| Research Article | ||
Open Vet. J.. 2025; 15(3): 1279-1288 Open Veterinary Journal, (2025), Vol. 15(3): 1279-1288 Research Article Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) modelWurlina Wurlina1, Dewa Ketut Meles2, Imam Mustofa1*, Aswin Rafif Khairullah3, Dewa Made Sucipta Putra4, Niluh Suwasanti5, Adeyinka Oye Akintunde6, Suzanita Utama1, Sri Mulyati1, Wasito Wasito3, Ricadonna Raissa7, Riza Zainuddin Ahmad3, Julaeha Julaeha8 and Fitrine Ekawasti31Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Dr. R. Soedjono Selong Hospital, East Lombok, Indonesia 5Department of Clinical Pathology, Faculty of Medicine, Universitas Katolik Widya Mandala Surabaya, Surabaya, Indonesia 6Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo State, Nigeria 7Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 8Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 07/12/2024 Accepted: 15/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
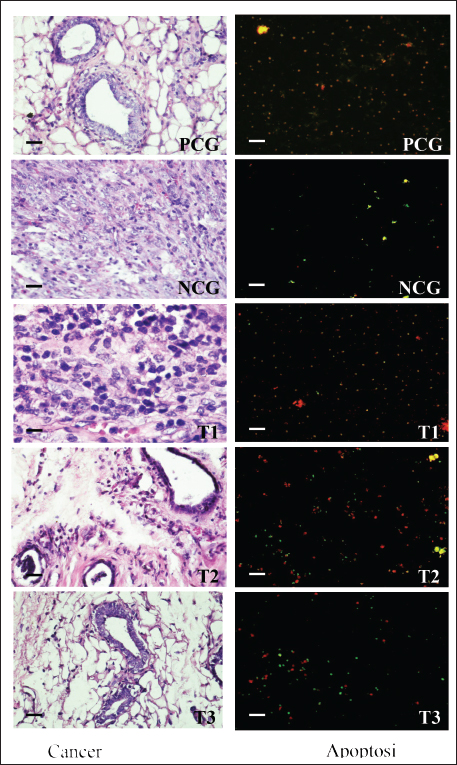
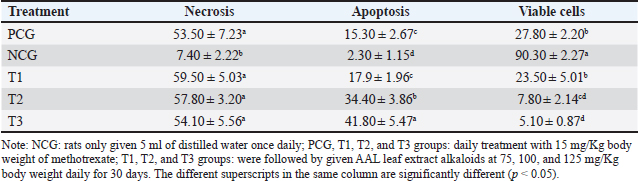
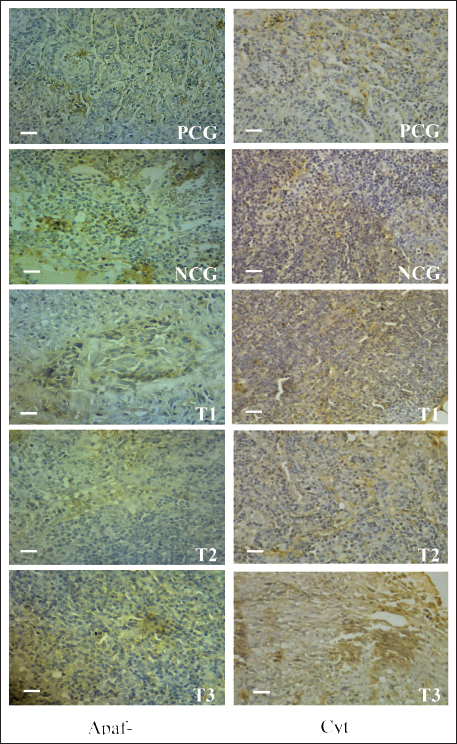
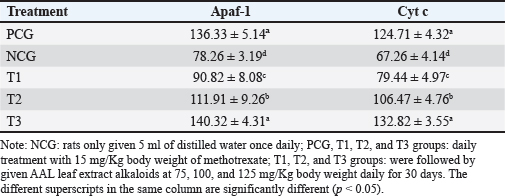
AbstractBackground: Breast cancer affects women of various ages, and its recurrence is a significant cause of death. The search for potent anticancer compounds of herbal origin with well-defined mechanisms of action is an essential focus of current research. Aim: This study aimed to investigate the effects of alkaloids in Achyranthes aspera Linn (AAL) leaf extract on necrosis, apoptosis, and related molecular markers, namely, cyclin-dependent kinase 1, Bcl-2 associated X-protein (Bax), rat sarcoma virus (Ras), cytochrome (Cyt) c, and apoptotic activating factor-1 (Apaf-1), in mice models. Methods: Thirty mice with breast cancer were randomly divided into five groups. The negative control group only received distilled water daily. Mice in the positive control group (PCG) were administered methotrexate (15 mg/Kg) daily. The T1, T2, and T3 groups received oral orally at 75, 100, and 125 mg/Kg body weight daily for 30 days, respectively. On day 31, all mice were euthanized for the preparation of histological specimens of the mammary glands. The negative control group had the lowest number of apoptotic cells, Apaf-1, Cyt C, and Bax expression, and the highest number of viable cancer cells and Ras expression. Results: The percentages of necrotic cells and breast cancer-expressed CDK-1 were not significantly (p > 0.05) different among groups. The percentage of apoptotic cells, Apaf-1, and Cyt c, was highest in T3. Conversely, the percentage of viable cells and breast cancer-expressing Ras was lowest in T3. Conclusion: Treatment with 125 mg/Kg AAL suppressed cancer cell growth in breast cancer-bearing mice. Further research is necessary to determine the complete signaling mechanism. Keywords: Herbal medicine, Cancer, Bax, Cyt c, Apaf-1. IntroductionBreast cancer affects women of various ages, and its recurrence is a significant cause of death (Contiero et al., 2023). Cancer treatment has not provided satisfactory results, especially for advanced cancers. However, in addition to killing cancer cells, cancer chemotherapy drugs can also cause the death of normal cells. Breast cancer can be treated with systemic or radiation therapy (Bhattacharyya et al., 2020), surgical lumpectomy, or mastectomy, with bilateral mastectomy being the last option (Watkins, 2019). Systemic therapy has side effects, such as fatigue, depression, low concentrations, pain, mucosal changes, skin rashes, and peripheral neuropathy (Haidinger and Bauerfeind, 2019). Systemic cancer therapy aims to increase cancer cell apoptosis. The balance between pro and antiapoptotic gene expression programs determines the progression of cancer cell apoptosis (Guo et al., 2018). Apoptosis efficiently removes damaged cells to maintain tissue homeostasis. The inhibition of cyclin-dependent kinase 1 (CDK1) attenuates apoptosis (Nie et al., 2022), whereas rat sarcoma virus (Ras) is an oncogenic protein involved in malignant transformation (Li et al., 2019). An increase in Cyt c stimulates the formation of apoptotic activating factor-1 (Apaf-1) and, followed by the activation of caspase-3, induces an increase in DNase activity, resulting in DNA fragmentation and cell apoptosis (Redza-Dutordoir and Averill-Bates, 2016). The release of Cyt c from the mitochondrial outer membrane is a crucial step in inducing the release of proapoptotic proteins Bcl-2-associated X (Bax) and Bad (Yue and López, 2020); Bax is an inducer of apoptosis (Manne et al., 2021). Cyt c released from the mitochondria can also form apoptosomes with Apaf-1 and procaspase-9, leading to the activation of caspases-3/7/9 and resulting in apoptosis (Roberts et al., 2022). The search for potent anticancer compounds of herbal origin with well-defined mechanisms of action is an essential focus of current research. The induction of apoptosis is the main cause of decreased cancer cell proliferation. The regulation of proapoptotic Bax is coupled with the regulation of antiapoptotic Bcl-2 expression (Venkatachalam and Nadumane, 2019). Achyranthes aspera Linn (AAL) is a traditional medicine in the tropical countries of Asia and Africa. The isolated constituents are mainly flavonoids, tannins, terpenoids, saponins, phytosterols, and phenolic compounds (Nargatti et al., 2021). The leaf extract of AAL contains 52.36% alkaloids (Meles et al., 2017), and the remaining are saponins, tannins, flavonoids, glycosides, steroids, essential oils, and fatty acids (Raju et al., 2022). Phenolic compounds, saponins, and alkaloids are potential cancer drugs that can induce p53 expression and decrease telomere length (Vakili et al., 2020). To the best of our knowledge, no study has examined the alkaloid fraction of AAL leaf extract as an antibreast cancer herbal medicine. Therefore, this study aimed to determine the influence of AAL leaf extract on necrosis, apoptosis, and other related molecular markers. Materials and MethodsPreparationThe extraction of AAL alkaloids was performed using the Indonesian Pharmacopeia method. The alkaloid fractionation process was performed using column chromatography with a silica gel stationary phase and a mixture of ethyl acetate, methanol, and water as the mobile phase. Alkaloids were detected using thin-layer chromatography, and the alkaloid contents of AAL were determined using high-performance liquid chromatography (Song et al., 2022). Experimental animalsThirty 2-month-old female mice (Mus musculus) were subcutaneously administered 10 mg/Kg body weight benzopyrene (Sigma-Aldrich, Darmstadt, Germany) into the mammary glands for 8 weeks to induce breast cancer (Sedeman et al., 2022). Breast cancer in mice was evaluated by digital palpation of the mammary glands and histological examination of cell proliferation on mammary gland biopsy under a light microscope (Nikon E200, Tokyo, Japan) at 400× magnification. Breast cancer-bearing mice were randomly divided into five groups. The negative control group (NCG) received 5 ml of distilled water once daily, whereas the positive control group (PCG) received daily treatment with 15 mg/Kg body weight of methotrexate (PT, OTTO Pharmaceutical Industries, Tangerang, Indonesia). Treatment groups 1, 2, and 3 (T1, T2, and T3) were administered with AAL leaf extract alkaloids at 75, 100, and 125 mg/Kg body weights daily (Meles et al., 2017), in 5 ml volume using a gastric probe daily for 30 days. On day 31, all mice were euthanized by cervical dislocation, and mammary glands were collected for histological preparation. Slide stainingThe micro-section was taken on a glass object and stained with hematoxylin and eosin (Feldman and Wolfe, 2014). The stained slides were observed using a light microscope at 400× (Nikon Eclipse E800, Tokyo, Japan). Cell proliferation was indicated by 2–3 nuclei stained with blue purplish (Mirzayans et al., 2018). Acridine orange-stained slides were examined under a fluorescence microscope at 100× (Nikon Eclipse E800). Apoptotic cells were yellow to reddish, and necrotic cells were brownish–orange (Liu et al., 2015) without bright spots, whereas viable cells appeared green (Byvaltsev et al., 2019). Avidin–biotin complex (ABC) stainingABC staining was performed to evaluate Apaf-1, Cyt c, Cdk1, Bax, and Ras expression under a light microscope (Nikon E200) at 400x magnification. A negative expression is indicated by a bluish/greenish stain. The positive expression of Apaf-1, Cyt c (Zlobec et al., 2006), Cdk1 (Clement et al., 2015), Bax (Yuan et al., 2022), and Ras (Calaf and Abarca-Quinones, 2016) were indicated by brown stains. Statistical analysisData were analyzed using a one-way analysis of variance, followed by Tukey’s honestly significant difference test at a 95% confidence level (IBM SPSS Statistics for Windows version 23, IBM Corp., Armonk, NY, USA). Ethical approvalThe Animal Care and Use Committee of Airlangga University, Surabaya, Indonesia (No. 158/HRECC.FODM/XI/2022). Animal treatment was conducted with minimum pain or discomfort according to the guidelines established by the Institutional Animal Ethics Committee. ResultBreast cancer was characterized by marked lumps in the mammary glands, and cell proliferation (2–3 nuclei) was observed in mammary gland slides (Fig. 1). The lowest percentage of necrotic cells (p < 0.05) was found in the NCG group, while there was no significant difference between the PCG, T1, T2, and T3 groups (p > 0.05). The percentage of apoptotic cells in breast cancer lesions was the highest in T3, even higher than that in the PCG, and the lowest in the NCG. Conversely, the percentage of viable breast cancer cells was the lowest in T3, even lower than that in the PCG, and the highest in the NCG (Fig. 1 and Table 1). The treatment of breast cancer-bearing mice with the alkaloid fraction of AAL increased the expression of Cyt c and Apaf-1, which were higher in the T3 group than in the NCG (p < 0.05), but not significantly (p > 0.05) different from that in the PCG (Fig. 2 and Table 2). The percentage of breast cancer cells expressing Bax was the highest in T3, even higher than that in the PCG, whereas it was significantly (p < 0.05) the lowest in the NCG. On the contrary, the percentage of breast cancer cells expressing Ras was the lowest in T3, even lower than that in the PCG; however, it was significantly (p < 0.05) the highest in the NCG (Fig. 3 and Table 3).
Fig. 1. The proliferation status of breast cancer cells (left) and apoptotic breast cancer (right). Table 1. Percentage of apoptosis, necrosis, and viable breast cancer cells after treatment with or without the alkaloid fraction of AAL compared with the findings after methotrexate treatment.
DiscussionBenzopyrene induces breast cancer cells in vivo (Guo et al., 2015). Mice with breast cancer were physically and histologically characterized by lumps in the mammary glands and high cell proliferation observed in mammary gland biopsies (Schmitt et al., 2017). Cancer treatment aims to suppress as much cell proliferation as possible through apoptotic death (Pfeffer and Singh, 2018). Regulated cell death in cancer can be marked by its signal transduction, including CDK1, Bax, Ras, Cyt c, and Apaf-1, which can be used as indicators of therapeutic progress (Peng et al., 2022). Necrosis is associated with aggressive breast cancer and poor prognosis (Tata et al., 2016). Tumor necrosis factor (TNF) induces necrosis in cancer cells (Mercogliano et al., 2020). Elevated levels of TNF-α in the serum are correlated with metastasis and poor prognosis of breast cancer (An et al., 2020). The treatment of malignant breast cancer by programmed induction of necrosis (necroptosis) can be considered when breast cancer cell apoptosis does not occur as expected (Thakur et al., 2019). However, in this study, the percentage of necrotic breast cancer cells was similar among breast cancer-bearing mice treated with placebo, various AAL doses, and methotrexate (Table 1). No comparative data were found on the use of methotrexate in breast cancer mouse models. However, the use of methotrexate on HeLa cancer cell lines was associated with higher (76.4%) viable cancer cells, lower (18.2%) necrotic cancer cells, and lower (5.34%) apoptotic cancer cells (Faraji et al., 2022) compared with the rates reported in this study (Table 1). A close interaction was observed between the metabolic and mitochondrial apoptotic signaling pathways (Daniels et al., 2021). In cancer cells, ROS play a role in inhibiting cancer growth, metastasis, and apoptosis; however, they are also involved in the development of cancer, including its malignant transformation (Wang et al., 2021). ROS is enhanced by increased metabolic rate, gene mutations, and relative hypoxia in cancer cells. However, ROS also triggers programmed cell death (Perillo et al., 2020). Cancer cells cause ROS accumulation, further stimulating cancer cell proliferation, death avoidance, angiogenesis, invasiveness, and metastasis (Hecht et al., 2016). ROS induces a decrease in mitochondrial membrane potential, Cyt c release, Bcl-2 dysregulation, and caspase-3 activation, leading to cancer cell apoptosis (Ponraj et al., 2018); thereby, ROS has the potential to be a target in breast cancer therapy (Sarmiento-Salinas et al., 2019). The extract of Achyranthes aspera leaves effectively reduces ROS formation by inhibiting CYP2E1 activity (Deshpande and Une, 2021) and reduces the growth of Dalton’s lymphoma via apoptosis through the mitochondrial pathway (Singh et al., 2021). The highest percentage of apoptotic breast cancer cells was observed in mice treated with an AAL dose of 125 mg/Kg body weight, which was even higher than that in mice treated with 15 mg/Kg body weight methotrexate (Table 1). The lowest percentage of breast cancer cells expressed Apaf-1 and Cyt c (Table 2), indicating low apoptosis in breast cancer cells. Apaf-1 expression is low in most cancer cells compared with that in normal cells (Loginov et al., 2017). AAL leaf extract effectively inhibits the course of Dalton’s lymphoma by attenuating the PKCα signaling pathway and increasing apoptosis through the mitochondrial cascade (Singh et al., 2021). The extract from Achyranthes aspera inhibited the antiproliferative activity of pancreatic cancer cells by selectively suppressing the transcription of metalloproteases (MMP-1 and -2), MMP inhibitors (TIMP-2), and angiogenic factors (VEGF-A and VEGF-B) (Subbarayan et al., 2010). AAL pharmacological properties made it a promising cancer treatment option for thyroid carcinoma in the docking experiments (Alamri et al., 2023). Based on the 2,2-diphenylpicrylhydrazyl radical scavenging assess antioxidant activity, AAL leaves exhibit significant promise as an anticancer drug (Bashir et al., 2024).
Fig. 2. Apaf-1 (left) and Cyt c (right) expression in breast cancer cells. Low Apaf-1 expression causes cancer cells to be protected from apoptosis (Bakhshoudeh et al., 2021). APAF-1 plays an essential role in DNA damage-induced apoptosis (Eskandari-Nasab and Hashemi, 2017). Furthermore, Apaf-1 is a crucial molecule that determines whether Cyt c is released from mitochondria to form apoptosomes (Shakeri et al., 2017). Competitive binding between Apaf-1 and Cyt c may inhibit apoptosis in breast cancer cells (Jemmerson et al., 2021). The 125 mg/Kg body weight dose of AAL alkaloid fraction increased Cyt c and Apaf-1 expression in breast cancer-bearing mice. This effect was comparable to that observed after treatment with 15 mg/Kg body weight of methotrexate (Table 2). High Apaf-1 expression inhibits the viability and colony formation ability and promotes apoptosis of breast cancer cells (Fang et al., 2019). Cyt c is oxidized by ROS with Apaf-1 to form apoptosomes (Matsuura et al., 2016). In addition, Cyt C acts to transfer electrons in the mitochondrial redox process, activates caspase cascades, and triggers apoptosis in breast cancer cells (Abramczyk et al., 2022). Table 2. Breast cancer cells express cytochrome c and Apaf-1 proteins.
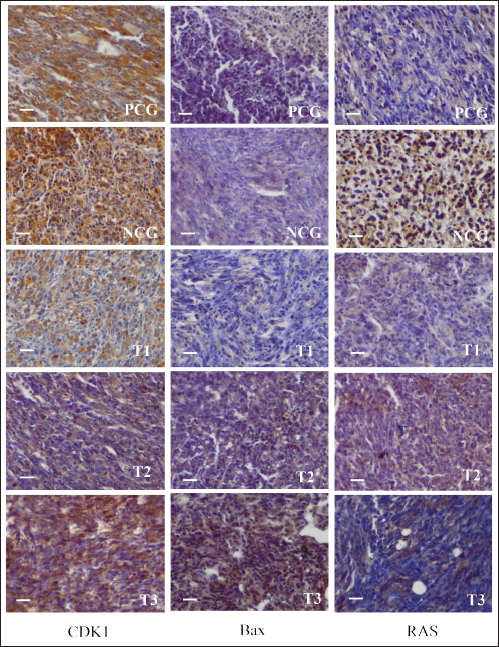
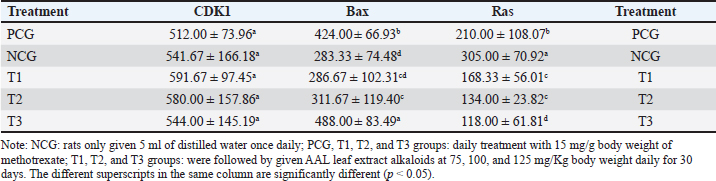
Fig. 3. CDK1 (left), Bax (center), and Ras (right) expression in breast cancer cells. CDK1 is a major cell cycle protein kinase regulator that regulates the normal function of cell mitosis (Diril et al., 2012) by combining cell proliferation with protein synthesis (Haneke et al., 2020). CDK1 initiates and elevates protein synthesis in cells related to mitotic division and cytokinesis (Kalous et al., 2020). In this study, no difference in CDK1 expression was observed after treatment of breast cancer-bearing mice with placebo, methotrexate, or AAL. This indicates that breast cancer cell apoptosis induced by AAL treatment did not involve CDK1 (Table 3). These results are comparable to the results of an attempt to induce apoptosis using solid lipid nanoparticles in tamoxifen without cell cycle arrest in breast cancer (Abbasalipourkabir et al., 2016). Table 3. Breast cancer cells expressing CDK1, Bax, and Ras proteins.
BAX is a proapoptotic member of the Bcl-2 gene family encoding the Bax-alpha protein. The Bax-alpha association forming the Bcl-2 heterodimer functions as an apoptosis activator (Saddam et al., 2024) by increasing the opening of mitochondrial voltage-dependent anion channels, followed by decreasing membrane potential and Cyt C release (Grosser et al., 2021). Lower Bax protein expression in breast cancer cells may lead to the avoidance of apoptosis of cancer cells. A correlation was reported between the Bax, p53, and caspase-3 proteins and apoptotic mechanisms in breast cancer cells (Pluta et al., 2011). Low levelBaxession can be a risk factor for breast cancer malignant tumors (Kholoussi et al., 2014). Breast cancer-bearing mice treated with a placebo exhibited the lowest Bax expression. The low expression levels of Bax indicate apoptosis and high breast cancer malignant tumors (Chen et al., 2021). The highest percentage of breast cancer cells expressing Bax was noted in breast cancer-bearing mice given the 125 mg/Kg body weight alkaloid fraction of AAL, which was even higher than that in mice receiving the 15 mg/Kg body weight methotrexate treatment (Table 3). These results are comparable to those of capsaicin, which increases Bax protein expression, activates caspase-3, induces apoptosis, and inhibits breast cancer proliferation (Chen et al., 2021). The experimental breast cancer-bearing mice in the placebo group exhibited the highest Ras expression. The percentage of breast cancer cells with the lowest Ras expression was detected in breast cancer-bearing mice given the 125 mg/Kg body weight alkaloid fraction of AAL, which was even lower than that observed in mice treated with 15 mg/Kg body weight methotrexate treatment (Table 3). Ras functions as an ON and OFF switch during signal transduction. Mutations in the Ras regulator cause normal cells to undergo malignant transformation (Simanshu et al., 2017). Pathologically, RAS mutations occur in breast cancer because of the overexpression of growth factor receptors. High mitogen-activated protein kinase activity correlates with Ras overexpression in breast cancer (von Lintig et al., 2000). Oncogenic Ras plays a role in the development and spread of metastases and resistance to therapy in breast cancer (Galie, 2019; Gimple and Wang, 2019). Ras oncogene p21, an essential component of the Ras signaling pathway, is hyperactivated in breast cancer, resulting in poor prognosis. Thus, low Ras expression is a target in breast cancer therapy (Banys-Paluchowski et al., 2018; Prior et al., 2020). ConclusionOverall, oral treatment of breast cancer-bearing mice with the 125 mg/Kg body weight alkaloid fraction of AAL extract daily for 30 days resulted in the highest rate of cancer cell apoptosis. This finding was supported by the highest expression of Cyt c, Apaf-1, and Bax and the lowest expression of Ras. However, the mechanism of apoptotic induction by AAL is not known. Therefore, further research into the complete signaling mechanism is necessary. AcknowledgmentsThe researchers would like to thank the Faculty of Veterinary Medicine, Airlangga University, for all the facilities provided during the research. Author’s contributionsWW, DKM, SM, and IM: conceived the idea and manuscript drafting. ARK, DMSP, JJ, RR, and NS: acquisition, analysis, and interpretation of data. AOA, RZA, SU, FE, and WW: The manuscript was critically read and revised for intellectual content. All authors have read and approved the final manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Conflict of interestThe authors declare no conflict of interest. FundingThe authors express their gratitude for the support provided by Universitas Airlangga, Indonesia (contract number: 314/LF.FKH.UA/2021. The authors thank Ulul Khoiriyah for providing technical support. We wish to thank the Center for Journal Development and Scientific Publication of Airlangga University and Enago English editing services for proofreading the manuscript. Data availabilityAll data are available in the manuscript. ReferencesAbbasalipourkabir, R., Salehzadeh, A. and Abdullah, R. 2016. Tamoxifen-loaded solid lipid nanoparticle-induced apoptosis in breast cancer cell lines. J. Exp. Nanosci. 11(3), 161–174. Abramczyk, H., Brozek-Pluska, B. and Kopeć, M. 2022. Double face of cytochrome c in cancers by Raman imaging. Sci. Rep. 12(1), 2120. Alamri, AA. M. Alkhilaiwi, FF. A. Khan, N.U. and Tasleem, M. 2023. In silico screening and validation of Achyranthes aspera as a potential inhibitor of BRAF and NRAS in controlling thyroid cancer. Anticancer Agents Med. Chem. 23(19), 2111–2126. An, L., Dou, X., Wang, M., Luo, W., Ma, Q. and Liu, X. 2020. Involvement of TNF-alpha and IL-10 in breast cancer and patient survival. Trop. J. Pharm. Res. 19(10), 2033–2039. Bakhshoudeh, M., Mehdizadeh, K., Hosseinkhani, S. and Ataei, F. 2021. Upregulation of apoptotic protease activating factor-1 expression correlates with antitumor effects of taxane drug. Med. Oncol. 38(8), 88. Banys-Paluchowski, M., Fehm, T., Janni, W., Aktas, B., Fasching, P.A., Kasimir-Bauer, S., Milde-Langosch, K., Pantel, K., Rack, B., Riethdorf, S., Solomayer, E.F., Witzel, I. and Müller, V. 2018. Elevated serum RAS p21 level is an independent prognostic factor in metastatic breast cancer. BMC Cancer 18(1), 541. Bashir, H., Sadia, S., Saddiqe, Z., Munir, M., Bai, X., Jia, M. and Ahmad, K.S. 2024. Application of microscopy and spectroscopyto investigate the anticancerr potential of Achyranthes aspera L leaves. Microsc. Res. Tech. 87(5), 1031–1043. Bhattacharyya, G.S., Doval, D.C., Desai, C.J., Chaturvedi, H., Sharma, S. and Somashekhar, S.P. 2020. Overview of breast cancer and the implications of overtreatment for early-stage breast cancer: an indian perspective. JCO Glob. Oncol. 6(1), 789–798. Byvaltsev, V.A., Bardonova, L.A., Onaka, N.R., Polkin, R.A., Ochkal, S.V., Shepelev, V.V., Aliyev, M.A. and Potapov, A.A. 2019. Acridine orange: a review of novel applications for surgical cancer imaging and therapy. Front. Oncol. 9(1), 925. Calaf, G.M. and Abarca-Quinones, J., 2016. Ras protein expression as a marker for breast cancer. Oncol. Lett. 11(6), 3637–3642. Chen, M., Xiao, C., Jiang, W., Yang, W., Qin, Q., Tan, Q., Lian, B., Liang, Z. and Wei, C. 2021. Capsaicin inhibits proliferation and induces apoptosis in breast cancer by down-regulating FBI-1-mediated NF-κB pathway. Drug Des. Devel. Ther. 15(1), 125–140. Clement, T.M., Inselman, A.L., Goulding, E.H., Willis, W.D. and Eddy, E.M. 2015. Disrupting cyclin dependent kinase 1 in spermatocytes causes late meiotic arrest and infertility in mice. Biol. Reprod. 93(6), 137. Contiero, P., Boffi, R., Borgini, A., Fabiano, S., Tittarelli, A., Mian, M., Vittadello, F., Epifani, S., Ardizzone, A., Cirilli, C., Boschetti, L., Marguati, S., Cascone, G., Tumino, R., Fanetti, A.C., Giumelli, P., Candela, G., Scuderi, T., Castelli, M., Bongiorno, S., Barigelletti, G., Perotti, V., Veronese, C., Turazza, F., Crivaro, M., Tagliabue, G. and MAPACA Working Group. 2023. Causes of death among women with breast cancer: risks and rates in a population-based cohort. Front. Oncol. 13(1), 1270877. Daniels, V.W., Zoeller, J.J., van Gastel, N., McQueeney, K.E., Parvin, S., Potter, D.S., Fell, G.G., Ferreira, V.G., Yilma, B., Gupta, R., Spetz, J., Bhola, P.D., Endress, J.E., Harris, I.S., Carrilho, E., Sarosiek, K.A., Scadden, D.T., Brugge, J.S. and Letai, A. 2021. Metabolic perturbations sensitize triple-negative breast cancers to apoptosis induced by BH3 mimetic. Sci. Signal 14(686), eabc7405. Deshpande, T.C. and Une, H.D. 2021. Effects of Achyranthes aspera Linn. Leave extract on reactive oxygen species (ROS) in diabetes-induced Rats by flow cytometry and possible molecular mechanism through molecular docking. Curr. Enzyme Inhib. 17(1), 71–81 Diril, M.K., Ratnacaram, C.K., Padmakumar, V.C., Du, T., Wasser, M., Coppola, V., Tessarollo, L. and Kaldis, P. 2012. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division andthe suppressionn of DNA rereplication, but not for liver regeneration. Proc. Natl. Acad. Sci. U S A 109(10), 3826–3831. Eskandari-Nasab, E. and Hashemi, M. 2017. Promoter methylation and mRNA expression of the APAF-1 gene in breast cancer. Gene Cell. Tissue 4(1), e13332. Fang, H., Jiang, W., Jing, Z., Mu, X. and Xiong, Z. 2019. miR-937 regulates proliferation and apoptosis by targeting APAF1 in breast cancer. Onco Targets Ther. 12(1), 5687–5699. Faraji, P., Araj-Khodaei, M., Jafari, A., Ghaffari, M., Mohammadinasab, R., Hamedeyazdan, S., de la Guardia, M. and Dolatabadi, J.E.N. 2022. Anticancer effects of methotrexate in combination with Melissa officinalis on the HeLa cancer cell line. Crescent J. Med. Biol. Sci. 9(4), 189–194. Feldman, A.T. and Wolfe, D., 2014. Tissue processing and hematoxylin and eosin staining. Methods: Mol. Biol. 1180(1), 31–43. Galiè, M. 2019. RAS as a supporting actor in breast cancer. Front. Oncol. 9(1), 1199. Gimple, R.C. and Wang, X., 2019. RAS: striking at the core of the oncogenic circuitry. Front. Oncol. 9(1), 965. Grosser, J.A. Maes, M.E. and Nickells, R.W. 2021. Characteristics of the intracellular propagation of mitochondrial BAX recruitment during apoptosis. Apoptosis 26(1–2), 132–145. Guo, J., Xu, Y., Ji, West, Song, L., Dai, C. and Zhan, L 2015. Effects of exposure to benzo[a]pyrene on metastasis of breast cancer are mediated through ROS-ERK-MMP9 axis signaling. Toxicol. Lett. 234(3), 201–210. Guo, X., Xiang, C., Zhang, Z., Zhang, F., Xi, T. and Zheng, L 2018. Displacement of Bax by BMF mediates STARD13 3′UTR-induced breast cancer cell apoptosis in an miRNA-dependent Manner. Mol. Pharm. 15(1), 63–71. Haidinger, R. and Bauerfeind, I., 2019. Long-term adverse effects of adjuvant therapy in patients with primary breast cancer: results of a web-based survey. Breast Care (Basel) 14(2), 111–116. Haneke, K., Schott, J., Lindner, D., Hollensen, A.K., Damgaard, C.K., Mongis, C., Knop, M., Palm, W., Ruggieri, A. and Stoecklin, G. 2020. CDK1 couples proliferation with protein synthesis. J. Cell. Biol. 219(3), e201906147. Hecht, F., Pessoa, C.F., Gentile, L.B., Rosenthal, D., Carvalho, D.P. and Fortunato, R.S. 2016. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 37(4), 4281–4291. Jemmerson, R., Staskus, K., Higgins, L., Conklin, K. and Kelekar, A. 2021. Intracellular leucine-rich alpha-2-glycoprotein-1 competes with Apaf-1 for binding cytochrome c to protect MCF-7 breast cancer cells from apoptosis. Apoptosis 26(1–2), 71–82. Kalous, J., Jansová, D. and Šušor, A. 2020. Role of cyclin-dependent kinase 1 in translational regulation of the M-Phase. Cells 9(7), 1568. Kholoussi, N.M., El-Nabi, S.E., Esmaiel, N.N., Abd El-Bary, N.M. and El-Kased, A.F. 2014. Evaluation of bax and bak gene mutations and expression in breast cancer. Biomed. Res. Int. 2014(1), 249372. Li, L., Fan, Y., Huang, X., Luo, J., Zhong, L., Shu, X.S., Lu, L., Xiang, T., Chan, A.T.C., Yeo, W., Chen, C., Chan, W.Y., Huganir, R.L. and Tao, Q. 2019. Tumor suppression of ras GTPase-activating protein RASA5 via antagonizing ras signaling perturbation in Carcinomas. iScience 21(1), 1–18. Liu, K., Liu, P.C., Liu, R. and Wu, X. 2015. Dual AO/EB staining for detecting apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 21(1), 15–20. Loginov, V.I., Pronina, I.V., Burdennyi, AM, Pereyaslova, E.A., Braga, E.A., Kazubskaya, T.P. and Kushlinskii, N.E. 2017. Role of methylation in the regulation of the apoptosis genes APAF1, DAPK1, and BCL2 in breast cancer. Bull. Exp. Biol. Med. 162(6), 797–800. Manne, R.K., Agrawal, Y., Malonia, S.K., Banday, S., Edachery, S., Patel, A., Kumar, A., Shetty, P. and Santra, M.K. 2021. FBXL20 promotes breast cancer malignant tumor by inhibiting apoptosis through degradation of PUMA and BAX. J. Biol. Chem. 297(4), 101253. Matsuura, K., Canfield, K., Feng, W. and Kurokawa, M. 2016. Metabolic regulation of apoptosis in cancer. Int. Rev Cell. Mol. Biol. 327(1), 43–87. Meles, D.K., Wurlina, W. and Adnyana, D.P.A. 2017. Measurement of alkaloids Achyranthes aspera Linn level using thin layer chromatography method and high-performance liquid chromatography. KnE Life Sci. 3(6), 378–385. Mercogliano, M.F., Bruni, S., Elizalde, P.V. and Schillaci, R. 2020. Tumor necrosis factor α blockade: an opportunity to tackle breast cancer. Front. Oncol. 10(1), 584. Mirzayans, R., Andrais, B. and Murray, D. 2018. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers (Basel) 10(4), 118. Nargatti, P., Patil, S. and Wadkar, K. 2021. Phytochemical profile and pharmacological aspects of Achyranthes aspera linn: an overview. J. Pharm. Res. Int. 33(34B), 187–206. Nie, B.X., Zhao, G., Yuan, X.F., Yu, L.X., Zhang, J., Yuan, Y., Liu, Y., Hu, J., Song, E., Zhou, Y.C. and Shu, J. 2022. Inhibition of CDK1 attenuates neuronal apoptosis and autophagy and confers neuroprotection after chronic spinal cord injury in vivo. J. Chem. Neuroanat. 119(1), 102053. Peng, F., Liao, M., Qin, R., Zhu, S., Peng, C., Fu, L., Chen, Y. and Han, B. 2022. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct. Target Ther. 7(1), 286. Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., Castoria, G. and Migliaccio, A. 2020. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 52(2), 192–203. Pfeffer, C.M. and Singh, A.T.K., 2018. Apoptosis: a target for anticancer therapy. Int. J. Mol. Sci. 19(2), 448. Pluta, P., Smolewski, P., Pluta, A., Cebula-Obrzut, B., Wierzbowska, A., Nejc, D., Robak, T., Kordek, R., Gottwald, L., Piekarski, J. and Jeziorski, A. 2011. Significance of Bax expression in patients with breast cancer. Pol. Przegl. Chir. 83(10), 549–553. Ponraj, T., Vivek, R., Paulpandi, M., Rejeeth, C., Babu, V.N., Vimala, K., Anand, K., Sivaselvam, S., Vasanthakumar, A., Ponpandian, N. and Kannan, S. 2018. Correction: Mitochondrial dysfunction-induced apoptosis in breast carcinoma cells through a pH-dependent intracellular quercetin NDDS of PVPylated-TiO2NPs. J. Mater. Chem. B. 6(27), 4539–4550. Prior, I.A., Hood, F.E. and Hartley, J.L. 2020. The frequency of ras mutations in cancer. Cancer Res. 80(14), 2969–2974. Raju, S.K., Kumar, S., Sekar, P., Murugesan, M., Karthikeyan, M., Elampulakkadu, A. and Arthanari, M. 2022. Therapeutic and pharmacological efficacy of Achyranthes aspera Linn.: an updated review. Drug Deliv. Ther. 12(3), 202–214. Redza-Dutordoir, M. and Averill-Bates, D.A. 2016. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta. 1863(12), 2977–2992. Roberts, J.Z., Crawford, N. and Longley, D.B. 2022. The role of Ubiquitination in apoptosis and necroptosis. Cell Death Differ. 29(1), 272–284. Saddam, M., Paul, S.K., Habib, M.A., Fahim, A., Mimi, A., Islam, S., Paul, B. and Helal, M.M.U. 2024. Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and antiapoptotic context. Egypt J. Med. Hum. Genet. 25(1), 12. Sarmiento-Salinas, F.L., Delgado-Magallón, A., Montes-Alvarado, J.B., Ramírez-Ramírez, D., Flores-Alonso, J.C., Cortés-Hernández, P., Reyes-Leyva, J., Herrera-Camacho, I., Anaya-Ruiz, M., Pelayo, R., Millán-Pérez-Peña, L. and Maycotte, P. 2019. Breast cancer subtypes present different reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Front. Oncol. 9(1), 480. Schmitt, E.E., Barhoumi, R., Metz, R.P. and Porter, W.W., 2017. Circadian regulation of Benzo[a]Pyrene metabolism and DNA adduct formation in breast cells and the mouse mammary gland. Mol. Pharmacol. 91(3), 178–188. Sedeman, M., Christowitz, C., de Jager, L. and Engelbrecht, AM 2022. Obese mammary tumor-bearing mice are highly sensitive to doxorubicin-induced hepatotoxicity. BMC Cancer 22(1), 1240. Shakeri, R., Kheirollahi, A. and Davoodi, J. 2017. Apaf-1: regulation and function in cell death. Biochimie 135(1), 111–125. Simanshu, D.K., Nissley, D.V. and McCormick, F. 2017. RAS proteins and their regulators in human disease. Cell 170(1), 17–33. Singh, R.K., Verma, P.K., Kumar, A., Kumar, S. and Acharya, A. 2021. Achyranthes aspera L leaf extract induces anticancer effects on dalton’s Lymphoma via regulation of PKCα signaling pathway and mitochondrial apoptosis. J. Ethnopharmacol. 274(1), 114060. Song, C., Zhang, Y., Manzoor, M.A. and Li, G. 2022. Identification of alkaloids and related intermediates of D. officinale by solid-phase extraction coupled with high-performance liquid chromatography tandem mass spectrometry. Front. Plant Sci. 13(1), 952051. Subbarayan, P.R., Sarkar, M., Impellizzeri, S., Raymo, F., Lokeshwar, B.L., Kumar, P., Agarwal, R.P. and Ardalan, B. 2010. Antiproliferative and anticancer properties of Achyranthes aspera: specific inhibitory activity against pancreatic cancer cells. J. Ethnopharmacol. 131(1), 78–82. Tata, A., Woolman, M., Ventura, M., Bernards, N., Ganguly, M., Gribble, A., Shrestha, B., Bluemke, E., Ginsberg, H.J., Vitkin, A., Zheng, J. and Zarrine-Afsar, A. 2016. Rapid detection of necrosis in breast cancer with desorption electrospray ionization mass spectrometry. Sci. Rep. 6(1), 35374. Thakur, B., Kumar, Y. and Bhatia, A. 2019. Programmed necrosis and its role in management of breast cancer. Pathol. Res. Pract. 215(11), 152652. Vakili, S.A., George, A., Ayatollahi, S.A., Martorell, M., Ostrander, E.A., Salehi, B., Martins, N. and Sharifi-Rad, J. 2020. Phenolic compounds, saponins,, and alkaloids in cancer progression: effects of p53 expression and telomere length. Cell. Mol. Biol. (Noisy-le-grand) 66(4), 110–119. Venkatachalam, P. and Nadumane, V.K. 2019. Modulation of the Bax and Bcl-2 genes by secondary metabolites produced by Penicillium rubens JGIPR9 causes the apoptosis of cancer cell lines. Mycology 12(2), 69–81. Von Lintig, F.C., Dreilinger, A.D., Varki, N.M., Wallace, AM, Casteel, D.E. and Boss, G.R. 2000. Ras activation in human breast cancer. Breast Cancer Res. Treat. 62(1), 51–62. Wang, Y., Qi, H., Liu, Y., Duan, C., Liu, X., Xia, T., Chen, D., Piao, H.L. and Liu, H.X. 2021. Double-edged roles of ROS in cancer prevention and therapy. Theranostics 11(10), 4839–4857. Watkins, E.J. 2019. Overview of breast cancer. JAAPA 32(10), 13–17. Yuan, L., Cai, Y., Zhang, L., Liu, S., Li, P. and Li, X. 2022. Promoting apoptosis as a promising way to treat breast cancer with natural products: a comprehensive review. Front. Pharmacol. 12(1), 801662. Yue, J. and López, J.M. 2020. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 21(7), 2346. Zlobec, I., Steele, R., Michel, R.P., Compton, C.C., Lugli, A. and Jass, J.R. 2006. Scoring of p53, VEGF, Bcl-2, and APAF-1 immunohistochemistry and interobserver reliability in colorectal cancer. Mod. Pathol. 19(9), 1236–1242. | ||
| How to Cite this Article |
| Pubmed Style Wurlina W, Meles DK, Mustofa I, Khairullah AR, Putra DMS, Suwasanti N, Akintunde AO, Utama S, Mulyati S, Wasito W, Raissa R, Ahmad RZ, Julaeha J, Ekawasti F. Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model. Open Vet. J.. 2025; 15(3): 1279-1288. doi:10.5455/OVJ.2025.v15.i3.19 Web Style Wurlina W, Meles DK, Mustofa I, Khairullah AR, Putra DMS, Suwasanti N, Akintunde AO, Utama S, Mulyati S, Wasito W, Raissa R, Ahmad RZ, Julaeha J, Ekawasti F. Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model. https://www.openveterinaryjournal.com/?mno=232024 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.19 AMA (American Medical Association) Style Wurlina W, Meles DK, Mustofa I, Khairullah AR, Putra DMS, Suwasanti N, Akintunde AO, Utama S, Mulyati S, Wasito W, Raissa R, Ahmad RZ, Julaeha J, Ekawasti F. Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model. Open Vet. J.. 2025; 15(3): 1279-1288. doi:10.5455/OVJ.2025.v15.i3.19 Vancouver/ICMJE Style Wurlina W, Meles DK, Mustofa I, Khairullah AR, Putra DMS, Suwasanti N, Akintunde AO, Utama S, Mulyati S, Wasito W, Raissa R, Ahmad RZ, Julaeha J, Ekawasti F. Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1279-1288. doi:10.5455/OVJ.2025.v15.i3.19 Harvard Style Wurlina, W., Meles, . D. K., Mustofa, . I., Khairullah, . A. R., Putra, . D. M. S., Suwasanti, . N., Akintunde, . A. O., Utama, . S., Mulyati, . S., Wasito, . W., Raissa, . R., Ahmad, . R. Z., Julaeha, . J. & Ekawasti, . F. (2025) Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model. Open Vet. J., 15 (3), 1279-1288. doi:10.5455/OVJ.2025.v15.i3.19 Turabian Style Wurlina, Wurlina, Dewa Ketut Meles, Imam Mustofa, Aswin Rafif Khairullah, Dewa Made Sucipta Putra, Niluh Suwasanti, Adeyinka Oye Akintunde, Suzanita Utama, Sri Mulyati, Wasito Wasito, Ricadonna Raissa, Riza Zainuddin Ahmad, Julaeha Julaeha, and Fitrine Ekawasti. 2025. Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model. Open Veterinary Journal, 15 (3), 1279-1288. doi:10.5455/OVJ.2025.v15.i3.19 Chicago Style Wurlina, Wurlina, Dewa Ketut Meles, Imam Mustofa, Aswin Rafif Khairullah, Dewa Made Sucipta Putra, Niluh Suwasanti, Adeyinka Oye Akintunde, Suzanita Utama, Sri Mulyati, Wasito Wasito, Ricadonna Raissa, Riza Zainuddin Ahmad, Julaeha Julaeha, and Fitrine Ekawasti. "Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model." Open Veterinary Journal 15 (2025), 1279-1288. doi:10.5455/OVJ.2025.v15.i3.19 MLA (The Modern Language Association) Style Wurlina, Wurlina, Dewa Ketut Meles, Imam Mustofa, Aswin Rafif Khairullah, Dewa Made Sucipta Putra, Niluh Suwasanti, Adeyinka Oye Akintunde, Suzanita Utama, Sri Mulyati, Wasito Wasito, Ricadonna Raissa, Riza Zainuddin Ahmad, Julaeha Julaeha, and Fitrine Ekawasti. "Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model." Open Veterinary Journal 15.3 (2025), 1279-1288. Print. doi:10.5455/OVJ.2025.v15.i3.19 APA (American Psychological Association) Style Wurlina, W., Meles, . D. K., Mustofa, . I., Khairullah, . A. R., Putra, . D. M. S., Suwasanti, . N., Akintunde, . A. O., Utama, . S., Mulyati, . S., Wasito, . W., Raissa, . R., Ahmad, . R. Z., Julaeha, . J. & Ekawasti, . F. (2025) Alkaloid fraction of Achyranthes aspera Linn triggers breast cancer apoptosis in mice (Mus musculus) model. Open Veterinary Journal, 15 (3), 1279-1288. doi:10.5455/OVJ.2025.v15.i3.19 |