| Research Article | ||
Open Vet. J.. 2025; 15(3): 1264-1278 Open Veterinary Journal, (2025), Vol. 15(3): 1264-1278 Research Article Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical featuresNike Sari Oktavia1,2, Arifa Mustika3* and Afif Nurul Hidayati4,5,61Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Midwifery, Polytechnic of Health Ministry of Health Padang, Padang, Indonesia 3Department of Anatomy, Histology, and Pharmacology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Dermatology Venereology and Aesthetics, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 5Department of Dermatology and Venereology, Dr. Soetomo Academic General Hospital, Surabaya, Indonesia 6Department of Dermatology and Venereology, Faculty of Medicine, Universitas Airlangga Teaching Hospital, Surabaya, Indonesia *Corresponding Author: Arifa Mustika. Department of Anatomy, Histology, and Pharmacology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: arifa-m [at] fk.unair.ac.id Submitted: 04/11/2024 Accepted: 04/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
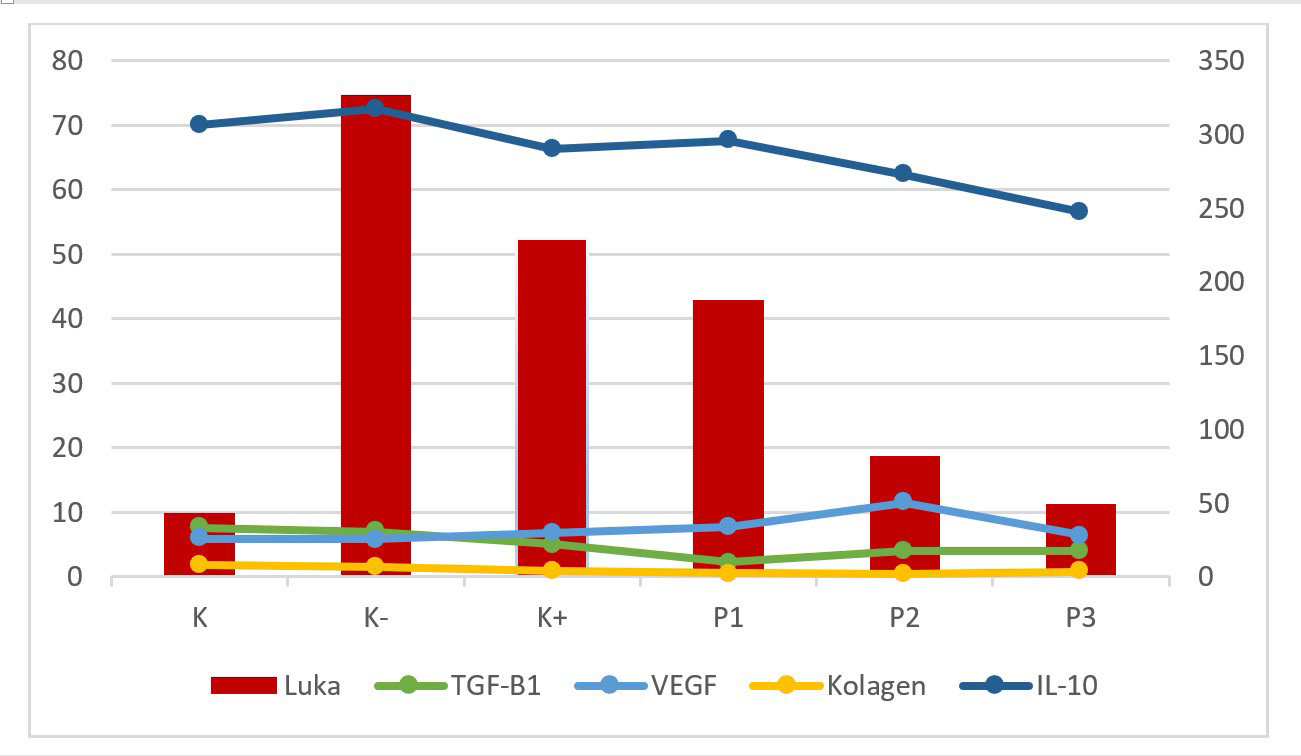
AbstractBackground: The incidence of wound complications after breast cancer surgery is > 30%. Delayed wound healing increases the risk of systemic recurrence up to three-fold after excisional surgery for primary breast cancer. Patients with breast malignant tumors exhibit immune system dysfunction while T cells play a major role in the inflammatory process of wound healing. The time required to achieve wound closure depends on the severity of the wound. The content of Piper crocatum ethanol extract contains alkaloids, flavonoids, tannins, steroids, and polyphenols. Flavonoid and polyphenol compounds are antioxidant, antidiabetic, anticancer, antiseptic, and anti-inflammatory. Aim: To determine whether P. crocatum extract increases interleukin-10 (IL-10) levels, TGF-β1 and VEGF expression, collagen density, and the clinical features of wounds, and how the mechanism works on wound healing of Wistar rats after excision of mammary tumors. Methods: This study used 35 female Wistar rats, 30 mammary tumor models by injecting 3-5% benzopyrene as much as 50 mg/Kg.BW in 5 injections into the mammary glands/mammae every 2 days as much as 10 mg/kg.BW. Thirty rats were divided into 5 groups; red betel extract gel 50%, 25%, and 12.5%; povidone iodine group as a positive control; and carboxymethyl cellulose natrium (CMC na) group as a negative control, 5 normal wounds as controls. Treatment was given 2 times a day for 14 days. Results: Multiple linear regression results; P. crocatum extract decreased IL-10 levels by −0.64 (p=0.025), decreased TGF-β1 expression −0.832 (p=0.001), increased VEGF expression by 0.638 (p=0.026), and decreased collagen density by −0.605 (p=0.037) in mammary tumor post-excision rat wounds. Conclusion: There is an effect of P. crocatum extract on the wound of Wistar rats after excision of mammary tumor by the mechanism of decreased IL-10 levels and TGF-β1 expression, increased VEGF expression, and decreased collagen density through increased VEGF expression is studied in this article. Keywords: Piper crocatum extract, IL-10 level, TGF-β1 expression, VEGF expression, Collagen density. IntroductionDelayed wound healing is associated with an increased rate of subsequent systemic recurrence after excisional surgery for primary mammary cancer with a threefold risk (Savioli et al., 2020). Wound-related complications can cause significant delays in the commencement of adjuvant therapy, often resulting in aesthetic compromise, patient distress, and financial loss (Rizvi et al., 2020). Postoperative infection is one of the most costly healthcare problems with an estimated USD 3.3 billion/year (CDC USA gov, 2022). Cancer prognosis may be related to the status of immune system function. Campbell et al.’s study in 85 mammary cancer patients showed generalized immune system dysfunction in patients relative to a shift in the balance of type 1 and type 2 cells (Lister et al., 2020). Mammary cancer patients with larger tumors have a greater cytokine response pressure (Alqathama, 2024). Red betel leaf extract (Piper crocatum) is a traditional ingredients that has long been used because it contains antibacterials such as flavonoids, alkaloids, tannins, steroids, triterpenoids, phenols, essential oils, polyphenolic, and saponins (Li et al., 2019; Rosyadi et al., 2020; Oktavia et al., 2022). Flavonoid and polyphenolic compounds are antioxidant, antidiabetic, anticancer, antiseptic, and anti-inflammatory. Some studies have reported that the methanol extract of red betel leaf has anti-inflammatory effects (Lister et al., 2020; Gong et al., 2021). Piper crocatum at low concentrations has anti-inflammatory potential, as shown by the inhibition of the activity of inflammatory mediators including, TNF-α, interleukin-1β, interleukin-6, and nitric oxide, reactive oxygen species levels, increased apoptosis, and the percentage of living cells significantly (Ginting et al., 2020; Lister et al., 2020; Gong et al., 2021; Lely et al., 2023; Maslikah et al., 2023; Maslikah et al., 2025). The cytokine IL-10 has emerged as a key mediator of the pro- to anti-inflammatory transition that counteracts collagen deposition in scar tissue (Singampali et al., 2020). IL-10 influences the composition of the extracellular matrix in fetal wounds, leading to reduced scar formation. Research involving animal models has demonstrated that the administration of IL-10 or IL-10 gene therapy can significantly improve wound healing outcomes. These interventions have been shown to reduce scar formation, encourage tissue regeneration, and enhance the wound healing process (Mahmoud et al., 2024). TGF-β1 is also called pleiotropic, which means that it plays more than one role in modifying growth factor β1. TGF-β1 plays a role in wound healing, fibrosis, inflammation, and immunity. TGF-β1 facilitates the resolution of proinflammatory responses and triggers fibroblast activation and the development of ECM-producing myofibroblasts that facilitate repair and promote the process of scar tissue formation or fibrosis (Lodyga and Hinz, 2020; Mahmoud et al., 2024). VEGF stimulates wound healing through several mechanisms, including collagen deposition, angiogenesis, and epithelialization. Angiogenesis wound healing involves several steps, including vasodilatation, basement membrane degradation, migration, and proliferation of endothelial cells. Subsequently, capillary vessel formation occurs, followed by anastomosis of new parallel capillaries (loop formation), and finally formation of a new basement membrane. VEGF is involved in some of these processes (White Michael et al., 2021). One way to accelerate the appearance of angiogenesis is to reduce the amount of free radicals that appear in the tissue after trauma. Factors that reduce free radicals include various types of antioxidants (Xu et al., 2020). Research by Tonahi et al. (2014) stated that red betel leaf extract (Piper crocatum) has an inhibitory concentration 50% (IC50) value of 47.45 ppm and is included in a very strong antioxidant group, almost the same as that of vitamin C at 49.20 ppm, so it can be used as a natural antioxidant substance (Tonahi et al., 2014; Oktavia et al., 2022). Supported by previous research comparing ethanol extracts and water extracts for fish meat marinades, the results of fish meat with the best organoleptic properties on day 19 were in the 50% P. crocatum ethanol extract marinade and 25% P. crocatum ethanol extract (Oktavia et al., 2022). Başaran et al. stated that saponins are components that develop collagen that functions to heal wounds (Başaran et al., 2017). Robinson (1963) stated that saponins also have the ability to act as antiseptic for healing open wounds (Wurlina et al., 2019). Piper crocatum increases collagen deposition and wound closure by up to 98% through increased expression of alpha-smooth muscle actin (αSMA), superoxide dismutase 1 (SOD1), and E-cadherin and decreased expression of protein 53 (p53) (Setyawati et al., 2021). The basic collagen fibers and new blood vessels began infiltrating the wound. Once collagen has formed, there is a rapid increase in the strength of the wound bed. In the remodeling phase, the collagen that has formed will fuse together, compressing the blood vessels during wound healing, so that the scar becomes flat and thin. This study demonstrated that red betel extract accelerates wound healing (Setyawati et al., 2021). This study aimed to determine the effect and mechanism of red betel extract (P. crocatum) on the wounds of Wistar rats post-excision with mammary tumors based on IL-10 levels, TGF-β1 expression, VEGF expression, collagen amount, and clinical features. Material and MethodsPreparation of Piper crocatum extract gelThe red betel leaves (P. crocatum) used in this study came from Purwodadi Regency, East Java, Indonesia. Piper crocatum is dried at room temperature and then blended into a fine powder. Piper crocatum powder was macerated with 96% ethanol and evaporated until a thick extract (Oktavia et al., 2023). The thick extract is then mixed with a gel. The basic material of the gel is a mixture of 2% carboxymethylcellulose natrium (CMC na) and sterile distilled water at a ratio of 1:10. Piper crocatum extract gel is made by mixing the thick extract of red betel leaves with a gel according to the desired percentage, 12.5%, 25%, and 50% (Wurlina et al., 2019; Oktavia et al., 2022). Preparation of experimental animalThe samples of this study were 35 female white rats (Rattus novergicus) of Wistar strain with mammary tumors of minimum size 0.5–1 cm, 8–11 weeks of age, body weight 150–200 grams, virgin (never mated), healthy, marked by fluffy fur, shining eyes, not limping, active movement, non-soggy stools, and no scars. The rats in this study were obtained from a Wistar rat breeder in Surabaya City, who usually supplies rats for trials and research in the Anatomy, Histology, and Pharmacology Laboratory, Faculty of Medicine, Universitas Airlangga. Rats were acclimated for 7 days in the laboratory of the experimental animal, Anatomy, Histology, and Pharmacology, Faculty of Medicine, Universitas Airlangga (Habibi et al., 2020). Rats were placed in cages with a total of 5–6 rats in each cage. The size of the cage around was approximately 50 centimeters × 50 cm. Rats were given food and drink ad libitum, 12 hours of light and 12 hours of dark, and normal room temperature (Habibi et al., 2020). The cage has walls and a wire bottom so that dirt does not accumulate on the floor to prevent infection in rat. The cage was cleaned every 2–3 d. Method section 1.Thirty rats were induced with 3%–5% benzopyrene in powder form dissolved in olive oil with 30 mg/10 ml composition, given as 50 mg/Kg.BW in 5 times intramammary injections every 2 d. The rats were maintained and waited for 10–18 weeks until mammary tumors formed. Tumor tissue removal was performed after mammary tumors were established (Amadou et al., 2021). Five rats with the same inclusion criteria were included in the normal wounds as the control group. Rats were anaesthetized intramuscularly using ketamine hydrochloride and xylazine hydrochloride at a dose of 50 mg/Kg.BW at a 1:1 ratio. Method section 2.Thirty post-excision mammary tumor rats were divided into 5 groups of 6 each; K- was the CMC na gel treatment group, K+ was the povidone iodine 10% treatment group, P1 was the 12.5% P. crocatum extract gel treatment group, P2 was the 25% P. crocatum extract gel treatment group, and P3 was the 50% P. crocatum extract gel treatment group. Five rats as the control group were a normal wound treated with NaCl solution. All rats were treated twice a day with an interval of 11–12 hours for 14 consecutive days. To determine the clinical picture of the wound, on days 1st, 7th, and 14th of the treatment, the rat wounds were photographed and measured with J image to determine the wound area in 3 measurements to determine the healing. On day 15th, the post-excision skin tissue from the mammary tumor and the treated normal wound were removed. Each sample was divided into 2, namely for ELISA examination, cleaned with RL, placed into a tube, and immediately placed into a cooling box, for further ELISA examination. A partial sample of the rat skin was placed between filter paper so as not to roll up and then put into neutral buffer formalin (NBF) 10% for further immunohistochemistry (IHC) and Masson’s trichrome (MT) processing. ELISA and IL-10 analysisRinse the skin tissues of the rats in ice-cold PBS (pH 7.4) to remove excess blood thoroughly and then weigh them before homogenization. Tissues were homogenized in PBS (tissue weight (g): PBS volume (ml)=1:9) with a glass homogenizer on ice. The scouring results of the scouring were sonicated with a long sonicator at a frequency of 400 Hz for 2–3 minutes so that the tissue was homogeneous. The results of sonication were centrifuged to prepare for the examination of IL-10 in skin tissue using the ELISA technique. Furthermore, IL-10 was examined in the skin tissue using ELISA kits (BT Lab) and the results were read using an ELISA reader (Bessera et al., 2020). Immunohistochemistry process and analysis of TGF-β1 and VEGF expressionTissues of rats skin were immersed in 10% NBF solution for 24 hours and then placed in 70%, 80%, 90%, 96%, and 96% absolute alcohol solution for 1–1.5 hours each. The tissue was placed into xylol solution for 3 times, xylol I for 1 hour, xylol II and xylol III for 1.5 hours, impregnated and immersed in liquid paraffin at 60°C four times 1 hour. The next step was to embed the tissue in a paraffin block molded with bismuth. The frozen paraffin block is cut using a microtome with a thickness of 5–10 µm. The cut ribbons are placed in a water bath at 37°C–40°C, wait until the ribbon expands then take 1–2 paraffin ribbons to be placed on a polylysine object glass, labelled, and placed on a hot plate (at 60°C) for 1 hour so that the tissue pieces stick perfectly/do not fall off, and then the immunohistochemical staining process is carried out. After the IHC slide had dried, it was covered with a glass covering. Furthermore, the preparations were examined using a trinocular microscope. TGF-β1 expression is shown as a brown color in rat skin (Rattus norvegicus) using polyclonal TGF-β1 antibody Santa Cruz in immunohistochemical method. The brownish color was then calculated as the percentage using immunoratio by averaging five fields of view of the percentage of TGF-β1 expression area with a magnification of 400× (Sofii et al., 2021). VEGF measurement is done by looking at the number of large blood vessels that have brown endothelium visible on rats’ skin slices under a trinocular microscope and manually counting the number recorded on each sample examined and sealed according to the intervention given. Examination with immunohistochemical techniques to identify cellular or tissue constituents (antigens) through antigen-antibody interactions (Bessera et al., 2020). This study used the VEGF antibody Santa Cruz. Observed using a microscope with a magnification of 400×. IHC staining and microscopic examination were performed out in the Anatomy and Pathology Laboratory and the Integrated Medicine Laboratory of Airlangga. Masson’s trichrome staining to assess collagen densitySkin samples were fixed with alcohol, formalin, and acetic acid and processed in paraffin. The number of blood vessels and deposition of collagen fibers were assessed by MT staining, respectively. Collagen density was examined by observing the percentage of blue fibers on Masson’s trichrome staining. Masson’s trichrome is a three-color stain used in histology to distinguish between collagen and smooth muscle, as well as to observe an increase in collagen. This stain uses three colors that act selectively on muscle, collagen fibers, fibrin, and erythrocytes. The stain produces red keratin and muscle fibers, blue or green collagen, bone, red or pink cytoplasm, and dark brown to black cell nuclei. Collagen density was categorized by the percentage of collagen area pixels compared to other tissue area pixels; 0=no collagen fibers found, 1=low collagen fiber density (25%), 2=medium collagen fiber density (50%), 3=dense collagen fiber density (75%), and 4=very dense fiber density (100%) (Rachmanita et al., 2019; Beserra et al., 2020). Collagen density was observed using a microscope with a magnification of 400×. MT staining and microscopic examination were performed at the Anatomy and Pathology Laboratory of Universitas Airlangga. Statistical analysisOne-way ANOVA to compare the mean values of more than two groups if the data distribution is normal. If the data distribution was not normal, the nonparametric Kruskal−Wallis test was used. Post hoc analysis (LSD) to determine which groups were significantly different from the ANOVA test results. The post hoc analysis for Kruskal–Wallis is the Man−Whitney test. Multiple path analysis is used to determine the mechanisms between variables. The significance level of p < 0.05 indicates that any observed difference between the groups was considered statistically significant (SPSS, version 25) (Ghozali, 2018). Ethical approvalEthical clearance was obtained from the Institutional Ethical Committee, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia (No: 2.KEH.094.06.2023). ResultsThe lowest IL-10 levels were observed in the 50% P. crocatum extract gel group=247,515. The lowest TGF-B1 expression observed in the 25% P. crocatum extract gel group=4,033. The highest VEGF expression was observed in the 25% P. crocatum extract gel group=11.45. The highest collagen density was found in the 50% Piper crocatum extract gel group=0.833, and the smallest percentage of wound area approaching normal wounds was in the 50% P. crocatum extract gel group, which was 11,338 (Fig. 1). TGF-β1 expression is shown as brown color in rat skin (Rattus norvegicus), seen in the 50% P. crocatum extract gel group (P3), with the lowest value=4 (Fig. 2). Based on IHC examination using Santa Cruz antibodies, the VEGF expression was observed in large blood vessels with brown endothelium visible in the skin of rats in the 25% P. crocatum extract gel group (P2), with the highest number 13 (Fig. 3). The group with the highest collagen density was the 50% P. crocatum extract gel (P3)=0.833 (Fig. 4). The group with wound healing approaching normal wounds was the 50% P. crocatum extract gel group (P3) even though almost all of them were diagnosed with malignant tumors (Figs. 5 and 6). Path analysis of the direct and indirect effects of P. crocatum extract (Fig. 7) and mechanism of effect of P. crocatum extract on IL-10 levels, TGF-β1 expression, VEGF expression, and collagen density in post-excision wounds of mammary tumors of female Wistar rats (Fig. 8).
Fig. 1. Graph of the mean of IL-10 levels, TGF-β1 expression, VEGF expression, collagen density, and percentage of wound area in each group.
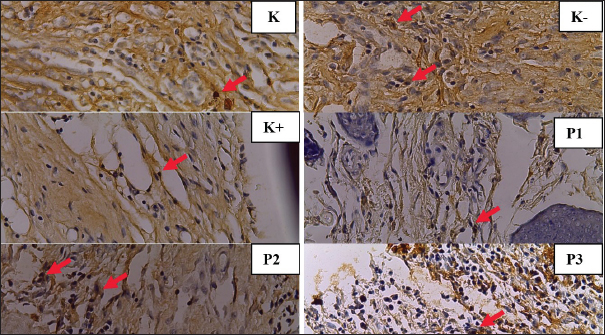
Fig. 2. Histopathological features of the skin of Wistar rats post-excision of mammary tumors after treatment for 14 days in each group. TGF-β1 expression shown by macrophage cells (⇧) in Figure K (7.8), K- (7.4), K+ (5.7), P1 (1.7), P2 (4.7), and P3 (4) (HE staining, magnification of 400).
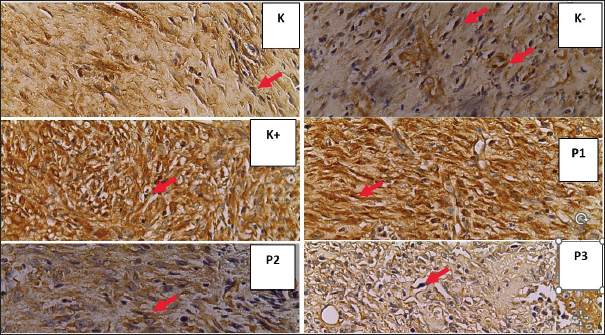
Fig. 3. Histopathological features of Wistar rat skin post-excision of mammary tumor after treatment for 14 days in each group. VEGF expression shown in endothelial cells (⇧) in figure K (5.3), K- (6.1), K+ (8.5), P1 (7.5), P2 (13), and P3 (5.9) (HE staining, magnification of 400). DiscussionEffect of P. crocatum extract on interleukin-10 (IL-10) levels on post-excision wound areas of female Wistar rat mammary tumorsThe results of the ELISA examination on IL-10 levels statistically showed p=0.051 (> 0.05), indicating that there was no statistical difference in the treatment groups. IL-10 acts as an anti-inflammatory agent so that it will increase in the inflammatory phase. At the end of the proliferation phase or the beginning of the remodeling phase, IL-10 plays a role in reducing collagen density so as not to form scarsdale and scars, another role as an immunostimulant in certain conditions, one of which is cancer conditions. In wounds with tumors or mammary cancer, the role of IL-10, in addition to reducing collagen density in wound healing, also acts as an immunostimulant, which stimulates cytokines that maintain the body’s immune system to fight tumors or cancer cells. The goal is for the wound to heal and no severe conditions occur in the tumor or cancer (Zhao et al., 2021). If IL-10 levels are measured during the inflammatory phase, IL-10 levels will increase because of its anti-inflammatory role in preventing wound inflammation. In this study, IL-10 levels were lower in all betel leaf extract gel groups than in the CMC na gel group. The higher the percentage of red betel leaf extract, the lower the IL-10 levels. The P. crocatum 50% extract gel group had the lowest average IL-10 level of 247,515. This decrease may be due to measurements taken at the end of the proliferation phase on day 15 after tumor excision or wound formation. In this phase, IL-10 functions both as a cytokine that promotes wound healing and as an immunostimulant.
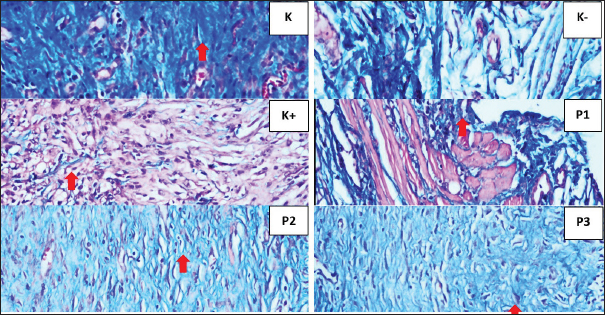
Fig. 4. Histopathological features of Wistar rat skin post-excision of mammary tumors after treatment for 14 days in each group, as indicated by collagen (⇧) K (scoring 3) K- (scoring 0), K+ (scoring 1), P1 (scoring 2), P2 (scoring 1), and P3 (scoring 3) (HE staining, magnification of 400×).
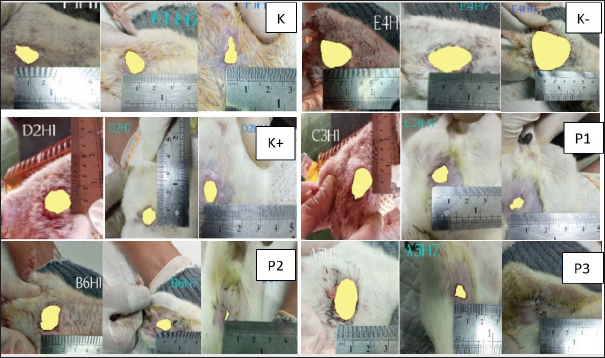
Fig. 5. Image J measurements on days 1st, 7th, and 14th in each treatment group.
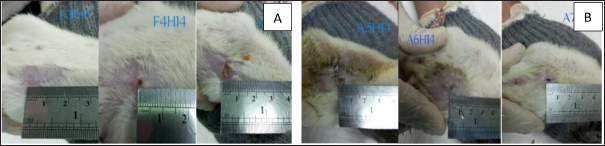
Fig. 6. Appearance of wounds in the control group (A) and the Piper50% extract gel group (B) on the 14th day of treatment.
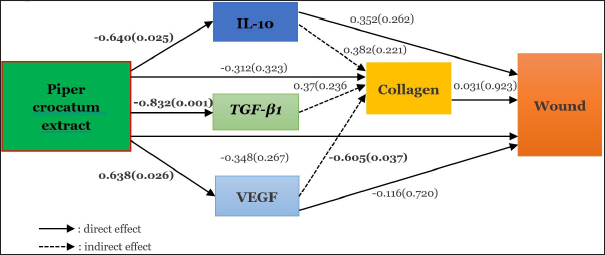
Fig. 7. Path analysis of direct and indirect effects of Piper crocatum extract on IL-10 levels, VEGF expression, TGF-β1 expression, collagen density, and clinical features of post-excision mammary tumor wounds in female Wistar rats.
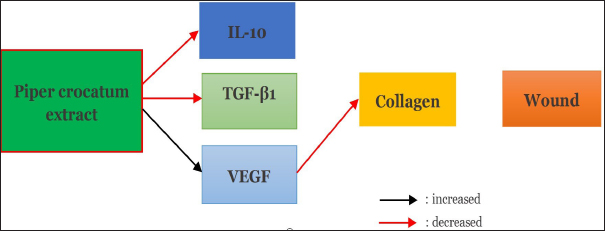
Fig. 8. Mechanism of effect of Piper crocatum extract on IL-10 levels, TGF-β1 expression, VEGF expression, and collagen density in post-excision wound of mammary tumor of female Wistar rats. IL-10 is a pleiotropic cytokine that plays a fundamental role in modulating inflammation and maintaining cell homeostasis. It primarily acts as an anti-inflammatory cytokine and protects the body from uncontrolled immune responses. On the other hand, IL-10 can also exert immunostimulatory effects under certain conditions. Given the important role of IL-10 in immune modulation, this cytokine could have relevant implications in pathologies characterized by hyperinflammatory states, such as cancer and infectious diseases as in the case of Coronavirus-19 (COVID-19) and post-COVID-19 syndrome. Recent evidence suggests that IL-10 is a predictor of disease severity and mortality in patients with acute or postacute severe acute respiratory syndrome coronavirus-2 infection. In this context, IL-10 may act as an endogenous danger signal released by damaged tissues in an attempt to protect the organism from harmful hyperinflammation. Pharmacological strategies aimed at potentiating or restoring the immunomodulatory actions of IL-10 may be a promising new approach to counter the cytokine storm arising from hyperinflammation and effectively reduce severe complications (Carlini et al., 2023).
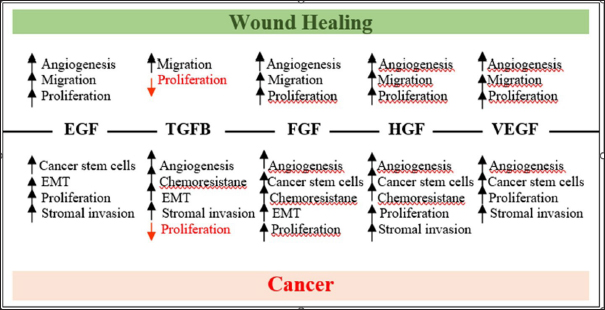
Fig. 9. Schematic representing the common growth factor signaling pathways that regulate key cellular processes required for wound healing and cancer (Sundaram et al., 2018). In this study, the average IL-10 levels analyzed by the ELISA method obtained the lowest number in the P. crocatum 50% extract gel treatment group, namely 247,515, in the P. crocatum 25% extract group as much as 272,733, and the P. crocatum 12.5% extract group is 295,605, while in the normal group, namely normal wounds without mammary tumors, the average IL-10 reached 306,487. The decrease in average IL-10 levels at greater concentrations of the P. crocatum extract gel indicates an increased role of IL-10 in the gel. The role of IL-10 as an anti-inflammatory and immonostimulant allows it to protect by preventing hyperinflammation in tumor conditions found in the post-excision skin of rat mammary tumors. Cytokines are small soluble proteins that instruct and mediate the communication between immune cells and nonimmune cells. A portfolio of cytokines is essential for macrophages to guard the innate immune system and mediate the transition from innate to adaptive immunity. Along with other mediators, cytokines bias the fate of macrophages into a spectrum of “classically activated” inflammation-inducing macrophages, to anti-inflammatory or “alternatively activated” macrophages. Deregulated cytokine secretion has been implicated in several disease conditions, ranging from chronic inflammation to allergy (Liu et al., 2021). Circulating IL-10 levels were found to be elevated in the serum of various cancer patients, often accompanied by an increase in other inflammatory markers, and correlated with poor prognosis. On the other hand, IL-10 mediates tumor inhibition with the important activities of recruiting and stimulating cytotoxic CD8+ T cells and NK cells in the tumor microenvironment by promoting lymphocyte and antibody-dependent immune memory, canceling the inflammatory macrophage-Th17 macrophage M1 T cell axis, decreasing the synthesis of pro-angiogenic factors, and suppressing the local pro-inflammatory release of cytokines that support tumor growth, survival, and invasion (Morris et al., 2022). Cytokines have a fundamental influence on the immune system and health, and dysregulation in their secretion or signaling. Cytokines are involved in the origin and/or progression of several human pathological conditions, including cancer, infectious, and immune diseases, and usually represent important biomarkers and targets (Kany et al., 2019). The role of IL-10 with its up- and down-regulation. Effect of P. crocatum extract on TGF-β1 expression on post-excision wound area of female Wistar rat mammary tumorsThe results of the IHC examination on TGF-β1 expression in all treatment groups were carried out using the one-way ANOVA test. The results showed that p =0.00 (< 0.05) there were significant differences in TGF-β1 expression in several treatment groups. The results showed that the control group, which had a normal wound and the CMC na group, which was a negative control, showed differences in TGF-β1 expression in all the P. crocatum extract gel treatment groups. This indicates that P. crocatum extract at all percentages has an effect on TGF-β1 expression in the post-excision wounds of female Wistar rat mammary tumors. The CMC na gel group (negative control) and the P. crocatum 12.5% extract gel group showed the strongest difference (p =0.00). TGF-β1 is a key regulator of tissue repair, inflammation, and fibrosis. TGF-β1 is a homeostatic factor that maintains the balance of the immune system and regulates the complex process of tissue repair after injury or infection in all organs. The failure of TGF-β1 to regulate the body’s repair mechanisms is one of the commonalities of pathological conditions such as cancer and fibrosis, which both involve dysregulated immunity, inflammation, exacerbated stromal cell activation, and extracellular matrix (ECM) overproduction. TGF-β can be produced by all cells, but the three most important cells are monocytes, fibroblasts, and platelets, and it plays an important role in the wound healing process. TGF-β1 facilitates the resolution of the pro-inflammatory response. In the remodeling phase, wound contraction and collagen remodeling occur. Wound contraction occurs due to the activity of fibroblasts that differentiate under the influence of the cytokine TGF-β1 into myofibroblasts, which contain intracellular actin microfilament components. Myofibroblasts will express α-smooth muscle action, which will make the wound contract. The intracellular matrix will undergo maturation and hyaluronic acid, and fibronectin will be degraded. ECM-producing myofibroblasts facilitate repair and promote scar tissue formation or fibrosis. Increased synthesis of TGF-β1 is important for tissue repair (Setyawati et al., 2021). According to Stuelten et al., from a study conducted on rats, it was concluded that the wound microenvironment is conducive to cancer development (Stuelte et al., 2008; Lopez et al., 2022). A large number of growth factors influence wound healing and metastasis, including epithelial growth factor, fibroblast growth factor, TGF-β, and hepatocyte growth factor. An explanation can be provided in Figure 9 (Sundaram et al., 2018). The effects of each growth factor on various physiological processes during wound healing are described later. The bottom panel describes the role of growth factors in cancer growth and metastasis. Note that TGF-β decreases epithelial cell proliferation during wound healing and cancer and promotes keratinocyte migration during wound healing and tumor-initiating processes in cancer. In this study, the administration of 50% red betel leaf extract gel had the effect of reducing TGF-β1 expression at the end of the proliferation phase or remodeling phase. This condition can be caused because TGF-β1, which plays a role in encouraging the process of scar tissue formation or fibrosis, limits the formation of collagen so as not to form excessive scar tissue so that a scar is formed. Piper crocatum 50% extract gel containing flavonoids, alkaloids, saponins, and tannins as natural antioxidants can reduce TGF-β1 expression to inhibit the tumor-inducing processes in post-excision mammary tumor skin cancer mice. Antioxidants work double on TGF-β1 expression in addition to helping fibroblasts synthesize collagen, and they can also inhibit the growth of cancer cells or malignant tumors in mouse skin. In the 50% red betel leaf extract gel treatment group, there were 2 benign tumors and 4 malignant tumors in the skin of Wistar rats post-excision with mammary tumors. All P. crocatum extract gel treatment groups with hematoxylin–eosin results after treatment in the form of benign tumors and malignant tumors had low TGF-β1 expression compared with the CMC na gel group as a negative control. This result demonstrates that red betel leaf extract reduces TGF-β1 expression in post-excision wounds of female Wistar rat mammary tumors. TGF-β1 is the most potent growth factor known to induce myofibrogenesis (Lodyga and Hinz, 2020). Active TGF-β1 levels measured in the skin are a good indicator of skin fibrosis, and overexpression of constitutive TGF-β1 receptors causes spontaneous skin fibrosis in transgenic animals. Active TGF-β1 assembles and acts through the TGF-β1 receptor complex to regulate myofibroblast activation and actions via Smad 2/3 “canonical” and/or JNK “non-canonical” signaling (Yu and Feng, 2019; Lodyga and Hinz, 2020). Numerous cell culture studies and animal models of wound healing and fibrosis have shown that inhibition of TGF-β1 signaling at all levels of the pathway effectively prevents myofibroblast formation and fibrosis progression. Deviations in the TGFβ signaling machinery can also participate in tumourigenesis, as cancer cells will use all the help they can get. TGFβ is a powerful tumor suppressor in the context of premalignant cells but an enhancer of invasion and metastasis in the context of more advanced carcinoma cells (Sundaram et al., 2018). This dichotomy may seem paradoxical, but there is logic to it. TGFβ has cytostatic effects that are important for tissue regeneration and homeostasis. When cells undergo oncogenic mutations and reach a premalignant state, the suppressive effects mediated by TGFβ become more dramatic, as in this context TGFβ triggers apoptosis (Batlle and Massague, 2019). Effect of P. crocatum extract on VEGF expression in the post-excision wound area of female Wistar rat mammary tumorsThe results of the Kruskal−Wallis test showed that p=0.016 (< 0.05), there were significant differences in VEGF expression in several treatment groups. The LSD post hoc test was performed to determine which groups exhibited differences. The statistical results of the difference in VEGF expression between the groups with the lowest p value or the strongest difference is the CMC na gel group and the P. crocatum 50% extract gel group, which is 0.019 (p < 0.05). This result demonstrated that the P. crocatum 50% extract has an effect on VEGF expression in post-excision wounds of female Wistar rat mammary tumors. VEGF stimulates wound healing through several mechanisms, including collagen deposition, angiogenesis, and epithelialization. Angiogenesis wound healing involves several steps, including vasodilatation, basement membrane degradation, and migration and proliferation of endothelial cells. Subsequently, capillary vessel formation occurs, followed by anastomosis of new parallel capillaries (loop formation), and finally formation of a new basement membrane. VEGF is involved in some of these processes (White Michael et al., 2021). One way to accelerate the onset of angiogenesis is to reduce the amount of free radicals present in the tissue after trauma. Factors that can reduce the number of free radicals include various types of antioxidants. Research by Tonahi et al. (2014) states that P. crocatum extract has a 50% inhibitory concentration value (IC50) of 47.45 ppm and is included in the class of very strong antioxidants, almost the same as that of vitamin C of 49.20 ppm so that it can be used as a natural antioxidant substance (Tonahi et al., 2014; Oktavia et al., 2022). Supported by previous research comparing ethanol extracts and water extracts for fish meat marinades, the results of fish meat with the best organoleptic properties on day 19 were in the P. crocatum 50% ethanol extract marinade and then P. crocatum 25% ethanol extract (Oktavia et al., 2022). Based on the results of previous studies, this study used P. crocatum extract at concentrations of 25% and 50%. This study also proved that 50% extract gel from P. crocatum is the best for wound healing post-excision of female Wistar rat mammary tumors. In all treatment groups, the P. crocatum extract gel had higher VEGF expression values in benign tumors and malignant tumors than the VEGF values in benign tumors of the CMC na gel group. Effect of P. crocatum extract on collagen density in post-excision wound areas of female Wistar rat mammary tumorsStatistical results using SPSS version 25 showed no difference in the amount of collagen in all treatment groups (p value=0.164, p > 0.05). This condition may be due to the condition of the post-excision wound of mammary tumors of mice experiencing malignancy in the red betel extract treatment group, whereas in the negative control group or CMC na gel treatment, there was no malignant tumor. This condition causes collagen in the CMC na group to develop more rapidly because there is no malignant tumor disorder, even though there are no bioactives that increase collagen density. Although the P. crocatum extract gel treatment groups had almost all benign or malignant tumors, even the P. crocatum 50% extract gel group had the most malignant tumors and had no normal or inflammatory skin conditions. The results showed no high collagen density in the post-excision skin of mammary tumors diagnosed as normal, which was almost the same in the skin with malignant tumors. Skin with benign tumors also had low collagen density with only one sample in the P. crocatum 50% extract gel treatment having a collagen density of 3, whereas all samples with a diagnosis of inflammation had a higher collagen density. The collagen value was 0 in all samples, indicating that the wound healing process has occurred or that the wound has healed. The results of the phytochemical test of red betel leaves in this study showed that saponins were the most bioactive, species at 11.53%. Robinson (1963) stated that saponins also have the ability to act as antiseptics for healing open wounds (Wurlina et al., 2019). Başaran, et al. (2017) stated that saponins are a component of collagen that functions to heal wounds (Başaran et al., 2017). Robinson (1963) stated that saponins also have the ability to act as antiseptics for healing open wounds (Wurlina et al., 2019). In this study, besides saponins, flavonoids, alkaloids, and tannins are also antioxidants. Piper crocatum increases collagen deposition and wound closure by up to 98% through increased expression of αSMA, SOD1, and E-cadherin and decreased expression of p53 (Setyawati et al., 2021). Research by Latuheru et al. showed that the basic substance, collagen fibers, and new blood vessels began to infiltrate the wound. Once collagen has formed, there is a rapid increase in the strength of the wound bed. In the remodeling phase, the collagen that has been formed will fuse together, suppressing blood vessels in wound healing, resulting in a flat and thin. This study demonstrated that P. crocatum extract accelerates wound healing (Setyawati et al., 2021). Collagen density assessment was conducted on day 15 or after 14 days of treatment in all groups. It is likely that the 15th day of wound healing is at the end of the proliferation phase or the initial stage of remodeling, so that collagen density has begun to decrease or decrease because if collagen fibers are too dense, scarring will occur. The wounds in this study mostly closed well, with little or no scarring. The post-excision wounds of the mammary tumors treated with 50% red betel leaf extract gel twice a day for 14 days were almost completely closed on day 14, even though the images were in the form of benign and malignant tumors. Piper crocatum can heal wounds well even in tumor conditions. This proves that well-formed collagen fibers are neither deficient nor excessive, so the wound closes with little or no scarring. Effect of Piper crocatum extract on the improvement of clinical features of post-excision wound areas of female Wistar rat mammary tumorsThe ImageJ measurements were performed on days 1, 7, and 14 of treatment. On day 14 or measurement 3, the average percentage of wound area in the group of Wistar rats with post-excision mammary tumor treated with P. crocatum 50% extract gel had the lowest value of 11.338%. This value is closest to the percentage of normal wound area, which was 9.922%. The percentage of wound area in the P. crocatum 50% extract gel group showed a rather far figure with the next lowest group, the P. crocatum 25% extract gel, with a percentage of wound area of 18.682%. The content of red betel leaf extract in this study includes phenolics, steroids, saponins, alkaloids, flavonoids, and tannins. Phenolics, steroids, alkaloids, and tannins act as proinflammatory agents in the inflammatory phase. Inflammation plays an important role in wound healing. Meanwhile, alkaloids, flavonoids, saponins, and tannins act as antioxidants in the proliferation phase, which helps accelerate the process of fibroblasts becoming collagen so that ECM is formed, and wound contraction occurs, which indicates a healing process. Figure 9 shows that the clinical improvement of the post-excision wound of the mammary tumor was good at a percentage of P. crocatum 50% extract, even though the wound was in the form of a tumor. The clinical features of the wound on day 14 between the control group or normal wound and the P. crocatum 50% extract gel group were almost similar, although the HE results on the rat skin in the group given P. crocatum 50% extract gel were all tumors, namely 2 samples of benign tumors and 4 samples of malignant tumors, while all control samples were normal wounds in the form of inflammation. Wound healing in tumors was similar to normal wounds, although there was a decrease in IL-10 levels and TGF-β1 expression, an increase in VEGF expression, and a decrease in collagen density. However, the clinical picture of the wound showed good closure compared with the other groups. Antioxidant substances contained in betel leaves are able to suppress and fight cancerous substances by increasing angiogenesis for fibroblast formation and turning them into myofibroblasts that form collagen. Collagen together with proteoglycans and elastin produce the ECM that causes wound contraction to initiate the healing process. Mechanisms of the influence of P. crocatum extract on IL-10 levels, TGF-β1 expression, VEGF expression, collagen density, and clinical features in Wistar rat wounds post-excision of mammary tumorsThe results of the abovementioned calculations show that there are many other variables and factors that affect post-excision wound healing of mammary tumors in female Wistar rats besides IL-10 levels, TGF-β1 expression, VEGF expression, and collagen density. One of the variables that can be identified from this study is the mammary tumor cells that still exist or develop. This was evident in the HE results of the post-excision skin tissue of rats with mammary tumors treated for 14 days with wound conditions in each treatment group. There were well-healed wounds characterized by wound closure. Various supporting evidence suggests that wound healing and tumor genesis are two sides of the same coin. All major pathways involved in wound healing are also active in cancer (Savioli et al., 2020). Inflammation is instrumental in both cancer and wound healing, and it may be that inflammatory cells, in part, have these pivotal roles by being key sensors of altered microenviromental conditions, for example, hypoxia, and as mediators of changes to metabolic signaling in other cells (MacCourthy-Morrogh and Martin, 2020). Remodeling of the extracellular matrix during the later stages of wound healing is also similar with tumor remodeling; cancerous tissue can alter the surrounding matrix to support its own growth and invasion (Sundaram et al., 2018). Many hallmarks and supporting characteristics of cancer are reflected in wound healing. For some processes, similarities are obvious, whereas others are not. There are clues that there may be similarities. MacCourthy-Morrogh and Martin proposed three new prospective supporting characteristics or traits that contribute to or are a consequence of inflammation and may apply to both cancer and wound healing, namely matrix deposition changes, fat cells, and microbiome changes (dysbiosis) (MacCourthy-Morrogh and Martin, 2020). The hallmark contributions and characteristics that enable wound healing are then mapped onto the skin wound healing scheme. Cell migration and proliferation drive re-epithelialization, which may also depend on changes in cellular energy. The injured epithelium must also resist cell death and avoid damage caused by inflammatory cells infiltrating the wound. Damage signals from the microbiome and fat cells contribute to the inflammatory response, which in turn regulates wound angiogenesis and matrix deposition (MacCourthy-Morrogh and Martin, 2020). Besides having many cellular and molecular mechanisms in common, tissue damage and cancer are often juxtaposed in the clinic, as tissue biopsies are a key ingredient in the screening and assessment of various cancers, and surgery is still one of the most effective ways to cure patients with cancer. In the past few decades, a number of studies have addressed how cancer biopsy and surgery, which essentially damage tissue and, thus, trigger a wound inflammatory response, may affect remaining cancer cells (MacCourthy-Morrogh and Martin, 2020). Several clinical studies have described tissue-damaging cancer treatments may locally or systemically influence the growth or progression of cancer into malignant tumors. These local influences have been reflected in basic science studies that began with the observation that chicks injected with Rous sarcoma virus tended to develop tumors only at the site of injection; similarly, a wound was required to trigger tumorigenesis in v-jun transgenic rats (MacCourthy-Morrogh and Martin, 2020). Other studies using various animal models have shown that wound-induced cancer initiation is mediated by inflammation (Krall et al., 2018; MacCourthy-Morrogh and Martin, 2020; López-Cortés et al., 2021). There is clear clinical evidence that localized tissue damage in patients with melanoma can exacerbate cancer progression and worsen patient prognosis (Zhao et al., 2021). Conversely for some other cancers, including basal cell carcinoma, tissue damage and localized activation of the inflammatory response have sometimes been reported to cause cancer regression and are even sometimes used as a therapeutic strategy, especially in elderly patients when surgery is not possible (MacCourthy-Morrogh and Martin, 2020). Local wounds can sometimes inhibit cancer development. This is supported by a study in mice showing that for some human cancers grafted into mice, the presence of ulcers or ischemic wounds in the environment can inhibit tumor growth (MacCourthy-Morrogh and Martin, 2020). In addition to local effects, tissue damage can activate the cancer growth system. For example, in breast cancer, reconstructive plastic surgery is believed to sometimes trigger the “revival” of inactive lung micrometastases due to inflammation. This “inactive” and T-cell-restrained cancer activation has been replicated in rats and shown to be mediated by the systemic mobilization of innate immune inflammatory cells, and it can be overcome by temporary treatment with anti-inflammatory drugs (Krall et al., 2018). In this study, although there were several skin samples in the form of benign and malignant tumors, skin healing on day 14 was good, especially in the P. crocatum 50% extract gel group, which had the best percentage. The general objective of this study was to determine the concentration of P. crocatum extract gel that can accelerate wound healing after mammary tumor excision in female Wistar rats and not cure mammary tumors experienced by female Wistar rats. In Figure 4.11 from the pathway analysis, it can be seen that only VEGF expression has an effect on the amount of collagen, whereas IL-10 levels, TGF-β1 expression do not affect the amount of collagen and all three also do not affect wound healing through collagen processing. A stronger substance or component that can work on wound healing and also plays a role in killing cancer cells is necessary. At this time, the group of scientists still lacks and has not achieved the context of the specific set of genes and proteins that enable the wound healing mechanism and involve cancer (López-Cortés et al., 2021). Research is ongoing to ensure that the overlap between wound healing and cancer cell development is not in line. Lopez-Cortez et al. identified 21 proteins involved in wound healing and solid tumor formation. The results of their study showed that wound healing and cancer-associated proteins are involved in modifying the microenvironment of solid tumor tissue and regulating the immune system. The proteins involved in wound healing formed an interaction network of 233 wound healing proteins with at least one interaction with a high confidence level (cutoff=0.9). The darkest nodes represent proteins with the highest degree of centrality (mean=11.2). The wound healing protein–protein interaction network (WH-PPi) included 35 cancer-driving proteins with an average degree centrality of 11.3. The Mann−Whitney test showed a correlation of degree centrality between wound-healing nodes and cancer-driving nodes (p > 0.05). The network of 233 WH-PPi proteins was then visualized using Cytoscape v.3.7.134 software (López-Cortés et al., 2021). MacCarthy-Morrogh and Martin highlighted how tissue repair and cancer share cellular effects and molecular processes of cancer that are regulated in wounds but misregulated in cancer. They proposed eight prospective features that may apply to cancer and wound healing, namely (1) avoiding immune damage, (2) increasing wound healing inflammation, (3) allowing invasion and migration, (4) inducing angiogenesis, (5) resisting cell death, (6) maintaining proliferative signals, (7) avoiding growth suppression, and (8) deregulating cellular energy (MacCourthy-Morrogh and Martin, 2020; López-Cortés et al., 2021). From the research network Lopez-Cortez et al. showed TGF-β and VEGF have green color, indicating that these proteins play a significant role in wound healing. Key proteins involved in cancer and wound healing hallmarks can be seen. Each set of proteins was ranked based on the shortest path from the wound healing protein to the cancer phenotype hallmark. Positive regulatory pathway scores are considered to avoid growth suppression, maintain proliferative signaling, deregulate cellular energy, induce angiogenesis, activate invasion and migration, and cause wound inflammation; negative regulatory pathway scores are considered to resist cell death and avoiding immune damage does not show pathway score information. Twenty-one proteins were identified and eight which were highly associated with wound healing and cancer hallmarks. These proteins may represent critical nodes in the network of controlled wound healing processes or dysregulated interactions leading to tumor progression. Interestingly, the identified proteins are expressed only in solid tumors because cancer cells must use them to modify the tissue microenvironment and induce cellular regulatory immunity. These proteins may be specifically required to reorganize injured tissues and promote regeneration. However, in cancer, these proteins induce immune escape and survival. Placing evidence of shared proteins between wound healing and cancer leads to proteins primarily involved in sustained proliferation, invasion, angiogenesis, and other interconnected and overlapping pathways between health and disease (López-Cortés et al., 2021). Further interdisciplinary studies in vivo or patients will support the in silico role of the identified and overlapping proteins. Interdisciplinarity is important because it brings together specialists in bioinformatics, healthcare professionals, and technical experts, leading to the development of better screening methods for early diagnosis and therapy (Barba et al., 2021). These studies will allow the targeting of therapies and the development of specific pharmacological compounds that will stop the development of malignant tumors/cancers without affecting the healing process (López-Cortés et al., 2021). ConclusionBased on the results of the study and after processing the data using SPSS version 25, there are several conclusions as follows: P. crocatum extract decreased IL-10 levels in Wistar rat wounds post-excision of mammary tumors, P. crocatum extract decreased TGF-β1 expression in Wistar rat wounds post-excision of mammary tumors, P. crocatum extract increased VEGF expression of Wistar rat wound post-excision mammary tumors, P. crocatum extract decreased collagen density of Wistar rat wound post-excision mammary tumors, and P. crocatum extract accelerates the improvement of the clinical feature of Wistar rat wound post-excision mammary tumors. The mechanism of the effect of P. crocatum extract on the wounds of Wistar rats post-excision of mammary tumors through decreased IL-10 levels and TGF-β1 expression, increased VEGF expression, and decreased collagen density through increased VEGF expression is studied in this article. List of AbbreviationsCMC Na, Carboxymethylcellulose natrium; COVID-19, Coronavirus disease-19; ECM, Extracellular matrix; EGF, Epithelial growth factor; FGF, Fibroblast growth factor; HGF, Hepatocyte growth factor; HE, Hematoxylin–eosin; IC50, Inhibitory concentration 50%; IL, Interleukin; MT, Masson’s trichrome; NO, Nitric oxide; ROS, Reactive oxygen species; SARSCoV-2, Severe acute respiratory syndrome coronavirus-2; SOD1, Superoxide dismutase1; TGF-β1, Transforming growth factor-beta1; TNF-α, Tumor necrosing factor-alfa; VEGF, Vascular endothelial growth factor; αSMA, Alpha smooth muscle action. AcknowledgmentsNone. Conflict of interestAll authors declare that they have no conflicts of interest. FundingThis research was supported by the Ministry of Health of Indonesia. Author contributionsNSO contributed to conception, design, acquisition, analysis, and interpretation, drafted the manuscript, and approved the final version. AM and ANH contributed to the study design, analysis, and interpretation and critically revised the manuscript. All authors have read and approved the final manuscript. Data availabilityAll data were provided in the manuscript. ReferencesAlqathama A. 2024. Natural product as promising modulators of breast cancer immunotherapy. Front. Immunol. Available via https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1410300/full Amadou A, Praud D, Coudon T, Deygas F, Grassot L, Faure E, Couvidat, F., Caudeville, J., Bessagnet, B., Salizzoni, P., Gulliver, J., Leffondré, K., Severi, G., Mancini, F.R. and Fervers, B. 2021. Risk of breast cancer associated with long-term exposure to benzo[a]pyrene (BaP) air pollution: evidence from the French E3N cohort study. Environ. Int. 149, 106399. Barba, D., León-Sosa, A., Lugo, P., Suquillo, D., Torres, F., Surre, F., Trojman, L. and Caicedo, A. 2021. Breast cancer, screening and diagnostic tools: all you need to know. Crit. Rev Oncol. Hematol. 157, 103174. Başaran N, Evliyaoğlu O, Sucu V, Bulut L, Dikker O, Tezcan F, and Vardar M. 2017. Changing of Uric Acid Levels by Age and Sex in Patients with Diabetes Mellitus. Journal of Clinical and Experimental Investigations. 21;7(1). Batlle, E. and Massague, J. 2019. Transforming growth factor-B signaling in immunity and cancer. Immunity. 50, 924–940. Available via https://doi.org/10.1016/j.immuni.2019.03.024 Bessera, F.P., Sergio Gushiken, L.F., Vieira, A.J., Bergamo, D.A., Bergamo, P.L., Oliviera de Souza, M., Alberto Hussni, C., Kiomi Takahira, R., Henrique Nóbrega, R., Monteiro Martinez, E.R., John Jackson ,C., Lemos de Azevedo Maia, G., Leite Rozza, A. and Helena Pellizzon, C. 2020. From inflammation to cutaneous repair: topical application of lupeol improves skin wound healing in rats by modulating the cytokine levels, NF-κB, Ki-67, growth factor expression, and distribution of collagen fibers. Int. J. Mol. Sci. 21, 4952. Available via https://pubmed.ncbi.nlm.nih.gov/32668794/ Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Carlini, V., Noonan, D.M., Abdalalem, E., Goletti, D., Sansone, C., Calabrone, L. and Albini, A. 2023. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. Front. Media S.A. 14, 1161067. CDC, Ncezid, DHQP. 2022. Surgical Site Infection Event (SSI). USA: USA gov. Available via https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/ImportingProcedureData.pdf Ginting, C.N., Lister, I.N.E., Girsang, E., Widowati, W., Yusepany, D.T., Azizah, A.M. and Kusuma, H.S.W. 2020. Hepatotoxicity prevention in Acetaminophen-induced HepG2 cells by red betel (Piper crocatum Ruiz and Pav) extract from Indonesia via antioxidant, anti-inflammatory, and anti-necrotic. Heliyon 6, e05620. Gong, Y., Li, H.X., Guo, A.H., Widowati, W., Kim, Y.H., Yang, S.Y. and Kim, Y.R. 2021. Anti-allergic inflammatory components from the leaves of Piper crocatum Ruiz & Pav. Biol. Pharm. Bull. 44, 245–250. Available via https://www.jstage.jst.go.jp/article/bpb/44/2/44_b20-00726/_pdf/-char/en Ghozali, I. 2018. Aplikasi Analisis multivariate dengan Program IBM SPSS 25. 9th ed. Semarang, Indonesia: Badan Penerbit Universitas Diponegoro. Habibi, E., Shokrzadeh, M., Ahmadi, A., Chabra, A., Naghshvar, F., Haghi-Aminjan, H. and Salehi F. 2020. Pulmonoprotective action of Zataria multiflora ethanolic extract on cyclophosphamide-induced oxidative lung toxicity in mice. Chin. J. Integr. Med. 26(10):754–761. Available via https://doi:10.1007/s11655-018-2984-4 Kany, S., Vollrath, J.T. and Relja, B. 2019. Cytokines in inflammatory disease. Int. J. Mol. Sci. 20, 6008. Krall, J.A., Reinhardt, F., Mercury, O.A., Pattabiraman, D.R., Brooks, M.W., Dougan, M., Lambert, A.W., Bierie, B., Ploegh, H.L., Dougan, S.K. and Weinberg, R.A. 2018. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 10(436), eaan3464. Latuheru JO, Tambajong W J, and Posangi J. 2013. Efek Daun Sirih (Piper Betle L) terhadap Penyembuhan Luka Insisi Kelinci (Orictolagus cuniculus). eBM. 1(2), 802–5. Lely, N., Dachriyanus, Aldi, Y., Almahdy. and Wahyuni, F.S. 2023. Anti-inflammatory effect of isolate compounds from ethyl acetate fraction of Piper crocatum Ruiz and Pav leaves on lipopolysaccharide-induced RAW 264.7 Cells. Hayati J Biosci. 30(1), 28–34. Available via https://journal.ipb.ac.id Li, H.X., Yang, S.Y., Kim, Y.H. and Li, W. 2019. Isolation of two new compounds and other constituents from leaves of Piper crocatum and study of their soluble epoxide hydrolase activities. Molecules. 24(3), 489. Lister, I.N.E., Ginting, C.N., Girsang, E., Nataya, E.D., Azizah, A.M. and Widowati, W. 2020. Hepatoprotective properties of red betel (Piper crocatum Ruiz and Pav) leaves extract towards H2O2-induced HepG2 cells via anti-inflammatory, antinecrotic, antioxidant potency. Saudi Pharm. J. 28(10), 1182–1189. Liu, C., Chu, D., Kalantar-Zadeh, K., George, J., Young, H.A. and liu G. 2021. Cytokines: from clinical significance to quantification. Adv. Sci. 8, 20044332021; doi: https://doi.org/10.1002/advs.202004433 Lodyga, M. and Hinz, B. 2020. TGF-β1-A truly transforming growth factor in fibrosis and immunity. Seminars in Cell and Developmental Biology. Vol. 101, pp: 123–139. Available via https://www.sciencedirect.com/science/article/pii/S1084952118302787 López-Cortés, A., Abarca, E., Silva, L., Velastegui, E., León-Sosa, A., Karolys, G., Cabrera, F. and Caicedo, A. 2021. Identification of key proteins in the signaling crossroads between wound healing and cancer hallmark phenotypes. Sci. Rep. 11(1), 17245. Lopez, T., Wendremaire, M., Lagarde, J., Duquet, O., Alibert, L., Paquette, B., Garrido, C. and Lirussi, F. 2022. Wound healing versus Metastasis: role of oxidative stress. Biomedicines 10, 2784. Available via https://doi.org/10.3390/biomedicines10112784 MacCarthy-Morrogh, L. and Martin, P. 2020. The hallmarks of cancer are also the hallmarks of wound healing [Internet]. Sci. Signal. 13, eaay8690. Available from: https://www.science.org Mahmoud, N.N., Hamad, K., Shibitini, A.A., Juma, S., Sharifi, S., Gould, L. and Mahmoudi, M. 2024. Investigating inflammatory markers in wound healing: understanding implications and identifying artifacts. ACS Pharmacol. Transl. Sci. 7, 18–27. Available via https://pmc.ncbi.nlm.nih.gov/articles/PMC10789122/pdf/pt3c00336.pdf Maslikah , S.I., Fatchiyah, Widyananda, M.H., Rifa’i, M., Warsito, and Djati MS. 2025. Molecular docking and dynamic simulation of sesamin from Piper crocatum Ruiz & Pav as an inhibitor of canonical NF-κB: potential anti-inflammatory mechanism. J. Pharm. Pharmacogn. Res. 13(3), 848–856. Available via https://jppres.com/jppres/pdf/vol13/jppres24.2070_13.3.848.pdf Maslikah , S.I., Fatchiyah, Widyananda, M.H., Rifa, M., Warsito, Rifaí, M. et al. 2025. Molecular docking and dynamic simulation of sesamin from Piper crocatum Ruiz & Pav as an inhibitor of canonical NF-κB: potential anti-inflammatory mechanism. J. Pharm. Pharmacogn. Res. 13(3), 848–856. Available via https://jppres.com/jppres/pdf/vol13/jppres24.2070_13.3.848.pdf Morris, R.M., Mortimer, T.O. and O’Neill, K.L. 2022. Cytokines: can cancer get the message? Cancers. 14. Oktavia, N.S., Faridah, Armin, F. and Mustika, A. 2022. Utilization of natural ingrediens red betel (Piper crocatum) extract and white galangal (Alpinia galanga) extract as raw ingredients hand sanitizers (Research Phase 1). In: Rahim, R. and Marbun, N. editors. Proceeding of the 2nd Biennal International Conference on Safe Community. BandarLampung. Bratislava, Slovakia: EAI;pp:56-64. Rachmanita, R.T., Primarizky, H., Fikri, F., Setiawan, B., Agustono, B. and Saputro, A.L. 2019. Efektivitas Ekstrak Daun Afrika (Vernonia amygdalina) Secara Topikal Terhadap Kepadatan Kolagen dalam Penyembuhan Luka Insisi Pada Tikus Putih (Rattus norvegicus). Jurnal Medik Veteriner. 2(1), 36. Rizvi, F.H., Khan, M.K., Almas, T., Ullah, M., Shafi, A., Murad, M.F., Ali, A. and Nadeem, F. 2020. Early postoperative outcomes of breast cancer surgery in a developing Country. Cureus 12(8), e9941. Available via https://pmc.ncbi.nlm.nih.gov/articles/PMC7505669/pdf/cureus-0012-00000009941.pdf Rosyadi, A., Faizah, R.N., Nuri, N. and Puspitasari, E. 2020. Anticancer properties of methanolic extract of Piper crocatum leaf using BST and cytotoxicity on HeLa cell lines. Ann. Trop. Med. Public Health 23(3), 01–09. Savioli, F., Edwards, J., McMillan, D., Stallard, S., Doughty, J. and Romics, L. 2020. The effect of postoperative complications on survival and recurrence after surgery for breast cancer: a systematic review and meta-analysis. Clin. Rev. Oncol. Hematol. 155, 103075. Available via https://www.sciencedirect.com/science/article/pii/S1040842820302110?via%3Dihub Setyawati, A., Wahyuningsih, M.S.H., Nugrahaningsih, D.A.A., Effendy, C., Fneish, F. and Fortwengel, G. 2021. Piper crocatum Ruiz & Pav. ameliorates wound healing through p53, E-cadherin and SOD1 pathways on wounded hyperglycemia fibroblasts. Saudi J. Biol. Sci. 28(12), 7257–7268. Singampalli, K.L., Balaji, S., Wang, X., Parikh, U.M., Kaul, A., Gilley, J., Birla, R.K., Bollyky, P.L. and Keswani, S.G. 2020. The role of an IL-10/Hyaluronan axis in dermal wound healing. Front. Cell Dev Biol. Front. Media S.A.; 8, 636. Sofii, I., Kalembu, R.S., Fauzi, A.R., Makrufardi, F. and Makhmudi, A. 2021. TGF-β expression on different suturing technique for abdominal skin wound closure in rats. Ann. Med. Surg. 67, 102521; doi: https://doi.org/10.1016/j.amsu.2021.102521 Stuelten CH, Barbul A, Busch JI, Sutton E, Katz R, Sato M, et al. 2008. Acute wounds accelerate tumorigenesis by a T cell-dependent mechanism. Cancer Res. 68(18),7278–82. Sundaram, G.M., Quah, S. and Sampath, P. 2018. Cancer: the dark side of wound healing. FEBS J. 285, 4516–4534. Tonahi JMM, Nuryanti S, and Suherman. 2014. Antioksidan dari Daun Sirih Merah (Piper crocatum). J Akad Kim. 3(3),158–64. White Michael , J.V., Briquez, P.S., White, D.A.V. and Hubbel, J.A. 2021. VEGF-A, PDGF-BB and HB-EGF engineered for promiscuous super affinity to the extracellular matrix improve wound healing in a model of type 1 diabetes. NPJ Regen. Med. 6(1), 76. Available via www.nature.com/articles/s41536-021-00189-1.pdf Wurlina, Meles, D., Anom Adnyana, I., Sasmita, R. and Putri, C. 2019. Biological study of Piper crocatum Leaves ethanol extract improving the skin histopatology of wistar rat wound infected Staphylococcus Aureus. Eurasia J. Biosci. 13, 219–221. Xu, Z., Han, S., Gu, Z. and Wu, J. 2020. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthcare. Mater. 9, 1901502. Available via https://www.researchgate.net/publication/338808517_Advances_and_Impact_of_Antioxidant_Hydrogel_in_Chronic_Wound_Healing Yu, Y. and Feng, X.H. 2019. TGF-b signaling in cell fate control and cancer. Curr. Opin. Cell Biol. 61, 56–63. Available via https://doi.org/10.1016/j.ceb.2019.07.007 Zhao, H., Wu, L., Yan, G., Chen, Y., Zhou, M., Wu, Y. and Li, Y. 2021. Inflammation and tumor progression: signaling pathways and targeted intervention. Sig. Transduc. Target Ther. 6, 263. Available via https://www.nature.com/articles/s41392-021-00658-5 | ||
| How to Cite this Article |
| Pubmed Style Oktavia NS, Mustika A, Hidayati AN. Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features. Open Vet. J.. 2025; 15(3): 1264-1278. doi:10.5455/OVJ.2025.v15.i3.18 Web Style Oktavia NS, Mustika A, Hidayati AN. Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features. https://www.openveterinaryjournal.com/?mno=231522 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.18 AMA (American Medical Association) Style Oktavia NS, Mustika A, Hidayati AN. Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features. Open Vet. J.. 2025; 15(3): 1264-1278. doi:10.5455/OVJ.2025.v15.i3.18 Vancouver/ICMJE Style Oktavia NS, Mustika A, Hidayati AN. Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1264-1278. doi:10.5455/OVJ.2025.v15.i3.18 Harvard Style Oktavia, N. S., Mustika, . A. & Hidayati, . A. N. (2025) Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features. Open Vet. J., 15 (3), 1264-1278. doi:10.5455/OVJ.2025.v15.i3.18 Turabian Style Oktavia, Nike Sari, Arifa Mustika, and Afif Nurul Hidayati. 2025. Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features. Open Veterinary Journal, 15 (3), 1264-1278. doi:10.5455/OVJ.2025.v15.i3.18 Chicago Style Oktavia, Nike Sari, Arifa Mustika, and Afif Nurul Hidayati. "Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features." Open Veterinary Journal 15 (2025), 1264-1278. doi:10.5455/OVJ.2025.v15.i3.18 MLA (The Modern Language Association) Style Oktavia, Nike Sari, Arifa Mustika, and Afif Nurul Hidayati. "Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features." Open Veterinary Journal 15.3 (2025), 1264-1278. Print. doi:10.5455/OVJ.2025.v15.i3.18 APA (American Psychological Association) Style Oktavia, N. S., Mustika, . A. & Hidayati, . A. N. (2025) Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features. Open Veterinary Journal, 15 (3), 1264-1278. doi:10.5455/OVJ.2025.v15.i3.18 |