| Review Article | ||
Open Vet. J.. 2025; 15(3): 1091-1100 Open Veterinary Journal, (2025), Vol. 15(3): 1091-1100 Review Article Jembrana disease in Indonesia: An updated reviewDewa Ketut Meles1, Aswin Rafif Khairullah2, Suzanita Utama3, Wurlina Wurlina3, Sri Mulyati3, Imam Mustofa3*, Rimayanti Rimayanti3, Tita Damayanti Lestari3, Ikechukwu Benjamin Moses4, Syahputra Wibowo5, Bantari Wisynu Kusuma Wardhani6, Dea Anita Ariani Kurniasih7, Muhammad Khaliim Jati Kusala2, Riza Zainuddin Ahmad2, Ima Fauziah2, Wasito Wasito2 and Adeyinka Oye Akintunde81Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 5Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 7Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 8Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 02/12/2024 Accepted: 13/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
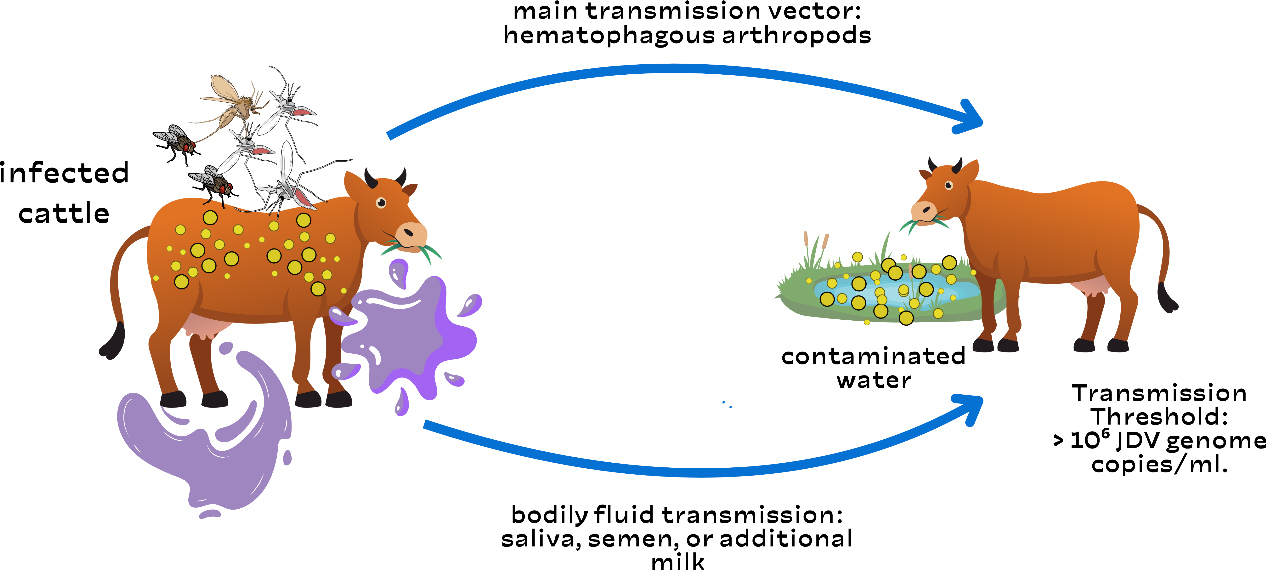
AbstractJembrana illness is an infectious disease that affects Balinese cattle in Indonesia. This disease is caused by the Jembrana disease virus (JDV), a lentivirus of the Retroviridae family. It was initially detected in 1964 in Jembrana Regency, Bali, Indonesia. Bali cattle have been widely disseminated throughout the Indonesian archipelago, and there is evidence that Jembrana disease has affected Bali cattle on the islands of Bali, Sumatra, and Java. During the acute stage, JDV is present in secreted fluids such as saliva, milk, and nasal secretions. There is no evidence of clinical illness recurrence in animals that have survived acute JDV infection. The clinical symptoms of Jembrana illness in cattle include elevated fever, stool bloody diarrhea, increased salivation, and enlarged lymph nodes. Jembrana illness is transmitted through direct contact between sick and healthy cows. This disease is also believed to be spread by blood-sucking insects, including flies, fleas, and mosquitoes. The most common risk factors are unregulated animal traffic movements that are not even under the supervision of animal health officers. Livestock producers suffered immediate financial losses as a result of the local epidemic of the Jembrana disease. Although there is currently no known cure for Jembrana disease, it can be prevented with vaccination. To stop the spread of the disease, livestock travel must also be considered. Keywords: Bali cattle, Infectious disease, JDV, Jembrana, Virus. IntroductionJembrana illness is an infectious disease that affects Balinese cattle (it is not communicable to other types of cattle) in Indonesia (Wilcox et al., 1992). This disease is caused by the Jembrana disease virus (JDV), a lentivirus of the Retroviridae family (Chen et al., 1999). Other species, such as buffalo, goats, sheep, and pigs, are immune to the disease and do not exhibit clinical signs, although they can carry the virus that causes Jembrana sickness for six months (Soeharsono et al., 1990). Meanwhile, experimental animals like rabbits, mice, white rats, and guinea pigs are resistant to Jembrana illness (Cooper et al., 2021). The youngest afflicted Bali cattle were 4 weeks old, while the oldest were 9 years old, both male and female. Bali cattle that recover from Jembrana sickness are immune to reinfection, but they can still spread the virus (Hartaningsih et al., 1994). Jembrana illness was detected in the Sankaragung district of Bali in December 1964, causing widespread deaths in Bali cattle (Soeharsono et al., 1990). The term “Jembrana” is derived from the name of the district where the infection first occurred, Jembrana Regency, Bali Province, Indonesia. This illness causes approximately 20% mortality in Bali cattle (Desport et al., 2007). The illness is currently endemic in Indonesia, particularly in Java, Kalimantan, and Sumatra. This demonstrates that Jembrana illness has been widespread since 2013. Prior to the outbreak of Jembrana disease, trading in Balinese cattle from Bali to other Indonesian islands, or even shipment to Hong Kong and Singapore, was unrestricted; now, inter-island trade in Balinese cattle from disease-endemic areas is prohibited (Hartaningsih et al., 1993). Jembrana disease is not a zoonotic disease, although it can result in significant economic losses because of its high morbidity and fatality rates. Cattle infected with Jembrana disease exhibit clinical symptoms such as fever, lethargy, anorexia, swelling of the superficial lymph nodes, bloody sweat, diarrhea with blood in the stool, and profuse salivation (Soesanto et al., 1990). This condition also causes leukopenia, thrombocytopenia, anemia, elevated blood urea levels, and decreased plasma protein (Tenaya et al., 2012). JDV infection is unique among lentivirus infections because it causes a severe and often deadly illness condition in Bali cattle and has a short incubation period. Bali cattle die only 1–2 weeks after infection (Kusumawati et al., 2014a). Jembrana illness is transmitted through direct contact between sick and healthy cows. Vertical transmission does not occur; therefore, cows affected with Jembrana disease can still have normal progeny (Soeharsono et al., 1995a). This disease is also believed to be spread by blood-sucking insects, including flies, fleas, and mosquitoes. This is due to the transmission of viruses by insects (Ditcham et al., 2009). Insect infections are frequent in farm-raised cattle. For many years, JDV titration in susceptible Bali cattle has been the only method for measuring virus load (VL); however, indirect approaches such as capture enzyme-linked immunosorbent assay (ELISA), Western immunoblotting, immunohistochemistry, and polymerase chain reaction (PCR) have recently been developed, allowing for a more in-depth analysis of virus dynamics across all stages of infection (Lewis et al., 2009). This makes Indonesia the sole country where Jembarana sickness exists. This occurrence is unusual and interesting and warrants further investigation and the attention of all parties involved in the development of Balinese cattle. The purpose of this review article is to explain the etiology, history, epidemiology, host range, pathogenesis, immune response, pathology, clinical symptoms, diagnosis, transmission, risk factors, economic impact, vaccination, and control of Jembrana disease. This review is designed to provide information that can be used by multiple stakeholders to determine the risks of Jembrana infection. EtiologyJembrana disease is caused by the JDV, which belongs to the Lentivirus and Retroviridae family (Chen et al., 1999). This virus can cause acute and occasionally fatal illness in afflicted animals. JDV is a virus that contains genetic material in the form of single-stranded ribonucleic acid, which has an icosahedral structure and 7732 base pairs. Similarly, this virus has an 80–120 nm envelope including four key proteins: p26, p16, p100, and p38-42-45 (Kusumawati et al., 2014b). This virus is classified as a retrovirus because it possesses reverse transcriptase activity. The JDV reverse transcriptase gene shares approximately 68% amino acid sequence similarity with bovine immunodeficiency virus (BIV); hence, BIV is referred to as bovine lentivirus type 1, and the membrane virus as bovine lentivirus type 2 (Chen et al., 1999). JDV only affects Bali cattle, whereas other breeds are more resistant to the virus (Wilcox et al., 1992). JDV infects target cells by adhering to their surfaces and injecting RNA genetic material into them. The viral RNA will be reverse-transcribed into complementary DNA (cDNA). The viral DNA will travel from the cytoplasm to the nucleus, where the integrase enzyme will integrate it with the host cell DNA to form a provirus (Stewart et al., 2008). The provirus DNA is detected in the blood of infected animals, such as Balinese cattle. The generated provirus will remain in the cow’s body, making the cow a carrier (Soeharsono et al., 1990). When these carrier cows become ill and their humoral immunity begins to wane, it is expected that the virus cDNA will become reactive and infect adjacent animals (Hartaningsih et al., 2001). JDV provirus DNA has a normal retrovirus genome structure with gag, pol, and env genes, as well as long terminal repeats at the 5′ and 3′ terminals (Anson, 2004). The env gene encodes two proteins present on the outermost surface of the Jembrana disease virus: the surface unit (SU) and the transmembrane protein (Kusumawati et al., 2003). The SU protein is involved in the early stages of replication and interacts with host cells by binding Jembrana virus particles to their surfaces (Deng et al., 2006). In addition, viruses contain a number of regulatory genes that encode proteins, some of which regulate viral gene expression. JDV is susceptible to chloroform and ether but resistant to sodium deoxycholate (1:1000). This virus is similarly inert against formalin and sensitive to severe pH (3.0–12.0). When heated to 55°C for 15 minutes, this virus becomes rapidly denatured (Metharom et al., 2001). JDV is resistant to antibiotics. JDV can live in the blood and tissues of patients for a long time (Chen et al., 2000). This virus can only grow in experimental animals, primarily Balinese cattle. The virus cannot grow and develop in primary cell cultures such as lungs, kidneys, testes, spleen, fetal muscles, peripheral blood macrophages, Vero (vero-E6 and CV1) (African green monkey), bovine embryonic tubule, Madin-Darby bovine kidney, HeLa, and baby hamster kidney, and it has no cytopathogenic effects (Desport et al., 2009). HistoryJembrana disease is an acute and contagious disease affecting Balinese cattle. It was first detected in 1964 in Jembrana Regency, Bali, Indonesia (Soeharsono et al., 1990). The sickness spread quickly to the surrounding areas, and within 9 months, it had reached the entire islands. In 2 years, an estimated 26,000 Bali cattle died out of a total Bali cattle population of roughly 300,000; in Jembrana Regency, Bali Island, 19,000 out of 31,000 Bali cattle died (Budiarso and Rikihisa, 1992). There were no other reports of the disease until 1971 and 1972, when it was discovered again in Tabanan Regency, Bali. The clinical indications and histology were identical to those observed in 1965, and the morbidity rate in Bali cattle in the area was 0.83%, with a mortality rate of 13%, which was lower than that reported during the initial epidemic (Soesanto et al., 1990). Since 1972, a more enzootic but comparable disease has affected Bali cattle throughout the island. In May 1976, the same disease struck Rama Dewa village in Seputih Raman district, Lampung Regency, Lampung Province. This illness is also known as Rama Dewa’s disease. The first incidence of Jembrana disease on Java Island was reported in Banyuwangi Regency, East Java, in May 1976. Various teams of domestic and foreign scientists were organized between 1989 and 1992 to determine the disease’s causal agent. Rickettsia was suspected of causing Jembrana disease until a team formed that year determined that the causative agent was a virus from the Retroviridae family, genus Lentivirus (Soeharsono et al., 1995a). EpidemiologyBali cattle have been widely disseminated throughout the Indonesian archipelago, and there is evidence that Jembrana disease has affected Bali cattle on the islands of Bali, Sumatra, and Java (Hartaningsih et al., 1993). Antibodies to JDV were found in cattle from the island of Bali, numerous districts in the provinces of Lampung and West Sumatra on Sumatra Island, and the province of East Java on Java Island, but not in other parts of Indonesia (Desport and Lewis, 2010). Clinical Jembrana illness, also known as Jembrana-like disease, has been found in Bali cattle in all places where antibodies have been discovered, but not in other areas. The first outbreak of the disease in each location was associated with significant mortality rates in Bali cattle, comparable to those recorded during the initial epidemic of Jembrana disease in Bali, and the disease in each area became endemic with only modest mortality rates (Soesanto et al., 1990). Hartaningsih et al. (1993) presented serological data showing minimal JDV transmission from locations where the disease was prevalent to cattle in nearby areas. Jembrana disease outbreaks were reported in West Sumatra in 1992, South Kalimantan in 1993, and Bengkulu in 1995. Jembrana disease was endemic throughout Bali from 1978 to 1988, with the exception of Nusa Penida Island, Lembongan Island, and Ceningan Island, where no cases had been reported (Soeharsono et al., 1995a). The majority of instances occurred in the Buleleng, Tabanan, and Jembrana districts, with the latest occurrence occurring in Badung district in 2005. The Jembrana outbreak returned to Riau Province between February and September of 2013. According to the Ministry of Agriculture of the Republic of Indonesia, Jembrana disease was reported in 10 provinces in 2015: Bali, West Sumatra, Riau, East Kalimantan, East Java, Jambi, South Kalimantan, Lampung, and North Kalimantan. Host rangeJembrana virus exclusively affects Bali cattle, both male and female. The youngest diseased cow was 4 weeks old, while the oldest was 9 years old. Surviving cattle will have the virus for at least 2 years following recovery from clinical cases, although their role in disease transmission is uncertain (Wareing et al., 1999). In studies by Soeharsono et al. (1990), Ongole cattle, Indian cattle, goats, Holstein Friesian cattle, sheep, buffalo, and pigs developed minor fever after being infected with the Jembrana virus, but no other clinical indications were observed. Buffalo, pigs, goats, and sheep can carry the Jembrana virus for up to 6 months despite the absence of obvious clinical symptoms. PathogenesisThe pathogenesis of Jembrana disease was first studied in 18-month-old Bali cattle after intravenous vaccination, with 10-fold dilutions of peripheral blood drawn periodically after experimental infection, resulting in the onset of clinical signs of disease 5–12 days post-infection lasting approximately 7 days (Berata, 2010). The dilution of blood inoculated into cattle was shown to have a linear relationship with the time taken for clinical signs of illness to appear. Lesions in Bali cattle with Jembrana illness are characterized by an intensive lymphoproliferative response; however, most cattle recover after the acute stage of the disease (Budiarso and Rikihisa, 1992). During the acute stage of infection, high viral loads in the plasma of infected Bali cattle were found to be 1010 to 1011 viral genome copies/ml (Kusumawati et al., 2015a). Inoculated buffaloes, sheep, Ongole, and Friesian cattle demonstrated chronic viremia. JDV remains in buffalo blood or spleen for at least 9 months. In other animals, the virus lives for less than 9 months, which is shorter. The inoculated crossbred cattle developed viremia for 3 months (Soeharsono et al., 1995b). However, resistant (surviving or recovered) animals had chronic viremia for at least 25 months following recovery, despite a significant drop in viral titers to 101 infectious units/ml (Soeharsono et al., 1995a). Because antibodies are not created until several weeks after infection, the humoral immune response plays a minor role in acute illness recovery (Hartaningsih et al., 1994). During the acute stage, the virus is present in secreted fluids such as saliva, milk, and nasal secretions. After 60 days of recovery, the virus was no longer found in secretions (Dharma et al., 1991). In situ hybridization was used to investigate the localization of JDV in various tissues or organs using a probe of DIG-labeled cDNA (digoxigenin) generated from the pol gene. Because of high levels of circulating viremia, numerous infected cells are seen in many organs early in the disease course during the feverish phase (Soesanto et al., 1990). The spleen had the most infected cells, but there were also many in the lymph nodes, lungs, bone marrow, liver, kidneys, cells in the general circulation, and intravascular lesions in the lungs (Chadwick et al., 1998). The presence of infected cells in these tissues explains the degenerative alterations observed in different tissues and organs. Immune responseThere is no evidence of clinical illness recurrence in animals that have survived acute JDV infection (Tenaya et al., 2012). Cattle infected with Jembrana disease had been experimentally induced for up to 22 months and showed no clinical indications when rechallenged with the infectious agent (Ardiawan et al., 2023). To detect antibodies against JDV in infected Bali cattle, an ELISA was devised. The antigen was a plasma-derived virus isolated by sucrose gradient centrifugation. ELISA identified antibodies in all infected cattle, but the antibody response to the virus was delayed; antibodies were not discovered until after acute disease recovery, and in most infected cattle, they were not detected until 11 weeks later (Desport et al., 2005). The peak antibody response is detected in 23–33 weeks following infection. Antibodies remained detectable at 59 weeks after infection. During the acute stage, there is a decrease in IgG-containing cells in lymphoid organs and a lower ratio of bovine CD4/bovine CD8 lymphocytes in lymph node follicles, indicating transient immunosuppression (Dharma et al., 1994). The inability of infected cattle to identify antibodies until they have recovered from acute clinical illness suggests that antibodies are unlikely to play a role in the recovery mechanism. PathologyWhen JDV infects experimentally infected Bali cattle, clear clinical symptoms result. This disease is general in nature, except in the central nervous system. Major hematologic abnormalities include leukopenia, moderate neutropenia, lymphopenia, eosinopenia, mild thrombocytopenia, elevated blood urea content, normocytic normochromic anemia, and decreased total plasma protein (Teuscher et al., 1981). These hematologic alterations point to aberrant hematopoietic system function. The cytopathological reaction is multifaceted and occurs in stages. The lymphoid organs experience widespread lymphoreticular reaction during the initial period. According to studies, lymphoblastoid cells and reticulum cells (dendritic cells) exhibit a strong non-follicular proliferative response as their main characteristic (Dharma et al., 1991). T-lymphocyte responses are thought to play a major role in the acute stage of proliferative alterations in the lymphoid system (Desport et al., 2007). The liver, kidneys, adrenal medulla, lungs, and other organs are affected in the second stage. Hypercellular glomerular edema occurs in the kidneys (Cleary et al., 2010). The lesions regress after infection but remain visible for up to 60 days. The lung alveolar cells in the lungs respond strongly to infection by swelling and proliferation, particularly in the anterior lobe (Bals and Hiemstra, 2004). This process is also accompanied by the invasion of mononuclear cells. Severe vascular lesions were observed in the lungs during morphological evaluation. These lesions are dispersed throughout the lungs and have a pulmonary granulomatous appearance (Budiarso and Rikihisa, 1992). The lumen of the pulmonary vasculature, with a diameter of 20–200 microns, is occupied by a significant number of intravascular macrophages, excluding other blood cell components (both in arteries and veins). Around arteries and veins, concentrated layers of peripheral cells, including plasma cells and macrophages, can occasionally be seen (Budiarso and Rikihisa, 1992). Rarely does tissue or blood vessel damage, or necrosis occur. Every damaged tissue has pleomorphic intracytoplasmic and basophilic inclusions (Budiarso and Rikihisa, 1992). Although they can still be detected in trace amounts for over 8 weeks, this process mostly takes place between the second and the fifth week of infection. Retinal cells, macrophages (Kupffer cells), pulmonary alveolar cells, lymphoblasts, and rarely vascular endothelium are known to have both massive intravacuolar inclusions and small basophilic granular forms. Lesions can be used to diagnose Jembrana disease after death because they are indicative of JDV infection (Kusumawati et al., 2014a). Clinical symptomsThe clinical symptoms of Jembrana illness in cattle include elevated fever, stool bloody diarrhea, increased salivation, and lymph node enlargement (Soeharsono et al., 1995a). De Pablo-Maiso et al. (2018) reported fever, anorexia, frostbite, lymphadenopathy, and lymphopenia as clinical signs of Jembrana illness in cattle. The Jembrana virus can weaken the immune system, increasing the body’s susceptibility to illness. This disease can also result in operative infections, which can lead to mortality. Other symptoms include blood spots on the skin that resemble bloody perspiration (Krisnayanti et al., 2020). According to Kusumawati and Fatimah (2018), seven of nine cows in Kinali district, West Pasaman Regency, were positive for Jembrana disease infection using PCR analysis. The clinical signs in the cow were fever, decreased appetite, bloody sweat, superficial lymph node swelling, hypersalivation, and hyperlacrimation. However, according to Anggy and Srihanto’s (2019) study on Balinese cattle deaths in Pubian district, Central Lampung Regency, up to 15 Balinese cattle who were pronounced dead tested positive for Jembrana disease in laboratory tests. Clinical signs, including anorexia, diarrhea, hypersalivation, and collapse, were present in the cow before death. Jembrana disease causes acute illness with a mortality rate of 20% in Balinese cattle (Su et al., 2018). The mortality rate in experimentally infected Bali cattle was 21%, which occurred 1–2 weeks after infection (Kusumawati et al., 2015b). In Balinese cattle, the disease takes 5–12 days to incubate. The most common sign of Jembrana illness is bloody sweat, which occurs in 93% of cases. Sweating blood can occur on the legs, scrotum, abdomen, back, and flanks. However, cows experimentally infected in pens lacking blood-sucking insects did not exhibit the signs of bloody sweat. This demonstrates the connection between blood-sucking insects and the bloody sweat of Balinese cattle with the Jembrana illness (Soesanto et al., 1990). Platelet counts can decrease as a result of Jembrana disease. According to the detection results, almost every organ was bleeding, and even wounds were crushed by the bites of blood-sucking insects, resulting in symptoms such as bloody perspiration (Chadwick et al., 1998). In addition to thrombocytopenia, this illness also results in leukopenia, anemia, elevated blood urea levels, and decreased plasma protein (Tenaya et al., 2012). DiagnosisThere are two kinds of observations that can be used to diagnose Jembrana disease: laboratory observations, which involve performing serological and antigen/virus detection tests, and field observations, which involve examining clinical symptoms and the disease’s pathology. In the field, the Jembrana illness can be easily diagnosed by looking for clinical signs, including bloody diarrhea, enlarged outer defense glands, and high temperatures, especially in infested areas (Soesanto et al., 1990). In the event that the afflicted animal passes away, a carcass can be dissected to determine pathological changes, such as splenic swelling and bleeding in all organs, particularly in organs or lymphoid tissue (Chadwick et al., 1998). According to histopathology, the growth of lymphoreticular cells within the lymphoid tissue causes the swelling and obstruction of the lymphoid organs (Elmore, 2006). A serological test called ELISA is used to detect antibodies. A recombinant main/dominant protein of the JDV or a complete virus extracted from the blood plasma of infected cows can be employed as the Jembrana viral antigen (p26) (Burkala et al., 1998). A microplate containing the antigen of the Jembrana virus is reacted with the cow’s antiserum. The antigen–antibody complex is then treated with an enzyme-labeled antibovine IgG conjugate. Following the addition of the substrate, the color of both the positive samples and the positive controls changed. Although the ELISA test has a very high sensitivity, its specificity is limited because the antigen can cross-react with different lentivirinae viruses (Drake and Levine, 2005). Western immunoblotting (WIB) is based on the examination of the separation of proteins that comprise the antigen of the Jembrana virus according to their molecular weight (Mahmood and Yang, 2012). The antigen protein that was separated on the gel was then transferred to cellulose paper as an antigen after the JDV protein was first separated using SDS-PAGE. The presence of a colorful line on the cellulose paper indicates a favorable reaction. The presence of numerous proteins in JDV can be demonstrated using the WIB test. The major proteins P26 and P16, minor proteins P33 and P45, and minor glycoprotein (GP 100) are among these proteins (Bianchi et al., 2016). The western blotting test is far more sensitive and specific than the ELISA test and can be used to confirm good results from the ELISA test (Santana et al., 2018). The Jembrana disease agent can be found in organs or tissues from affected animals using immunohistochemistry, a test that is comparable to the ELISA test (Helmi et al., 2020). The JDV in infected tissue cells can be identified by observing color changes in the cells using monoclonal anti-JDV serum. The diagnosis of Jembrana disease from tissue organs sent from the field has been made possible by this test (Desport et al., 2009). The PCR test is used to validate laboratory diagnoses in a more sensitive and specific manner (Helmi et al., 2020). This test was performed based on the detection of cDNA from the Jembrana disease virus by amplifying certain primers (JDV-1 and JDV-3) using PCR equipment. A commercially available DNAse Kit can be used to separate the cDNA of the JDV from the white blood cells (lymphocytes) that are needed for this test. This primer combination yielded a positive PCR result of approximately 360 bp (Lewis et al., 2009). Three days after infection, throughout the acute phase, and 6 months after recovery, PCR can identify animals infected with Jembrana disease, potentially even when the carrier animal is still alive. TransmissionThere have been reports that JDV is transmitted similarly to equine infectious anemia virus (EIAV), most likely through hematophagous arthropods when the virus is at its most severe and blood titers are high, as described in Figure 1 (Ditcham et al., 2009; De Pablo-Maiso et al., 2018). This is corroborated by epidemiological data that indicate the frequency of Jembrana disease is higher during Bali’s rainy season, which is associated with a high number of biting arthropods, and that infection happens following close contact between infected and susceptible animals (Horri et al., 2023). Transmission from infected cattle to animals in contact has been clearly observed in experimentally infected cattle (Soeharsono et al., 1990). There is evidence that JDV is shed into the milk of infected cattle during the acute phase of infection, and intranasal, conjunctival, and oral infections have been experimentally reproduced. However, other routes of transmission, such as via bodily fluids (milk, semen, or saliva), have not been thoroughly studied (Soeharsono et al., 1995a). Controlling disease spread during outbreaks requires an understanding of how viruses spread, and VL data produced using quantitative reverse transcription polymerase chain reaction have recently been used to determine the threshold at which the probability of virus transmission increases (Stewart et al., 2005). A plasma VL of 106 JDV genome copies/ml is calculated as the threshold for transmission based on the finding that blood meal residue in the mouthparts of tabanid flies can be identified in amounts as high as 4–10 nl and that a fly can transmit an EIAV titer of 106 ID50/ml in the blood (Foil et al., 1987). This is roughly equal to a 104 ID50/ml infectious titer or less than 1 ID50/ml per tabanid fly for EIAV.
Fig. 1. Jembrana disease virus: transmission via bodily fluids and hematophagous arthropods. Although cattle remain viremic for at least 2 years after recovery, virus titers rapidly decrease after the acute stage of disease to 100 ID50/ml at 32 days post-infection and further decrease to 10 ID50/ml at 72 days, raising questions about the role of animals recovering from persistent viremia in virus transmission (Soeharsono et al., 1995a). According to a recent field survey conducted in Bali, only 21% of seropositive cattle had provirus DNA levels in their peripheral blood mononuclear cells, suggesting that recovered calves have low provirus loads (Lewis et al., 2009). Risk factorsJembrana illness can spread either directly or indirectly through contact between ill and healthy livestock. The location on the edge of the village and district roads, the movement of livestock without animal health officers’ supervision, the ignorance of livestock farmers regarding Jembrana disease, and the state of the village environment, which will create puddles of water whenever it rains, are some risk factors that contribute to the transmission and spread of Jembrana disease (Desport and Lewis, 2010). The most common risk factors are unregulated animal traffic movements that are not even under the supervision of animal health officers. Economic impactLivestock producers suffered immediate financial losses as a result of the local epidemic of the Jembrana disease. Indirect economic losses include lost revenue as a result of a decline in trade commodities, whereas direct losses include the number of livestock mortality, sickness rates, animal health care, and population decline, lower reproduction, changes in population structure, and decreased feed efficiency (Unsunnidhal et al., 2021). The economic loss resulting from an infection involving 5,000 Bali cattle with 100% mortality is equal to 5,000 cattle times Indonesian Rupiah (IDR) 10 million, which is the typical price of normal cattle in the absence of an outbreak. A total of IDR 50 billion IDR were lost as a result of animal deaths. A 2% death rate, or 100 cows, is another assumption. In this case, the loss amounts to IDR 1 billion. Farmers typically sell sick or disease-incubating cattle to local traders for a low price (assuming 50% of the regular price), which results in an economic loss for the farmers equal to 50% of the normal cattle price times the number of cattle (Sieng et al., 2022). This can create issues in other locations or regions because the infection can spread to nearby areas if sick livestock are killed in slaughterhouses elsewhere. Cattle brought to the animal market are at risk of contracting diseases from other animals there (Rahman et al., 2020). Cattle have the potential to spread illness to free populations, making them vulnerable when imported or bought by other farmers to be kept in disease-free areas (Renault et al., 2021). Because of the disease’s approximately 10% morbidity rate, treating cattle will cost more money. This includes paying for supportive vitamins, medicines to stop secondary infections, and higher operating expenses for animal health technicians (Horri et al., 2023). VaccinationThere is no treatment for Jembrana disease because it comes from a virus. Nonetheless, antigens from animals that have recovered from JDV can be used for vaccination by extracting the serum (antigen) and subsequently causing the animal to produce more antibodies or immunity (Wilcox et al., 1992). Since immunization with this virus only lowers the length and severity of the disease to varying/certain levels, efforts to identify antigens from dormant viruses as the primary element for JDV vaccination have been challenging. The current vaccination against Jembrana disease is a whole inactivated vaccine prepared from spleen tissue that has been emulsified with an adjuvant. An adjuvant is used to emulsify the virus after it is extracted from the spleens of infected animals and concentrated into a solution. Triton X-100 was then used to inactivate the virus (Ishak et al., 2019). Because vaccination is a dead vaccination, a booster is essential to raise blood immunity while also activating the body’s defense system’s memory cells (Boretti, 2024). Two vaccinations must be administered, separated by 1 month from one another. The production of this kind of vaccine is still extremely restricted because it requires healthy Balinese cows as donors (Unsunnidhal et al., 2021). Furthermore, because other coinfections can potentially develop, vaccine safety cannot be guaranteed. Other forms of Jembrana vaccines, such as recombinant vaccines, which are safer and can be made in huge quantities, have been created to address these problems (Nascimento and Leite, 2012). On a larger scale, however, this vaccine is still limited despite its excellent laboratory development. When distributing Bali livestock from free areas to endemic areas, the cattle must be vaccinated three days prior to departure and again 3–4 weeks following the initial vaccination (Richeson et al., 2019). The vaccination campaign in endemic areas is followed by further vaccination. Similarly, Balinese cattle must be vaccinated before they are transferred from endemic areas (Lestari et al., 2022). Meanwhile, it is forbidden to drive from endemic countries to free zones. Routine vaccination for three consecutive years (on the same cattle) is the method used to control endemic areas. Two vaccinations are administered annually, separated by a month. ControlThe spread and losses of livestock must be minimized by anticipatory initiatives. The primary tactics that can be used are vaccination, counseling through information, education, and communication, and cattle transportation monitoring (Desport and Lewis, 2010). As preventive measures, environmental control measures, including vector management, biosecurity, biosafety, and multivitamin distribution, can also be implemented (Rasyid et al., 2023). It is anticipated that initiatives involving communication, information, and education will raise livestock farmers’ awareness and knowledge of managing their livestock enterprises (Horri et al., 2023). The reason for this is that many farmers still do not know enough about predicting Jembrana disease. To stop the spread of the disease, livestock travel must also be considered. Officers should keep an eye on cattle entering and leaving a territory, particularly in endemic areas. Livestock must undergo quarantine protocols before being mobilized (Soeharsono et al., 1995a). ConclusionThe viral illness known as “Jembrana disease” only affects Balinese cattle and is caused by lentiviruses belonging to the Retoviridae family. Bali cattle have a 20% mortality rate from this disease. Insect vectors and direct contact are the two modes of spreading of this disease. Although there is currently no known cure for Jembrana disease, it can be prevented with vaccination. To stop the spread of the disease, livestock travel must also be considered. AcknowledgmentsThe authors are grateful to Universitas Airlangga and Badan Riset dan Inovasi Nasional. Conflict of interestThe authors declare no conflict of interest. Author’s contributionsDKM, ARK, SW, MKJK, and BWKW drafted the manuscript. WW, RZA, IBM, and AOA revise and edit the manuscripts. SU, IF, WW, and DAAK participated in preparing and critical checking this manuscript. RR, TDL, IM, and SM edit the references. All authors have read and approved the final manuscript. FundingThe authors thank Universitas Airlangga for managerial support, Salma Firdausya Qurrotunnada Noor, Eunice Wong Hui Wen, Joo Jia Yin, and Rahma Novhira for technical support. This research was funded by the Directorate of Research and Community Service, Deputy for Strengthening Research and Technology, Ministry of Research and Technology/National Research and Innovation Agency for the 2022 fiscal year, Chancellor’s Decree number: 770/UN3.14/PT/2022. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAnggy, F.P. and Srihanto, E. 2019. Investigasi kematian Sapi Bali oleh Virus Jembrana di Kecamatan Pubian, Kabupaten Lampung Tengah Tahun 2019. Velabo 14(1), 6–9. Anson, D.S. 2004. The use of retroviral vectors for gene therapy: what risks are there? a review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet. Vaccines Ther. 2(1), 9. Ardiawan, F., Poetri, O.N., Hidayanto, N.K., Rumekso, A., Pradana, D. and Setiyaningsih, S. 2023. Antibody response to Jembrana disease virus capsid following field vaccination of Bali cattle in Sarolangun District, Jambi. Acta Vet. Indones. 11(2), 167–174. Bals, R. and Hiemstra, P.S. 2004. Innate immunity in the lungs: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23(2), 327–333. Berata, I.K. 2010. Studies of the pathogenesis of Jembrana disease based on the characteristics of infected cells in lymphoid tissues and peripheral blood. Bull. Vet. Udayana 2(1), 35–44. Bianchi, V., Bulek, A., Fuller, A., Lloyd, A., Attaf, M., Rizkallah, P.J., Dolton, G., Sewell, A.K. and Cole, D.K. 2016. A molecular switch abrogates glycoprotein 100 (gp100) t-cell receptor (TCR) targeting of a human melanoma antigen. J. Biol. Chem. 291(17), 8951–8959. Boretti, A. 2024. mRNA vaccine boosters and impaired immune system response in immune-compromised individuals: a narrative review. Clin. Exp. Med. 24(1), 23. Budiarso I.T. and Rikihisa, Y. 1992. Vascular lesions in the lungs of Bali cattle with Jembrana disease. Vet. Pathol. 29(3), 210–215. Burkala, E.J., Narayani, I., Hartaningsih, N., Kertayadnya, G., Berryman, D.I. and Wilcox, G.E. 1998. Recombinant Jembrana disease virus proteins as antigens for the detection of antibodies to bovine lentiviruses. J. Virol. Methods 74(1), 39–46. Chadwick, B.J., Desport, M., Brownlie, J., Wilcox, G.E. and Dharma, D.M. 1998. Detection of Jembrana disease virus in spleen, lymph nodes, bone marrow, and other tissues by in situ hybridization of paraffin-embedded sections. J. Gen. Virol. 79(1), 101–106. Chen, H., He, J., Fong, S., Wilcox, G. and Wood, C. 2000. Jembrana disease virus Tat can regulate HIV long terminal repeat-directed gene expression and can substitute for HIV Tat in viral replication. J. Virol. 74(6), 2703–2713. Chen, H., Wilcox, G., Kertayadnya, G. and Wood, C. 1999. Characterization of the Jembrana disease virus tat gene and the cis- and transregulatory elements in its long terminal repeats. J. Virol. 73(1), 658–666. Cleary, C.M., Moreno, J.A., Fernández, B., Ortiz, A., Parra, E.G., Gracia, C., Blanco-Colio, L.M., Barat, A. and Egido, J. 2010. Glomerular hematuria, renal interstitial hemorrhage and acute kidney injury. Nephrol. Dial. Transplant. 25(12), 4103–4106. Cooper, T.K., Meyerholz, D.K., Beck, A.P., Delaney, M.A., Piersigilli, A., Southard, T.L. and Brayton, C.F. 2021. Research-relevant conditions and pathology of laboratory mice, rats, gerbils, guinea pigs, hamsters, naked mole rats, and rabbits. ILAR J. 62(1–2), 77–132. de Pablo-Maiso, L., Doménech, A., Echeverría, I., Gómez-Arrebola, C., de Andrés, D., Rosati, S., Gómez-Lucia, E. and Reina, R. 2018. Prospects for innate immune responses as potential control strategies against non-primate lentiviruses. Viruses 10(8), 435. Deng, G., Qiao, W., Su, Y., Sha, R., Geng, Y. and Chen, Q. 2006. Internalization of the Jembrana disease virus Tat: possible pathway and implication. Virus Res. 121(2), 122–133. Desport, M. and Lewis, J. 2010. Jembrana disease virus: host responses, viral dynamics, and disease control. Curr. HIV Res. 8(1), 53–65. Desport, M., Stewart, M.E., Mikosza, A.S., Sheridan, C.A., Peterson, S.E., Chavand, O., Hartaningsih, N. and Wilcox, G.E. 2007. Sequence analysis of Jembrana disease virus strains revealed a genetically stable lentivirus. Virus Res. 126(1–2), 233–244. Desport, M., Stewart, M.E., Sheridan, C.A., Ditcham, W.G., Setiyaningsih, S., Tenaya, W.M., Hartaningsih, N. and Wilcox, G.E. 2005. Recombinant Jembrana disease virus gag proteins identify several different antigenic domains but do not facilitate serological differentiation of JDV and nonpathogenic bovine lentiviruses. J. Virol. Methods 124(1–2), 135–142. Desport, M., Tenaya, I.W., McLachlan, A., McNab, T.J., Rachmat, J., Hartaningsih, N. and Wilcox, G.E. 2009. In vivo infection of IgG-containing cells by Jembrana disease virus during acute infection. Virology 393(2), 221–227. Dharma, DD. M. Budiantono, A., Campbell, R.S. and Ladds, P.W. 1991. Studies on experimental Jembrana disease in Bali cattle. III. Pathology. J. Comp. Pathol. 105(4), 397–414. Dharma, D.M., Ladds, P.W., Wilcox, G.E. and Campbell, R.S. 1994. Immunopathology of experimental Jembrana disease in Bali cattle. Vet. Immunol. Immunopathol. 44(1), 31–44. Ditcham, W.G., Lewis, J.R., Dobson, R.J., Hartaningsih, N., Wilcox, G.E. and Desport, M. 2009. Vaccination reduces the viral load and risk of transmission of the Jembrana disease virus in Bali cattle. Virology 386(2), 317–324. Drake, C. and Levine, R.A. 2005. Sensitivity, specificity, and other diagnostic measures for multiple sites per unit. Contemp. Clin. Trials 26(2), 252–259. Elmore, S.A. 2006. Histopathology of the lymph nodes. Toxicol. Pathol. 34(5), 425–454. Foil, L.D., Adams, W.V., McManus, J.M. and Issel, C.J. 1987. Bloodmeal residues on mouthparts of Tabanus fuscicostatus (Diptera: Tabanidae) and the potential for mechanical transmission of pathogens. J. Med. Entomol. 24(6), 613–616. Hartaningsih, N., Dharma, D.M., Soeharsono, S. and Wilcox, G.E. 2001. Induction of protective immunity against Jembrana disease in cattle by vaccination with inactivated tissue-derived virus antigens. Vet. Immunol. Immunopathol. 78(2), 163–176. Hartaningsih, N., Wilcox, G.E., Dharma, D.M. and Soetrisno, M. 1993. Distribution of Jembrana disease in cattle in Indonesia. Vet. Microbiol. 38(1–2), 23–29. Hartaningsih, N., Wilcox, G.E., Kertayadnya, G. and Astawa, M. 1994. Antibody response to the Jembrana disease virus in Bali cattle. Vet. Microbiol. 39(1–2), 15–23. Helmi,,E., Yuherman, Rahmadani, I. and Subekti, D.T., 2020. Polyclonal antibody utilization for the detection of the Jembrana antigen in Bali cattle in West Sumatra Province. Indones. J. Vet. Sci. 14(1), 21–24. Horri, M., Suyanto and Jusnita, R.A.E. 2023. Disease control management strategy in Bali cattle. Int. J. Sci. Rev 5(1), 333–342. Ishak, J., Unsunnidhal, L., Martien, R. and Kusumawati, A. 2019. In vitro evaluation of Chitosan-DNA plasmid complex encoding Jembrana disease virus Env-TM protein as a vaccine candidate. J. Vet. Res. 63(1), 7–16. Krisnayanti, N.P.E., Pharmawati, M., Narayani, I. and Agustini, N.L.P., 2020. Monitoring the proviral DNA of the Jembrana disease virus in Bali cattle using PCR. Metamorfosa J. Biol. Sci. 7(1), 14. Kusumawati, A. and Fatimah. 2018. Combination of one-step reverse transriptase polymerase chain reaction (RT-PCR) and NALF (nucleic acid lateral flow) methods to detect the Env-tm gene of the Tabanan 1987 strain. J. Sain Vet. 36(2), 137–143. Kusumawati, A., Martien, R., Mangkoewidjojo, S. and Widada, J.S. 2003. Isolation and cloning of the Env-tm subunit gene of the Jembrana disease virus in the prokaryotic expression vector Pgex-2t. Indones. J. Vet. Sci. 21(2), 33–38. Kusumawati, A., Wanahari, T.A., Asmara., W., Prihatno, S.A., Mappakaya, B.A. and Hariono, B. 2015b. Immunodiagnosis in Jembrana disease: a review. Am. J. Immunol. 11(3), 102–107. Kusumawati, A., Wanahari, T.A., Astuti, P., Kurniasih, Mappakaya, B.A. and Wuryastuty, H. 2015a. Vaccination against the Jembrana disease virus infection: a summary findings. Am. J. Immunol. 11(3), 68–73. Kusumawati, A., Wanahari, T.A., Putri, R.F., Mappakaya, B.A. and Tampubolon, I.D. 2014b. The structure and function of the Jembrana disease virus genome. J. Inf. Mol. Biol. Sci. 2(2), 26–29. Kusumawati, A., Wanahari, T.A., Putri, R.F., Untari, T., Hartati, S., Mappakaya, B.A. and Putro, P.P. 2014a. Clinical and pathological perspectives of Jembrana disease virus infection: a review. Biosci. Biotech. Res. Asia 11(3), 1221–1225. Lestari, VV. S. Rahardja, D.P. and Sirajuddin, S.N. 2022. The willingness of beef cattle farmers to undergo vaccination from the animal health service unit in Bone regency, South Sulawesi province. IOP Conf. Ser. Earth Environ. Sci. 977(1), 012126. Lewis, J., McNab, T., Tenaya, M., Hartaningsih, N., Wilcox, G. and Desport, M. 2009. Comparison of immunoassay and real-time PCR methods for the detection of Jembrana disease virus infection in Bali cattle. J. Virol. Methods 159(1), 81–86. Mahmood, T. and Yang, P.C. 2012. Western blot: technique, theory, and trouble shooting. North Am. J. Med. Sci. 4(9), 429–434. Metharom, P., Takyar, S., Xia, H.Q., Ellem, K.A., Wilcox, G.E. and Wei, M.Q. 2001. Development of disabled, replication-defective gene transfer vectors of the Jembrana disease virus, a novel infectious agent of cattle. Vet. Microbiol. 80(1), 9–22. Nascimento I.P. and Leite, L.C. 2012. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 45(12), 1102–1111. Rahman, M.T., Sobur, M.A., Islam, M.S., Ievy, S., Hossain, M.J., El Zowalaty, M.E., Rahman, A.T. and Ashour, H.M. 2020. Zoonotic diseases: etiology, impact, and control. Microorganisms 8(9), 1405. Rasyid, I., Syarif, I. and Ashar, N.R. 2023. Prevention and management of Jembrana disease in beef cattle in Maros Regency, South Sulawesi Province, Indonesia. Adv. Environ. Biol. 17(5), 9–12. Renault, V., Humblet, M.F., Pham, P.N. and Saegerman, C. 2021. Biosecurity at cattle farms: strengths, weaknesses, opportunities and threats. Pathogens 10(10), 1315. Richeson, J.T., Hughes, H.D., Broadway, P.R. and Carroll, J.A. 2019. Vaccination management of beef cattle: delayed vaccination and endotoxin stacking. Vet. Clin. North Am. Food Anim. Pract. 35(3), 575–592. Santana, A.E., Taborda, C.P., Severo, J.S., Rittner, G.M.G., Muñoz, J.E., Larsson, C.E. Jr. and Larsson, C.E. 2018. Development of enzyme immunoassays (ELISA and Western blot) for the serological diagnosis of dermatophytosis in symptomatic and asymptomatic cats. Med. Mycol. 56(1), 95–102. Sieng, S., Patrick, I.W., Walkden-Brown, S.W. and Sar, C. 2022. A cost–benefit analysis of foot and mouth disease control program for smallholder cattle farmers in Cambodia. Transbound. Emerg. Dis. 69(4), 2126–2139. Soeharsono, S., Hartaningsih, N., Soetrisno, M., Kertayadnya, G. and Wilcox, G.E. 1990. Experimental Jembrana disease in Bali cattle. I. Transmission and persistence of the infectious agent in ruminants and pigs, and resistance of recovered cattle to reinfection. J. Comp. Pathol. 103(1), 49–59. Soeharsono, S., Wilcox, G.E., Dharma, D.M., Hartaningsih, N., Kertayadnya, G. and Budiantono, A. 1995b. Species differences in the reaction of cattle to Jembrana disease virus infection. J. Comp. Pathol. 112(4), 391–402. Soeharsono, S., Wilcox, G.E., Putra, A.A., Hartaningsih, N., Sulistyana, K. and Tenaya, M. 1995a. Transmission of the lentivirus disease Jembrana in Bos javanicus cattle. Epidemiol. Infect. 115(2), 367–374. Soesanto, M., Soeharsono, S., Budiantono, A., Sulistyana, K., Tenaya, M. and Wilcox, G.E. 1990. Experimental Jembrana disease in Bali cattle. II. Clinical signs and hematological changes. J. Comp. Pathol. 103(1), 61–71. Stewart, M., Desport, M., Hartaningsih, N. and Wilcox, G. 2005. TaqMan real-time reverse transcription-PCR and JDVp26 antigen capture enzyme-linked immunosorbent assay to quantify Jembrana disease virus load during the acute phase of in vivo infection. J. Clin. Microbiol. 43(11), 5574–5580. Stewart, M.E., Desport, M., Setiyaningsih, S., Hartaningsih, N. and Wilcox, G.E. 2008. Analysis of Jembrana disease virus mRNA transcripts produced during acute infection revealed a complex transcription pattern. Virus Res. 135(2), 336–339. Su, X., Wang, H., Zhou, X., Li, Z., Zheng, B. and Zhang, W. 2018. Jembrana disease virus Vif antagonizes the inhibition of bovine APOBEC3 proteins through ubiquitin-mediate protein degradation. Virology 519(1), 53–63. Tenaya, I.W., Heel, K., Stumbles, P.A. and Wilcox, G.E. 2012. Flow cytometric analysis of lymphocyte subset kinetics in experimentally infected Bali cattle with Jembrana disease virus. Vet. Immunol. Immunopathol. 149(3–4), 167–176. Teuscher, E., Ramachandran, S. and Harding, H.P. 1981. Observations on the pathology of “Jembrana disease” in Bali cattle. Zentralbl. Veterinarmed. A 28(8), 608–622. Unsunnidhal, L., Wasito, R., Setyawan, E.M.N., Warsani, Z. and Kusumawati, A. 2021. Potential of polylactic-co-glycolic acid (PLGA) for delivery Jembrana disease DNA vaccine Model (pEGFP-C1-tat). J. Vet. Sci. 22(6), e76–e77. Wareing, S., Hartaningsih, N., Wilcox, G.E. and Penhale, W.J. 1999. Evidence of immunosuppression associated with Jembrana disease virus infection of cattle. Vet. Microbiol. 68(1–2), 179–185. Wilcox, G.E., Kertayadnya, G., Hartaningsih, N., Dharma, D.M., Soeharsono, S. and Robertson, T. 1992. Evidence of the viral etiology of Jembrana disease in Bali cattle. Vet. Microbiol. 33(1–4), 367–374. | ||
| How to Cite this Article |
| Pubmed Style Meles DK, Khairullah AR, Utama S, Wurlina W, Mulyati S, Mustofa I, Rimayanti R, Lestari TD, Moses IB, Wibowo S, Wardhani BWK, Kurniasih DAA, Kusala MKJ, Ahmad RZ, Fauziah I, Wasito W, Akintunde AO. Jembrana disease in Indonesia: An updated review. Open Vet. J.. 2025; 15(3): 1091-1100. doi:10.5455/OVJ.2025.v15.i3.3 Web Style Meles DK, Khairullah AR, Utama S, Wurlina W, Mulyati S, Mustofa I, Rimayanti R, Lestari TD, Moses IB, Wibowo S, Wardhani BWK, Kurniasih DAA, Kusala MKJ, Ahmad RZ, Fauziah I, Wasito W, Akintunde AO. Jembrana disease in Indonesia: An updated review. https://www.openveterinaryjournal.com/?mno=231131 [Access: January 16, 2026]. doi:10.5455/OVJ.2025.v15.i3.3 AMA (American Medical Association) Style Meles DK, Khairullah AR, Utama S, Wurlina W, Mulyati S, Mustofa I, Rimayanti R, Lestari TD, Moses IB, Wibowo S, Wardhani BWK, Kurniasih DAA, Kusala MKJ, Ahmad RZ, Fauziah I, Wasito W, Akintunde AO. Jembrana disease in Indonesia: An updated review. Open Vet. J.. 2025; 15(3): 1091-1100. doi:10.5455/OVJ.2025.v15.i3.3 Vancouver/ICMJE Style Meles DK, Khairullah AR, Utama S, Wurlina W, Mulyati S, Mustofa I, Rimayanti R, Lestari TD, Moses IB, Wibowo S, Wardhani BWK, Kurniasih DAA, Kusala MKJ, Ahmad RZ, Fauziah I, Wasito W, Akintunde AO. Jembrana disease in Indonesia: An updated review. Open Vet. J.. (2025), [cited January 16, 2026]; 15(3): 1091-1100. doi:10.5455/OVJ.2025.v15.i3.3 Harvard Style Meles, D. K., Khairullah, . A. R., Utama, . S., Wurlina, . W., Mulyati, . S., Mustofa, . I., Rimayanti, . R., Lestari, . T. D., Moses, . I. B., Wibowo, . S., Wardhani, . B. W. K., Kurniasih, . D. A. A., Kusala, . M. K. J., Ahmad, . R. Z., Fauziah, . I., Wasito, . W. & Akintunde, . A. O. (2025) Jembrana disease in Indonesia: An updated review. Open Vet. J., 15 (3), 1091-1100. doi:10.5455/OVJ.2025.v15.i3.3 Turabian Style Meles, Dewa Ketut, Aswin Rafif Khairullah, Suzanita Utama, Wurlina Wurlina, Sri Mulyati, Imam Mustofa, Rimayanti Rimayanti, Tita Damayanti Lestari, Ikechukwu Benjamin Moses, Syahputra Wibowo, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, Riza Zainuddin Ahmad, Ima Fauziah, Wasito Wasito, and Adeyinka Oye Akintunde. 2025. Jembrana disease in Indonesia: An updated review. Open Veterinary Journal, 15 (3), 1091-1100. doi:10.5455/OVJ.2025.v15.i3.3 Chicago Style Meles, Dewa Ketut, Aswin Rafif Khairullah, Suzanita Utama, Wurlina Wurlina, Sri Mulyati, Imam Mustofa, Rimayanti Rimayanti, Tita Damayanti Lestari, Ikechukwu Benjamin Moses, Syahputra Wibowo, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, Riza Zainuddin Ahmad, Ima Fauziah, Wasito Wasito, and Adeyinka Oye Akintunde. "Jembrana disease in Indonesia: An updated review." Open Veterinary Journal 15 (2025), 1091-1100. doi:10.5455/OVJ.2025.v15.i3.3 MLA (The Modern Language Association) Style Meles, Dewa Ketut, Aswin Rafif Khairullah, Suzanita Utama, Wurlina Wurlina, Sri Mulyati, Imam Mustofa, Rimayanti Rimayanti, Tita Damayanti Lestari, Ikechukwu Benjamin Moses, Syahputra Wibowo, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, Riza Zainuddin Ahmad, Ima Fauziah, Wasito Wasito, and Adeyinka Oye Akintunde. "Jembrana disease in Indonesia: An updated review." Open Veterinary Journal 15.3 (2025), 1091-1100. Print. doi:10.5455/OVJ.2025.v15.i3.3 APA (American Psychological Association) Style Meles, D. K., Khairullah, . A. R., Utama, . S., Wurlina, . W., Mulyati, . S., Mustofa, . I., Rimayanti, . R., Lestari, . T. D., Moses, . I. B., Wibowo, . S., Wardhani, . B. W. K., Kurniasih, . D. A. A., Kusala, . M. K. J., Ahmad, . R. Z., Fauziah, . I., Wasito, . W. & Akintunde, . A. O. (2025) Jembrana disease in Indonesia: An updated review. Open Veterinary Journal, 15 (3), 1091-1100. doi:10.5455/OVJ.2025.v15.i3.3 |