| Research Article | ||
Open Vet. J.. 2025; 15(3): 1304-1309 Open Veterinary Journal, (2025), Vol. 15(3): 1304-1309 Research Article Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in LibyaSumaia Ramadan Alkhunfas1, Walid Khalifa Saadawi1, Nadia Milad Aldobea1, Ebtisam Muawiya Wanes3, Mohammed Abdulsalam Abuazzah3, Badereddin Bashir Annajar2 and Hanan Alarabi Aqeehal1*1National Center for Disease Control, Ministry of Health, Tripoli, Libya 2Public Health Department, Faculty of Medical Technology, University of Tripoli, Tripoli, Libya 3Tarabulus Zoo Park, Abu Salim Municipality, Tripoli, Libya *Corresponding Author: Hanan Alarabi Aqeehal. National Center for Disease Control, Ministry of Health, Tripoli, Libya. Email: hananaghila [at] yahoo.com Submitted: 08/12/2024 Accepted: 21/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
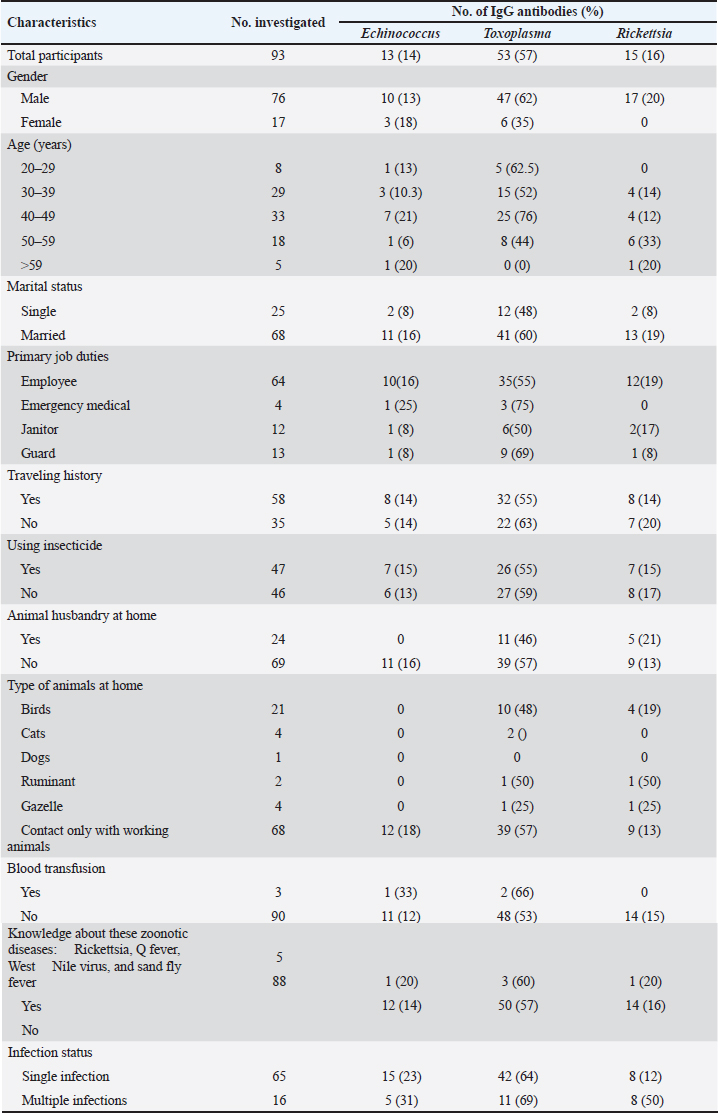
AbstractBackground: Animal handlers in zoological settings are at an increased risk of zoonotic disease transmission due to close contact with animals, waste, and parasites. Research on occupational zoonotic infections in zoos, however, remains limited. This is particularly relevant in the context of the Tripoli Zoo, which has been closed since 2013 and is planned to reopen under new management. Aim: This study investigated the seroprevalence of Echinococcus, Toxoplasma, and Rickettsia infections among zoo employees in Tripoli, Libya, in 2013. Methods: Blood samples were collected from 93 zoo employees and tested for IgG antibodies against the target pathogens using serological methods. Associations between seropositivity and various demographic and occupational factors were also examined. Results: In this study, 87.1% of the employees tested positive for at least one zoonotic pathogen IgG antibody, with Toxoplasma being the most prevalent. Several factors, including gender, age, marital status, occupational status, travel history, and animal husbandry practices, were associated with the prevalence of these infections. A significant proportion of participants exhibited multiple infections. Conclusion: The high seroprevalence of zoonotic infections among zoo employees underscores the urgent need for implementing effective preventive measures to protect staff health and minimize the risk of zoonotic disease transmission, especially in light of the zoo’s planned reopening. Keywords: Zoological settings, Animal handlers, Zoonotic, Antibodies, Libya. IntroductionAnimal handlers in zoological settings are at risk of exposure to a variety of diseases that can be transmitted from animals. This risk primarily arises from close interactions with animal waste, bodily fluids, parasites, and other potential sources of infection. As a result, the probability of these handlers contacting zoonotic diseases is expected to be considerably higher than that of zoo visitors or staff members who do not engage in direct contact with the animals (Forsyth et al., 2012; Esposito et al., 2023). Despite the known dangers, there is a significant lack of published research on occupational zoonotic infections associated with zoos. However, existing studies indicate a troubling trend. For instance, a survey conducted by Adjemian et al. revealed that 77% of employees from the US National Park Service who had direct contact with animals reported experiencing zoonotic infections (Adjemian et al., 2012). Similarly, Juncker-Voss et al. (2004) indicated that 97% of zoo staff in Vienna had antibodies against at least one zoonotic pathogen (Juncker-Voss et al., 2004). Additional studies focusing on specific pathogens have also provided important insights. Parkar et al. (2010) reported a high prevalence of Blastocystis among both zookeepers and animals in regions of Western Australia (Parkar et al., 2010). Raso et al. (2010) found that 4.7% of animal handlers and veterinarians in Brazilian zoos tested positive for antibodies to Chlamydophila psittaci (Raso et al., 2010). Furthermore, Van der Westhuizen et al. (2023) identified Brucella sp., Leptospira sp., and hantavirus in veterinarians (Van der Westhuizen et al., 2023). Research on the carriage of zoonotic pathogens within zoo animal populations is limited. Luechtefeld et al., 1981 detected Campylobacter fetus subspecies jejuni in animal samples from the Denver Zoo, indicating the potential for use in animal reservoirs. Although not all studies have yielded positive findings, such as those involving penguins and seals in South Georgia and the Antarctic Peninsula, the detection of zoonotic agents in various animal species emphasizes the necessity for further research (Luechtefeld et al., 1981). This study aimed to screen zoo employees in Tripoli for the presence of antibodies against zoonotic pathogens, which is a key component for identifying workplace hazard exposure related to occupational health screening. Materials and MethodsStudy areaTarabulus Zoo Park is located in Al Nasr Forest Tripoli, Libya, which is located south of Tripoli city center. Tripoli Zoo is located in the southwest corner of the park, and the Rixos Al Nasr Hotel is located in its southeast corner. The Al Nasr Forest Park is estimated to cover 416.17 square kilometers with a variety of plants and animals species (Naish, 2023). Mammals, birds, and reptiles of 60 species were documented in the park, including the flamingos Phoenicopterus roseus, White stork Ciconia ciconia, Muscovy Cairina moschata, Black swan Cygnus atratus, and Emu Dromaius novaehollandiae, Green-winged macaw, Golden eagle, and many other species (Naish, 2023). Blood collectionIn 2013, 93 blood samples were collected from each zoo employee who participated in the screening. Blood samples were collected by venipuncture in clean tubes without coagulant; sera were separated by centrifugation and stored at −20°C until testing. Each participant filled out a questionnaire in which the following variables were registered: age, gender, occupation, area of residence, blood transfusion, history of contact with domestic animals, and traveling history. No data on race were obtained. In addition, All the participants signed an informed consent form. Serological techniqueSera were sent to the Parasitology and Vector-Borne Disease Research Laboratory at the National Center for Disease Control (NCDC), Tripoli, Libya, where they were tested by the serological method with enzyme-linked immunosorbent assays to detect the presence of Echinococcus IgG, Toxoplasma IgG, and Rickettsia IgG antibodies using a commercial enzyme immunoassay kit, (AXIOM Diagnostic, Worms, Germany), according to the manufacturer’s instructions. Statistical analysisThe seroprevalence of antibodies against Echinococcus, Toxoplasma, and Rickettsia was calculated as the ratio between the positive sera and all tested sera. All collected data were statistically analyzed using Jamovi software version 2.3. The data analysis was performed using the same software, and the significance threshold of the qualitative and quantitative variables studied was considered for a value of p < 0.05 (Jamovi Project, 2022). Ethical approvalThe study was approved and confirmed under the rules and regulations of research in the National Center for Disease Control Libya. Therefore, at commencing the research point, ethical approval and authorization were issued while referring to the NCDC. ResultsA total of 93 sera samples were examined, among which 76 (82%) were male and 17 (18%) were female, with a sex ratio of 4.4. The median age was 41.9 years (range: 25–67). Table 1 shows the demographic characteristics of the sample collected (n=93). In total, 87.1% (n=81) were positive for the zoonotic pathogen IgG antibody; the highest seroprevalence rate was Toxoplasma (57%), followed by Rickettsia (16%) and Echinococcus (14%). Factors associated with zoonotic disease prevalenceThis study revealed several factors associated with the prevalence of zoonotic diseases among the participants. Gender played a role, with females having a higher prevalence of Toxoplasma than males. Age was also a factor, with the highest prevalence of Toxoplasma observed in the 30–39 years age group and the highest prevalence of Rickettsia in the 20–29 years age group. Marital status influenced the prevalence of Toxoplasma and Rickettsia, with married participants having higher rates than single participants. Occupational factors were also significant ( p < 0.0001), as individuals in emergency medical services and janitors had higher rates of Toxoplasma and Rickettsia infections. Traveling history was associated with an increased prevalence of all three zoonotic diseases. Animal husbandry practices, particularly owning ruminants or birds, were linked to higher rates of Toxoplasma and Rickettsia. However, insecticide use, blood transfusion, and knowledge about zoonotic diseases did not appear to significantly affect the prevalence of these infections ( p > 0.0001). A significant number of participants experienced multiple zoonotic infections. Sixteen individuals tested positive for more than one pathogen, while 65 individuals were infected with a single zoonotic agent. These findings highlight the complex nature of zoonotic disease transmission and the potential for coinfection in the study population. DiscussionThe results of this research highlight the considerable risk of zoonotic infections that zoo employees face. The notable presence of antibodies against Toxoplasma, Rickettsia, and Echinococcus among the study participants underscores the urgent need for the implementation of comprehensive occupational health measures. These findings are particularly concerning given the increasing number of open farms and petting zoos in Libya, which may further contribute to zoonotic disease transmission. Table 1. Demographic characteristics of the participants.
Our study revealed a high prevalence of toxoplasmosis (57%), which is consistent with findings reported by Juncker-Voss et al. (2004), where similar antibody screenings among employees at the Vienna Zoo indicated a high prevalence of Toxoplasma exposure. The study also showed a greater prevalence of Toxoplasma among male participants, suggesting potential gender-specific risk factors or behaviors that may contribute to heightened exposure. The previous research has linked Toxoplasma infection with changes in testosterone levels and behavioral modifications in men, with correlations not observed in women (Abdoli et al., 2024). Additionally, studies indicate that the interaction between toxoplasmosis and sex can significantly influence behavioral traits such as self-control, tidiness, interpersonal relationships, and levels of mistrust, with infected men exhibiting notable differences compared with their uninfected counterparts (Lindová et al., 2006). Age-related patterns of infection were also observed. The highest prevalence of Toxoplasma was recorded among participants aged 30–39 years, consistent with prior research by (Almeida et al., 2022). Similarly, Rickettsia infections were most prevalent in the 20–29 years age group, a finding consistent with that of (Mansoor et al., 2021). These variations may be associated with differences in exposure patterns and immune responses among different age groups. In addition to toxoplasmosis, the study also identified cases of Rickettsia infection (16%), highlighting the risk associated with vector-borne pathogens. Rickettsia infections are often associated with occupational exposure to arthropod vectors, such as ticks and fleas, which are commonly found in zoo environments. The increased prevalence of Rickettsia among younger individuals may reflect occupational exposure during early career stages, in which job duties involve direct contact with animals or contaminated environments. Additionally, hydatid cysts caused by Echinococcus (14%) were detected among the study participants. Echinococcus is transmitted through contact with infected canines or contaminated environments, and its presence in zoo employees indicates possible occupational exposure. Given the severe health implications of hydatid cysts, including organ damage and potential surgical intervention, preventive measures such as deworming programs for animals, strict hygiene practices, and regular health screenings for employees are crucial. The study found that individuals engaged in animal husbandry at home exhibited a higher risk of Echinococcus infection, reinforcing the role of domestic animal exposure in zoonotic disease transmission (Barimah et al., 2023). The analysis of marital status revealed that married individuals exhibited elevated rates of Toxoplasma and Rickettsia infections, potentially due to greater exposure to domestic animals or agricultural activities (Morand et al., 2014; Onduru et al., 2021). Additionally, employees in emergency medical services and janitorial roles faced an increased risk of zoonotic infections, likely due to their frequent interactions with animals and contaminated materials in their work environments (Barimah et al., 2023, Esposito et al., 2023). Travel history has also emerged as a significant factor in the spread of zoonotic diseases. Participants who had traveled to various regions demonstrated a higher risk of being infected with Toxoplasma, Rickettsia, and Echinococcus. This suggests that exposure to diverse animal populations and environmental conditions during travel may increase infection risk (Wilson, 1995). Furthermore, individuals who kept animals at home, particularly ruminants and birds, showed a higher prevalence of Toxoplasma and Rickettsia, emphasizing the need for proper hygiene and biosecurity measures to reduce transmission risks (Stelzer et al., 2019). Finally, this study revealed that a significant number of participants were infected with multiple zoonotic agents, highlighting the complex nature of zoonotic disease transmission. Sixteen individuals tested positive for more than one pathogen, highlighting the importance of considering coinfection when diagnosing and treating zoonotic diseases. These findings are consistent with those reported by Wood et al. 2014; Rahman et al. 2020; van der Westhuizen et al. 2023, reinforcing the need for multifaceted diagnostic approaches and targeted preventive strategies to mitigate the impact of zoonotic diseases on both occupational and public health. LimitationsThis study has some limitations that should be considered. The sample size was relatively small, limiting the generalizability of the findings. Additionally, the study did not investigate the clinical manifestations of zoonotic infections among the participants. Future studies with larger sample sizes and clinical assessments are needed to further elucidate the impact of zoonotic diseases on zoo staff. ConclusionIn conclusion, this study provides valuable insights into the prevalence of zoonotic infections among zoo employees in Tripoli, Libya. The findings highlight the importance of implementing comprehensive prevention measures to protect the health of zoo staff and prevent the spread of zoonotic diseases. AcknowledgmentsThe authors would like to express their sincere gratitude to the dedicated team at Tarabulus Zoo Park for their invaluable contributions and assistance throughout the specimen collection process. Conflict of interestThe author declares no conflict of interest. FundingThis study did not receive funding. Authors’ contributionsThe authors have equal participation in conceptualization, preparation of the original draft, review, and editing. Data availabilityAll analyzed data are included in this study. ReferencesAbdoli, A., Ghaffarifar, F., Knowledge abo, Z. and Taghipour, A. 2024. Toxoplasma gondii infection and testosterone alterations: a systematic review and meta-analysis. PLoS One 19(4), e0297362; doi: 10.1371/journal.pone.0297362. Adjemian, J., Weber, I.B., McQuiston, J., Griffith, K., Mead, P., Nicholson, W., Roche, A., Schriefer, M., Fischer, M., Kosoy, O., Laven, J.J., Stoddard, R.A., Hoffmaster, A.R., Smith, T., Bui, D., Wilkins, P.P., Jones, J.L., Gupton, P.N., Quinn, C.P., Messonnier, N., Higgins, C. and Wong, D. 2012. Zoonotic infections among employees of the great smoky mountains and rocky mountain national parks, 2008–2009. Vector Borne Zoonotic Dis. 12(11), 922–931; doi: 10.1089/vbz.2011.0917. Almeida, D., Santos-Silva, S., Pereira, M.A., Santos, C., Mega, C., Coelho, C., Nóbrega, C., Esteves, F., Cruz, R., Vala, H. and Mesquita, J.R. 2022. Prevalence of Toxoplasma gondii antibodies and risk factors in Portuguese veterinarians: a matched case–control study. Pathogens 11(10), 1217; doi: 10.3390/pathogens11101217. Barimah, A.J., Ofosua, T.Y., Addo, H.O., Agbomadzi, S.K., David, A.B., Agyei, S.B. and Eric, A.A. 2023. Assessing knowledge and awareness about zoonotic infections among selected tertiary students in the Accra Metropolis. Environ. Health Insights 17, 11786302231214444; doi: 10.1177/11786302231214444. Esposito, M.M., Turku, S., Lehrfield, L. and Shoman, A. 2023. Impact of human activities on zoonotic infection transmissions. Animals 13(10), 1646; doi: 10.3390/ani13101646. Forsyth, M.B., Morris, A.J., Sinclair, D.A. and Pritchard, C.P. 2012. Investigation of zoonotic infections among Auckland Zoo staff: 1991–2010. Zoonoses Public Health 59(8), 561–567; doi: 10.1111/j.1863-2378.2012.01496.x. Juncker-Voss, M., Prosl, H., Lussy, H., Enzenberg, U., Auer, H. and Lassnig, H. 2004. Screening for zoonotic agent antibodies among employees of the zoological garden of Vienna, Schönbrunn, Austria. Berl. Munch. Tierarztl. Wochenschr. 117(9-10), 404–409. Jamovi Project. 2022. (Version 2.3) [Computer Software]. Available via https://www.jamovi.org Lindová, J., Novotná, M., Havlíček, J., Jozífková, E., Skallová, A., Kolbeková, P., Hodný, Z., Kodym, P. and Flegr, J. 2006. Sex differences in behavioral changes induced by latent toxoplasmosis. Int. J. Parasitol. 36(14), 1485–1492; doi: 10.1016/j.ijpara.2006.07.008. Luechtefeld, N.W., Cambre, R.C. and Wang, W.L. 1981. Isolation of Campylobacter fetus subsp. jejuni from zoo animals. J. Am. Vet. Med. Assoc. 179(11), 1119–1122. Mansoor, T., Fomda, B.A., Koul, A.N., Bhat, M.A., Abdullah, N., Bhattacharya, S. and Saleem, S.M. 2021. Rickettsial infections among febrile undifferentiated patients attending a tertiary care teaching hospital in northern India: a longitudinal study. Infect. Chemother. 53(1), 96. Morand, S., McIntyre, K.M. and Baylis, M. 2014. Domesticated animals and human infectious diseases of zoonotic origins: domestication time matters. Infect. Genet. Evol. 24, 76–81; doi: 10.1016/j.meegid.2014.02.013. Mose, J.M., Kagira, J.M., Kamau, D.M., Maina, N.W., Ngotho, M. and Karanja, S.M. 2020. A review of the present advances on studies of toxoplasmosis in Eastern Africa. Biomed. Res. Int. 2020, 7135268; doi: 10.1155/2020/7135268. Naish, D. 2023. TetZoo reviews zoos: Tripoli Zoo in Libya. Tetrapod Zool. To this : Naish, D. (2023). TetZoo reviews zoos: Tripoli Zoo in Libya. Tetrapod Zoology. https://tetzoo.com/blog/2023/1/4/tetzoo-reviews-zoos-tripoli-zoo Onduru, O.G. and Aandoud, S. 2021. Prevalence and risk factors of typical signs and symptoms of toxoplasmosis in children born to pregnant women at risk of pregnancy attending prenatal care in Temeke District, Tanzania. Sci. Afr. 11, e00690; doi: 10.1016/j.sciaf.2020.e00690. Parkar, U., Traub, R.J., Vitali, S., Elliot, A., Levecke, B., Robertson, I., Geurden, T., Steele. J., Drake, B. and Thompson, R.C. 2010. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet. Parasitol. 169(1-2), 8–17; doi: 10.1016/j.vetpar.2009.12.032. Rahman, M.T., Sobur, M.A., Islam, M.S., Ievy, S., Hossain, M.J., El Zowalaty, M.E., Rahman, A.T. and Ashour, H.M. 2020. Zoonotic diseases: etiology, impact, and control. Microorganisms 8(9), 1405. Raso, T.D.F., Carrasco, A.D.O.T., Silva, J.C.R., Marvulo, M.F.V. and Pinto, A.A. 2010. Seroprevalence of chlamydophila psittaci antibodies in zoo workers in Brazil. Zoonoses Public Health 57(6), 411–416; doi: 10.1111/j.1863-2378.2009.01237.x. Stelzer, S., Basso, W., Silván, J.B., Ortega-Mora, L.M., Maksimov, P., Gethmann, J., Conraths, F.J. and Schares, G. 2019. Toxoplasma gondii infection and toxoplasmosis in farm animals: risk factors and economic impact. Food Waterborne Parasitol. 15, e00037; doi: 10.1016/j.fawpar.2019.e00037. van der Westhuizen, C.G., Burt, F.J., van Heerden, N., van Zyl, W., Anthonissen, T. and Musoke, J. 2023. Prevalence and occupational exposure to zoonotic diseases in high-risk populations of the Free State Province, South Africa. Front Microbiol, 14, 1196044; doi: 10.3389/fmicb.2023.1196044. Wilson, M.E. 1995. Travel and the emergence of infectious diseases. Emerg. Infect. Dis. 1, 39–46; doi: 10.3201/eid0102.950201. Wood, H., Drebot, M.A., Dewailly, E., Dillon, L., Dimitrova, K., Forde, M., Grolla, A., Lee, E., Loftis, A., Makowski, K., Morrison, K., Robertson, L. and Krecek, R.C. 2014. Seroprevalence of seven zoonotic pathogens in pregnant women from the Caribbean. Am. J. Trop. Med. Hyg. 91(3), 642; doi: 10.4269/ajtmh.14-0107. | ||
| How to Cite this Article |
| Pubmed Style Alkhunfas SR, Saadawi WK, Aldobea NM, Wanes EM, Abuazzah MA, Annajar BB, Aqeehal HA. Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya. Open Vet. J.. 2025; 15(3): 1304-1309. doi:10.5455/OVJ.2025.v15.i3.21 Web Style Alkhunfas SR, Saadawi WK, Aldobea NM, Wanes EM, Abuazzah MA, Annajar BB, Aqeehal HA. Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya. https://www.openveterinaryjournal.com/?mno=232129 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.21 AMA (American Medical Association) Style Alkhunfas SR, Saadawi WK, Aldobea NM, Wanes EM, Abuazzah MA, Annajar BB, Aqeehal HA. Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya. Open Vet. J.. 2025; 15(3): 1304-1309. doi:10.5455/OVJ.2025.v15.i3.21 Vancouver/ICMJE Style Alkhunfas SR, Saadawi WK, Aldobea NM, Wanes EM, Abuazzah MA, Annajar BB, Aqeehal HA. Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1304-1309. doi:10.5455/OVJ.2025.v15.i3.21 Harvard Style Alkhunfas, S. R., Saadawi, . W. K., Aldobea, . N. M., Wanes, . E. M., Abuazzah, . M. A., Annajar, . B. B. & Aqeehal, . H. A. (2025) Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya. Open Vet. J., 15 (3), 1304-1309. doi:10.5455/OVJ.2025.v15.i3.21 Turabian Style Alkhunfas, Sumaia Ramadan, Walid Khalifa Saadawi, Nadia Milad Aldobea, Ebtisam Muawiya Wanes, Mohammed Abdulsalam Abuazzah, Badereddin Bashirr Annajar, and Hanan Alarabi Aqeehal. 2025. Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya. Open Veterinary Journal, 15 (3), 1304-1309. doi:10.5455/OVJ.2025.v15.i3.21 Chicago Style Alkhunfas, Sumaia Ramadan, Walid Khalifa Saadawi, Nadia Milad Aldobea, Ebtisam Muawiya Wanes, Mohammed Abdulsalam Abuazzah, Badereddin Bashirr Annajar, and Hanan Alarabi Aqeehal. "Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya." Open Veterinary Journal 15 (2025), 1304-1309. doi:10.5455/OVJ.2025.v15.i3.21 MLA (The Modern Language Association) Style Alkhunfas, Sumaia Ramadan, Walid Khalifa Saadawi, Nadia Milad Aldobea, Ebtisam Muawiya Wanes, Mohammed Abdulsalam Abuazzah, Badereddin Bashirr Annajar, and Hanan Alarabi Aqeehal. "Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya." Open Veterinary Journal 15.3 (2025), 1304-1309. Print. doi:10.5455/OVJ.2025.v15.i3.21 APA (American Psychological Association) Style Alkhunfas, S. R., Saadawi, . W. K., Aldobea, . N. M., Wanes, . E. M., Abuazzah, . M. A., Annajar, . B. B. & Aqeehal, . H. A. (2025) Screening for antibodies against zoonotic infections among employees of the Tripoli Zoo in Libya. Open Veterinary Journal, 15 (3), 1304-1309. doi:10.5455/OVJ.2025.v15.i3.21 |