| Research Article | ||
Open Vet. J.. 2025; 15(3): 1244-1252 Open Veterinary Journal, (2025), Vol. 15(3): 1244-1252 Research Article Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotypeDmitrij Oleynikov*Veterinary Clinic “Belij Klyk”, Moscow, Russian Federation *Corresponding Author: D. Oleynikov. Veterinary clinic “Belij Klyk”, Moscow, Russia. Email: wolfberg.guard [at] gmail.com Submitted: 04/12/2024 Accepted: 20/20/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
AbstractBackground: In humans and cats hypertrophic cardiomyopathy (HCM) is a cause of sudden cardiac death. This is usually associated with arrhythmias, based on myocardial fibrosis and electric impulse propagation disturbances. Restrictive cardiomyopathy (RCM) is a CM associated with excessive fibrosis which is predisposed to arrhythmic episodes. Electric coupling in the myocardium is based on the His-Purkinje system and cardiomyocytes cell-to-cell contacts. Cell connection is based on gap junctions and their structural proteins—connexins. Today there is a lack of information in the literature regarding these functional units and their distribution in cats. Aim: Discover the presence of connexin43 (Cx43) in myocardial tissues of cats and to differentiate in HCM and RCM phenotypes. Methods: Retrospective analysis of materials collected from cats with CM. Results: Animals with the histological animals with histological and immunohistochemical markers of HCM and RCM. Cx43 was distributed in the myocardial tissue of a healthy cat in a typical pattern to other described animals (rats, mice, and human). In HCM, Cx43 was decreased and lateralized near the fibrotic zones, and it was absent in the scar tissue. In RCM, there was a similar pattern, but loci with spontaneously altered expression of Cx43 were also observed, forming lacunas in the gap junction or presented as an intermittent granulated mass. Conclusion: Cx43 has different expression patterns in different CM phenotypes; however, the role of this fact in arrhythmogenesis needs to be studied. Keywords: Feline, Myocardium, Hypertrophic cardiomyopathy, Restrictive cardiomyopathy, Connexin 43 IntroductionHeart failure is one of the most common causes of veterinary hospital visits among feline patients. Cardiomyopathies (CMs) are highly represented in the population with heart failure. Feline CMs are mostly associated with the following nosologies: dilated CM (DCM), hypertrophic CM hypertrophic cardiomyopathy (HCM), restrictive CM restrictive cardiomyopathy (RCM), and unclassified CM. HCM is a typical disease in feline patients. This nosology is associated with hypertrophy of the myocardium, myocardial disarray, developing fibrosis, small myocardial vessel pathology, and episodes of ischemia (Payne et al., 2015; Kittleson and Cote, 2021). In humans and cats, HCM can cause sudden cardiac death. This phenomenon is usually associated with arrhythmias, which are followed by myocardial fibrosis and electric impulse propagation disturbances. Another CM associated with excessive fibrosis is RCM, which is highly predisposed to arrhythmic episodes. Electric coupling in the myocardium is based on the His-Purkinje system and cardiomyocytes cell-to-cell contacts. Cell connection is based on gap junctions and their structural proteins. Gap junctions are transmembrane structures that sustain direct cytoplasmic compartments connection. These structures present as mirroring connexons—hemichannels on the outer membrane of cardiomyocytes. Connexons structurally consist of several types of connexins (Cx): Cx40, Cx43, and Cx45. These proteins are expressed in myocyte-type specific proportions, reflecting their electrical features. This distribution is also associated with heart chambers and structures. Atrial cardiomyocytes usually have relatively equivocal expressions of Cx40 and Cx43, whereas ventricle myocardium has only Cx43, except Purkinje fibers, which express Cx40 and Cx45, and sinus nodes represented with mostly Cx45 (Severs et al., 2008). Cardiac gap junctions are very adaptive due to the short life of Cx (several hours), which changes trafficking, degradation, channel permeability, and gating under different conditions to support normal electricity. However, in cases of hypertrophy, ischemia, and inflammation, this adaptivity decreases and predisposes individuals to arrhythmogenesis (Verheule and Kaese, 2013). Cardiac remodeling associated with CM results in alterations of electrical and structural properties. At the gap junction level, one of the most important changes is Cx expression, leading to a decrease in protein levels and heterogeneous distribution. These abnormalities lead to decreased conduction speed and coupling and may be arrhythmogenic (Billur et al, 2022). In the modern literature, there is a lack of information about gap junctions and Cxs distribution in the myocardial tissues of diseased and healthy felines. This study aimed to elucidate some data about Cx43 representation in feline myocardium in the context of heart disease (HCM and RCM phenotype). Material and MethodsTwenty-two cats were included in this study: 21 with CM and 1 healthy cat. We compared animals with a typical HCM phenotype (n=10) visualized during the echocardiography study with RCM-phenotype cats (n=11). Instrumental diagnosticsAll patients included in the study underwent a full clinical study, as well as standard blood analyses, and troponin. In addition, an echocardiographic study was conducted (or performed) by an experienced operator using the standard protocol. After euthanasia, gross pathology was performed, and the heart was analyzed. Following the macroscopic study, tissue samples were collected. The heart sections were fixed in 10% buffered formalin. The tissue samples were then sent to the pathomorphological laboratory for histological and immunohistochemical analyses. anti-Cx43 antibody (Rabbit polyclonal antibody to Cx43/GJA1, AB_2837824, Affinity Biosciences Cat# AF5339, RRID:AB_2837824). The process was performed using the Leica bond system and buffered saline as negative controls.
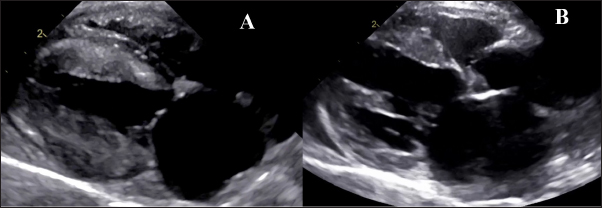
Fig. 1. Echocardiography. A- HCM group with hypertrophy of left ventricle walls, dilated left atrium, B— RCM-phenotype with hyperechogenic endomyocardium and loci in myocardial walls and dilated left atrium.
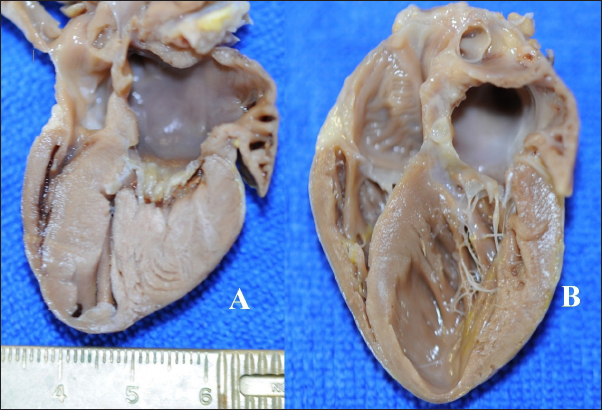
Fig. 2. Gross heart pathology. A—HCM-phenotype, hypertrophied left ventricular walls and papillary muscle, dilated left atria. B—RCM-phenotype, patchy pale and thickened endomyocardium, excessive tendons, dilated left atria with thickened endomyocardial layer.
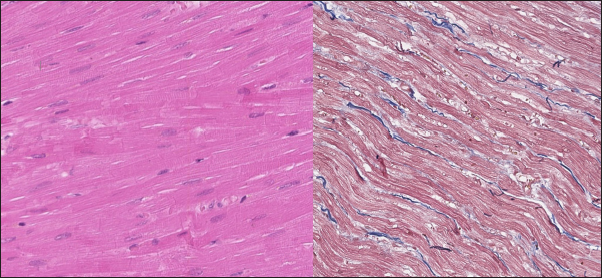
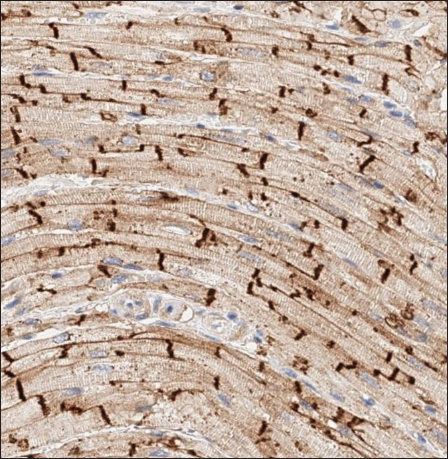
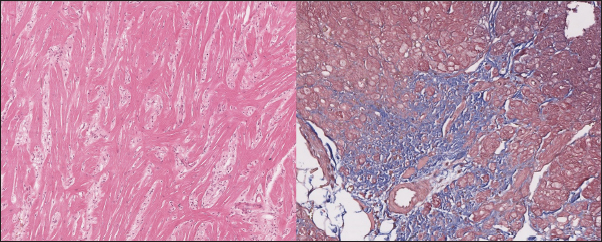
Fig. 3. Histomorphology. Left—Normal myocardium of the cat. Stained with H&E. Right—Normal myocardium of the cat. Red- myocardium; blue—collagen fibers. Stained with Masson’s trichrome. ResultsAnimal echocardiography showed signs of typical morphology within the phenotypes. The HCM group had signs of ventricular wall hypertrophy (more than 6 mm, symmetrically or asymmetrically) as well as a dilated left atrium. The RCM group had a nonhypertrophied myocardium, hyperechogenic zones in the myocardial tissue, and a thickened endomyocardial layer, combined with dilated atria (Fig. 1). Gross pathologyThe cats with HCM showed hypertrophied left ventricular walls. Dilated left atria with pale and thick endomyocardium. In some cases, mild obstruction of the left ventricle outflow tract was noted. In the RCM group, we found quite normal myocardial walls with zones of pale and thickened endomyocardium, excessive proliferation of tendons, sometimes forming net-like structures across the left ventricle, and dilated left atria with a thickened endomyocardial layer (Fig. 2). HistopathologyIn the myocardial samples of healthy cats, we did not find any significant structural alterations. The cardiomyocytes were presented as cell stripes with one or two centrally located nuclei, Z disks were easily recognized, and striation was presented. The interstitial spaces were not expanded, and the capillary lumen was noticed. Masson’s trichrome staining for fibrosis did not reveal a significant increase in collagen fibers, with sporadic fibers observed in interstitial spaces and near vessels (Fig. 3). Immunohistochemical staining for Cx43 revealed the typical distribution of proteins accumulated in cell-to-cell connections via intercalated disks. Weak-to-absent signals were observed in the lateral wall of the cardiomyocytes. The immunoadsorption zone exhibited a solid line without disruptions or lesions (Fig. 4). In the myocardium of cats with HCM we found a typical myocardial fibers disarray, with myocytes presented as thickened stripes branching in chaotic ways. Moreover, significant interstitial fibrosis was observed. Some myocytes were hypertrophied with multiple eccentrically located nuclei. Masson’s trichrome-stained samples showed myocardial fibrosis with interstitial and patchy patterns distributed around arteriolas and deep in the tissues (Fig. 5). Immunohistochemical staining with anti-Cx43 antibodies showed almost intact intercalated disks morphology in unaffected zones, but Cx43 expression began to fade near areas of developed fibrosis. In the transverse axis, we found increased expression of Cx43 on the lateral membrane (Fig. 6). In cardiomyocytes directly connected to the fibrotic tissue, cell-to-cell contacts were pale with almost no staining (Fig. 7).
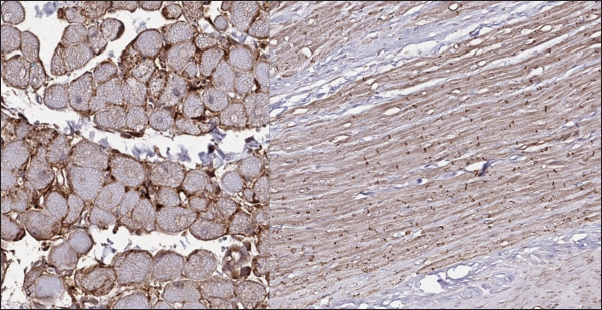
Fig. 4. Immunohistochemistry. Normal myocardium of the cat. Cx43 proteins located in zones of cell-to-cell contact. Stained with Cx43 antibodies.
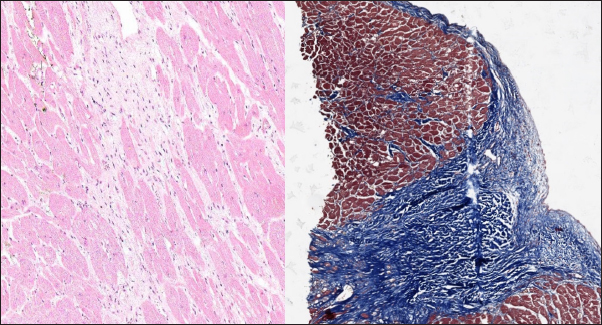
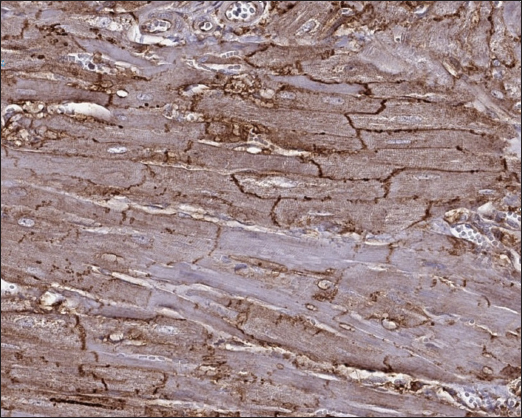
Fig. 5. Histomorphology. HCM group. Left—Myocardial fibers disarray, atypical branching, interstitial fibrosis. Stained with H&E. Right—Interstitial and patchy fibrosis, cardiomyocytes vacuolar dystrophy. Redmyocardium; bluecollagen fibers. Stained with Masson’s trichrome. In the myocardial samples of the cats with RCM, we found zones of slightly dystrophic cardiomyocytes surrounded by areas of diffuse or patchy fibroses, and some loci had compact fibrosis associated with subendocardial spaces. Slides stained with Masson’s trichrome showed areas of significant interstitial, compact subendocardial fibrosis (Fig. 8). Immunohistochemical staining with anti-Cx43 antibodies showed the partial absence of Cx-43 positive staining despite the presence of fibrotic tissue (Fig. 9). Additionally, some loci showed increased lateral expression of the Cx43 proteins. In certain longitudinal slides, we observed intermittent patterns in the stained structures of intercalated disks. In some cardiomyocytes, Cx43 immunostaining revealed granular structures within intercalated discs and the cytoplasm (Fig. 10).
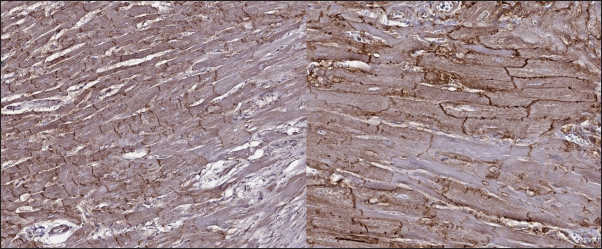
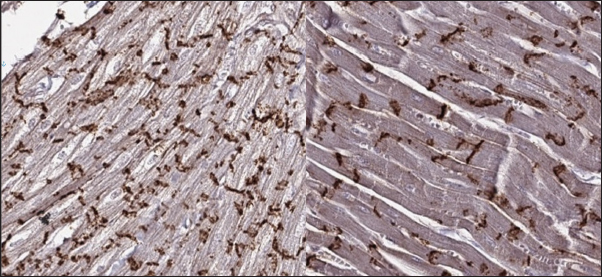
Fig. 6. Immunohistochemistry. HCM group. Left—Transverse section, Cx43 proteins accumulated in the outer membrane periphery of the cardiomyocytes. Stained with Cx43 antibodies. Right—Longitudinal section, Cx43 proteins found in zones of cell-to-cell contact, but reduced in fibrotic and para-fibrotic zones. Stained with Cx43 antibodies.
Fig. 7. Immunohistochemistry. HCM group. Longitudinal section, Cx43 proteins found in zones of cell-to-cell contact, but reduced in fibrotic and para-fibrotic zones. Stained with Cx43 antibodies. DiscussionIn this study, we describe for the first time the myocardial expression of Cx43 proteins in felines. We also illustrate some differences between Cx43 distribution in patients with different phenotypes of suspected CM. Myocardial remodeling plays a pivotal role in CM development. At certain stages, heart diseases can be associated with arrhythmias, and sometimes it is difficult to differentiate between the inherited cause and the acquired. Despite the variable phenotype (dilated or hypertrophic), different genetic CM share similar genes (Kittleson et al., 2015; Vaykhanskaya et al., 2019; Jordan et al., 2021). Data from human medicine indicate that not only HCM or DCM share the same genetic features, but the RCM phenotype can be found in the same genetic abnormalities (Chen et al., 2001; Mogensen et al., 2003; Kaski et al., 2008; Menon et al., 2008; Gannon et al., 2021). In particular cases, RCM could be a desminopathy with a high rate of arrhythmic episodes (Clemen et al., 2013). In summary, this information reveals high arrhythmogenicity in CM regardless of the etiology and ongoing process of myocardial remodeling.
Fig. 8. Histomorphology. RCM group. Left—Myocardial dystrophy, interstitial and patchy fibrosis. Stained with H&E. Right—Interstitial, patchy and compact fibrosis in the left ventricle wall, cardiomyocytes vacuolar dystrophy. Red- myocardium; blue—collagen fibers. Stained with Masson’s trichrome.
Fig. 9. Immunohistochemistry. RCM group. Longitudinal section, Cx43 proteins found in zones of cell-to-cell contact and laterally, but could be stochastically reduced in other cells. Stained with Cx43 antibodies. In the medical literature, we can find several studies describing gap junction changes during heart failure. Some of these data describe Cx43 modulations. Experiments in mice recently showed that changes in the expression of Cx43 channels promoted ventricular arrhythmia, which was provoked by calcium overload (isoproterenol infusion) (Lillo et al., 2023). Another mouse study showed that fibrosis associated with chronic heart failure leads to an uneven distribution of gap junction structures, including Cx43, in the myocardium (Liu et al., 2022). What is more, rat models showed that the depletion of Cx43 expression and its lateralization to the outer membrane led to decreased conduction velocity in the myocardium and proarrhythmogenic state (Emdad et al., 2001; Jin et al., 2010). Additionally, an experimental rat model with insulin resistance revealed that this state is associated with Cx43 reduction, and their trafficking alterations, presented with proteins lateralization and internalization in the cytoplasm (Billur et al., 2022). In another animal model (dog) with the DCM phenotype, the Cx43 decrease led to prolonged conduction speed, increased QT, and predisposition to polymorphic ventricular tachycardia (Akar et al., 2004).
Fig. 10. Immunohistochemistry. RCM group. Longitudinal section, Cx43 proteins found in zones of cell-tocell contact but do not form solid bands in contact zones, while presented in intermittent pattern or as granules on the cell’s pole/ in cytoplasm. Stained with Cx43 antibodies. In the literature review by Fontes et al, we found data on variations in Cx43 myocardial expression under different conditions. In biopsies obtained from human patients with HCM, Cx43 was a differential marker of compensated or decompensated status. Compensated myocardial function was associated with reserved or increased Cx43 expression in cardiomyocytes, whereas a worsening state was associated with its depletion. In DCM, left ventricle biopsies from human patients showed decreased Cx43 expression in zones of fibrosis and uneven distribution in the surroundings, whereas distant areas had normal expression. A correlation was observed between ventricular tachycardia, late potentials in the myocardium, and decreased Cx43 presence. Moreover, ischemia is associated with depleted Cx43 expression and inside-cell trafficking. The significance of this process increases in cases of isolated cardiomyocytes captured in stunned myocardium or scar tissue (Akar et al., 2007; Fontes et al., 2012). Interestingly, the reduction in Cx43 expression does not linearly decrease conduction velocity, but a loss of 50% leads to electrical uncoupling leading to higher arrhythmogenicity, and this effect is potentiated by fibrosis (Fontes et al., 2012). In this study, we found that the changes in cats with HCM are similar to those in human hearts with a hypertrophic phenotype caused by aortic stenosis. We observed loss of intercalated disk structures and Cx43 lateralization in the zones affected by fibrosis. In human patients, researchers found a straight-correlated hypertrophy-dependence of Cx43 remodeling. In the first stage, Cx43 proteins are overexpressed as a compensatory reaction to increased stretch and pulsatile left ventricular work. This state stimulates angiotensin II, endothelin I, and vascular growth factors, which affect the Cx43 biocycle. However, in the late stages, overstimulation depresses protein synthesis and leads to Cx subtype heterogeneity and deficit (Kostin et al., 2004). The findings of the RCM phenotype revealed large zones of fibrotic tissue and significantly dispersed Cx43 expression. There is an additional significance to such changes; they should be much more predisposed to arrhythmic episodes. This conclusion is supported by experimental data from mouse models. In a 2016 study using microelectrode techniques, in scar tissue and in Cx43 absent mice impulse propagation, conduction velocity, and membrane potential were decreased. At the same time, the ability of fibroblasts to connect with the myocardium via disorganized and stochastic Cx43 channels can sustain the re-entry circle (Mahoney et al., 2016). In conclusion, this study is the first to show the expression of Cx43 in feline myocardium and to demonstrate differences between the presence of protein in the myocardium of healthy and cats with different CM-phenotypes (RCM and HCM). LimitationThis study is retrospective. In this study, we did not correlate histopathological signs with the severity of heart failure and arrhythmia because this requires a more complex study. Conflict of interestNo. AcknowledgmentsNo. FundingNo. ReferencesAkar, F.G., Nass, R.D., Hahn, S., Cingolani, E., Shah, M., Hesketh, G.G., DiSilvestre, D., Tunin, R.S., Kass, D.A. and Tomaselli, G.F. 2007. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 293(2):H1223–230. Akar, F.G., Spragg, D.D., Tunin, R.S., Kass, D.A. and Tomaselli, G.F. 2004. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ. Res. 95(7), 717–725. Billur, D., Olgar, Y. and Turan, B. 2022. Intracellular redistribution of left ventricular connexin 43 contributes to the remodeling of electrical properties of the heart in insulin-resistant elderly rats. J. Histochem. Cytochem. 70(6), 447–462. Chen, S-C., Balfour, I.C. and Jureidini, S. 2001. Clinical spectrum of restrictive cardiomyopathy in children. J. Heart Lung Transplant. 20, 90–92. Clemen, C.S., Herrmann, H., Strelkov, S.V. and Schroder, R. 2013. Desminopathies: pathology and mechanisms. Acta Neuropathol. 125, 47–75. Emdad, L., Uzzaman, M., Takagishi, Y., Honjo, H., Uchida, T., Severs, N.J., Kodama, I. and Murata, Y. 2001. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: prevention by angiotensin II type 1 receptor blockade. J. Mol. Cell Cardiol. 33(2), 219–231. Fontes, M.S., van Veen, T.A., de Bakker, J.M. and van Rijen, H.V. 2012. Functional consequences of abnormal Cx43 expression in the heart. Biochim. Biophys. Acta. 1818(8), 2020-9. Gannon, M.P. and Link, M.S. 2021. Phenotypic variation and targeted therapy of hypertrophic cardiomyopathy using genetic animal models. Trends Cardiovasc. Med. 31(1), 20–31. Jin, H., Chemaly, E.R., Lee, A., Kho, C., Hadri, L., Hajjar, R.J. and Akar, F.G. 2010. Mechanoelectrical remodeling and arrhythmias during progression of hypertrophy. FASEB J. 24(2), 451–463. Jordan, E., Peterson, L., Ai, T., Asatryan, B., Bronicki, L., Brown, E., Celeghin, R., Edwards, M., Fan, J. and Ingles, J. 2021. An evidence-based assessment of genes in dilated cardiomyopathy. Circulation 144(1), 7–19. Kaski, J.P., Syrris, P., Burch, M., Tomé-Esteban, M.T., Fenton, M. and Christiansen, M. 2008. Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart 94, 1478–1484. Kittleson, M.D. and Côté, E. 2021. The feline cardiomyopathies: 1. General concepts. J Feline Med Surg. 23(11), 1009–1027. Kittleson, M.D., Meurs, K.M., Harris, S.P. 2015. The genetic basis of hypertrophic cardiomyopathy in cats and humans. J. Vet. Cardiol. 17 Suppl 1(Suppl 1), S53–73. Kostin, S., Dammer, S., Hein, S., Klovekorn, W.P., Bauer, E.P. and Schaper, J. 2004. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc. Res. 62(2), 426–436. Lillo, M.A., Muñoz, M., Rhana, P., Gaul-Muller, K., Quan, J., Shirokova, N., Xie, L.H., Santana, L.F., Fraidenraich, D. and Contreras, J.E. 2023. Remodeled connexin 43 hemichannels alter cardiac excitability and promote arrhythmias. J. Gen. Physiol. 155(7):e202213150. Liu, S., Lan, Y., Zhao, Y., Zhang, Q., Lin, T., Lin, K., Guo, J. and Yan, Y. 2022. Expression of connexin 43 protein in cardiomyocytes of heart failure mouse model. Front. Cardiovasc Med. 9:1028558 Mahoney, V.M., Mezzano, V., Mirams, G.R., Maass, K., Li, Z., Cerrone, M., Vasquez, C., Bapat, A., Delmar, M. and Morley, G.E. 2016. Connexin43 contributes to electrotonic conduction across scar tissue in the intact heart. Sci. Rep. 6:26744. Menon, S.C., Michels, V.V., Pellikka, P.A., Ballew, J.D., Karst, M.L. and Herron, K.J. 2008. Cardiac troponin T mutation in familial cardiomyopathy with variable remodeling and restrictive physiology. Clin. Genet. 74, 445–454. Mogensen, J., Kubo, T., Duque, M., Uribe, W., Shaw, A., Murphy, R., Gimeno, J.R., Elliott, P. and McKenna, W.J. 2003. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J. Clin. Invest. 111, 209–216. Payne, J.R., Brodbelt, D.C. and Luis Fuentes V. 2015. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J. Vet. Cardiol. 17 Suppl 1, S244–257. Severs, N.J., Bruce, A.F., Dupont, E. and Rothery, S. 2008. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc. Res. 80(1), 9–19. Vaykhanskaya, T.G., Sivitskaya, L.N., Kurushko, T.V., Levdansky, O.D. and Danilenko, N.G. 2019. Dilated cardiomyopathy: reconceptualization of the problem. Russian J. Cardiol. 4, 35–47. Verheule, S. and Kaese, S. 2013. Connexin diversity in the heart: insights from transgenic mouse models. Front. Pharmacol. 4, 81. | ||
| How to Cite this Article |
| Pubmed Style Dmitrij Oleynikov. Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype. Open Vet. J.. 2025; 15(3): 1244-1252. doi:10.5455/OVJ.2025.v15.i3.16 Web Style Dmitrij Oleynikov. Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype. https://www.openveterinaryjournal.com/?mno=231565 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.16 AMA (American Medical Association) Style Dmitrij Oleynikov. Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype. Open Vet. J.. 2025; 15(3): 1244-1252. doi:10.5455/OVJ.2025.v15.i3.16 Vancouver/ICMJE Style Dmitrij Oleynikov. Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1244-1252. doi:10.5455/OVJ.2025.v15.i3.16 Harvard Style Dmitrij Oleynikov (2025) Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype. Open Vet. J., 15 (3), 1244-1252. doi:10.5455/OVJ.2025.v15.i3.16 Turabian Style Dmitrij Oleynikov. 2025. Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype. Open Veterinary Journal, 15 (3), 1244-1252. doi:10.5455/OVJ.2025.v15.i3.16 Chicago Style Dmitrij Oleynikov. "Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype." Open Veterinary Journal 15 (2025), 1244-1252. doi:10.5455/OVJ.2025.v15.i3.16 MLA (The Modern Language Association) Style Dmitrij Oleynikov. "Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype." Open Veterinary Journal 15.3 (2025), 1244-1252. Print. doi:10.5455/OVJ.2025.v15.i3.16 APA (American Psychological Association) Style Dmitrij Oleynikov (2025) Myocardial expression of connexin 43 in cats with hypertrophic and restrictive cardiomyopathy phenotype. Open Veterinary Journal, 15 (3), 1244-1252. doi:10.5455/OVJ.2025.v15.i3.16 |