. Parasitol. 199(3–4), 255–258. Baszler, T.V., Gay, L.J., Long, M.T. and Mathison, B.A. 1999. Detection of Neospora caninum in fetal tissues from spontaneous bovine abortions by polymerase chain reaction. J. Clin. Microbiol. 37(12), 4059–4064. Benavides, J., González-Warleta, M., Arteche-Villasol, N., Pérez, V., Mezo, M. and Gutiérrez-Expósito, D. 2022. Ovine neoporosis: the current global situation. Animals 12(16), 2074. Benavides, J., Katzer, F., Maley, S.W., Bartley, P.M., Cantón, G., Palarea-Albaladejo, J., Purslow, C.A., Pang, Y., Rocchi, M.S., Chianini, F., Buxton, D. and Innes, E.A. 2012. High rate of transplacental infection and transmission of Neospora caninum following experimental challenge of cattle on day 210 of gestation. Vet. Res. 43(1), 83. Bjerkås, I. and Dubey, J.P. 1991. Evidence that Neospora caninum is identical to the toxoplasma-like parasite of Norwegian dogs. Acta Vet. Scand. 32(3), 407–410. Bjerkås, I., Mohn, S.F. and Presthus, J. 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z. Parasitenkd. 70(2), 271–274. Cantón, G.J., Katzer, F., Maley, S.W., Bartley, P.M., Benavides-Silván, J., Palarea-Albaladejo, J., Pang, Y., Smith, S.H., Rocchi, M.S., Buxton, D., Innes, E.A. and Chianini, F. 2014. Inflammatory infiltration into placentas of Neospora caninum-challenged cattle correlates with clinical outcome of pregnancy. Vet. Res. 45(1), 11. Cerqueira-Cézar, C.K., Calero-Bernal, R., Dubey, J.P. and Gennari, S.M. 2017. All aboutneosporrosiss in Brazil. Rev. Bras. Parasitol. Vet. 26(3), 253–279. Ciuca, L., Borriello, G., Bosco, A., D’Andrea, L., Cringoli, G., Ciaramella, P., Maurelli, M.P., Di Loria, A., Rinaldi, L. and Guccione, J. 2020. Seroprevalence and clinical outcomes of Neospora caninum, Toxoplasma gondii, and Besnoitia besnoiti Infections in Water Buffaloes (Bubalus bubalis). Animals (Basel) 10(3), 532. Corbellini, L.G., Pescador, C.A., Frantz, F., Wunder, E., Steffen, D., Smith, D.R. and Driemeier, D. 2006. Diagnostic survey of bovine abortion with special reference to Neospora caninum infection: importance, repeated abortion and concurrent infection in aborted fetuses in Southern Brazil. Vet. J. 172(1), 114–120. Correia, A., Ferreirinha, P., Botelho, S., Belinha, A., Leitão, C., Caramalho, Í., Teixeira, L., González-Fernandéz, Á., Appelberg, R. and Vilanova, M. 2015. Predominant role of interferon-γ in the host protective effect of CD8(+) T cells against Neospora caninum infection. Sci. Rep. 5(1), 14913. Cuteri, V., Nisoli, L., Preziuso, S., Attili A.R., Guerra, C., Lulla, D. and Traldi G. 2005. Application of a new therapeutic protocol against Neospora caninum-induced abortion in cattle: a field study. J. Anim. Vet. Adv. 4(5), 510–514. Dahourou, L.D., Gbati, O.B., Savadogo, M., Yougbare, B., Dicko, A., Combari, A.H.B. and Kamga-Waladjo, A.R. 2019. Prevalence of Toxoplasma gondii and Neospora caninum infections in households sheep “Elevage en case” in Dakar, Senegal. Vet. World 12(7), 1028–1032. Dirikolu, L., Yohn, R., Garrett, E.F., Chakkath, T. and Ferguson, D.C. 2009. Detection, quantification, and pharmacokinetics of toltrazuril sulfone (Ponazuril) in cattle. J. Vet. Pharmacol. Ther. 32(3), 280–288. Donahoe, S.L., Lindsay, S.A., Krockenberger, M., Phalen, D. and Šlapeta, J. 2015. A review of neospora caninum infection in wildlife. Int. J. Parasitol. Parasites Wildl. 4(2), 216–238. Dubey, J.P. 1989. Congenital neosporosis in a calf. Vet. Rec. 125, 486. Dubey, J.P. 1992. A review of Neospora caninum and Neospora-like infections in animals. J. Protozool. Res. 2(1), 40–52. Dubey, J.P. 2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41(1), 1–16. Dubey, J.P. Carpenter, J.L. Speer, C.A., Topper, M.J. and Uggla, A. 1988a. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 192(9), 1269–1285. Dubey, J.P., Hattel, A.L., Lindsay, D.S. and Topper, M.J. 1988b. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J. Am. Vet. Med. Assoc. 193(10), 1259–1263. Dubey, J.P., Schares, G. and Ortega-Mora, L.M. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20(2), 323–367. Dubey, J.P., Sreekumar, C., Knickman, E., Miska, K.B., Vianna, M.C., Kwok, O.C., Hill, D.E., Jenkins, M.C., Lindsay, D.S. and Greene, C.E. 2004. Biologic, morphologic, and molecular characterization of Neospora caninum isolates from littermate dogs. Int. J. Parasitol. 34(10), 1157–1167. Fávero, J.F., Da Silva, A.S., Campigotto, G., Machado, G., de Barros, L.D., Garcia, J.L., Vogel, F.F., Mendes, R.E. and Stefani, L.M. 2017. Risk factors for Neospora caninum infection in dairy cattle and their possible cause-effect relation for disease. Microb. Pathog. 110(1), 202–207. Fayisa, W.O. 2023. A current update on Neospora caninum. Microbiol. Res. J. Int. 33(11), 32–37. Fereig, R.M. and Nishikawa, Y. 2020. Signaling pathways to distinct immune responses: key factors for establishing or combating Neospora caninum infection in different susceptible hosts. Pathogens 9(5), 384. Fereig, R.M., AbouLaila, M.R., Mohamed, S.G.A., Mahmoud, H.Y.A.H., Ali, A.O., Ali, A.F., Hilali, M., Zaid, A., Mohamed, A.E.A. and Nishikawa, Y. 2016. Serological detection and epidemiology of Neospora caninum and Cryptosporidium parvum antibodies in cattle in southern Egypt. Acta Trop. 162(1), 206–211. Fisher, C., Seferidis, N., Zilli, J., Roberts, T. and Harcourt-Brown, T. 2024. Insights into the clinical presentation, diagnostics, and outcome of dogs with neurological signs secondary to infection with Neospora caninum: 41 cases (2014-2023). Small Anim. Pract. 65(7), 582–588. Furuta, P.I., Mineo, T.W.P., Carrasco, A.O.T., Godoy, G.S., Pinto, A.A. and Machado, R.Z. 2007. Neospora caninum infection in birds: experimental infections in chickens and embryonated eggs. Parasitology 134(14), 1931–1939. Gaitero, L., Añor, S., Montoliu, P., Zamora, A. and Pumarola, M. 2006. Detection of Neospora caninum tachyzoite in canine cerebrospinal fluid. J. Vet. Intern. Med. 20(2), 410–414. Gao, X. and Wang, H. 2019. Seroprevalence and risk factors of Neospora caninum infection in dogs in rural northeastern mainland China. Parasite 26(1), 32. Ghanem, M.E., Suzuki, T., Akita, M. and Nishibori, M. 2009. Neospora caninum and complex vertebral malformation as possible causes of bovine fetal mummification. Can. Vet. J. 50(4), 389–392. Gharekhani, J. and Yakhchali, M. 2019. Neospora caninum infection in dairy farms with a history of abortion in West of Iran. Vet. Anim. Sci. 8(1), 100071. Gharekhani, J., Yakhchali, M., Esmaeilnejad, B., Mardani, K., Sohrabi, A.M.G., Berahmat, R. and Alaei, M.H. 2018. Seroprevalence and risk factors of Neospora caninum and Toxoplasma gondii in small ruminants in the southwest of Iran. Arch. Razi. Inst. 73(4), 305310. Gondim, L.F.P. and McAllister, M.M. 2022. Experimental Neospora caninum infection in pregnant cattle: different outcomes between inoculation with tachyzoites and oocysts. Front. Vet. Sci. 9(1), 911015. González-Warleta, M., Castro-Hermida, J.A., Calvo, C., Pérez, V., Gutiérrez-Expósito, D., Regidor-Cerrillo, J., Ortega-Mora, L.M. and Mezo, M. 2018. Endogenous transplacental transmission of Neospora caninum during successive pregnancies across three generations of naturally infected sheep. Vet. Res. 49(1), 106. Guido, S., Katzer, F., Nanjiani, I., Milne, E. and Innes, E.A. 2016. Serology-based diagnostics for the control of bovine neosporosis. Trends Parasitol. 32(2), 131–143. Haddad, J.P., Dohoo, I.R. and VanLeewen, J.A. 2005. A review of Neospora caninum in dairy and beef cattle—a Canadian perspective. Can. Vet. J. 46(3), 230–243. Haerdi, C., Haessig, M., Sager, H., Greif, G., Staubli, D. and Gottstein, B. 2006. Humoral immune reaction of newborn calves congenitally infected with Neospora caninum and experimentally treated with toltrazuril. Parasitol. Res. 99(5), 534–540. Hemphill, A., Aguado-Martínez, A. and Müller, J. 2016. Approaches to vaccination and treatment of Neospora caninum infection in mice and ruminant models. Parasitology 143(3), 245–259. Hou, Y., Chen, M., Bian, Y., Zheng, X., Tong, R. and Sun, X. 2023. Advanced subunit vaccine delivery technologies: from vaccine cascade obstacles to design strategies. Acta Pharm. Sin. B 13(8), 3321–3338. Imhof, D., Hänggeli, K.P.A., De Sousa, M.C.F., Vigneswaran, A., Hofmann, L., Amdouni, Y., Boubaker, G., Müller, J. and Hemphill, A. 2024. Working toward the development of vaccines and chemotherapeutics against neosporosis—with all of its ups and downs—looking ahead. Adv. Parasitol. 124(1), 91–154. Jenkins, M.C., Parker, C., Hill, D., Pinckney, R.D., Dyer, R. and Dubey, J.P. 2007. Neospora caninum detected in feral rodents. Vet. Parasitol. 143(2), 161–165. Jiménez-Pelayo, L., García-Sánchez, M., Vázquez, P., Regidor-Cerrillo, J., Horcajo, P., Collantes-Fernández, E., Blanco-Murcia, J., Gutiérrez-Expósito, D., Román-Trufero, A., Osoro, K., Benavides, J. and Ortega-Mora, L.M. 2019. Early Neospora caninum infection dynamics in cattle after inoculation at midgestation with high (Nc-Spain7)- or low (Nc-Spain1H)-virulence isolates. Vet. Res. 50(1), 72. Jordan, K.A. and Hunter, C.A. 2010. Regulation of CD8+ T-cell responses to infection with parasitic protozoa. Exp. Parasitol. 126(3), 318–325. Jordan, K.A., Wilson, E.H., Tait, E.D., Fox, B.A., Roos, D.S., Bzik, D.J., Dzierszinski, F. and Hunter, C.A. 2009. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infect. Immun. 77(9), 3894–3901. Kamali, A., Seifi, H.A., Movassaghi, A.R., Razmi, G.R. and Naseri, Z. 2014. Histopathological and molecular analyses of Neospora caninum infection in bovine aborted fetuses. Asian Pac. J. Trop. Biomed. 4(12), 990–994. Kasap, S., Ertunc, S., Temizel, E.M. and Senturk, S. 2020. A study of Neospora caninum antibody seroprevalence ın dairy cows in Turkey. J. Hellenic Vet. Med. Soc. 71(1), 2018–2022. Keller, N., Naguleswaran, A., Cannas, A., Vonlaufen, N., Bienz, M., Björkman, C., Bohne, W. and Hemphill, A. 2002. Identification of a Neospora caninum microneme protein (NcMIC1) that interacts with sulfated host cell surface glycosaminoglycans. Infect. Immun. 70(6), 3187–3198. Khan, A., Shaik, J.S. Sikorski, P., Dubey, J.P. and Grigg, M.E. 2020. Neosporosis: an overview of its molecular epidemiology and pathogenesis. Engineering 6(1), 10–19. Klevar, S., Norström, M., Tharaldsen, J., Clausen, T. and Björkman, C. 2010. Prevalence and spatial clustering of Neospora caninum in dairy herds in Norway. Vet. Parasitol. 170(1–2), 153–157. Kraft, S., Buchenauer, L. and Polte, T. 2021. Mold, mycotoxins, and a dysregulated immune system: a combination of concern? Int. J. Mol. Sci. 22(22), 12269. Kritzner, S., Sager, H., Blum, J., Krebber, R., Greif, G. and Gottstein, B. 2002. An explorative study to assess the efficacy of toltrazuril-sulfone (ponazuril) in calves experimentally infected with Neospora caninum. Ann. Clin. Microbiol. Antimicrob. 1(1), 4. Landmann, J.K., Jillella, D., O’Donoghue, P.J. and McGowan, M.R. 2002. Confirmation of the prevention of vertical transmission of Neospora caninum in cattle by the use of embryo transfer. Aust. Vet. J. 80(8), 502–503. Lefkaditis, M., Mpairamoglou, R., Sossidou, A., Spanoudis, K. and Tsakiroglou, M. 2020. Neospora caninum, A potential cause of reproductive failure in dairy cows from Northern Greece. Parasitol. Reg. Stud. Rep. 19(1), 100365. Lindsay, D.S. and Dubey, J.P. 1989. Immunohistochemical diagnosis of Neospora caninum in tissue sections. Am. J. Vet. Res. 50(11), 1981–1983. Lindsay, D.S. and Dubey, J.P. 1990. Effects of sulfadiazine and amprolium on Neospora caninum (Protozoa: Apicomplexa) infections in mice. J. Parasitol. 76(2), 177–179. Lindsay, D.S. and Dubey, J.P. 2000. Canine neosporosis. Parasitol. 87(1), 1–11. Liu, Y., Reichel, M.P. and Lo, W.C. 2020. Combined control evaluation for Neospora caninum infection in dairy: economic perspective coupled with population dynamics. Vet. Parasitol. 277(1), 108967. Llano, H.A.B., Guimarães, M.S., Soares, R.M., Polo, G. and da Silva, A.C. 2018. Seroprevalence and risk factors for Neospora caninum infection in cattle from the eastern Antioquia, Colombia. Vet. Anim. Sci. 6(1), 69–74. Lyon, C. 2010. Update on the diagnosis and management of Neospora caninum infections in dogs. Top. Companion Anim. Med. 25(3), 170–175. Mahajan, V., Banga, H.S. and Filia, G. 2020. Patho-epidemiological and risk factor studies for detection of Neospora-associated abortion in cattle and buffaloes in Punjab, India. Rev Sci. Tech. 38(3), 801–808. Malaguti, J.M., Cabral, A.D., Abdalla, R.P., Salgueiro, Y.O., Galleti, N.T., Okuda, L.H., Cunha, E.M., Pituco, E.M. and Del Fava, C. 2012. Neospora caninum asa causativee agent of bovine encephalitis in Brazil. Rev. Bras. Parasitol. Vet. 21(1), 48–54. Manca, R., Ciccarese, G., Scaltrito, D. and Chirizzi, D. 2022. Detection of anti-Neospora caninum antibodies on dairy cattle farms in Southern Italy. Vet. Sci. 9(2), 87. Mansilla, F.C., Franco-Mahecha, O.L., Lavoria, M.Á., Moore, D.P., Giraldez, A.N., Iglesias, M.E., Wilda, M. and Capozzo, A.V. 2012. Immune enhancement of a novel soy lecithin/β-glucans based adjuvant on native Neospora caninum tachyzoite extract vaccine in mice. Vaccine 30(6), 1124–1131. Mansilla, F.C., Moore, D.P., Quintana, M.E., Cardoso, N., Hecker, Y.P., Gual, I., Czepluch, W., Odeón, A.C. and Capozzo, A.V. 2015. Safety and immunogenicity of a soluble native Neospora caninum tachyzoite-extract vaccine formulated with a soy lecithin/β-glucan adjuvant in pregnant cattle. Vet. Immunol. Immunopathol. 165(1–2), 75–80. Marugan-Hernandez, V. 2017. Neospora caninum and bovine neosporosis: current vaccine research. J. Comp. Pathol. 157(2–3), 193–200. Mazuz, M.L., Leibovitz, B., Savitsky, I., Blinder, E., Yasur-Landau, D., Lavon, Y., Sharir, B. and Tirosh-Levy, S. 2021. Effect of vaccination with Neospora caninum live-frozen tachyzoites on the abortion rate of naturally infected pregnant cows. Vaccines (Basel) 9(4), 401. McFarland, M.M., Zach, S.J., Wang, X., Potluri, L.P., Neville, A.J., Vennerstrom, J.L. and Davis, P.H. 2016. Review of experimental compounds demonstrating anti-toxoplasma activity. antimicrob. Agents Chemother. 60(12), 7017–7034. Monney, T., Debache, K. and Hemphill, A. 2011. Vaccines against the major cause of abortion in cattle, Neospora caninum infection. Animals 1(3), 306–325. Muñoz-Zanzi, C.A., Thurmond, M.C. and Hietala, S.K. 2004. Effects of bovine viral diarrhea virus infection on the fertility of dairy heifers. Theriogenology 61(6), 1085–1099. Naguleswaran, A., Müller, N. and Hemphill, A. 2003. Neospora caninum and Toxoplasma gondii: a novel adhesion/invasion assay revealed distinct differences in tachyzoite-host cell interactions. Exp. Parasitol. 104(3–4), 149–158. Nam, N.H., Aiumlamai, S., Chanlun, A. and Kanistanon, K. 2011. Epidemiology of Neospora caninum infection in animals. J. Sci. Dev. 9 (1), 28–40. Nasir, A., Ashraf, M., Khan, M.S., Yaqub, T., Javeed, A., Avais, M. and Akhtar, F. 2011. Seroprevalence of Neospora caninum in Dairy Buffaloes in Lahore District, Pakistan. J. Parasitol. 97(3), 541–543. Nazari, N., Shojaee, S., Salimi, M., Mohebali, M., Ahmadifard, N., Hamzavi, Y., Zarei, Z., Farahmand-Rad, R., Bozorgomid, A. and Heydarian, P. 2020. Serological survey of Neospora caninum and Toxoplasma gondii co-infection in rodents in Northwestern Iran. Iran. J. Parasitol. 15(2), 253–258. Nolan, S.J., Romano, J.D., Luechtefeld, T. and Coppens, I. 2015. Neospora caninum recruits host cell structures to its parasitophorous vacuoles and salvages lipids from organelles. Eukaryot. Cell 14(5), 454–473. Noori, M., Rasekh, M., Ganjali, M. and Fard, S.R.N. 2019. Seroprevalence of Neospora caninum infection and associated risk factors in cattle of Sistan Areas, Southeastern Iran in 2016. Iran J. Parasitol. 14(2), 340–346. Ojo, K.K., Reid, M.C., Siddaramaiah, L.K., Müller, J., Winzer, P., Zhang, Z., Keyloun, K.R., Vidadala, R.S., Merritt, E.A., Hol, W.G., Maly, D.J., Fan, E., Van Voorhis, W.C. and Hemphill, A. 2014. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neoporosis therapy. PLoS One 9(3), e92929. Pabón, M., López-Gatius, F., García-Ispierto, I., Bech-Sàbat, G., Nogareda, C. and Almería, S. 2007. Chronic Neospora caninum infection and repeated abortion in dairy cows: a 3-year study. Vet. Parasitol. 147(1–2), 40–46. Packham, A.E., Sverlow, K.W., Conrad, P.A., Loomis, E.F., Rowe, J.D., Anderson, M.L., Marsh, A.E., Cray, C. and Barr, B.C. 1998. A modified agglutination test for Neospora caninum: development, optimization, and comparison with indirect fluorescent-antibody test and enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 5(4), 467–473. Paone, S. and Olivieri, A. 2022. Role of small host GTPases in apicomplexan parasite infection. Microorganisms 10(7), 1370. Peregrine, A.S., Duffield, T.F., Wideman, G., Kelton, D., Hobson, J., Cramer, G. and Hietala, S.K. 2004. Udder health of dairy cattle infected with Neospora caninum. Prev. Vet. Med. 64(2–4), 101–112. Pollo-Oliveira, L., Post, H., Acencio, M.L., Lemke, N., van den Toorn, H., Tragante, V., Heck, A.J., Altelaar, A.F. and Yatsuda, A.P. 2013. Unraveling the Neospora caninum secretome through the secreted fraction (ESA) andquantifyingf the discharged tachyzoite using high-resolution massspectrometry–based proteomics. Parasit Vectors 6(1), 335. Qian, W., Wang, H., Shan, D., Li, B., Liu, J. and Liu, Q. 2015. Activity of several kinds of drugs against Neospora caninum. Parasitol. Int. 64(6), 597–602. Regidor-Cerrillo, J., Arranz-Solís, D., Benavides, J., Gómez-Bautista, M., Castro-Hermida, J.A., Mezo, M., Pérez, V., Ortega-Mora, L.M. and González-Warleta, M. 2014. Neospora caninum infection during early pregnancy in cattle: how the isolate influences infection dynamics, clinical outcome, and peripheral and local immune responses. Vet. Res. 45(1), 10. Reichel, M.P., Ayanegui-Alcérreca, M.A., Gondim, L.F. and Ellis, J.T. 2013. What is the global economic impact of Neospora caninum in cattle? The billion dollar question. Int. J. Parasitol. 43(2), 133–142. Reichel, M.P., Mcallister, M.M., Pomroy, W.E., Campero, C., Ortega-Mora, L.M. and Ellis, J.T. 2014. Control options for Neospora caninum: is there anything new or are we going backward? Parasitology 141(11), 1455–1470. Reichel, M.P., Moore, D.P., Hemphill, A., Ortega-Mora, L.M., Dubey, J.P. and Ellis, J.T. 2015. A live vaccine against Neospora caninum abortions in cattle. Vaccine 33(11), 1299–1301. Reichel, M.P., Wahl, L.C. and Ellis, J.T. 2020. Research into Neospora caninum—what have we learnt over the last thirty years? Pathogens 9(6), 505. Romero, J.J., Breda, S.V., Vargas, B., Dolz, G. and Frankena, K. 2005. Effects of neosporosis on the productive and reproductive performance of dairy cattle in Costa Rica. Theriogenology 64(9), 1928–1939. Rosa, P.D., Fiorentino, M.A., Morrell, E.L., Scioli, M.V., Paolicchi, F.A., Moore, D.P., Cantón, G.J. and Hecker, Y.P. 2021. Neospora caninum and Toxoplasma gondii as causes of reproductive losses in commercial sheep flocks in Argentina. Curr. Res. Parasitol. Vector Borne Dis. 1(1), 100057. Rosbottom, A., Gibney, H., Kaiser, P., Hartley, C., Smith, R.F., Robinson, R., Kipar, A. and Williams, D.J. 2011. Upregulation of the maternal immune response in the placenta of cattle naturally infected with Neospora caninum. PLoS One 6(1), e15799. Sánchez-Sánchez, R., Vázquez, P., Ferre, I. and Ortega-Mora, L.M. 2018. Treatment of toxoplasmosis and neosporosis in farm ruminants: state of knowledge and future trends. Curr. Top. Med. Chem. 18(15), 1304–1323. Sánchez-Sánchez, R., Vázquez-Calvo, Á., Fernández-Escobar, M., Regidor-Cerrillo, J., Benavides, J., Gutiérrez, J., Gutiérrez-Expósito, D., Crespo-Ramos, F.J., Ortega-Mora, L.M. and Álvarez-García, G. 2021. Dynamics of Neospora caninum-associated abortions in a dairy sheep flock and results of a test-and-cull control programme. Pathogens 10(11), 1518. Selim, A., Alshammari, A., Gattan, H.S., Marzok, M., Salem, M. and Al-Jabr, O.A. 2023. Neospora caninum infection in dairy cattle in Egypt: a serosurvey and associated risk factors. Sci. Rep. 13(1), 15489. Semango, G., Hamilton, C.M., Kreppel, K., Katzer, F., Kibona, T., Lankester, F., Allan, K.J., Thomas, K.M., Claxton, J.R., Innes, E.A., Swai, E.S., Buza, J., Cleaveland, S. and de Glanville, W.A. 2019. Sero-epidemiology of Neospora caninum in Cattle in Northern Tanzania. Front. Vet. Sci. 6(1), 327. Şentürk, S., Temizel, E.M. and Kasap, S. 2020. Clinic congenital neosporosis in a calf. Turkiye Parazitol. Derg. 44(2), 109–111. Shaapan, R.M. 2016. Common zoonotic protozoal diseases causing abortion. J. Parasit. Dis. 40(4), 1116–1129. Silva, R.C. and Machado, G.P. 2016. Canine neosporosis: perspectives on pathogenesis and management. Vet Med (Auckl) 7(1), 59–70. Špilovská, S., Reiterová, K. and Antolová, D. 2015. Neospora caninum -associated abortions at the Slovak Dairy Farm. Iran. J. Parasitol. 10(1), 96–101. Ståhl, K., Björkman, C., Emanuelson, U., Rivera, H., Zelada, A. and Moreno-López, J. 2006. A prospective study of the effect of Neospora caninum and BVDV infections on bovine abortions in a dairy herd in Arequipa, Peru. Prev. Vet. Med. 75(3–4), 177–188. Staska, L.M., McGuire, T.C., Davies, C.J., Lewin, H.A. and Baszler, T.V. 2003. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect. Immun. 71(6), 3272–3279. Stenlund, S., Kindahl, H., Uggla, A. and Björkman, C. 2003. A long-term Neospora caninum infection in a Swedish Dairy Herd. Acta Vet. Scand. 44(1), 63–74. Tagwireyi, W.M., Thompson, P.N., Garcia, G.A., Morar-Leather, D. and Neves, L. 2024. Seroprevalence and associated risk factors for Neospora caninum infection in dairy cattle in South Africa. Parasitol. Res. 123(8), 298. Tamponi, C., Varcasia, A., Pipia, A.P., Zidda, A., Panzalis, R., Dore, F., Dessì, G., Sanna, G., Salis, F., Björkman, C. and Scala, A. 2015. ISCOM ELISA of milk for screening Neospora caninum in dairy sheep. Large Anim. Rev 21(5), 213–216. Uesaka, K., Koyama, K., Horiuchi, N., Kobayashi, Y., Nishikawa, Y. and Inokuma, H. 2018. A clinical case of neosporosis in a 4-week-old holstein friesian calf that developed hindlimb paresis postnatally. J. Vet. Med. Sci. 80(2), 280–283. Uggla, A., Stenlund, S., Holmdahl, O.J., Jakubek, E.B., Thebo, P., Kindahl, H. and Björkman, C. 1998. Oral Neospora caninum inoculation of neonatal calves. Int. J. Parasitol. 28(9), 1467–1472. Vanleeuwen, J.A., Haddad, J.P., Dohoo, I.R., Keefe, G.P., Tiwari, A. and Scott, H.M. 2010. Risk factors associated with Neospora caninum seropositivity in randomly sampled Canadian dairy cows and herds. Prev. Vet. Med. 93(2–3), 129–138. Wang, S., Li, L., Lu, Y., Zhang, H., Xie, Q. and Zhang, Z. 2018. Seroprevalence and risk factors of Neospora caninum infection in domestic sheep in Henan province, central China. Parasite 25(1), 15. Wang, S., Yao, Z., Zhang, N., Wang, D., Ma, J., Liu, S., Zheng, B., Zhang, B., Liu, K. and Zhang, H. 2016. Serological study of Neospora caninum infection in dogs in central China. Parasite 23(1), 25. Wang, X., Chen, J. and Zheng, J. 2022. The state of the art of extracellular vesicle research in protozoan infection. Front. Genet. 13(1), 941561. Weston, J.F., Heuer, C. and Williamson, NB. 2012. Efficacy of a Neospora caninum-killed tachyzoite vaccine in preventing abortion and vertical transmission in dairy cattle. Prev. Vet. Med. 103(2–3), 136–144. Williams, D.J., Guy, C.S., McGarry, J.W., Guy, F., Tasker, L., Smith, R.F., MacEachern, K., Cripps, P.J., Kelly, D.F. and Trees, A.J. 2000. Neospora caninum-associated abortion in cattle: time of experimentally induced parasitemia during gestation determines fetal survival. Parasitology 121(Pt 4), 347–358. Wilson, D.J., Orsel, K., Waddington, J., Rajeev, M., Sweeny, A.R., Joseph, T., Grigg, M.E. and Raverty, S. A. 2016. Neospora caninum is the leading cause of bovine fetal loss in British Columbia, Canada. Parasitol. 218(1), 46–51. Winzer, P., Müller, J., Imhof, D., Ritler, D., Uldry, A.C., Braga-Lagache, S., Heller, M., Ojo, K.K., Van Voorhis, W.C., Ortega-Mora, L.M. and Hemphill, A. 2020. Neospora caninum: differential proteome of multinucleated complexes induced by the bumped kinase inhibitor BKI-1294. Microorganisms 8(6), 801. | ||
| How to Cite this Article |
| Pubmed Style Rimayanti R, Khairullah AR, Utama S, Ahmad RZ, Mulyati S, Damayanti R, Lestari TD, Mustofa I, Hernawati T, Wasito W, Moses IB, Wardhani BWK, Kurniasih DAA, Kusumarini S, Wibowo S, Yanestria SM, Kusala MKJ, Lisnanti EF, Fauziah I. Review of neosporosis: Disease insights and control approaches. Open Vet. J.. 2025; 15(3): 1078-1090. doi:10.5455/OVJ.2025.v15.i3.2 Web Style Rimayanti R, Khairullah AR, Utama S, Ahmad RZ, Mulyati S, Damayanti R, Lestari TD, Mustofa I, Hernawati T, Wasito W, Moses IB, Wardhani BWK, Kurniasih DAA, Kusumarini S, Wibowo S, Yanestria SM, Kusala MKJ, Lisnanti EF, Fauziah I. Review of neosporosis: Disease insights and control approaches. https://www.openveterinaryjournal.com/?mno=229775 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.2 AMA (American Medical Association) Style Rimayanti R, Khairullah AR, Utama S, Ahmad RZ, Mulyati S, Damayanti R, Lestari TD, Mustofa I, Hernawati T, Wasito W, Moses IB, Wardhani BWK, Kurniasih DAA, Kusumarini S, Wibowo S, Yanestria SM, Kusala MKJ, Lisnanti EF, Fauziah I. Review of neosporosis: Disease insights and control approaches. Open Vet. J.. 2025; 15(3): 1078-1090. doi:10.5455/OVJ.2025.v15.i3.2 Vancouver/ICMJE Style Rimayanti R, Khairullah AR, Utama S, Ahmad RZ, Mulyati S, Damayanti R, Lestari TD, Mustofa I, Hernawati T, Wasito W, Moses IB, Wardhani BWK, Kurniasih DAA, Kusumarini S, Wibowo S, Yanestria SM, Kusala MKJ, Lisnanti EF, Fauziah I. Review of neosporosis: Disease insights and control approaches. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1078-1090. doi:10.5455/OVJ.2025.v15.i3.2 Harvard Style Rimayanti, R., Khairullah, . A. R., Utama, . S., Ahmad, . R. Z., Mulyati, . S., Damayanti, . R., Lestari, . T. D., Mustofa, . I., Hernawati, . T., Wasito, . W., Moses, . I. B., Wardhani, . B. W. K., Kurniasih, . D. A. A., Kusumarini, . S., Wibowo, . S., Yanestria, . S. M., Kusala, . M. K. J., Lisnanti, . E. F. & Fauziah, . I. (2025) Review of neosporosis: Disease insights and control approaches. Open Vet. J., 15 (3), 1078-1090. doi:10.5455/OVJ.2025.v15.i3.2 Turabian Style Rimayanti, Rimayanti, Aswin Rafif Khairullah, Suzanita Utama, Riza Zainuddin Ahmad, Sri Mulyati, Ratna Damayanti, Tita Damayanti Lestari, Imam Mustofa, Tatik Hernawati, Wasito Wasito, Ikechukwu Benjamin Moses, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, Shelly Kusumarini, Syahputra Wibowo, Sheila Marty Yanestria, Muhammad Khaliim Jati Kusala, Ertika Fitri Lisnanti, and Ima Fauziah. 2025. Review of neosporosis: Disease insights and control approaches. Open Veterinary Journal, 15 (3), 1078-1090. doi:10.5455/OVJ.2025.v15.i3.2 Chicago Style Rimayanti, Rimayanti, Aswin Rafif Khairullah, Suzanita Utama, Riza Zainuddin Ahmad, Sri Mulyati, Ratna Damayanti, Tita Damayanti Lestari, Imam Mustofa, Tatik Hernawati, Wasito Wasito, Ikechukwu Benjamin Moses, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, Shelly Kusumarini, Syahputra Wibowo, Sheila Marty Yanestria, Muhammad Khaliim Jati Kusala, Ertika Fitri Lisnanti, and Ima Fauziah. "Review of neosporosis: Disease insights and control approaches." Open Veterinary Journal 15 (2025), 1078-1090. doi:10.5455/OVJ.2025.v15.i3.2 MLA (The Modern Language Association) Style Rimayanti, Rimayanti, Aswin Rafif Khairullah, Suzanita Utama, Riza Zainuddin Ahmad, Sri Mulyati, Ratna Damayanti, Tita Damayanti Lestari, Imam Mustofa, Tatik Hernawati, Wasito Wasito, Ikechukwu Benjamin Moses, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, Shelly Kusumarini, Syahputra Wibowo, Sheila Marty Yanestria, Muhammad Khaliim Jati Kusala, Ertika Fitri Lisnanti, and Ima Fauziah. "Review of neosporosis: Disease insights and control approaches." Open Veterinary Journal 15.3 (2025), 1078-1090. Print. doi:10.5455/OVJ.2025.v15.i3.2 APA (American Psychological Association) Style Rimayanti, R., Khairullah, . A. R., Utama, . S., Ahmad, . R. Z., Mulyati, . S., Damayanti, . R., Lestari, . T. D., Mustofa, . I., Hernawati, . T., Wasito, . W., Moses, . I. B., Wardhani, . B. W. K., Kurniasih, . D. A. A., Kusumarini, . S., Wibowo, . S., Yanestria, . S. M., Kusala, . M. K. J., Lisnanti, . E. F. & Fauziah, . I. (2025) Review of neosporosis: Disease insights and control approaches. Open Veterinary Journal, 15 (3), 1078-1090. doi:10.5455/OVJ.2025.v15.i3.2 |