| Research Article | ||
Open Vet. J.. 2025; 15(3): 1289-1303 Open Veterinary Journal, (2025), Vol. 15(3): 1289-1303 Research Article Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigationsMohamed Tharwat1*, Hazem M. M. Elmoghazy2,3, Elhassan M. A. Saeed4, Abdulrahman A. Alkheraif41Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2University Veterinary Hospital, Qassim University, Buraidah, Saudi Arabia 3Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 4Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia *Corresponding Author: Mohamed Tharwat. Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia. Email: atieh [at] qu.edu.sa Submitted: 08/12/2024 Accepted: 16/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
AbstractBackground: Although renal abscessation is rarely reported in dromedary camels, it is increasingly detected in this species. Aim: This study aimed to investigate renal abscessation in camels with special emphasis on ultrasonographic findings and causative agents. Methods: Seventeen diseased camels (Camelus dromedarius) were examined. In addition, 10 healthy camels were used as the control group. A jugular puncture was performed to collect blood in EDTA tubes and serum samples. The thorax and abdomen were examined via transcutaneous and transrectal ultrasound. A free-hand ultrasound-guided aspiration technique using a 14G × 170 mm spinal biopsy needle was used for aspiration of the renal lesion content. Results: The signs of diseased camels included general gradual and/or progressive weakness, inappetence or anorexia, passage of dry feces, dysuria, bloody feces, lameness, bloody urine, and abdominal pain. Neutrophilic leukocytosis is the most significant hematological abnormality. Significant biochemical alterations included hyperproteinemia, azotemia, hyperglycemia, and increased serum alkaline phosphatase activity. Single or multiple renal abscesses were visualized sonographically compressing the renal parenchyma. Three abscesses measuring 5.2–15.0 cm were scanned in 2 camels; one affected the left and the other affected the right kidneys. In addition, two abscesses were imaged in 2 camels; both are affecting the left kidney. However, single abscesses measuring 3.6–14.0 cm in length were recorded in the remaining 13 camels; nine in the right and four in the left kidneys. The contents of the abscesses were hyperechogenic in 8 cases, hypoechoic in 4, isoechoic with hyperechoic foci in 3 cases, and heterogenous in 2. In 4 of the 17 diseased camels, hyperechoic urine and echogenic deposits were found within the urinary bladder. Bacteriological examination showed pure growth of Staphylococcus lugdunensis in 10 coagulase-negative isolates, Staphylococcus aureus in 5 coagulase-positive isolates, and 2 unidentified Staphylococcus species. Conclusion: Sonography is extremely helpful for verifying renal abscesses and is a good guide for abscess aspiration in dromedary camels. The isolation of S. lugdunensis from camel renal abscessation in this study was significant because of the zoonotic nature of this organism. Keywords: Camels, Kidney diseases, Renal abscessation, Staphylococcus, Ultrasound. IntroductionIn human medicine, renal abscessation is a rare condition, and if discovered, presentation is always non-specific, which delays the detection of the lesion; thus, severe complications, such as loss of the kidney and terminal death, may occur (Jiménez et al. 2022). Predisposing causes of infection of the urinary tract include diabetes mellitus and the presence of an underlying condition (Lim and Ng 2011; Tang and Cheong 2017). Bilateral renal abscesses were diagnosed by ultrasonography (US) and computed tomography (CT) in a 16-year-old girl; differential diagnosis included polycystic kidneys with infected cysts (Tang and Cheong 2017). The use of clinical symptoms and diagnostic imaging techniques, such as CT, magnetic resonance imaging, and the US, results in early detection and consequently improves prognosis (Rubilotta et al., 2014). In a report describing 56 consecutive cases of perirenal and renal abscesses, the causative organism in 44% of the cases was Escherichia coli (E. coli) and Klebsiella pneumoniae in 28% of the patients (Lee et al. 2008). In another 11-year retrospective investigation, Staphylococcus aureus was the causative organism in 50% of the cases, whereas E. coli was the etiological pathogen in 29% of the patients (Buschel et al. 2022). In veterinary medicine, the diagnosis of renal abscesses was reported to be linked to diabetes mellitus in a 9-year-old female Dalmatian. The dog in the latter case was diagnosed with abdominal US and aspiration of the abscess under ultrasound guidance and was treated by nephrectomy (Hess and Ilan 2003). Nephrectomy was also performed unilaterally in foal with a renal abscess (Trotter et al. 1984). Ultrasonography (US) and ultrasound-guided aspiration of the abscess were also performed for the diagnosis of renal abscesses in Boerboel dogs (Kitshoff et al. 2011). Bilateral renal abscessation caused by E. coli was previously reported in a male dromedary camel (Tharwat et al. 2018a). In another 6-year-old female dromedary camel reported with chronic suppurative pyelonephritis caused by Staphylococcus lugdunensis, unilateral surgical nephrectomy was performed (Tharwat et al. 2018b). In the latter 2 case reports, the results have stressed the usefulness of sonography for the detection and verification of renal abscesses and have also shown the effectiveness of ultrasound guidance for obtaining samples of kidney lesions. Ultrasound has also proven useful for the early detection and verification of different diseases affecting the dromedaries, as well as the scanning of healthy camels (Tharwat, 2024). This study was conducted to prospectively investigate the clinical presentation, sonographic and hematobiochemical findings, causative organisms, diagnosis, and treatment of 17 dromedary camels with right and left kidney abscesses. Materials and MethodsAnimals and clinical examinationsWe examined 17 camels (Camelus dromedarius) (15 females and 2 males) aged 7–15 years were examined at the Veterinary Hospital, Qassim University between 2019 and 2024. The camels presented with different complaints, including inappetence or anorexia, gradual or progressive decrease in body weight, fits of abdominal pain, weakness, dry feces, chronic lameness, and voiding of bloody urine. The duration of the disease ranged from 3 weeks to 6 months. Previous medications included antibiotics, anti-inflammatories, appetite stimulants, multi-minerals, and vitamins. Each camel was clinically examined upon admission. Examination includes measurement of respiratory and pulse rates and rectal temperature, inspection of visible mucosae, complete auscultation of the thorax and gastrointestinal tract, and transrectal ultrasonographic examination. Ten healthy dromedary camels from the university farm were used as the control group. Blood sampling and hematobiochemical parameter determinationFrom both diseased and healthy animals, a jugular puncture was performed to collect 2 blood samples. The first was on EDTA tubes for determination of leukogram (white blood cell count and its differentials) and hemogram (erythrocytes count, hemoglobin concentration, hematocrit percent, and erythrocytes indexes) parameters. The second sample was collected in plain tubes to harvest sera for the measurement of the concentrations of total protein, calcium, blood urea nitrogen (BUN), creatinine, inorganic phosphorus, glucose, and the activity of alkaline phosphatase (ALP). Thoracic and abdominal USThe thorax, including the heart and its major vessels, lungs, and pleurae, were visualized transcutaneously using a 3.5-MHz convex transducer (SonoScape, Sonoscape Medical Corp., China), as previously reported (Tharwat, 2024). In addition, the digestive system, including stomach compartments and small and large intestines, liver, peritoneum, and urinary system, including kidneys and urinary bladder, were visualized transcutaneously and per rectum using 3.5-MHz convex and 7.5-MHz linear probes, respectively, using the same ultrasound scanner (Tharwat et al. 2012a,b,c,d,e; Tharwat 2020a,b,c; Tharwat 2024; Tharwat et al. 2025a,b,c). Ultrasound-guided aspiration of lesion contentsImmediately after obtaining written approval from the owners for the purpose of aspiration of the abscesses, lesions were aspirated using a free-hand ultrasound-guided aspiration technique using a 14G × 170 mm spinal biopsy needle (Kurita Co., Ltd, Tokyo, Japan) as reported in cattle (Mohamed et al. 2002; Mohamed et al., 2003a,b,c; Mohamed and Oikawa, 2008). Initially, the right and left flanks were clipped, shaved, and non-septically prepared for aspiration of the right and left kidney abscesses, respectively. Two milliliters of Xylazine 2% (0.2 mg/Kg BW) (Xylased, Bioveta, Komenskeho, Czech Republic) were injected intravenously, and another 10 milliliters of procaine Hydrochloride 2% (Lidocaine Hydrochloride, Pharmaceutical Solutions Industry, Jeddah, Saudi Arabia) as an anesthetic solution was infiltrated locally. The puncture site was selected to allow the needle to advance through the thickest part of the lesion. With ultrasound guidance, the spinal needle was inserted into the center of one lesion using a free-hand technique (Fig. 1). Twenty to two hundred ml of the pus were collected using a sterile syringe (Fig. 2). The pus samples were immediately transferred to the bacteriology laboratory for the isolation and identification of the causative bacteria. The samples were enriched in Brain Heart Infusion Broth and then streaked on Sheep Blood Agar, which was aerobically incubated at 37°C for 48 hours. Gram-stained smears were made and examined for microscopic features. Pure colonies were examined for catalase and tube-coagulase reactions and subjected to biochemical identification using a VITEK 2 Compact system (bioMérieux, Craponne, France).
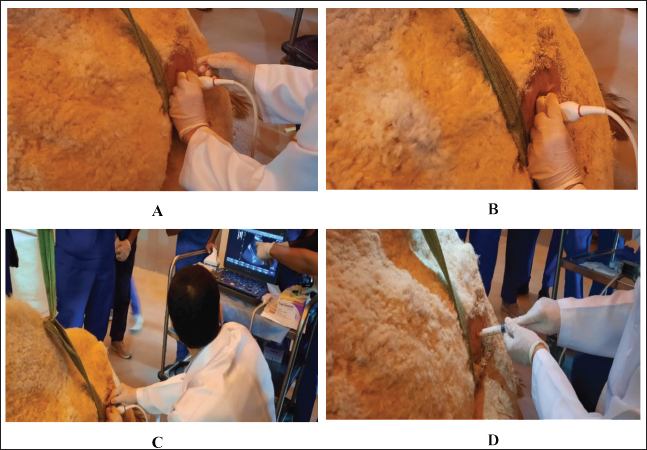
Fig. 1. Ultrasound-guided aspiration of abscess contents in the left kidney of camel number 14. (A). The needle was placed perpendicular to the transducer (B) and then inserted into the center of the abscess (C). Finally, the pus sample was collected (D).
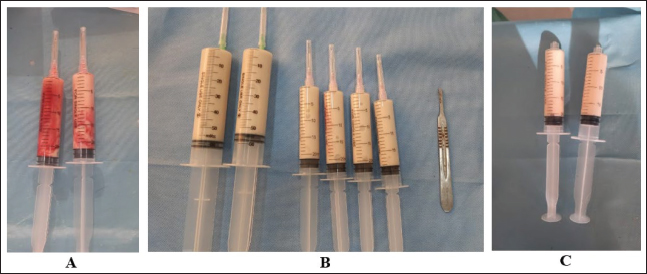
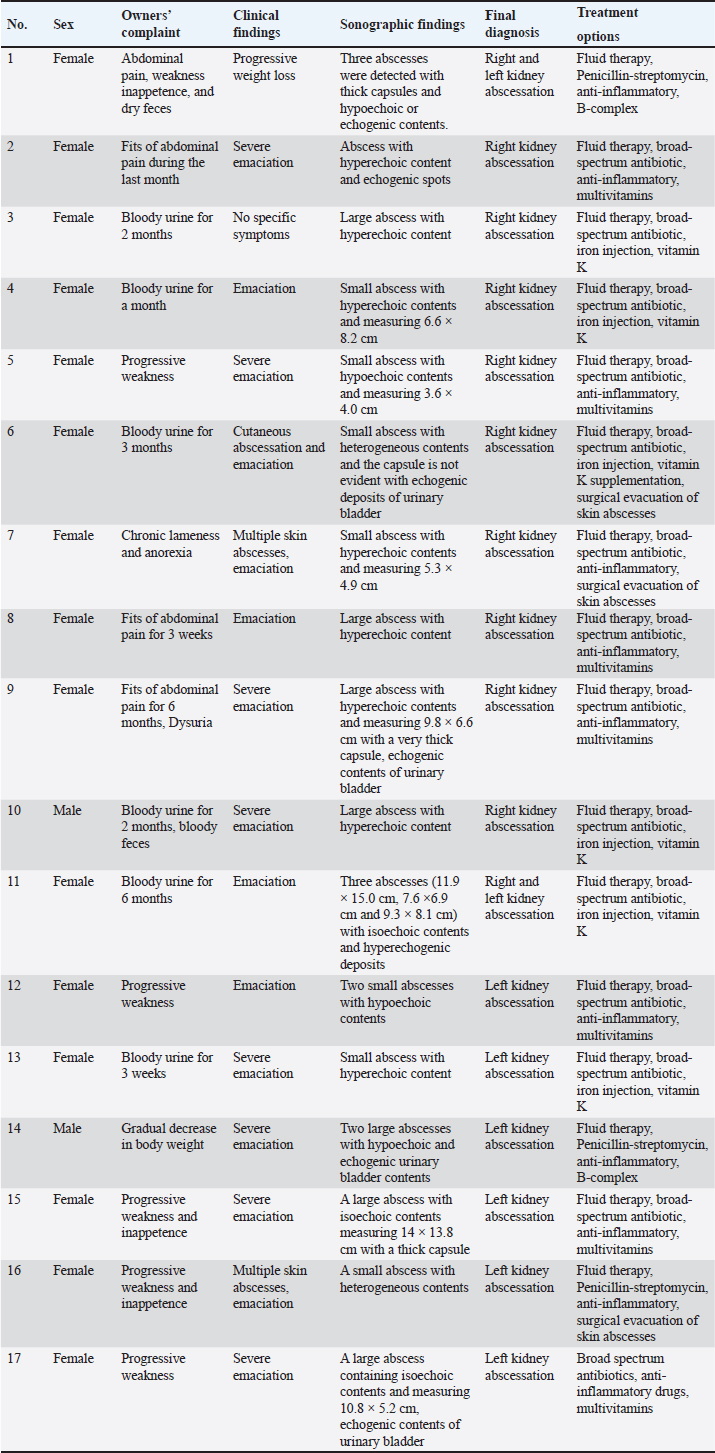
Fig. 2. Aspirated contents of abscess lesions in camels with abscessation of the kidneys. Figure A shows an approximately 50 ml pus sample collected from the right kidney abscess in camel number 1. Image B shows a large amount of pus (about 200 ml) aspirated from the left kidney abscess in camel number 14. Figure C shows approximately 40 ml of abscess lesions collected from the left kidney abscess of camel number 15. Ethical approvalCamels were handled and treated based on the Guidelines of Laboratory Animal Control Ethics of Qassim University, which were basically aligned with the Guide for the Use and Care and Laboratory Animals of the National Institutes of Health in the USA (US Department of Health and Human Services, 1996). ResultsComplete details including owner complaints, clinical symptoms, sonographic findings, diagnosis, and treatment options for the 17 dromedary camels with right and left kidney abscesses are presented in Table 1. Different presenting history was recorded for the diseased camels. Presenting signs included general gradual and/or progressive weakness, inappetence and/or anorexia, the passage of dry feces, dysuria, bloody feces, and lameness. Six of the examined camels (35.3%) had a history of bloody urine for periods ranging from 3 weeks to 6 months. In addition, four other camels (23.5%) were reported to have signs of abdominal pain. The clinical findings in the diseased camels included multiple skin abscesses in three camels (17.6%) and severe emaciation in 15 camels (88.2%) (Fig. 3). Table 2 shows the hematobiochemical parameters in camels with right and left kidney abscessation compared with healthy controls. Neutrophilic leukocytosis was the most significant hematological abnormality (p < 0.01). The hemogram profiles did not reveal significant differences. Significant biochemical alterations included hyperproteinemia (p < 0.05), azotemia (p < 0.01), hyperglycemia (p < 0.01), and increased serum activity of ALP (p < 0.01). Other biochemical serum variables, including calcium, creatinine, and inorganic phosphorus did not show any significant alterations. Table 1. Owner complaints, symptoms, sonographic findings, diagnosis, and treatment in 17 dromedary camels with right and left kidney abscesses.
Single or multiple renal abscesses were visualized sonographically compressing the renal parenchyma. Three abscesses measuring 5.2–15.0 cm were scanned in 2 camels (11.8%); one affected the left and the other affected the right kidneys. In addition, 2 abscesses were imaged in 2 camels; both affected the left kidney. However, single abscesses measuring 3.6–14.0 cm in length were recorded in the remaining 13 camels; 9 in the right and 4 in the left kidneys. The contents of the abscesses were hyperechogenic in 8 cases (47.1%), hypoechoic in 4 (23.5%), isoechoic with hyperechoic foci in 3 cases (17.6%), and heterogenous in 2 (11.8%). In 4 of the diseased camels (23.5%), hyperechoic urine together with echogenic deposits was found within the urinary bladder (Fig. 6 and Fig. 10). Overall, unilateral lesions were found in the right kidneys only in 9 cases (52.9%) and in the left kidneys only in 6 cases (35.3%), while bilateral affection of both kidneys was found in 2 cases (11.7%). In the control camels, both kidneys appeared subjectively normal with no detectable lesions.
Fig. 3. Progressive body weight loss and emaciation in camels with both right and left (number 1), right (number 9) and left (number 13 and 14) kidney abscessation. Table 2. Hematobiochemical parameters of camels with right and left kidney abscessation compared with healthy controls.
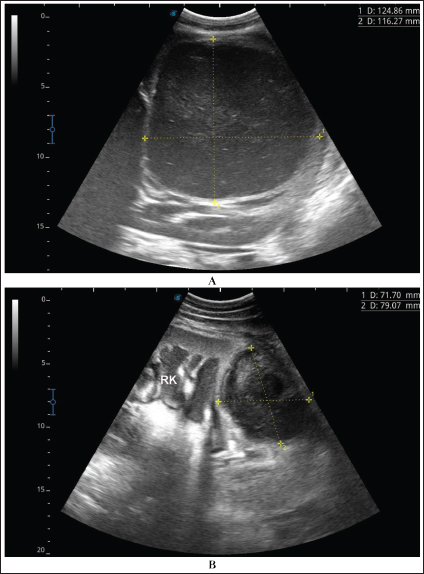
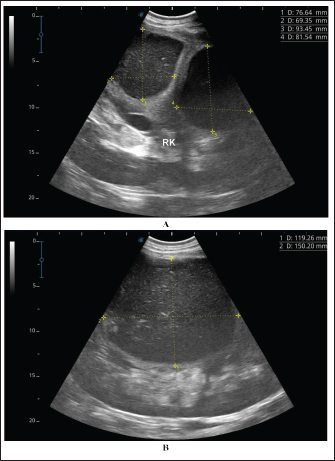
Fig. 4. Ultrasonographic findings of camel number 1 with abscessation of the right kidney. A large abscess was found in image A measuring 12.5 × 11.6 cm with hypoechoic contents and a thick capsule. The abscess in image B compresses the parenchyma of the right kidney (RK) and measuring 7.2 × 7.9 cm with echogenic contents and a thick capsule. A detailed description of the renal abscesses is presented in Figures 4–10. Figure 4 shows sonographic findings in camel number 1 with abscessation of the right and left kidneys. A large abscess measuring 12.5 × 11.6 cm with hypoechoic contents and a thick capsule was detected. A second abscess in the same camel was imaged compressing the parenchyma of the right kidney and measuring 7.2 × 7.9 cm with echogenic contents and a thick capsule. Figure 5 shows ultrasonographic results in camel numbers 2 and 4 with right kidney abscesses. The lesions in camel number 2 appeared markedly large, compressing the renal parenchyma, with echogenic contents and a thickened capsule. The lesion in camel number 4 appeared to compress the renal parenchyma of the right kidney, measuring 6.7 × 8.2 cm with echogenic contents and a thick capsule. In addition, Figure 6. clarifies sonographic findings in camel number 6, where the abscess appeared within the right kidney. The contents were heterogeneous, but the capsule could not be imaged. The urinary bladder in the same animal was imaged with echogenic deposits that appeared highly echogenic compared with the echogenic urine. Ultrasonographic findings in camel number 9 with right kidney abscesses are shown in Figure 7, where the lesion appeared compressing the parenchyma of the right kidney. Its contents are hyperechoic, and its capsule is thick. Figure 8 shows sonographic findings in camel number 11 with abscessation of the right and left kidneys. Abscesses measured 7.6 × 6.9 cm and 9.3 × 8.1 cm with isoechoic contents. A third abscess in the same camel was found within the left kidney. It was relatively large and was isoechoic. Ultrasonographic findings in camels 15 and 16 with abscesses of the left kidney are shown in Figure 9. A large abscess was found in camel number 15 with isoechoic contents and a thick capsule, while a small abscess was imaged in the left kidney of animal number 16 with heterogeneous contents. Figure 10 shows the ultrasonographic findings in camel number 17 with abscessation of the left kidney. A large abscess was found compressing the parenchyma of the left kidney with isoechoic contents. The urinary bladder in the same camel contained echogenic deposits that appeared to be highly echogenic.
Fig. 5. Ultrasonographic findings in camel numbers 2 (A) and 4 (B) with right kidney abscesses. The abscess in image A appeared markedly large and compresses the renal parenchyma with echogenic contents and a thickened capsule (stars). The lesion in image B is compressing the parenchyma of the right kidney (RK) and measuring 6.7 × 8.2 cm with echogenic contents and a thick capsule. Unfortunately, all owners did not accept surgical interference for nephrectomy of the affected kidney. Therefore, supportive fluid therapy, broad-spectrum antibiotics, anti-inflammatories, multivitamins, iron, and vitamin K were prescribed for camels for 5 successive days. The camels were discharged from the clinic within 3–5 hours after completing examinations. Follow-up telephone calls 1 month later revealed that five (29.4%) recovered fully, 7 (41.2%) did not show any improvement and were, therefore, slaughtered, and two (11.8%) died but were not necropsied. However, we could not contact the owners of the remaining three cases. Unfortunately, attendance at slaughter procedures was not available.
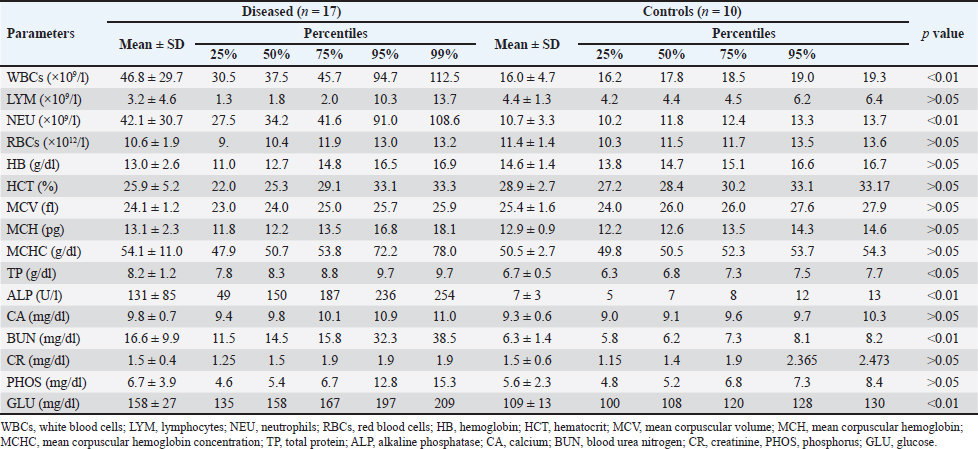
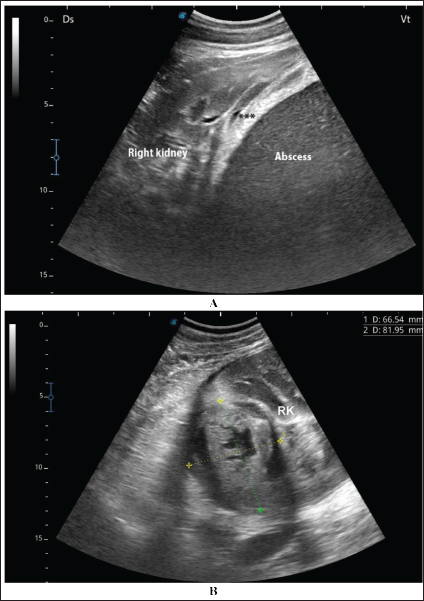
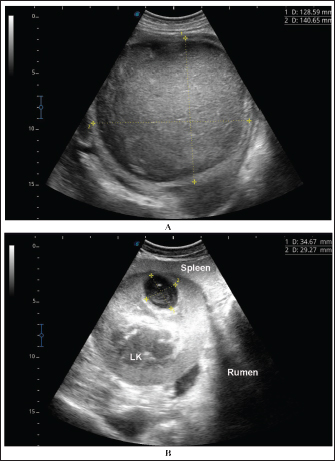
Fig. 6. Ultrasonographic findings in camel number 6. The lesion in image A appeared within the right kidney (RK). The contents are heterogenous, and the capsule is not evident. Image B shows the urinary bladder (UB) in the same animal with echogenic deposits that appeared highly echogenic compared with the echogenic urine. Bacteriological examination showed pure growth of a single type of colonies from all samples, which were Gram-positive cocci with grape-like clusters, catalase-positive (all samples), and coagulase-negative (12 samples). Results of biochemical identification by the Vitek 2 system revealed S. lugdunensis in 10 coagulase-negative isolates, S. aureus in 5 coagulase-positive isolates (probability of ≥ 98%), while 2 isolates (coagulase-negative) were unidentified. DiscussionPrevious studies by Tharwat et al. 2018b and 2018a reported signs like loss of appetite, body weight, and oliguria in camels with unilateral or bilateral kidney abscessation. In the current investigation, with the exception of 6 out of the 17 diseased camels that are presented with a history of bloody urine, remaining animals were presented with non-specific symptoms including general weakness, decrease or complete loss of appetite, emaciation, dry feces, dysuria, skin abscessation, bloody feces, and lameness. Four camels were also admitted with a history of abdominal pain. Therefore, camels with renal abscesses in the present study had various and diverse symptoms that did not indicate renal involvement, except in approximately one-third of the sick camels. Concerning hematological abnormalities, neutrophilic leukocytosis suggesting underlying bacterial infection was the only and most significant abnormality. Generally, studies in humans with renal abscessation reported that 80% of the patients had leukocytosis (Jiménez et al. 2022), and additionally, the total leukocytic count was significantly higher in those with renal and peri-renal abscesses (Lee et al. 2008; Tang and Cheong, 2017). In camels, severe neutrophilic leukocytosis was reported in two previously published 2 case reports (Tharwat et al. 2018a,b). Some of the tested biochemical metabolites differed significantly compared with the controls. Changes included hyperproteinemia, azotemia, hyperglycemia, and increased ALP serum activity. Increased serum protein concentrations, azotemia, and hyperglycemia were also found in 2 camels with renal abstention (Tharwat et al. 2018a,b). In cattle, Floeck (2007) observed azotemia in more than 50% of cows with pyelonephritis. Human studies have also reported hypoalbuminemia in patients with renal and perirenal abscesses and acute pyelonephritis (Lee et al. 2008; Lim and Ng, 2011). Interestingly, changes in creatinine concentration in the latter study and in our current investigation were non-significant compared with controls (Lee et al. 2008).
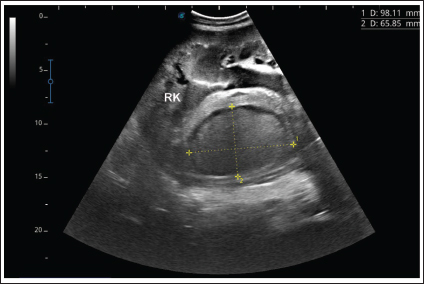
Fig. 7. Ultrasonographic findings in camel number 9 with abscessation of the right kidney-. The lesion appeared to compress the parenchyma of the right kidney (RK), its contents are hyperechoic, and its capsule is thick.
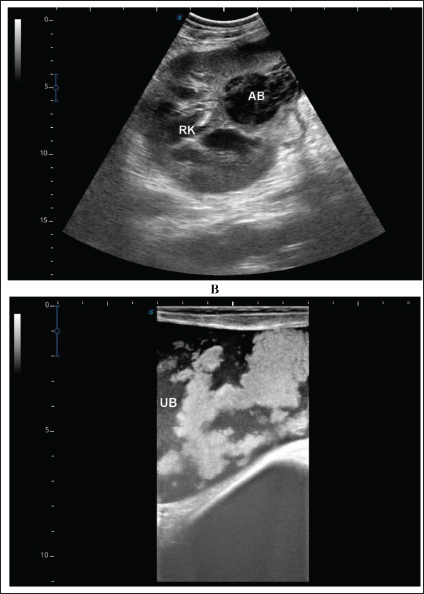
Fig. 8. Ultrasonographic findings in camel number 11 with right and left kidneys. Image A shows 2 abscesses within the right kidney (RK) and measuring 7.6 × 6.9 cm and 9.3 × 8.1 cm with isoechoic contents. Image B shows a relatively large abscess in the left kidney, and measuring 11.9 × 15.0 cm and with isoechoic contents.
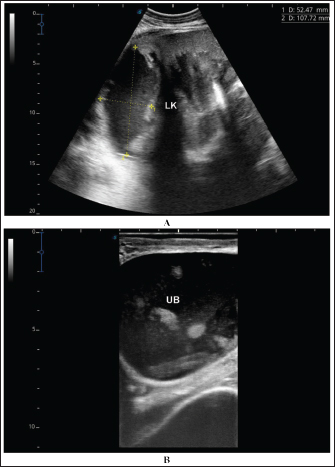
Fig. 9. Ultrasonographic findings in the camels with abscessation of the left kidney (LK). Image A shows a large abscess in camel number 15 with isoechoic contents and a thick capsule. Image B shows a small abscess in the left kidney of camel number 16 with heterogeneous contents.
Fig. 10. Ultrasonographic findings in camel number 17 with abscessation of the left kidney. Image A shows a large abscess compressing the parenchyma of the left kidney (LK) with isoechoic contents. Image B shows the urinary bladder (UB) in the same animal with echogenic deposits that appeared highly echogenic compared with the echogenic urine. In dromedary camel medicine, US has been found valuable in scanning normal thoracic and abdominal organs as well as for the diagnosis of various intra-abdominal disorders (Tharwat et al. 2012a,b,c,d,e,f; Tharwat 2013a,b; Tharwat et al. 2013; Tharwat and Al-Sobayil 2016a,b; Tharwat et al. 2017; Tharwat et al. 2018a,b,c; Tharwat 2019; Tharwat 2020a,b,c; Tharwat 2021; Tharwat and Al-Hawas 2021; Tharwat and El-Tookhy, 2021; Tharwat et al., 2023; Sadan et al., 2024; Tharwat; 2024; Tharwat et al. 2024a; Tharwat and Al-Hawas 2024; Tharwat and Tsuka 2024; Tharwat et al. 2025a,b). It was also proved that ultrasound is valuable in dromedary camels for assistance in the antemortem diagnosis of abdominal masses (Sadan et al. 2024; Tharwat et al. 2024b,c,d). In the present study, either one or more abscesses of various sizes and of different echogenicity were visualized within the kidney compressing its parenchyma, and all cases were unilateral except 2 cases that were bilateral. Most lesions in this investigation affected the right kidney, followed by the left kidney. Unilateral lesions were found in the right kidneys only in nine cases (52.9%) and in the left kidneys only in six cases (35.3%). Bilateral affection of both kidneys was found in two cases (11.7%). In approximately 25% of the diseased camels, the urinary bladder contained echogenic urine alongside hyperechoic deposits. The ultrasound-guided aspiration of renal lesions was very useful for collecting samples for bacteriological examination. The procedure was also safe and effective, ass post-aspiration complications were not observed in any of the aspirated camels. Several pathogens are reported to cause renal abscesses in humans and animals. In humans, E. coli, K. pneumonia, and S. aureus have been isolated (Lee et al. 2008., Buschel et al., 2022). In animals, a renal subcapsular abscess caused by S. pseudintermedius was reported in an 11-year-old Drahthaar Dog (Cola et al. 2020). In cattle, pyelonephritis is caused by a variety of pathogens, such as Salmonella dublin, Corynebacterium cystitidis, C. renale, and Mannheimia varigena (Floeck 2007; Braun et al. 2008; Komatsu et al. 2019; Taghipur et al. 2015; Smith et al. 2020). In dromedary camels, S. lugdunensis and E. coli were isolated from 2 animals with renal abscesses (Tharwat et al. 2018a,b). In this study, S. lugdunensis was isolated from 10 out of 17 cases, S. aureus from 5 cases, and unidentified catalase-positive and coagulase-negative Staphylococcus species from 2 cases. The high prevalence of S. lugdunensis is quite unusual. Primarily, it is a human opportunistic pathogen that causes skin and soft tissue infections; especially endocarditis (Frank et al. 2008). In animals, it has been reported to cause infections in companion animals (Rook et al., 2012), pet cats (Bierowiec 2020), cattle (Ardigò et al. 2014), and camels (Tharwat et al. 2018b). Our findings indicate that S. lugdunensis is an increasingly common camel renal abscess pathogen in the study area, which necessitates real estimation and, in turn, the development of specific vaccines to avoid high economic losses and public health impact. ConclusionTo the best of our knowledge, this is the largest study investigating renal abscesses in dromedary camels, as only two case studies have been reported. As demonstrated in this study, laboratory tests are usually nonspecific for confirming renal abscesses; however, US and ultrasound-guided aspiration have been found to be extremely helpful for verifying renal abscesses in the dromedary camels. AcknowledgmentsThe authors would like to thank Professor Sébastien Buczinski, Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Montreal, Canada, for critical reading of the manuscript and for his fruitful suggestions. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Author contributionMT: conceptualization, data collection, writing of the original manuscript draft, and preparation of figures and tables. HE: sharing of practical work and editing. HS: microbiological work and editing. AA: review and editing. All authors have approved the final manuscript for publication. Data availabilityAll data supporting the findings of this study are available in the manuscript, and no additional data sources are required. ReferencesArdigò, P., D’Incau, M. and Pongolini, S. 2014. Abortion in cattle due to infection with Staphylococcus lugdunensis. J. Vet. Diagn. Investig. 26, 818–820. Bierowiec, K. 2020. Crosssectional study of Staphyloccus lugdunensis prevalence in cats. Sci. Rep. 10, 15417. Braun, U., Nuss, K., Wehbrink, D., Rauch, S. and Pospischil, A. 2008. Clinical and ultrasonographic findings, diagnosis and treatment of pyelonephritis in 17 cows. Vet. J. 175, 240–248. Buschel, H., Leung, P., Stalewski, H., Carroll, D. and Mariyappa-Rathnamma, B. 2022. Renal abscesses in children: an 11-year retrospective study and review of the literature. ANZ. J. Surg. 92, 3293–3297. Cola, V., Foglia, A., Pisoni, L., Dondi, F., Avallone, G., Gruarin, M., Zanardi, S., Rinnovati, R. and Del Magno, S. 2020. Kidney-sparing surgery for renal subcapsular abscess caused by Staphylococcus pseudintermedius in a Dog. J. Am. Anim. Hosp. Assoc. 56, 242–247. Floeck, M. 2007. Sonographic application in the diagnosis of pyelonephritis in cattle. Vet. Vet. Radiol. Ultrasound. 48, 74–77. Frank, K.L., Del Pozo, J.L. and Patel, R. 2008. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. 2008 Clin. Microbiol. Rev. 21, 111–133. Hess, R.S. and Ilan, I. 2003. Renal abscess in a dog with transient diabetes mellitus. J. Small Anim. Pract. 44, 13–16. Jiménez, M., Gajardo, M., Bolte, L., Lazcano, A. and Salgado, I. 2022. Pediatric renal abscesses: a diagnostic challenge. Andes. Pediatr. 93, 222–228. Kitshoff, A.M., McClure, V., Lim, C.K. and Kirberger, R.M. 2011. Bilateral multiple cystic kidney disease and renal cortical abscess in a Boerboel. J. S. Afr. Vet. Assoc. 82, 120–124. Komatsu, T., Inaba, N., Watando, E., Sugie, K., Kimura, K., Katsuda, K. and Shibahara, T. 2019. Pyelonephritis caused by Mannheimia varigena in a Holstein calf. J. Vet. Med. Sci. 81, 1113–1116. Lee, B.E., Seol, H.Y., Kim, T.K., Seong, E.Y., Song, S.H., Lee, D.W., Lee, S.B. and Kwak, I.S. 2008. Recent clinical overview of renal and perirenal abscesses in 56 consecutive cases. Korean J. Intern. Med. 23, 140–148. Lim, S.K. and Ng, F.C. 2011. Acute pyelonephritis and renal abscesses in adults-correlating clinical parameters with radiological (computer tomography) severity. Ann. Acad. Med. Singap. 40, 407–413. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2002. Echo-guided studies on portal and hepatic blood in cattle. J. Vet. Med. Sci. 64, 23–28. Mohamed, T., Oikawa, S., Nakada, K., Kurwasawa, T., Sawamukai, Y. and Sato, H. 2003a. Percutaneous ultrasound-guided over-the-wire catheterization of the portal and hepatic vessels in cattle. J. Vet. Med. Sci. 65, 813–816. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2003b. Transcutaneous ultrasound-guided pancreatic biopsy in cattle and its safety: a preliminary report. Vet. J. 166, 188–193. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2003c. Ultrasound-guided biopsy of the pancreas of cattle. Vet. Rec. 153, 467. Mohamed, T. and Oikawa, S. 2008. Efficacy and safety of ultrasound-guided percutaneous biopsy of the right kidney in cattle. J. Vet. Med. Sci. 70, 175–179. Rook, K.A., Brown, D.C., Rankin, S.C. and Morris, D.O. 2012. Case-control study of Staphylococcus lugdunensis infection isolates from small companion animals. Vet. Dermatol. 23, 476–e90. Rubilotta, E., Balzarro, M., Lacola, V., Sarti, A., Porcaro, A.B. and Artibani, W. 2014. Current clinical management of renal and perinephric abscesses: a literature review. Urologia 81, 144–147. Sadan, M., Tharwat, M., Alkhedhairi, S., Refaai, W., Moghazy, H.M.E.L., Khodier, M.M., Alkhamiss, A.S. and Ghallab, A. 2024. Abdominal pedunculated leiomyoma in a male dromedary camel: clinical, hematobiochemical, ultrasonographic and pathologic findings. Int. J. Vet. Sci. 13, 458–462. Smith, J.S., Krull, A.C., Schleining, J.A. Derscheid, R.J., Kreuder, A.J. 2020. Clinical presentations and antimicrobial susceptibilities of Corynebacterium cystitidis associated with renal disease in four beef cattle. J. Vet. Intern. Med. 34, 2169–2174. Taghipur Bazargani, T., Khodakaram-Tafti, A., Ashrafi, I. and Abbassi, A.M. 2015. Giant hydronephrosis and secondary pyelonephritis induced by Salmonella dublin in a Holstein calf. Iran. J. Vet. Res. 16, 114–116. Tang, R.Y. and Cheong, B.M. 2017. Multiple bilateral renal abscesses in a previously healthy young patient. Med. J. Malaysia. 72, 250–251. Tharwat, M., Al-Sobayil, F., Ali, A. and Buczinski, S. 2012a. Transabdominal ultrasonographic appearance of the gastrointestinal viscera of healthy camels (Camelus dromedarius). Res. Vet. Sci. 93, 1015–1020. Tharwat, M., Al-Sobayil, F., Ali, A. and Buczinski, S. 2012b. Ultrasonographic evaluation of abdominal distension in 52 camels (Camelus dromedarius). Res. Vet. Sci. 93, 448–456. Tharwat, M., Al-Sobayil, F. and Buczinski, S. 2012c. Ultrasound-guided hepatic and renal biopsy in camels (Camelus dromedarius): technique development and assessment of the safety. Small Rum. Res. 103, 211–219. Tharwat, M., Al-Sobayil, F., Ali, A., Hashad, M. and Buczinski, S. 2012d. Clinical, ultrasonographic, and pathologic findings in 70 camels (Camelus dromedarius) with Johne’s disease. Can. Vet. J. 53, 543–548. Tharwat, M., Al-Sobayil, F., Ali, A. and Buczinski, S. 2012e. Ultrasonography of the liver and kidneys of healthy camels (Camelus dromedarius). Can. Vet. J. 53, 1273–1278. Tharwat, M., Al-Sobayil, F., Ali, A. and Buczinski, S. 2012f. Echocardiography of the normal camel (Camelus dromedarius) heart: technique and cardiac dimensions. BMC Vet. Res. 8, 130. Tharwat, M. 2013a. Ultrasonographic findings in camels (Camelus dromedarius) with trypanosomiasis. J. Camel Pract. Res. 20, 283–287. Tharwat, M. 2013b. Ultrasonography of the lungs and pleura in healthy camels (Camelus dromedarius). Acta Vet. Hung. 61, 309–318. Tharwat, M., Ali, A., Al-Sobayil, F. and Buczinski, S. 2013. Ultrasound-guided collection of peritoneal fluid in healthy camels (Camelus dromedarius) and its biochemical analysis. Small Rum Res. 113, 307–311. Tharwat, M. and Al-Sobayil, F. 2016a. Ultrasonographic findings in camels (Camelus dromedarius) with abdominal disorders. J. Camel Pract. Res. 23, 291–299. Tharwat, M. and Al-Sobayil, F. 2016b. Ultrasonographic findings in camel calves (Camelus dromedarius) with thoracic affections. J. Camel Pract. Res. 23, 287–290. Tharwat, M., Al-Sobayil, F., Ali, A., Derar, D. and Khodeir, M. 2017. Renal cell carcinoma in a female Arabian camel: clinical, hematobiochemical, ultrasonographic and pathologic findings. J. Camel Pract. Res. 24, 61–66. Tharwat, M., Sadan, M., El-shafaey, E., Saeed, E. and Al-Hawas, A. 2018a. Bilateral renal abscessation and chronic active pyelonephritis in a male camel (Camelus dromedarius) caused by Escherichia coli. J. Vet. Med. Sci. 80, 778–783. Tharwat, M., Sadan, M., El-Shafaey, E., Al-Hawas, A.A. and Saeed, E. 2018b. Unilateral nephrectomy in a female dromedary camel with pyelonephritis caused by Staphylococcus lugdunensis. Pakistan Vet. J. 38, 116–118. Tharwat, M., El-Shafaey, E., Sadan, M., Ali, A., Al-Sobayil, F. and Al-Hawas, A. 2018c. Omaso-abomasal adenocarcinoma in a female Arabian camel (Camelus dromedarius). J. App. Anim. Res. 46, 1268–1271. Tharwat, M. 2019. Chronic peritonitis in dromedary camels: clinical, hematobiochemical, ultrasonographic and pathologic findings. J. Camel Pract. Res. 26, 169–172. Tharwat, M. 2020a. Ultrasonography of the abdomen in healthy and diseased camels (Camelus dromedaries). J. App. Anim. Res. 48, 300–312. Tharwat, M. 2020b. Ultrasonography of the liver in healthy and diseased camels (Camelus dromedaries). J. Vet. Med. Sci. 82, 399–407. Tharwat, M. 2020c. Ultrasonography of the kidneys in healthy and diseased camels (Camelus dromedarius). Vet. Med. Intern. 2020, 7814927. Tharwat, M. 2021. Obstructive urolithiasis in dromedary camels: clinical, ultrasonographic and postmortem findings. J. Camel Pract. Res. 28, 85–93. Tharwat, M. and Al-Hawas, A. 2021. Ultrasound detection of cosmic fillers injection of lips in camel beauty pageants: first report in Veterinary Medicine. Trop. Anim. Health Prod. 53, 53. Tharwat, M. and El-Tookhy, O. 2021. Ocular ultrasonography in camels (Camelus dromedarius): a review. J. Camel Pract. Res. 28, 185–190. Tharwat, M., El Moghazy, H.M. and Oikawa, S. 2023. Ultrasonographic verification of hepatic hydatidosis in a female dromedary camel: a case report. J. Vet. Med. Sci. 85, 1286–1290. Tharwat, M. 2024. Fundamentals of diagnostic ultrasound in dromedary camel medicine. Int. J. Vet. Sci. 13, 1–6. Tharwat, M. and Al-Hawas, A. 2024. The emerging topic of cosmetic medicine in dromedary camels. Int. J. Vet. Sci. 13, 139–146. Tharwat, M. and Tsuka, T. 2024. Diagnostic utility of ultrasonography for thoracic and abdominal bacterial and parasitic diseases in ruminants: a comprehensive overview. Front. Vet. Sci. 11, 1435395. Tharwat, M., El-Ghareeb, W.R. and Almundarij, T.I. 2024a. Depraved appetite in dromedary camels: clinical, ultrasonographic, and postmortem findings. Open Vet. J. 14, 652–663. Tharwat, M., Haridy, M., Elmoghazy, H.M.M., Elnahas, A. and Alkheraif, A.A. 2024b. Abdominal fat necrosis in a female dromedary camel: clinical, hematobiochemical, sonographic, and pathologic findings. Open Vet. J. 14, 1726–1732. Tharwat, M., El-Shafaey, E. and Alkheraif, A.A. 2024c. Ultrasonographic evaluation of thoracic and abdominal neoplasia in domestic ruminants: a systemic review. Open Vet. J. 14, 1751–1760. Tharwat, M., Alkhedhairi, S. and Marzok, M. 2024d. Intestinal obstruction in dromedary camels: clinical and ultrasonographic findings as well as variations in acid-base balance, blood gases and hematobiochemical profiles. IJAB. 13, 500–504. Tharwat, M., Ali, H. and Alkheraif, A.A. 2025a. Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): a review. Open Vet. J. 15, 8–17. Tharwat, M., Alkheraif, A.A., Elmoghazy, H.M.M., Haridy, M. and Marzok, M. 2025b. Disseminated pyogranulomas in a female dromedary camel: hematobiochemical, sonographic and pathologic investigations. Int. J. Vet. Sci. 14, 120–124. Tharwat, M. and Al-Hawas, A. 2025c. Identification of tampering methods among 12,385 Arabian camels using different diagnostic imaging techniques. Open Vet. J. In press. Trotter, G.W., Brown, C.M. and Ainsworth, D.M. 1984. Unilateral nephrectomy for treatment of a renal abscess in a foal. J. Am. Vet. Med. Assoc. 184, 1392–1394. US Department of Health and Human Services. Guide for the Care and Use of Laboratory Animals; NIH Publication No. 86-23, Revised 1996; Public Health Services, National Institutes of Health: Washington, DC, 1996. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Elmoghazy HMM, Saeed EMA, Alkheraif AA. Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations. Open Vet. J.. 2025; 15(3): 1289-1303. doi:10.5455/OVJ.2025.v15.i3.20 Web Style Tharwat M, Elmoghazy HMM, Saeed EMA, Alkheraif AA. Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations. https://www.openveterinaryjournal.com/?mno=232104 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.20 AMA (American Medical Association) Style Tharwat M, Elmoghazy HMM, Saeed EMA, Alkheraif AA. Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations. Open Vet. J.. 2025; 15(3): 1289-1303. doi:10.5455/OVJ.2025.v15.i3.20 Vancouver/ICMJE Style Tharwat M, Elmoghazy HMM, Saeed EMA, Alkheraif AA. Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1289-1303. doi:10.5455/OVJ.2025.v15.i3.20 Harvard Style Tharwat, M., Elmoghazy, . H. M. M., Saeed, . E. M. A. & Alkheraif, . A. A. (2025) Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations. Open Vet. J., 15 (3), 1289-1303. doi:10.5455/OVJ.2025.v15.i3.20 Turabian Style Tharwat, Mohamed, Hazem M. M. Elmoghazy, Elhassan M. A. Saeed, and Abdulrahman A. Alkheraif. 2025. Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations. Open Veterinary Journal, 15 (3), 1289-1303. doi:10.5455/OVJ.2025.v15.i3.20 Chicago Style Tharwat, Mohamed, Hazem M. M. Elmoghazy, Elhassan M. A. Saeed, and Abdulrahman A. Alkheraif. "Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations." Open Veterinary Journal 15 (2025), 1289-1303. doi:10.5455/OVJ.2025.v15.i3.20 MLA (The Modern Language Association) Style Tharwat, Mohamed, Hazem M. M. Elmoghazy, Elhassan M. A. Saeed, and Abdulrahman A. Alkheraif. "Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations." Open Veterinary Journal 15.3 (2025), 1289-1303. Print. doi:10.5455/OVJ.2025.v15.i3.20 APA (American Psychological Association) Style Tharwat, M., Elmoghazy, . H. M. M., Saeed, . E. M. A. & Alkheraif, . A. A. (2025) Renal abscessation in dromedary camels: Clinical, ultrasonographic, hematobiochemical, and etiological investigations. Open Veterinary Journal, 15 (3), 1289-1303. doi:10.5455/OVJ.2025.v15.i3.20 |