| Research Article | ||
Open Vet. J.. 2025; 15(3): 1387-1396 Open Veterinary Journal, (2025), Vol. 15(3): 1387-1396 Research Article In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultryPawi Bawitlung Lalthanpuii1, Lalrosangpuii1,2 and Kholhring Lalchhandama1*1Department of Life Sciences, Pachhunga University College, Aizawl, India 2Department of Biochemistry, Government Zirtiri Residential Science College, Durtlang, India *Corresponding Author: Kholhring Lalchhandama. Department of Life Sciences, Pachhunga University College, Aizawl, India. Email: chhandama [at] pucollege.edu.in Submitted: 02/12/2024 Accepted: 04/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
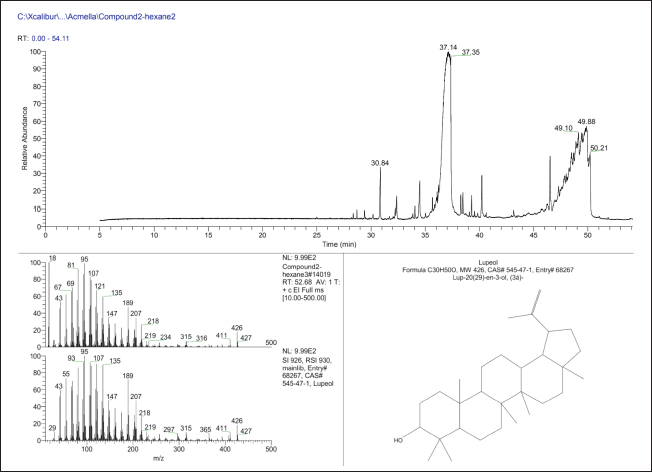
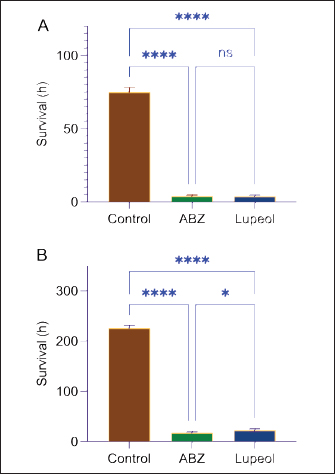
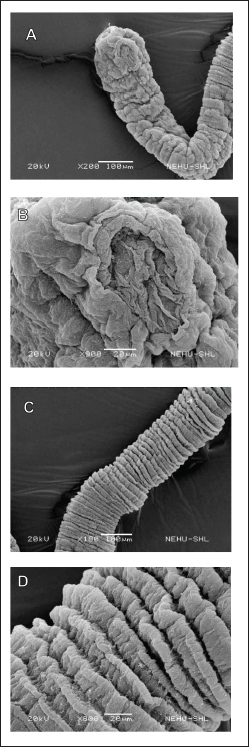
AbstractBackground: The management of helminthiasis remains a significant challenge in clinical and veterinary practice because the most important parasites have acquired extensive drug resistance in animal infections and reduced efficacy in human conditions. The toothache plant (Acmella oleracea) is a medicinal plant used in the Mizo traditional system of India for deworming intestinal parasites in both humans and domesticated animals. Therefore, it is important to understand the bioactive components and biological effects of the plant. Aim: Experiments were set up to characterize the bioactive compound of the plant and assess its antiparasitic activity against intestinal cestodes and nematodes. Methods: The aerial parts of the plants were extracted with hexane in a Soxhlet apparatus. The extract was concentrated and fractionated using column chromatography. The elution was performed with n-hexane and ethyl acetate. The major compound was characterized by thin-layer chromatography (TLC) and gas chromatography-mass spectrometry (GC-MS). The antiparasitic activity was evaluated against parasitic cestodes and nematodes of the chicken intestine. The antiparasitic effects were studied by scanning electron microscopy. Results: A single compound was eluted from column chromatography as indicated by TLC at an Rf value of 0.56 from a mobile phase consisting of 0.1% ethyl acetate in n-hexane. GC-MS data showed that the isolated compound exhibited the chemical properties of lupeol (fagarasterol). This is the first report of a lupane-type pentacyclic triterpene isolated from the toothache plant. The compound was effective against both cestode and nematode. Scanning electron microscopy revealed hallmark anthelmintic effects on the parasites. Distortion of the tegument, destruction of the suckers, and removal of spines were noted for the cestode; while fracture of the mouth region, loss of sensory papillae, and deflation of the cuticle throughout the body were observed on the nematode. Conclusion: This study presents a showcase of the isolation of lupeol from the toothache plant and demonstrates the utility of the compound as a broad-spectrum antiparasitic molecule against cestodes and nematodes. The findings of this study encourage further investigations on the mode of action, molecular interactions, and other pharmacological properties of the compounds for drug development. Keywords: Cestode, Chromatography, Medicinal plant, Nematode, Scanning electron microscopy. IntroductionParasitic worm infections, or helminthiases, remain a major global burden of infectious diseases in human and animal cases. Nematodes, which cause soil-transmitted helminthiases, alone are responsible for an estimated 1.5 billion infections in humans worldwide (Chen et al., 2024). If other helminths like cestodes and trematodes are taken into account, helminthiasis has become the leading infectious disease; schistosomiasis alone accounting for millions of cases every year (Zhang and Ming, 2024). Mass deworming programs and global actions to mitigate infections are at a lagging pace as the situation is compounded by the spread of drug resistance in animals and reduced drug efficacy in humans (Nixon et al., 2020; Fissiha and Kinde, 2021). The emergence of anthelmintic resistance has led to increased infections in veterinary animals and is becoming the primary factor of massive economic losses in the livestock industry (Ola-Fadunsin et al., 2020; Khan et al., 2023). In poultry farming, the transmission of major helminths, such as nematodes (Ascaridia galli and Heterakis gallinarum) and cestodes (Raillietina spp.), is increasing in different parts of the world (Shifaw et al., 2021; Muñoz-Gómez et al., 2024). The quest for novel anthelmintic compounds and improved drugs is as alive as ever. Among the potential lead molecules for anthelmintic development, pentacyclic triterpenes are among the promising botanical compounds. Several of these compounds have been shown to have valuable biological activities. For example, α- and β-amyrin, asiatic acid, bardoxolone, carbenoxolone, clerkdom, corosolic acid, categoric acid, glycyrrhetinic acid, oleanolic acid, tripterine, and ursolic acid are documented to have antidiabetic, anti-inflammatory, and cardiac protective activities (Sharma et al., 2018; Miranda et al., 2022). Lupane, oleanane, and ursane groups, betulinic acid, and oleanolic acid are shown to have anticancer properties against several cancer cell types (Nistor et al., 2023; Banerjee et al., 2023; Torres-Sanchez et al., 2024). These natural compounds and their semi-synthetic derivatives are also potential antiviral and antimicrobial agents, specifically against drug-resistant microbes (Wu et al., 2021; Li et al., 2023). Few compounds, including celastrol and grandiflora, are reportedly effective against helminth parasites (Cui et al., 2023; Soto-Sánchez, 2024). We previously reported that extracts of Acmella oleracea prepared with methanol, chloroform, and hexane showed anthelmintic activities in both cestode and nematode models (Lalthanpuii and Lalchhandama, 2020a; Lalthanpui et al., 2020a,b). The plant species is known by the common name “toothache plants” for its traditional use as a treatment for oral infections. The analgesic and botulinum neurotoxin-like effects of this plant are exploited in the development of commercial dental health care products and as alternatives to costly Botox in cosmetic applications. For the latter, a popular name, herbal Botox, is given to the plant (Lalthanpuii et al., 2020c). It is well known in the South American and Asian cultures as a versatile medicinal plant and is used in the treatment of cancer, flatulence, indigestion, liver abscess, malaria, urinary problems, and gastric ulcer (Aktar et al., 2024; Jerônimo et al., 2024). It is also used as an antimicrobial in injuries, antivenom to snake bites, and an insecticide (Uthpala and Navaratne, 2021). In Mizo traditional medicine, the plant is used to treat dysentery, rheumatism, speech impediment in children, and gastrointestinal infections (Lalthanpuii et al., 2020c). Based on the medicinal application of the anthelmintic plant and experimental evidence showing antiparasitic activities, we attempted to isolate a compound of A. oleracea that could be an anthelmintic agent. Using chromatographic separation and detection, this study aimed to identify the exact compound. The isolated compounds were then tested on two parasite models, a cestode and nematode, collected from the intestine of chickens. Materials and MethodsChemicals and drugsChemicals of standard analytical grade were procured from HiMedia Laboratories Private Limited, Mumbai, India. Acetonitrile and tetramethylsilane were obtained from Merck Life Science Private Limited, Mumbai, India. Albendazole was manufactured by UNI-PEX Pharmaceutical Private Limited, New Delhi, India. Plant specimen and extractionThe aerial parts of A. oleracea were collected from an agricultural plantation in Ngopa, Saitual district, Mizoram, India, which is located between 23.8861°N and 93.2119°E. The voucher specimen was validated at the Botanical Survey of India, Eastern Regional Office, Shillong, India (verification no BSI/ERC/Tech/Ident/2017/312) and cataloged (PUC-A-17-1) in the botanical collection at Pachhunga University College. The hexane extract of the plant was prepared in a 5-l Soxhlet apparatus. The crude extract was desolventized and concentrated in Buchi Rotavapor® R-210 (Flawil, Switzerland) under reduced pressure. Compound isolationThe plant extract was loaded with activated silica gel (230–400 mesh) packed with hexane in a 60 cm-long glass column with a 4 cm bore size. The column was continuously eluted with hexane and then hexane:ethyl acetate with increasing concentrations of ethyl acetate. Sixty fractions of 250 ml each were collected in conical flasks and analyzed using thin layer chromatography (TLC) with hexane:ethyl acetate at varying ratios as the mobile phase. The fractions collected from 0.5% ethyl acetate in hexane were dried, mixed with 60–120 mesh silica gel, and loaded into a prefixed column packed with 200–430 mesh silica gel. The elution was performed with hexane followed by 0.1%, 0.2%, 0.3%, 0.4%, and 0.5% ethyl acetate in hexane. The isolated compound was visualized at 254 and 366 nm in an ultraviolet-iodine chamber. Thin layer chromatography (TLC)Every fraction of the plant extract obtained from column chromatography was analyzed for the presence of the target compound using TLC. For the detection of the compounds, fraction samples were spotted on pre-coated TLC plates having 20 × 20 cm size, 60 Å pore size, 200 μm thickness (Sigma-Aldrich®, Darmstadt, Germany). The TLC plates were developed in a mobile mixture of n-hexane:ethyl acetate at various ratios and were visualized in a UV cabinet under an absorbance range of 254 to 366 nm. Gas chromatography-mass spectrometry (GC-MS)The isolated compound was characterized using mass spectrometry. For the most direct identification, the compound was analyzed with GC-MS using Perkin Elmer AutoSystem™ XL chromatograph with TurboMass™ spectrometer (Waltham, USA). Acetonitrile was used to dissolve the sample. Elution was done through Elite-5MS (30 m × 0.25 mm × 0.25 µm) capillary column. In brief, injector temperature at 260°C; oven temperature started at 75°C and incrementally raised by 10°C up to 280°C; helium injected at 1 ml/min in a split mode of 1:50; electron ionization at 220°C with the electron energy at 70 eV; mass range scanned between 50 and 700 m/z. Data were generated using TotalChrom® software incorporated into the GC-MS system. Compound identification was performed using the chemical database of the National Institute of Standards and Technology (NIST, US Department of Commerce). Anthelmintic survivalIntestinal parasites were obtained from the intestine of freshly sacrificed chicken (Gallus gallus Linnaeus, 1758). The parasite models used for anthelmintic susceptibility were Davainea tetragona Molin, 1858 (family Davaineidae; class Cestoda; phylum Platyhelminthes), and Ascaris lineata Schneider, 1866 (family Ascaridiidae; class Chromoadorea; phylum Nematoda). Applying the procedure of helminth survival assay (Lalchhandama, 2010), the parasites were collected and cultured in a microbiological incubator maintained at 37±1°C using a medium composed of 0.9% neutral phosphate-buffered saline (PBS) supplemented with 1% dimethyl sulfoxide (DMSO). The parasites maintained in the culture medium served as negative controls. Positive control was performed using parasites treated with the standard anthelmintic, albendazole, at a concentration of 20 mg/ml. To test the efficacy of the isolated compound, a concentration corresponding to albendazole was prepared in PBS + DMSO, and a set of parasites was exposed to it. The duration of survival of the parasites in the three different culture conditions was recorded to assess anthelmintic susceptibility. The data were expressed as means ± standard deviations, and the significance of the efficacy against the negative control was analyzed using Student’s t-test, with p values considered significant at less than 0.05. Scanning electron microscopyBoth cestode and nematode parasites exposed to the isolated compounds were washed with PBS and prepared for scanning electron microscopy based on the normalized procedure for helminth parasites (Lalthanpuii and Lalchhandama, 2020b). In brief, the parasites were first fixed in 10% neutral-buffered formaldehyde at 4°C for 4 hours. Post-fixation treatment was performed with 1% osmium tetroxide at 4°C for 1 hour. The tissues were dehydrated using increasing concentrations of acetone and finally in pure acetone. The tissues were dried by treatment with tetramethylsilane for 10 min and then allowed to evaporate in air. The dried specimens were sputter coated with gold in JFC-1100 (JEOL Ltd., Tokyo, Japan), and electron micrographs were taken with a JSM-6360 scanning electron microscope (JEOL Ltd., Tokyo, Japan) under an electron accelerating voltage of 20 kV. Ethical approvalThe study involved helminth parasites (cestode and nematodes obtained from chicken intestine) and was approved by the Institutional Animal Ethics Committee of Pachhunga University College (PUC-IAEC-2016-Z2). ResultsCompound isolation and identificationHexane, having a polarity index of 0.1, is an extremely nonpolar solvent, and its extraction yields only specific molecules. Soxhlet extraction of A. oleracea yielded 4.07% extractive value, and the concentrated extract was an amorphous powder. A single compound was detected from TLC in each fraction of the column chromatography at a retention coefficient (Rf value) of 0.56 using a mixture of hexane and ethyl acetate as a mobile phase at a mixture ratio of 8:2 (Fig. 1). A comparison of the Rf value with standard compounds at the same value and mobile phase indicated that the compound was lupeol (also known as fagarasterol). From the GC-MS chromatogram and spectra, it was confirmed that the compound was indeed lupeol. The characteristic mass with 264 m/z was identified at several peaks, such as at retention times of 30.84, 37.14, 37.35, 49.10, 49.88, and 50.21 minutes, corresponding to relative abundance of 33.8%, 99.6%, 96.5%, 54.1%, 56.7%, and 41.8%, respectively (Fig. 2). All the identified peaks were those of lupeol (chemical formula C30H50O). Anthelmintic efficacyThe two helminth parasites survived for several days in the culture medium (negative control) composed of PBS with 1% DMSO maintained at 37 ± 1°C in an ambient environment without food supplementation. The more delicate cestode, D. tetragona, survived for three days, whereas the more robust nematode, A. lineata, survived for nine days. Both parasites showed anthelmintic susceptibility to albendazole at the manufactured dosage, i.e., 20 mg/ml. The drug caused complete inhibition of the cestode at 4.14 ± 0.91 hours, whereas the A. oleracea compound, lupeol, exerted total inhibition at 04.04 ± 0.93 hours (Table 1). Both of them were significantly different (at p < 0.05) in efficacy against the control, but not between them (at p =0.99). Thus, albendazole and lupeol are equally effective against the cestode as indicated in Figure 3A. Albendazole completely inhibited the nematode at 18.01 ± 2.73 hours, whereas lupeol caused total lethality at 23.32 ± 2.65 hours (Table 2). A significant difference in survival was observed between the control, albendazole, and lupeol (Fig. 3B); and lupeol was less effective than albendazole (at p =0.014). Anthelmintic effectsScanning electron microscopy was used to assess the structural alterations induced by lupeol in both cestodes and nematodes. The cestode body consists of three main parts: the anterior scolex, neck, and main body called the strobila, which is a series of body segments or proglottids. The entire body is covered with an external surface called the tegument. Lupeol-treated cestode displayed extensive shrinkage and convolution of the tegument all around the scolex and neck (Fig. 4A). The special parasitic attachment organs, the oval-shaped suckers on the scolex, were disfigured with tegumental folds. Tissue-piercing devices, the spines, normally attached to the suckers, completely disintegrated (Fig. 4B). Constriction extended throughout the body segments, as each proglottid exhibited abnormal folds (Fig. 4C). On closer view, each proglottid had clumps of debris indicating degeneration of the proteinaceous filaments (microtriches), which are the sensory and absorptive organs (Fig. 4D).
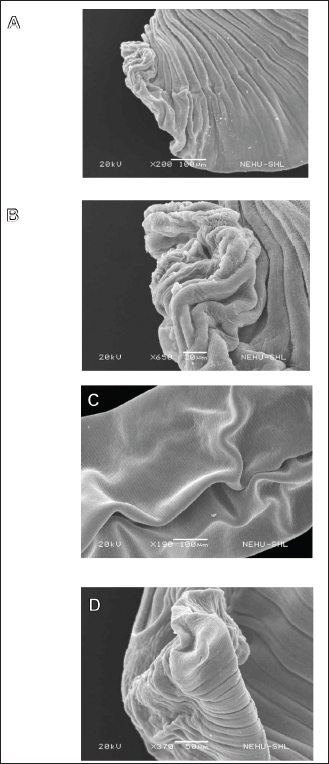
Fig. 1. TLC chromatogram of a compound isolated from Acmella oleracea. The mobile phase was hexane:ethyl acetate with a composition of 8:2. The nematode body is covered with a rigid cuticle and has three regions: the anterior cephalic region, the main body, and the posterior tail. Lupeol caused a massive disruption of the cuticle throughout the body. The cephalic region was shrunken and the characteristic bulbous mouthpart was completely disrupted (Fig. 5A). The denticle and lips were lost from view, and the sensory spots (amphids or papillae) appeared to disintegrate (Fig. 5B). The main body was deflated along the longitudinal axis of the body, and the transverse rings (annulations) were disrupted (Fig. 5C). The cuticular damage extended to the tail region (Fig. 5D). The anal opening (cloaca) and surrounding sensory organs (papillae) are invisible because of the heavy constriction of the cuticle. DiscussionAcmella species are common vegetables in South American and Asian cultures and are rich in fatty acid amides, of which a variety of unsaturated alkylamines appear to be the predominant compounds (Sharma et al., 2021; Savant and Kareppa, 2022). The N-alkylamide secondary metabolites are established as the chemical basis of their culinary uses as they produce a pungent flavor, tingling or burning sensation, and salivating effects (Elufioye et al., 2020). The compounds are also principal bioactive components in medicinal applications derived from different species. They have a range of therapeutic properties, including analgesic, anti-cancer, anti-inflammatory, antimicrobial, antimutagenic, antioxidant, antiprotozoal, insecticidal, and neuroprotective activities (Barbosa et al., 2016). In A. oleracea, the major bioactive alkylamines are N-isobutyl-(2E, 4Z, 8Z, 10E)-dodecatetraenamide and spilanthol; the latter was specifically identified as the molecule responsible for the anesthetic property and Botox-like effect for which the plant extract had been used in dental products and cosmetic practice (Silveira et al., 2018; Rondanelli et al., 2020).
Fig. 2. GC-MS chromatogram and mass spectra of lupeol isolated from A. oleracea. Structure of lupeol (bottom right) as generated using the NIST chemical library. Table 1. Efficacy of albendazole and lupeol from Acmella oleracea against chicken cestode.
Fig. 3. Comparison of the survival values of intestinal parasites, cestode (A) and nematode (B), in culture (negative control) and with those treated with albendazole (positive control) and lupeol from A. oleracea using one-way ANOVA and Turkey’s multiple comparison test. ABZ=albendazole; n=9; ****p < 0.0001; *p < 0.05; ns=not significant (p > 0.05). Table 2. Efficacy of albendazole and lupeol from Acmella oleracea against chicken nematode.
We have experimentally demonstrated that different extracts of A. oleracea exhibit antiparasitic activity against both intestinal nematode and cestode models. In an attempt to identify the anthelmintic compound, several N-alkylamides and other fatty acids were detected in the different extracts and fractions (Lalthanpuii and Lalchhandama, 2020a; Lalthanpui et al., 2020a,b). We performed comprehensive chemical analyses using an elemental analyzer, mass spectrometry, Fourier-transform nuclear magnetic resonance spectroscopy, and Fourier-transform infrared spectroscopy, the data of which are reported separately. We detected the pentacyclic triterpene lupeol (systematic name: 3β-lup-20(29)-en-3-ol) as the major anthelmintic component, as validated by the GC-MS data of the present analysis. The pentacyclic triterpene has been identified in many species of plants, including edible fruits, such as figs, guavas, mangoes, olives, red grapes, and strawberries, and common vegetables, such as aloe veras, carrots, cucumbers, white cabbages, peppers, peas, soy beans, and tomatoes (Liu et al., 2021; Ramsis et al., 2024).
Fig. 4. Scanning electron micrographs of chicken cestode treated with lupeol from A. oleracea. (A) Anterior region showing the scolex and neck region that indicates the mouth-like rostellum at the tip and two eye-like lateral suckers, with the general tegument folded all over the body. (B) The main parasitic attachment organ, a sucker focused indicating distortion and loss of spines. (C) The main body (strobila) showing a series of body segments (proglottids) with all the surfaces showing aberrant constrictions. (D) Magnification of the segments revealing fuzzy balls of threads on the surfaces indicating disintegration of the proteinaceous fibers (microtriches) of the tegument.
Fig. 5. Scanning electron micrographs of chicken nematode treated with lupeol from A. oleracea. (A) Anterior end showing the cephalic region that is highly convoluted and shrunk. (B) The mouth region shows collapse of mouth parts like the lips and denticles, with complete loss of amphids or papillae. (C) The main body showing a series of transverse rings (annulations) of the cuticle and the body being longitudinally constricted and deflated. (D) The extreme posterior end showing the precloacal sucker (top left circular structure) and the tail tip indicating massive deflation and degeneration. Lupeol has several valuable medicinal properties, including antidiabetic, anti-inflammatory, antimicrobial, anti-neurotic, and anti-protozoal activities. In addition, its potential as a treatment for cardiac, dermatological, digestive, nephrological, and neurological ailments has been documented (Sen et al., 2024). The most challenging but promising application seems to be in cancer management. There is preclinical and pharmacological evidence in animal and human cell lines that it selectively targets aberrant tumor cells while circumventing normal healthy cells (Liu et al., 2021; Fatma et al., 2024). The molecular basis of the anticancer and antiinflammatory activities has been established. It interacts with several key signaling molecules such as cFLIP, EGFR/STAT3, Fas, Kras, MAPK/ERK, nuclear factor kappa B (NFκB), p38/MAPK, phosphatidylinositol-3-kinase (PI3-K/Akt), prostaglandin E2 (PGE2), RhoA-ROCK1 and Wnt/β-catenin which are involved in cancer development and inflammatory reactions (Sohag et al., 2022; Sen et al., 2024). Until now, lupeol has not been recognized as a potential anthelmintic agent. In identifying the compound from A. oleracea, we demonstrated in this study that it is the major, if not sole, principle for the plant’s effectiveness in the treatment of intestinal parasitic infection, as claimed in the Mizo traditional medicine. Scanning electron microscopy is a reliable tool for assessing anthelmintic effects, as structural alterations can be revealed in detail (Spiegler et al., 2017; Kumar et al., 2024). Our data indicated that lupeol exerted extensive damage on both cestode and nematode, which are hallmark effects of antiparasitic drugs. The external coverings of helminths, the cuticle in nematodes, and the tegument in cestodes, are the primary targets of anthelmintics, as the parasite directly acquires any nutrient or drug molecules from their surrounding digested or semi-digested food particles of the host by passive diffusion (Sepúlveda-Crespo et al., 2020; Zamanian and Chan, 2021). The characteristic effects of albendazole on the cestode Raillietina echinobothrida were shown to include tegumental damage associated with surface erosion, degeneration of microtriches (the sensory and absorptive hair-like organs on the tegument), and distortion of the suckers and attachment organs (Lalchhandama, 2010). A related benzimidazole, flubendazole, destroys the tegumental tissue and rostellar complex, accompanied by the loss of microtriches in Echinococcus granulosus (Elissondo et al., 2006). A combined albendazole and praziquantel treatment caused scolex disfigurement marked by collapse of the suckers, removal of the spines, as well as wrinkling of the tegumental layer and excision of microtriches in E. granulosus and Mesocestoides corti (Urrea-Paris et al., 2000; Markoski et al., 2006). Albendazole and artemether induce swelling and disruption of the cuticle, distortion of the lips, and sensory papillae in the ascarid nematode Toxocara canis (Shalaby et al., 2009). Common destructive effects, such as shrinking of the cuticle, folding, and deformity of the mouth region, were induced by albendazole and praziquantel/pyrantel pamoate treatment of Ascaridia galli (Lalthanpuii and Lalchhandama, 2020b). The coadministration of albendazole and diethylcarbamazine in human infection with Wuchereria bancrofti resulted in cuticular destruction of the nematode (Oliveira-Menezes et al., 2007). Thickening and folding of the cuticle were observed on Haemonchus contortus after in vitro and in vivo exposure to tannins (Martínez-Ortíz-de-Montellano et al., 2013; del Carmen Acevedo-Ramírez et al., 2019). The findings of our study on the damaging effects of lupeol on different groups of helminth parasites are in agreement with the known effects of anthelmintics, thus substantiating the compound as a lead molecule for anthelmintic development. Exploration into the pharmacological properties of the compound will reveal the exact potential for clinical and veterinary applications. ConclusionThe pentacyclic triterpene lupeol was isolated from the hexane extract of A. oleracea and its chemical identity was confirmed by GC-MS. The compound was tested for antiparasitic activity in comparison with albendazole against cestode and nematode parasites. The compound was equally effective as the standard anthelmintic on cestodes, but less potent than the drug on nematodes. Scanning electron microscopy affirmed the classic anthelmintic effects. The lupeol-treated cestode exhibited comprehensive distortion, damage to the suckers, removal of the spines, and tegumental disintegration. The cuticle of the nematode showed extensive constrictions and folds, indicating collapse of the cuticular tissue, and damage to the mouth parts, including disappearance of the denticles and papillae. Our findings emphasize the importance of A. oleracea as an anthelmintic plant and that its bioactive component, lupeol, deserves research attention for drug development. Conflict of interestThe authors declare no conflict of interest. The authors are responsible for the accuracy and integrity of the paper content. FundingThis study was supported by the Department of Biotechnology, Government of India, under the scheme of DBT-BUILDER (grant number BT/INF/22/SP41398/2021). Chemical analysis facilities were provided by the Central Drug Research Institute, Lucknow, India. Authors’ contributionChemical analysis and electron microscopy: Pawi Bawitlung Lalthanpuii. Extract preparation and anthelmintic tests: Pawi Bawitlung Lalthanpuii and Lalrosangpuii. Writing: Lalrosangpuii and Kholhring Lalchhandama. Conceptualization resources, supervision, and data interpretation: Kholhring Lalchhandama. Data availabilityAll data presented in this study are included in the manuscript. ReferencesAktar, M.A., Bhuia, M.S., Molla, S., Chowdhury, R., Sarkar, C., Al Shahariar, M., Roy, P., Reiner, Ž., Sharifi-Rad, J., Calina, D. and Shakil, M.A.K. 2024. Pharmacological and phytochemical review of Acmella oleracea: a comprehensive analysis of its therapeutic potential. Discover Appl. Sci. 6(8), 412. Banerjee, J., Samanta, S., Ahmed, R. and Dash, S.K. 2023. Bioactive pentacyclic triterpenes trigger multiple signaling pathways for selective apoptosis, leading to anticancer efficacy: Recent updates and future perspectives. Curr. Protein Peptide Sci. 24(10), 820–842. Barbosa, A.F., Carvalho, M.G.D., Smith, R.E. and Sabaa-Srur, A.U. 2016. Spilanthol: occurrence, extraction, chemistry and biological activities. Rev. Bras. Farmacogn. 26(1), 128–133. Chen, J., Gong, Y., Chen, Q., Li, S. and Zhou, Y. 2024. Global burden of soil-transmitted helminth infections, 1990–2021. Poverty 13(1), 77(1–10). Cui, X., Ma, X., Li, C., Meng, H. and Han, C. 2023. A review: structure-activity relationship between saponins and cellular immunity. Mol. Biol. Rep. 50(3), 2779–2793. del Carmen Acevedo-Ramírez, P.M., Hallal-Calleros, C., Flores-Pérez, I., Alba-Hurtado, F., Mendoza-Garfías, M.B., Del Campo, N.C. and Barajas, R. 2019. Anthelmintic effect and tissue alterations induced in vitro by hydrolyzable tannins in the adult stage of the gastrointestinal nematode Haemonchus contortus. Vet. Parasitol. 266, 1–6. Elissondo, M., Dopchiz, M., Ceballos, L., Alvarez, L., Sánchez Bruni, S., Lanusse, C. and Denegri, G. 2006. In vitro effects of flubendazole on Echinococcus granulosus protoscoleces. Parasitol. Res. 98, 317–323. Elufioye, T.O., Habtemariam, S. and Adejare, A. 2020. Chemistry and pharmacology of alkylamines from natural origin. Rev. Bras. Farmacogn. 30, 622–640. Fatma, H., Jameel, M., Siddiqui, A.J., Kuddus, M., Buali, N.S., Bahrini, I. and Siddique, H.R. 2024. Chemotherapeutic potential of lupeol against cancer in preclinical model: a systematic review and meta-analysis. Phytomedicine. 132, 155777. Fissiha, W. and Kinde, M.Z. 2021. Anthelminic resistance and its mechanism: a review. Infect. Drug Resist. 14, 5403–5410. Jerônimo, L.B., Santos, P.V.L., Pinto, L.C., da Costa, J.S., de Aguiar Andrade, E.H., Setzer, W.N., da Silva, J.K.D.R., de Araújo, J.A.C. and Figueiredo, P.L.B. 2024. Acmella oleracea (L.) R.K. Jansen essential oils: chemical composition, antioxidant, and cytotoxic activities. Biochem. Syst. Ecol. 112, 104775. Khan, A., Jamil, M., Ullah, S., Ramzan, F., Khan, H., Ullah, N., Ali, M., Rehman, A.U., Jabeen, N. and Amber, R. 2023. The prevalence of gastrointestinal nematodes in livestock and their health hazards: a review. World Vet. J. 13(1), 57–64. Kumar, P., Bhatia, R. and Rangra, N.K. 2024. Scaffolds imparting anthelmintic activity: recent advancements and SAR studies. Mol. Divers. 2024, 1–34. Lalchhandama, K. 2010. In vitro effects of albendazole on the cestode of chicken, Gallus domesticus. J. Young Pharm. 2(4), 374–378. Lalthanpuii, P.B. and Lalchhandama, K. 2020a. Chemical composition and broad-spectrum anthelmintic activity of a toothache plant, Acmella oleracea, from Mizoram, India. Pharm. Biol. 58(1), 393–399. Lalthanpuii, P.B. and Lalchhandama, K. 2020b. Scanning electron microscopy study of the anthelmintic effects of some anthelmintic drugs on poultry nematode, Ascaridia galli. Adv. Anim. Vet. Sci. 8(8), 788–793. Lalthanpuii, P.B., Zokimi, Z. and Lalchhandama, K. 2020a. Anthelmintic activity of praziquantel and Spilanthes acmella extract against intestinal cestode parasite. Acta Pharm. 70(4), 551–560. Lalthanpuii, P.B., Zokimi, Z. and Lalchhandama, K. 2020b. The toothache plant (Acmella oleracea) exhibits anthelmintic activity on both parasitic tapeworms and roundworms. Pharmacogn. Mag. 16(68), 193−198. Lalthanpuii, P.B., Laldinpuii, Z.T., Lalhmangaihzuala, S., Vanlaldinpuia, K., Lalruatfela, B., Lalnunfela, C., Lalawmpuii, R., Lalhriatpuii, T.C., Lalhlenmawia, H. and Lalchhandama, K. 2020c. Chemical profiling of alkylamines from the “herbal Botox”, Acmella oleracea, cultivated in Mizoram and their pharmacological potentials. J. Environ. Biol. 41(4), 845–850. Li, Y., Wang, J., Li, L., Song, W., Li, M., Hua, X., Wang, Y., Yuan, J. and Xue, Z. 2023. Natural products of pentacyclic triterpenoids: from discovery to heterologous biosynthesis. Nat. Prod. Rep. 40(8), 1303–1353. Liu, K., Zhang, X., Xie, L., Deng, M., Chen, H., Song, J., Long, J., Li, X. and Luo, J. 2021. Lupeol and its derivatives as anticancer and antiinflammatory agents: molecular mechanisms and therapeutic efficacy. Pharmacol Res. 164, 105373. Markoski, M.M., Trindade, E.S., Cabrera, G., Laschuk, A., Galanti, N., Zaha, A., Nader, H.B. and Ferreira, H.B. 2006. Praziquantel and albendazole damage the in vitro developing Mesocestoides corti (Platyhelminthes: Cestoda). Parasitol. Int. 55(1), 51–61. Martínez-Ortíz-de-Montellano, C., Arroyo-López, C., Fourquaux, I., Torres-Acosta, J.F.J., Sandoval-Castro, C.A. and Hoste, H. 2013. Scanning electron microscopy of Haemonchus contortus exposed to tannin-rich plants in vivo and in vitro conditions. Exp. Parasitol. 133(3), 281–286. Miranda, R.D.S., de Jesus, B.D.S.M., da Silva Luiz, S.R., Viana, C.B., Adao Malafaia, C.R., Figueiredo, F.D.S., Carvalho, T.D.S.C., Silva, M.L., Londero, V.S., da Costa-Silva, T.A. and Lago, J.H.G. 2022. Anti-inflammatory activity of natural triterpenes—An overview from 2006 to 2021. Phytother. Res. 36(4), 1459–1506. Muñoz-Gómez, V., Ma, T., Li, Y., Rasmussen, P. and Torgerson, P.R. 2024. Global and regional prediction of coccidiosis and ascaridiosis prevalence in extensive backyard chickens in low-income and middle-income countries. Vet. Parasitol. 331, 110268. Nistor, M., Rugina, D., Diaconeasa, Z., Socaciu, C. and Socaciu, M.A. 2023. Pentacyclic triterpenoid phytochemicals with anticancer activity: updated studies on mechanisms and targeted delivery. Int. J. Mol. Sci. 24(16), 12923. Nixon, S.A., Welz, C., Woods, D.J., Costa-Junior, L., Zamanian, M. and Martin, R.J. 2020. Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics. Drugs Drug Resist. 14, 8–16. Ola-Fadunsin, S.D., Ganiyu, I.A., Rabiu, M., Hussain, K., Sanda, I.M., Baba, A.Y., Furo, N.A. and Balogun, R.B. 2020. Helminth infections of great concern among cattle in Nigeria: insight to its prevalence, species diversity, patterns of infections and risk factors. Vet. World 13(2), 338–344. Oliveira-Menezes, A., Lins, R., Norões, J., Dreyer, G. and Lanfredi, R.M. 2007. Comparative analysis of a chemotherapy effect on the cuticular surface of Wuchereria bancrofti adult worms in vivo. Parasitol. Res. 101, 1311–1317. Ramsis, T., Selim, H.M.R.M., Elseedy, H. and Fayed, E.A. 2024. The role of current synthetic and possible plant and marine phytochemical compounds in the treatment of acne. RSC Adv. 14(33), 24287–24321. Rondanelli, M., Fossari, F., Vecchio, V., Braschi, V., Riva, A., Allegrini, P., Petrangolini, G., Iannello, G., Faliva, M.A., Peroni, G. and Nichetti, M. 2020. Acmella oleracea for pain management. Fitoterapia 140, 104419. Savant, P.B. and Kareppa, M.S. 2022. A systemetic and scientific review on the Acmella oleracea and its traditional medical and pharmacological uses. Asian J. Pharm. Res. 12(1), 71–75. Sen, K., Das, S.K., Ghosh, N., Sinha, K. and Sil, P.C. 2024. Lupeol: a dietary and medicinal triterpene with therapeutic potential. Biochem. Pharmacol. 229, 116545. Sepúlveda-Crespo, D., Reguera, R.M., Rojo-Vázquez, F., Balaña-Fouce, R. and Martínez-Valladares, M. 2020. Drug discovery technologies: Caenorhabditis elegans as a model for anthelmintic therapeutics. Med. Res. Rev. 40(5), 1715–1753. Shalaby, H.A., Abdel-Shafy, S., Abdel-Rahman, K.A. and Derbala, A.A. 2009. Comparative in vitro effect of artemether and albendazole on adult Toxocara canis. Parasitol. Res. 105, 967–976. Sharma, H., Kumar, P., Deshmukh, R.R., Bishayee, A. and Kumar, S. 2018. Pentacyclic triterpenes: new tools to fight metabolic syndrome. Phytomedicine. 50, 166–177. Sharma, R. and Arumugam, N. 2021. N-alkylamides of Spilanthes (syn: Acmella): structure, purification, characterization, biological activities and applications–A review. Future Foods. 3, 100022. Shifaw, A., Feyera, T., Walkden-Brown, S.W., Sharpe, B., Elliott, T. and Ruhnke, I. 2021. Global and regional prevalence of helminth infection in chickens over time: a systematic review and meta-analysis. Poult. Sci. 100(5), 101082. Silveira, N., Sandjo, L.P. and Biavatti, M.W. 2018. Spilanthol-containing products: a patent review (1996–2016). Trends Food Sci. Technol. 74, 107–111. Sohag, A.A.M., Hossain, M.T., Rahaman, M.A., Rahman, P., Hasan, M.S., Das, R.C., Khan, M.K., Sikder, M.H., Alam, M., Uddin, M.J. and Rahman, M.H. 2022. Molecular pharmacology and therapeutic advances of the pentacyclic triterpene lupeol. Phytomedicine 99, 154012. Soto-Sánchez, J. 2024. Could natural terpenes be an alternative for the treatment of neglected tropical diseases? Chem. Biol. Drug Des. 103(2), e14470. Spiegler, V., Liebau, E. and Hensel, A. 2017. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Nat. Prod. Rep. 34(6), 627–643. Torres-Sanchez, A., Torres, G., Estrada, S., Perez, D., Garcia, C., Milian, M., Velazquez, E., Molina, V. and Delgado, Y. 2024. Unraveling the impact of six pentacyclic triterpenes regulating metabolic pathways on lung carcinoma cells. Pharmaceuticals 17(6), 694. Urrea-Paris, M.A., Moreno, M.J., Casado, N. and Rodriguez-Caabeiro, F. 2000. In vitro effect of praziquantel and albendazole combination therapy on the larval stage of Echinococcus granulosus. Parasitol. Res. 86, 957–964. Uthpala, T.G.G. and Navaratne, S.B. 2021. Acmella oleracea plant; identification, applications and use as an emerging food source – Review. Food Rev. Int. 37(4), 399–414. Wu, P., Tu, B., Liang, J., Guo, S., Cao, N., Chen, S., Luo, Z., Li, J., Zheng, W., Tang, X. and Li, D. 2021. Synthesis and biological evaluation of pentacyclic triterpenoid derivatives as potential novel antibacterial agents. Bioorg. Chem. 109, 104692. Zamanian, M. and Chan, J.D. 2021. High-content approaches to anthelmintic drug screening. Trends Parasitol. 37(9), 780–789. Zhang, Y. and Ming, Y. 2024. Burden of schistosomiasis in global, regional, and national 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Travel Med. Infect. Dis. 61, 102751. | ||
| How to Cite this Article |
| Pubmed Style Lalthanpuii PB, Lalrosangpuii , Lalchhandama K. In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry. Open Vet. J.. 2025; 15(3): 1387-1396. doi:10.5455/OVJ.2025.v15.i3.30 Web Style Lalthanpuii PB, Lalrosangpuii , Lalchhandama K. In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry. https://www.openveterinaryjournal.com/?mno=231192 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.30 AMA (American Medical Association) Style Lalthanpuii PB, Lalrosangpuii , Lalchhandama K. In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry. Open Vet. J.. 2025; 15(3): 1387-1396. doi:10.5455/OVJ.2025.v15.i3.30 Vancouver/ICMJE Style Lalthanpuii PB, Lalrosangpuii , Lalchhandama K. In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1387-1396. doi:10.5455/OVJ.2025.v15.i3.30 Harvard Style Lalthanpuii, P. B., Lalrosangpuii, . & Lalchhandama, . K. (2025) In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry. Open Vet. J., 15 (3), 1387-1396. doi:10.5455/OVJ.2025.v15.i3.30 Turabian Style Lalthanpuii, Pawi Bawitlung, Lalrosangpuii, and Kholhring Lalchhandama. 2025. In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry. Open Veterinary Journal, 15 (3), 1387-1396. doi:10.5455/OVJ.2025.v15.i3.30 Chicago Style Lalthanpuii, Pawi Bawitlung, Lalrosangpuii, and Kholhring Lalchhandama. "In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry." Open Veterinary Journal 15 (2025), 1387-1396. doi:10.5455/OVJ.2025.v15.i3.30 MLA (The Modern Language Association) Style Lalthanpuii, Pawi Bawitlung, Lalrosangpuii, and Kholhring Lalchhandama. "In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry." Open Veterinary Journal 15.3 (2025), 1387-1396. Print. doi:10.5455/OVJ.2025.v15.i3.30 APA (American Psychological Association) Style Lalthanpuii, P. B., Lalrosangpuii, . & Lalchhandama, . K. (2025) In vitro investigation of the antiparasitic effects of a pentacyclic triterpene from the toothache plant on intestinal worms of poultry. Open Veterinary Journal, 15 (3), 1387-1396. doi:10.5455/OVJ.2025.v15.i3.30 |