| Review Article | ||
Open Vet. J.. 2025; 15(3): 1101-1115 Open Veterinary Journal, (2025), Vol. 15(3): 1101-1115 Review Article The hidden threat of cysticercosis: A neglected public health problemKusnoto Kusnoto1, Aswin Rafif Khairullah2, Agus Sunarso1, Endang Suprihati1*, Suhita Aryaloka3, Dyah Haryuningtyas Sawitri2, Ikechukwu Benjamin Moses4, Dea Anita Ariani Kurniasih5, Syahputra Wibowo6, Bantari Wisynu Kusuma Wardhani7, Wasito Wasito2, Riza Zainuddin Ahmad2, Ima Fauziah2, Muhammad Khaliim Jati Kusala2, Sheila Marty Yanestria8, Julaeha Julaeha9, Kartika Afrida Fauzia9,10, Fitrine Ekawasti21Division of Veterinary Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Magister Program of Veterinary Agribusiness, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 5Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 7Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 8Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 9Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Department of Environmental and Preventive Medicine, Faculty of Medicine, Oita University, Yufu, Japan *Corresponding Author: Endang Suprihati. Division of Veterinary Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia. Email: endang-s [at] fkh.unair.ac.id Submitted: 12/12/2024 Accepted: 20/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
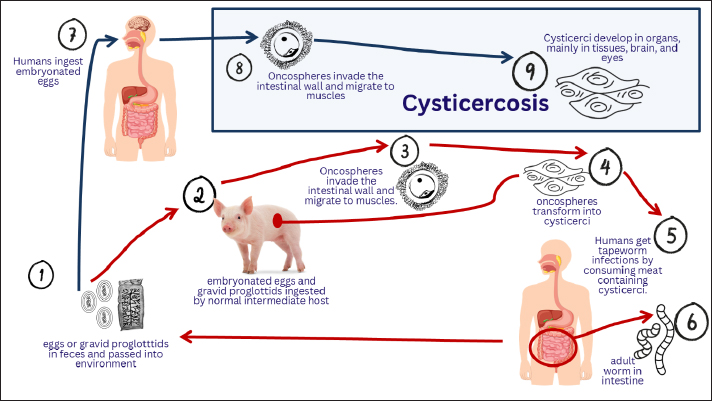
AbstractThe parasitic infection known as cysticercosis is caused by the larvae of the Taenia solium (pork tapeworm). Cysticercosis is spread by food. Humans can serve as both definitive and intermediate hosts, whereas pigs serve as intermediate hosts. This illness is one of the neglected tropical diseases that affect the public health of people from low-income backgrounds. Cysticercosis is endemic to Africa, China, India, Southeast Asia, and Latin America. When humans consume pork that has been contaminated and cooked incorrectly, cysticerci enter the small intestine where they are evaginated by digestive enzymes and stick to the intestinal wall. Cysticerci can reside in the host tissues of both humans and pigs without causing illness or inflammation. Cysts are most frequently observed in the cerebral hemispheres, particularly where gray and white matter meet. Cysticerci typically build nests in the muscles and subcutaneous fat of pigs. This parasite typically causes neurocysticercosis, a pleomorphic clinical condition, in humans by infecting the central nervous system. Neuroimaging, serological tests, and a thorough clinical examination are good methods for diagnosing cysticercosis. An infection with T. solium tapeworm can be transmitted by eating raw or undercooked pork that has been infected. Individuals with pork tapeworms in their intestinal lumen, pigs, poverty, and cultural factors are the main causes of this disease. It is possible to treat human tapeworm infections with niclosamide. Cysticercosis has been eliminated in more through improved sanitation and restrictions on domestic pig husbandry. Keywords: Cysticercosis, Human, Pig, Public health, T. solium. IntroductionThe parasitic infection known as cysticercosis is caused by the larvae of the Taenia solium (pork tapeworm), also known as cysticerci, which is a member of the Cestoda class of the Platyhelminthes phylum (García et al., 2003). There are other types of cysticercosis, including neurocysticercosis (a cysticerci in the brain), ocular cysticercosis (a cysticerci in the eye), muscular cysticercosis, and skin cysticercosis, which manifests as a soft lump beneath the skin (Meena et al., 2016; Satya et al., 2016; Dhiman et al., 2017; Rama et al., 2023). Because neurocysticercosis is the most deadly form of infection, it is well known. It is thought to be the cause of up to 56% of all epilepsy cases in developing nations, and it is frequently fatal due to complications from surgery to remove brain cysts (Bustos et al., 2021). Infection by T. solium larvae (cysticerci) is a neglected public health issue, particularly in places with inadequate livestock husbandry and poor sanitation (Aung and Spelman, 2016). Cysticercosis is spread by food. Humans can serve as both definitive and intermediate hosts, whereas pigs serve as intermediate hosts (Hossain et al., 2023). Since open housing management systems give pigs unrestricted access to human waste, particularly in places where latrines are either nonexistent or poorly built, they are considered to be one of the primary risk factors for cysticercosis (Chege et al., 2023). All phases of the parasite life cycle are affected by human activity. T. solium infections are spread to humans and pigs by humans carrying the parasite in their intestinal lumen, which contaminates the environment with T. solium eggs through open defecation (Jansen et al., 2021). Humans and pigs contract cysticercosis by consuming food and water tainted with the excrement of human carriers that contains T. solium eggs or gravid proglottids (Kabululu et al., 2023). In humans, neurocysticercosis is caused by cysticerci, which are typically found in the brain, eyes, subcutaneous tissue, and skeletal muscles (Meena et al., 2016). Pigs with cysticercosis in many predilection locations, including the masseter muscle, skeletal muscle, heart, and diaphragm, are not suitable for human consumption, and their diminished market value results in large financial losses (Shyaka et al., 2024). The surrounding cells will degenerate if cysticerci are present in the tissue (Garcia et al., 2020). Some or all of the pig carcasses must be destroyed if there are many cysts, since they could be harmful to human health. Furthermore, cysticercosis causes significant financial losses. Infected pigs cause losses of €10 million and US$18.6 to US$34.2 million annually, respectively, and account for 4.7% to 26.9% of overall pig farming expenses, according to a global economic impact research (Bulaya et al., 2015). The primary neurological signs of this condition include headaches, seizures, and focal impairments; however, the quantity and size of larvae, developmental stage, and location in the brain can cause a variety of clinical pleomorphic presentations (Garcia et al., 2014a). The condition may be diagnosed based on a combination of epidemiological characteristics, clinical history, neuroimaging, and immunological data. Many people who are carriers of the causal agent of cysticercosis (T. solium) may not exhibit any symptoms; instead, they become long-term carriers of the infection by reinfecting themselves and other members of the household (García et al., 2003). Meat inspection using visual inspection of meat pieces and lingual inspection of live animals can identify T. solium infection in pigs; however, this method is not very sensitive because cysts may be overlooked (Sithole et al., 2019). Cysticercosis is a neglected zoonotic disease due to underdiagnosis and underreporting of cases of the disease, resulting in low estimates of the number of cases and the global burden (Praet et al., 2009). Additionally, as more people migrate from regions where T. solium is endemic, the prevalence of cysticercosis is rising in developed nations (Soto et al., 2021). The purpose of this review article is to explain the etiology, history, life cycle, epidemiology, pathogenesis, immune response, pathology, clinical symptoms, diagnosis, transmission, risk factors, public health importance, economic impact, treatment, vaccination, and control of cysticercosis. The treatment of cysticercosis should be a primary focus because of its impact on public health, especially in poor countries. EtiologyTaenia solium is a member of the order Cyclophyllidea, family Taeniidae, phylum Platyhelminthesis, and subclass Eucestoda (Okello and Thomas, 2017). The scolopendra and strobila are 2- to 4-m-long adult tapeworms that live in the human gut (García et al., 2003). The scolopendra is composed of four suckers, a rostellum with 22–32 hooks, and a strobila with 700–1,000 proglottid segments. Taenia solium can produce up to 300,000 eggs every day, and each proglottid has roughly 40,000 eggs (Wandra et al., 2013). The egg is encased in an embryophore, which is composed of an oncosphere with six tiny hooks. When eggs are expelled from feces, they can spread to humans (Carabin and Traoré, 2014). The mature oncosphere is a round larva with a diameter of 30 μm. It has two penetration glands for migration and six distinctive tiny embryonic hooks (hexacanth embryo) (Alroy et al., 2018). In the intermediate host (pig), the oncosphere develops into the metacestode cysticercus (Del Brutto, 2013). The oncosphere quickly transforms from a solid larva to a bladder-like structure that is filled with fluid and contains cell clusters that eventually develop into an invaginating scolex (Venkat et al., 2016). The cysticercus is an ovoid bladder stage with an invaginating scolex that is filled with opalescent fluid (Bhardwaj and Rather, 2019). The adult tapeworm emerges from underneath the cysticercus scolex. HistoryCases of cerebral cysticercosis were identified after the Catholic Church ceased to condemn autopsies. It is widely acknowledged that the earliest known cases of neurocysticercosis were revealed by Panarolus in 1652, when he discovered similar vesicles in the corpus callosum of a priest who was experiencing seizures, and by Rumler in 1558, when he performed an autopsy on an epileptic patient who had fluid-filled vesicles attached to the meninges (Garcia et al., 2020). Before Malpighi characterized the scolex of T. solium in 1697, the parasitic nature of these vesicles was unknown (Hossain et al., 2023). In those years, Zeder assigned the vesicles to a new genus called Cysticercus (from Greek: kustis, cystis, bladder, and kerkos, cercos, and tail), while Gmelin named them Taenia cellulosae (Symeonidou et al., 2018). Since the word “cyst” is part of the etymological definition of “cysticercus,” the often used term “cysticercus cyst” is a pleonasm. Because of their propensity to flourish in connective tissue, cysticerci were once thought to be a distinct species of parasite and were categorized as Cysticercus cellulosae; this false designation is still frequently used today (Neethu et al., 2019). In 1792, Peruvian physician and journalist Hipólito Unanue wrote in the journal “El Mercurio” about a soldier who had taeniasis and died after suffering severe convulsions. This is likely the first case of taeniasis and cysticercosis occurring simultaneously in the same person (Del Brutto and García, 2015). As stated in the original publication, Unanue’s paper was published as a request to authorities to construct anatomical amphitheaters in communities so that the causes of mortality in the population may be more accurately identified, rather than to explain a particular medical disease. In the 19th century, German pathologists noticed that the head of an adult T. solium and the scolex of a cysticercus resembled each other morphologically. Küchenmeister also proved that eating pork cysticerci caused intestinal taeniasis in humans by giving a condemned man who had been given a death sentence sausages and noodle soup that contained cysticerci from a freshly killed pig (Galán-Puchades and Fuentes, 2013). Küchenmeister discovered “a small Taenia firmly attached by its proboscis to a piece of duodenal mucosa” during the autopsy, along with nine additional Taenia, one of which had a complete crown of 22 hooks in two rows, which is typical of T. solium’s rostellum. Soon after, studies conducted in Belgium and Germany added to our understanding of the life cycle of T. solium by demonstrating that pigs contracted cysticercosis following the ingestion of Taenia eggs derived from proglottids spread by human T. solium carriers (Gonzalez et al., 2006). This conclusion was reinforced by Yoshino’s significant research on the cestode life cycle, which involved infecting himself with T. solium cysticerci (Ito et al., 2020). Life cycleTo reproduce, T. solium requires two vertebrates as hosts. Both definitive and intermediate hosts. Humans can serve as both definitive and intermediate hosts, whereas pigs serve as intermediate hosts (Aung and Spelman, 2016). This worm’s life cycle starts when the intermediate host consumes eggs. The eggs will be broken down by stomach acid. The larvae of the tapeworm Taenia solium are the source of cysticercosis, which affects both humans and pigs, as shown in FIgure 1 (Sciutto et al., 2000). The consumption of eggs released from the feces of human tapeworm carriers is the cause of this sickness. Humans and pigs contract this disease by eating gravid proglottids or eggs (García et al., 2003). Humans can contract this disease through autoinfection or by eating excrement-tainted food. The latter scenario involves the ingestion of tapeworm eggs by humans infected with adult T. solium, either through fecal contamination or, perhaps, from proglottids transported to the stomach by reverse peristalsis (Pray et al., 2020). The hatched oncosphere enters the body through the blood vessels after the eggs are swallowed, travel through the intestines, assault the intestinal wall, and then move to the striated muscles, brain, heart, liver, eyes, and other tissues, where they mature into cysticerci (Lescano and Zunt, 2013).
Fig. 1. Life cycle of Taenia solium: cysticercosis. Humans ingest eggs that cause cysticercosis or cysticerci in pork, causing taeniasis, with the lifecycle involving eggs, tissue invasion, and adult tapeworm development. Cysts in humans can cause neurocysticercosis, a serious consequence if they are located in the brain (Garcia et al., 2014a). Consuming undercooked pork containing cysticerci completes the parasite’s life cycle and causes tapeworm infection in humans (Thomas et al., 2017). The cyst evaginates and uses its scolex to adhere to the small intestine. This parasite forms an adult worm after invaginating the small intestinal wall. Gravid proglottids are released by adult worms 2 months after infection. The adult tapeworm grows to a length of 2–7 m and produces less than 1,000 proglottids, each of which contains roughly 50,000 eggs. They then spend several years in the small intestine (Kassa and Zekarias, 2023). EpidemiologyThe World Health Organization (WHO) has prioritized 17 neglected tropical diseases (NTDs), among which cysticercosis is one of them (Yajima et al., 2023). This illness is one of the NTDs that affects the public health of individuals from low-income backgrounds. Many cases of cysticercosis remain unreported because infected individuals are unaware of their infection and can thus spread the infection to others. Without laboratory and sophisticated molecular testing, it is impossible to identify T. solium eggs, which are discovered in feces and may go undetected (Croker, 2015). Therefore, endemic locations do not report human cysticercosis. Porcine cysticercosis is not well known in endemic areas because of inadequate meat inspection services and the lack of clinical symptoms in afflicted pigs (Afonso et al., 2011). Cysticercosis is endemic to Africa (Gulelat et al., 2022), China (Qian et al., 2020), India (Ahmad et al., 2017), Southeast Asia (Aung and Spelman, 2016), and Latin America (Rodríguez-Rivas et al., 2022). Additionally, more attention needs to be paid to cysticercosis because of the rise in tourism and migration of tapeworm-carrying individuals. Consuming T. solium eggs from fecal-contaminated habitats exposes humans to cysticercosis (Carabin and Traoré, 2014). After being liberated from the egg by gastric acid in the gut, the invasive oncosphere (embryo) travels through the gut wall and can develop in the skin, muscle, eye, and central nervous system (CNS), growing to a size of roughly 1 cm in 2 to 3 months (Nkwengulila, 2014). Cysticercosis is a condition in which a person has cysticerci in their body. This illness is associated with early mortality, morbidity, and disability (Kungu et al., 2015). Qiu et al. (2023) asserted that individuals with impairments experience economic challenges because of their diminished capacity for productive work. However, it is a crippling illness, and those who suffer from neurological symptoms, particularly epilepsy, are stigmatized by society, which frequently results in social withdrawal (Hurła et al., 2024). Among the main issues brought on by cysticercosis are the diagnosis, management, and surveillance of epilepsy patients, as well as associated mishaps such as burns and drowning. The epidemiology of cysticercosis is associated with residence in endemic areas, frequent travel to endemic areas, and household contact (Zammarchi et al., 2013). The disease is focal because although it may not arise without an intermediate host, it typically centers on the human tapeworm carrier (Bustos et al., 2021). Migration from endemic locations can spread the tapeworm infection to non-endemic locations, where it can infect people who do not often eat pork (Pray et al., 2020). However, in endemic nations, the sociocultural milieu, free-range pig-rearing techniques, and inadequate sanitation continue to be the primary causes of this disease (Pondja et al., 2010). Approximately 20 million individuals worldwide suffer from T. solium cysticercosis, causing approximately 50,000 deaths each year (Aung and Spelman, 2016). High rates of cysticercosis have been reported in Asia (Bizhani et al., 2020), Africa (Melki et al., 2018), and Latin America (Rodríguez-Rivas et al., 2022). The prevalence of cysticercosis in the general population can reach 3.6% in some parts of Mexico (Fleury et al., 2012), 3.2% in Asia (Rajshekhar et al., 2003), and 2.8% in the Caribbean (Haiti) (Raccurt et al., 2009). The frequency of cysticercosis varies between 1.3% and 2.4% in West Africa (Zoli et al., 2003). Similarly, neurocysticercosis is estimated to affect over 30% of epileptics in sub-Saharan Africa (Owolabi et al., 2020). Because few studies have examined neurocysticercosis in people, little is known about its prevalence in East Africa. Swine cysticercosis, a disease that affects pigs, has received the majority of attention. Recent studies in Tanzania have revealed that between 14% and 56% of individuals with epilepsy have neurocysticercosis (Hunter et al., 2015). Nevertheless, research on individuals with epilepsy has revealed that cysticercosis is far more common. PathogenesisWhen humans consume pork that has been contaminated and cooked incorrectly, cysticerci enter the small intestine where they are evaginated by digestive enzymes and stick to the intestinal wall (Samorek-Pieróg et al., 2018). The proglottids then procreate and quickly mature into adults. Humans can potentially serve as intermediate hosts after ingesting T. solium eggs (Hossain et al., 2023). In this case, cysticercosis develops in humans. Cysticercosis is caught by humans either through autoinfection in those who have adult pork tapeworms in their intestinal lumen or by eating food and water tainted with tapeworm eggs (García et al., 2003). Cysts can form in any part of the human body; however, they most frequently impact the eyes, skeletal muscle, subcutaneous tissue, and CNS (Ezike et al., 2023). The bloodstream carries parasites into the CNS, where they typically settle in the water-rich gray matter. The parasite may then reach the ventricles or subarachnoid space after migrating to the choroid plexus (Garcia et al., 2011). The illness caused by cysticerci in the CNS is known as neurocysticercosis. The vesicular wall and scolex are the primary components of cysticerci. The cysticerci remain in a vesicular (viable) stage after entering the CNS, which is made up of an invaginated scolex, vesicular fluid, and a transparent membrane (Del Brutto, 2014). Cysticerci either degenerate as a result of the host’s immune system defense systems and calcify or remain alive for a number of years (Prodjinotho et al., 2020). The first stage of cysticerci involution, known as the colloid stage, is marked by hyaline degeneration and turbidity of the vesicular fluid and scolex. The next step, known as the granular stage, is the thickening of the cyst wall and the mineralization of the scolex. At this point, the parasite’s remnants manifest as calcified nodules (calcification stage), and the cysticerci are no longer viable (Fogang et al., 2015). Vesicular cysticerci cause the surrounding tissue to experience a noticeable, mild inflammatory response. Conversely, colloid cysticerci are encased in collagen capsules and exhibit a mononuclear inflammatory response containing the parasite (Christensen et al., 2016). Numerous inflammatory alterations, such as astrocyte gliosis, microglial proliferation, edema, neuronal degenerative changes, and perivascular lymphocyte infiltration, are present in the brain parenchyma (Sikasunge et al., 2009). The edema resolves as the parasite reaches the granular and calcified stages, while the surrounding tissue’s astrocytes undergo more noticeable alterations. Epithelioid cells also emerge, combine with one another, and form multinucleated giant cells (Zumaeta et al., 2024). Unusual thickening of the leptomeninges results from the development of cysticerci in the meninges, which cause an inflammatory response in the subarachnoid space and the formation of an exudate containing collagen fibers, multinucleated giant cells, lymphocytes, eosinophils, and hyalinized parasitic membranes (Takayanagui and Haes, 2022). In addition to small penetrating arteries that emerge from the circle of Willis and can result in cerebral infarction by obstructing the blood vessel lumen, this inflammatory response has the potential to disseminate and harm the optic chiasm and cranial nerves (Del Brutto, 2014). Obstructive hydrocephalus results from blockage of the Luschka and Magendie foramina caused by the parasite membrane and thickened leptomeninges (Del Brutto, 2012). Inflammatory changes may result from ventricular cysticerci connected to the choroid plexus or ventricular wall. Cerebrospinal fluid (CSF) transit is obstructed when damaged ependymal lining is projected into the ventricular cavity, particularly when the protrusion is at or close to the cerebral aqueduct or foramen of Monro (Mansour et al., 2023). The immunological diagnosis of cysticercosis is based on the development of certain antibodies that are triggered by specific cysticerci antigens. Patients with neurocysticercosis also exhibit cellular immunological dysfunction as a result of aberrant cytokine concentrations, abnormal lymphocyte proliferation, and CD8+ T lymphocyte accumulation (Coyle et al., 2024). It has been proposed that decreased cellular immunity could be the cause of the correlation between neurocysticercosis and immune-compromised conditions, as well as the development of gliomas; in these cases, it has been proposed that the growth of glial cells around the parasite, in conjunction with decreased cellular immunity, could impede the immunological surveillance of cancer and result in malignant transformation of astrocytes (Toribio et al., 2024). Immune responseCysticerci can reside in the host tissues of both humans and pigs without causing illness or inflammation. There are several reasons why living cysticerci might not trigger an immune response, including antigenic shift, molecular mimicry of host-like antigenic determinants, sequestration in immunologically privileged locations, host immunoglobulins obscuring cysticerci antigens, and host immune response modulation (DeMaio et al., 2022). The necessity for diagnostic blood tests has led to the study of the immunological response to cysticerci. It is evident that almost all cases of symptomatic cysticercosis are linked to a detectable immunological response, despite the fact that the literature contains a large number of serological tests conducted with differing levels of scientific rigor (Rodriguez et al., 2012). Increased serum immunoglobulin (Ig) production and the development of certain antibodies, primarily of the IgG class, are frequent outcomes of T. solium infection (Moss et al., 2018). IgM, IgA, and IgE antibodies are present in certain patients, but they are less frequent than IgG subclass responses (Dorny et al., 2003). The majority of infected hosts probably create many antibodies with varying specificities that manifest at various points after infection, seemingly in reaction to both qualitative and quantitative alterations in secreted, somatic, and excreted antigens at various stages of parasite development (Flisser et al., 1986). There are many fascinating questions in the field of cysticercosis immunology research. The reaction can be anything from a strong immunological reaction to total tolerance. At any point during the degenerative process, a patient may also have some dispersed calcifications in the brain, viable cysts without inflammation, and significant inflammation surrounding the cyst (Bustos et al., 2023). The majority of neurocysticercosis infections are found inadvertently during autopsy and are asymptomatic in survivors of traffic accidents and war (Ojo et al., 2024). In many cases, imaging studies have revealed some parenchymal calcifications by chance in asymptomatic subjects. These calcifications go through a degenerative or transitional phase after becoming live cysts (Nash and Garcia, 2011). People who have this parasite may exhibit a “silent immune response” and a surprising level of parasite tolerance (Fan et al., 2021). PathologyCysts are homogeneous, round, or oval vesicles that range in size from a few millimeters to 1-2 cm (developing cysts can occasionally reach several centimeters in diameter) (García et al., 2003). Cysts are most frequently observed in the cerebral hemispheres, particularly where gray and white matter meet. There are cysts in the brainstem, subarachnoid space, brainstem, cerebellum, ventricles, spine, and basal cisterns (Garcia et al., 2014a). The range of the number is from one to more than one thousand. The neural parenchyma surrounding the viable cyst remained unchanged upon gross inspection (Nash et al., 2020). The contents of vesicles change depending on the evolutionary stage. The scolex of a live cyst is visible as a tiny, 2-3-mm lump through its translucent membrane. The vesicular fluid becomes dense and opaque as the cyst starts to deteriorate, and the cyst’s edges become smaller and less regular (García et al., 2010). Afterward, a spherical, white, calcified nodule is left behind when calcification starts in the cephalic region and moves to the vesicular wall (García et al., 2003). Rarely, a single person can exhibit all stages of evolution. In the cavity, intraventricular cysticercosis is typically isolated and unattached (Sinha and Sharma, 2012). In rare cases, cysts may migrate between ventricular cavities. Classically, cysts are described as being more frequently located in the lower cavities: most commonly in the fourth ventricle, less commonly in the third ventricle, and least commonly in the lateral ventricles (Lobo et al., 2021). Intraventricular parasites may cause inflammation of the choroid plexus and ependyma or block the flow of CSF (Garcia et al., 2011). The racemose is a large, transparent vesicle that forms in the ventricles or at the base of the brain. It is frequently lobulated and lacks a scolex (Bansal et al., 2016). Occasionally, the stalk connected to the pia mater is encircled by a number of tiny vesicles that resemble clusters of grapes (García et al., 2003). The vesicles take over the basal cisterns, giving them an infiltrative appearance as they proliferate and adhere. The racemose form is associated with high mortality due to its association with hydrocephalus (Mahale et al., 2015). Clinical symptomsCysticerci typically build nests in the muscles and subcutaneous fat of pigs. The clinical signs of cysticercosis are infrequent, although some pigs may exhibit severe illness. The cysts did not develop to the degenerative stage at which they would subsequently manifest clinical symptoms in humans since the pigs were killed at 7 months of age (Arango-Londoño et al., 2024). This parasite typically causes neurocysticercosis, a pleomorphic clinical condition, in humans by infecting the CNS (Prodjinotho et al., 2020). Cysticerci can persist once they have entered the CNS and only slightly alter the inflammatory response of surrounding tissues (Mahanty et al., 2015). Because of their active immune evasion mechanisms and protection from the blood–brain barrier, cysticerci can persist for extended periods of time. The parasite experiences degeneration and related immune-mediated inflammation after an ambiguous and variable period of time (expected to be many years based on classic studies of British soldiers returning from India) (García et al., 2003). The majority of symptoms in neurocysticercosis are directly caused by the inflammatory process that follows cyst degeneration, although cysticerci can also produce symptoms by mass effect or by blocking the flow of CSF fluid (Nash et al., 2006). Therefore, individual variations in the quantity, size, and topography of lesions as well as the intensity of the host’s immune response to the parasite are linked to clinical symptoms. Signs and symptoms are generic and vary. The most prevalent sign of neurocysticercosis is epileptic seizures, which are typically the disease’s sole or predominant symptom (Nash and Garcia, 2011). Seizures are less prevalent in other forms of the disease, but they occur in 50%–80% of patients with brain parenchymal cysts or calcifications (El-Kady et al., 2021). The recent onset of seizures in a middle-aged, young adult, or otherwise healthy adolescent is very predictive of neurocysticercosis in endemic locations (Bustos et al., 2021). When examined neurologically, the majority of the patients are normal. Approximately 50% of patients with neurocysticercosis who initially reported with seizures developed more seizures (epilepsy), according to a series of tests that primarily included patients with moderate types of illness (Carpio and Roma, 2014). Patients with more severe forms of the disease are believed to have a higher rate of relapse. An additional 20%–30% of cases of neurocysticercosis are associated with intracranial hypertension, hydrocephalus, or both (the percentage varies by case origin, being higher in neurosurgical series) (El-Kady et al., 2021). The presence of the parasite itself, ependymal inflammation, or residual fibrosis are the three factors that induce this illness, which is linked to the parasite’s placement in the cerebral ventricles or basal cisterns, which impedes the circulation of CSF (Garcia et al., 2014a). Cysticercosis does not cause significant symptoms outside the CNS. Subcutaneous cysticercosis is characterized by small, movable, painless nodules most commonly seen on the arms or chest (Lobo et al., 2021). The nodules swell and hurt and become inflamed after a few months or even years, and then they eventually go away. In Asia and Africa, subcutaneous cysticercosis is extremely common, whereas it is uncommon in Latin America (Lobo et al., 2021). The diagnosis of cysticercosis can be confirmed by subcutaneous nodule cytology or fine needle biopsy (Kala and Khare, 2014). Muscle cysticercosis is caused by radiography performed for purposes unrelated to the underlying cause. It manifests as pinpoint or ellipsoidal calcifications that follow muscle bundles in the arm or thigh (Gupta et al., 2014). After several years of radiological follow-up, 75% of patients with neurocysticercosis displayed muscle calcification in a traditional series; however, this study has not been repeated using more sensitive computerized tomography (CT) scanning (Nash and Garcia, 2011). Rarely does a patient’s limbs grow due to high parasite burden (muscular pseudohypertrophy) (Bhalla et al., 2008). Approximately 5% of patients have an infection in their heart, which is another infrequent source of cysticercosis (Brunetti and White, 2012). Currently, there are no symptoms associated with cardiac cysticercosis. Taenia solium is the most prevalent intraorbital parasite, although ocular cysticercosis is far less frequent than neurocysticercosis, which affects 1%–3% of all infections (Gonzalez-Alcaide et al., 2023). Most frequently, intraocular cysts are found floating freely in the subretinal region or vitreous fluid (Dhiman et al., 2017). The severity of retinal tissue damage or the onset of chronic uveitis is associated with visual impairment. Additionally, cysticercosis can damage the conjunctiva, extraocular muscles, or anterior chamber (Verma et al., 2016). Cysts in the retroocular area can impact the optic nerve or cause proptosis in some patients with severe illness (Dhiman et al., 2017). The use of orbital ultrasonography is a useful and safe diagnostic technique (Babalola et al., 2013). Cerebral cysticercosis resulting in chiasm compression, hydrocephalus, or papilledema may also be linked to vision loss (Coyle and Tanowitz, 2009). DiagnosisNeuroimaging, serological tests, and a thorough clinical examination are good methods for diagnosing cysticercosis (Coyle and Tanowitz, 2009). Every technique has advantages and disadvantages; some are better at identifying different phases of cysticercosis infection (calcified cysts and cysts). Anti-T. solium antibodies or specific T. solium antigens can be detected in the blood, urine, and CNS using serological techniques (Rodriguez et al., 2009). Antibody testing specific to T. solium cannot differentiate between exposure to a prior infection and the present infection. Specific antibodies against the lentil lectin-purified glycoprotein antigen (LLGP-EITB) of T. solium were detected using enzyme-linked immunoelectrotransfer blotting (EITB) (Arroyo et al., 2018). EITB has an almost 100% sensitivity in patients with several parenchymal cysts or subarachnoid neurocysticercosis but only 60%–70% sensitivity in patients with a single calcified cyst or parenchymal lesion (White et al., 2018). IgG is the immunoglobulin target used in the enzyme-linked immunosorbent assay (ELISA) for the detection of T. solium antibodies using crude or refined parasite antigen extracts; nevertheless, its specificity and sensitivity are typically lower than those of EITB (Kabululu et al., 2020). However, specific ELISA is helpful for extraparenchymal cyst diagnosis and treatment evaluation (Garcia et al., 2014a). Monoclonal antibody-based antigen capture ELISA (Ag-ELISA) can detect circulating cysticercus antigens (Kabululu et al., 2020). This test only detects the presence of living, active cysts. Ag-ELISA can be used in conjunction with antibody detection tests to distinguish between dead larvae and live parasite infection in degenerating cysts; intraparenchymal neurocysticercosis is linked to low or undetectable antigen levels, whereas extraparenchymal neurocysticercosis is linked to high antigen levels. The gold standard for diagnosing neurocysticercosis is neuroimaging; however, this technology is either unavailable or too expensive in many regions where the disease is widespread (Butala et al., 2021). Cysticerci are visualized in the CNS via magnetic resonance imaging (MRI) or computed tomography (CT), which provides information on the number of cysts, lesion morphology, stage of cyst evolution, and degree of host inflammatory response to the parasite (Nash and Garcia, 2011). The most popular diagnostic imaging method when it is available is CT, particularly in underdeveloped nations. However, intraventricular cysts, which account for up to 22% of all cases of neurocysticercosis, are easier to detect with MRI than with CT (Butala et al., 2021). To improve diagnosis in non-endemic areas where neurocysticercosis is frequently disregarded and to eliminate false-positive diagnoses in endemic areas (from serological testing), Del Brutto et al. (2017) updated their diagnostic criteria for neurocysticercosis to incorporate neuroimaging. According to the updated diagnostic criteria, a definite diagnosis of neurocysticercosis requires the presence of the tapeworm soleus (head) on the scan, and neuroimaging is necessary to make this diagnosis. The high expense of imaging prevents early and sequential scanning; neuroimaging is unavailable in many endemic locations, and radiologists’ training for accurate scan interpretation can be challenging in underdeveloped nations (Dhesi et al., 2015). TransmissionAn infection with T. solium tapeworm can be transmitted by eating raw or undercooked pork that has been infected (Pray et al., 2020). There are not many clinical signs of tapeworm infections. Pigs can contract tapeworms from the eggs released in the feces of tapeworm carriers (Pray et al., 2017). Ingestion of T. solium eggs by a person (via the fecal–oral route, contaminated food or water, or close contact with a carrier) or close contact with a carrier can also result in infection with the larval parasite in the tissues, which causes human cysticercosis (Symeonidou et al., 2018). Additionally, a person infected with tapeworms may autoinfect themselves with the worms’ eggs, which might result in cysticercosis (Ito et al., 2016). The muscles, skin, eyes, and CNS can also harbor larvae (cysticerci) (Kraft, 2007). The disorder is known as neurocysticercosis, and it involves the formation of cysts in the brain. Risk factorsIn many underdeveloped nations, cysticercosis is more common because of a number of risk factors. This illness is caused by low socioeconomic status (Aung and Spelman, 2016). Individuals with pork tapeworms in their intestinal lumen, pigs, poverty, and cultural factors are the main causes of this disease (Kungu et al., 2015). Most cases of this disease occur in rural regions with poor sanitation and no facilities for meat inspection (Wilson et al., 2023). Numerous factors contribute to the disease’s human transmission, such as the use of untreated or partially treated human waste in agriculture, incorrect food handling, ignorance of the infection risk when visiting endemic areas, and eating raw or undercooked meat (Wu et al., 2017). All phases of the parasite life cycle are affected by human activity. Pork tapeworms in the intestinal lumen of humans are a cause of environmental contamination (Jansen et al., 2021). Some of the risk factors related to pigs include the type of diet, source of water supply, failure to deworm the pigs, procurement of replacement piglets, source of income for farmers, and way pigs handle raw feces (Kungu et al., 2017). One of the key aspects of pig breeding is pig farming, particularly in places with inadequate or nonexistent restrooms (Kajuna et al., 2022). Public health importanceAn estimated 50 million people worldwide suffer from cysticercosis, which is common in Latin America, Asia, and parts of Africa (Sorvillo et al., 2011). Immigrants from endemic regions, especially Latin America, frequently present with the disease when they arrive in the United States (Arsuaga et al., 2024). Cysticercosis has recently received considerable attention as a significant preventable cause of epilepsy and a neglected infection linked to poverty in the United States. Reports of autochthonous infections have highlighted the importance of this parasite infection as a public health issue in the United States (Hotez, 2008). Schantz et al. (1992) verified this tendency by describing a cysticercosis outbreak that occurred in 1990–1991 in an Orthodox Jewish community in New York City. Economic impactBecause neurocysticercosis mainly affects older adults and children, prolonged impairment is predicted to have serious economic repercussions. Pigs infected with T. solium are responsible for 4.7% to 26.9% of total pig farming expenses, resulting in losses of €10 million and US$18.6 to US$34.2 million annually, respectively, according to global economic impact research (Bulaya et al., 2015). According to Fleury et al. (2012), hospitalization of newly diagnosed cases of neurocysticercosis alone costs Mexico $15 million annually. The costs of continuing antiepileptic medication therapy for epilepsy caused by neurocysticercosis, as well as other long-term expenses, are not available. Even after being deemed cured, patients with calcified cysticercosis may continue to have seizures or other neurologic morbidities for years (Bustos et al., 2021). Taenia solium not only causes serious illness but also costs the swine business a lot of money (Lightowlers et al., 2016). For instance, Mexico has lost almost half of its national investment in pig production due to swine cysticercosis, and the projected annual costs from meat damage are $43 million (Bhattarai et al., 2019). TreatmentChemotherapy, surgical excision of the cyst, and symptomatic treatment (with or without cyst removal) are the available therapeutic options. Usually, therapy consists of giving a mix of drugs to treat the cyst and drugs to alleviate its symptoms. It is possible to treat human tapeworm infections using niclosamide (Chen et al., 2018). Treatment options for adult T. solium worms include 2 g of niclosamide, 5 mg/kg BW of paromomycin, 7–10 mg/kg BW of quinacrine, and 3–4 mg/kg BW of oxfendazole (Pawlowski, 2006). For older than 30 years, intestinal nematodes, cysticercosis, and schistosomiasis have been regularly managed with the anthelmintic medications praziquantel and albendazole (Hong, 2018). Albendazole and praziquantel are currently the only medications available for treating neurocysticercosis, despite modifications to the recommended dosages (Matthaiou et al., 2008). Pregnant women should not take albendazole; however, children older than 2 years can receive lower dosages (Gyorkos and St-Denis, 2019). Praziquantel is recommended for expectant mothers and can be taken by both children and pregnant women (Friedman et al., 2018). Due to inadequate absorption, neither medication is 100% effective; albendazole has an oral absorption rate of less than 5% (though this rises to 25% when taken with a high-fat meal), whereas praziquantel has an oral absorption rate of 80% (Butala et al., 2021). Praziquantel is quickly absorbed and is recommended at a dose of 50 mg/kg/day for 10–14 days. Albendazole is typically administered for 10–14 days at a dose of 15 mg/kg/day (White et al., 2018). A 30-day course of extended albendazole treatment may be required in severe cases and in certain parenchymal cases (Sugianto et al., 2023). In terms of reducing live cysticerci, albendazole was administered at levels with or better than praziquantel in comparative clinical trials. It has been demonstrated that the frequency of generalized seizures is decreased for 30 months following therapy with albendazole at a dose of 15 mg/kg/day for 10 days, followed by a dose of 6 mg dexamethasone to minimize inflammation (Butala et al., 2021). Albendazole, which is more often recommended than praziquantel, is more effective against extraparenchymal forms and penetrates the CSF more effectively (Matthaiou et al., 2008). These medications may exacerbate the symptoms of intracranial hypertension and cysticercotic encephalitis in certain patients. In certain situations, treatment with a combination of both medications may be the best option because some patients respond better to one medication than the other. According to Garcia et al. (2014b), albendazole plus praziquantel treatment enhanced the cysticidal effects of individuals with numerous brain cysts while reducing drug-induced adverse effects. However, frequent prescriptions of anthelminics can increase the risk of resistance. VaccinationDue to the occult nature of the infection, which makes it difficult to identify tapeworm carriers, and the low morbidity associated with intestinal infection, cysticercosis is not currently immunologically or logistically susceptible to vaccination (Hossain et al., 2023). Since little is known about the immunological response to human cysticercosis, protective or therapeutic vaccination against the disease has not been commonly regarded as an acceptable strategy in endemic locations (Singhi and Saini, 2016). Humans do not appear to have protective immunity against cysticercosis, and there is little chance that humans will ever be vaccinated against this parasite. Vaccinating pigs is an alluring way to disrupt the parasite life cycle. This endeavor has made significant strides. Vaccines derived from cysticercoid extracts have shown promise in field testing, and recombinant vaccines might work well in controlled settings (Toledo et al., 1999). Fast advancements in the sequencing and comparison of T. solium and related parasite antigens have led to the creation of more potent T. solium vaccines (Gauci et al., 2013). It is possible to prevent human infection and pig transmission by vaccinating pigs with a combination of the recombinant antigens TSOL16 and TSOL18 (Jayashi et al., 2012). ControlTaenia solium mainly spreads in rural settings where diseased pork is readily available and pigs have access to untreated human waste or feces (García et al., 2003). Symptomatic neurocysticercosis is believed to affect 400,000 people in Latin America alone (Bern et al., 1999). Cysticercosis has been eliminated in more through improved sanitation and restrictions on domestic pig husbandry (Kabululu et al., 2023). There is an urgent need for intervention methods for control and eradication because minimal socioeconomic development is anticipated in endemic areas in the foreseeable future. No intervention has effectively and sustainably blocked transmission to date. In the majority of endemic nations, diagnostic techniques are slaughterhouse inspections and seizures (Aung and Spelman, 2016). The majority of pigs in these regions are killed covertly. Slaughterhouse checks might miss the majority of mild illnesses because each carcass is only sliced a few times to preserve its commercial worth (García et al., 2003). This strategy does not prevent the spread of contaminated pork but rather incentivizes farmers to kill pigs illegally. In terms of transmission, only infected pork and tapeworm carriers were significant. Although neurocysticercosis is a public health concern, it does not represent a public health issue unless individuals also have intestinal tapeworms (Garcia et al., 2014a). Human cysticercosis can be prevented by treating the entire population or by identifying and treating tapeworm carriers (Sorvillo et al., 2011). Inspections at slaughterhouses can help stop the spread of the disease by preventing the sale and consumption of contaminated pigs (Edia-Asuke et al., 2014). In Mexico, health education tests alone have yielded encouraging but insufficient results (Bhattarai et al., 2019). Although there was a significant increase in knowledge about the disease, risky behaviors (such as hand washing, field defecation, and pig herding) remained constant (Ngowi et al., 2017). The long-term consequences of acquired knowledge motivate health education. Infections in pigs can be controlled by mass anthelmintic therapy or, if vaccination is effective, by immunizing the pig population (Ouma et al., 2021). Given that the target population is comprised of remote endemic areas, the high cost of vaccines will surely deter the vaccine strategy. To achieve sustained control, any combination of actions must guarantee community collaboration. ConclusionCysticercosis is a disease caused by the larvae of T. solium and is a significant public health problem, especially in developing countries, with serious health and economic impacts. Effective management of this disease is essential for reducing its prevalence and risk and for protecting public health. AcknowledgmentsThe authors are grateful to Universitas Airlangga and Badan Riset dan Inovasi Nasional. Conflict of interestThe authors declare no conflict of interest. FundingThis research was supported by Rencana Kerja dan Anggaran Tahunan Skema Mandat year 2020 (grant number: 384/UN3.14/PT/2020). Author’s contributionsKK, ARK, MKJK, and DHS drafted the manuscript. WW, DAAK, IBM, and IF revised and edited the manuscripts. AS, ES, SW, JJ, and RZA participated in preparing and critical checking this manuscript. FE, KAF, SA, SMY, and BWKW edited the references. All authors have read and approved the final manuscript. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAfonso, S.M., Vaz, Y., Neves, L., Pondja, A., Dias, G., Willingham, A.L., Vilhena, M., Duarte, P.C., Jost, C.C. and Noormahomed, E.V. 2011. Human and porcine Taenia solium infections in Mozambique: identifying research priorities. Anim. Health Res. Rev. 12(1), 123–129. Ahmad, R., Khan, T., Ahmad, B., Misra, A. and Balapure, A.K. 2017. Neurocysticercosis: a review on status in India, management, and current therapeutic interventions. Parasitol. Res. 116(1), 21–33. Alroy, K.A., Arroyo, G., Gilman, R.H., Gonzales-Gustavson, E., Gallegos, L., Gavidia, C.M., Verastegui, M., Rodriguez, S., Lopez, T., Gomez-Puerta, L.A., Alroy, J., Garcia, H.H., Gonzalez, A.E. and For The Cysticercosis Working Group In Peru. 2018. Carotid Taenia solium oncosphere infection: a novel porcine neurocysticercosis model. Am. J. Trop. Med. Hyg. 99(2), 380–387. Arango-Londoño, M.M., López-Osorio, S., Rojas-Bermudéz, F. and Chaparro-Gutiérrez, J.J. 2024. The frequency of porcine cysticercosis and factors associated with Taenia solium infection in the municipality of Tuchín-Córdoba, Colombia. Pathogens. 13(4), 311. Arroyo, G., Rodriguez, S., Lescano, A.G., Alroy, K.A., Bustos, J.A., Santivañez, S., Gonzales, I., Saavedra, H., Pretell, E.J., Gonzalez, A.E., Gilman, R.H., Tsang, V.C.W., Garcia, H.H. and Cysticercosis Working Group in Peru. 2018. Antibody banding patterns of the enzyme-linked immunoelectrotransfer blot and brain imaging findings in patients with neurocysticercosis. Clin. Infect. Dis. 66(2), 282–288. Arsuaga, M., De Miguel Buckley, R., De La Calle-Prieto, F. and Díaz-Menéndez, M. 2024. Imported infectious diseases in migrants from Latin America: a retrospective study from a referral centre for tropical diseases in Spain, 2017-2022. Travel Med. Infect. Dis. 59(1), 102708. Aung, A.K. and Spelman, D.W. 2016. Taenia solium taeniasis and cysticercosis in Southeast Asia. Am. J. Trop. Med. Hyg. 94(5), 947–954. Babalola, O., Adu, A. and Akano, A. 2013. Ocular cysticercosis in a 32-year-old man in Abuja: ultrasonic features as an aid in diagnosis. Clin. Ophthalmol. 7(1), 2275–2279. Bansal, R., Gupta, M., Bharat, V., Sood, N. and Agarwal, M. 2016. Racemose variant of neurocysticercosis: a case report. J. Parasit Dis. 40(2), 546–549. Bern, C., Garcia, H.H., Evans, C., Gonzalez, A.E., Verastegui, M., Tsang, V.C. and Gilman, R.H. 1999. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin. Infect. Dis. 29(5), 1203–1209. Bhalla, A., Sood, A., Sachdev, A. and Varma, V. 2008. Disseminated cysticercosis: a case report and review of the literature. J. Med. Case Rep. 2(1), 137. Bhardwaj, S. and Rather, G. 2019. Fine needle aspiration cytology of cysticercosis: a study of 30 cases. J. Cytol. 36(1), 18–21. Bhattarai, R., Carabin, H., Proaño, J.V., Flores-Rivera, J., Corona, T., Flisser, A., León-Maldonado, L. and Budke, C.M. 2019. The monetary burden of cysticercosis in Mexico. PLoS Negl. Trop. Dis. 13(7), e0007501. Bizhani, N., Hafshejani, S.H., Mohammadi, N., Rezaei, M. and Rokni, M.B. 2020. Human cysticercosis in Asia: a systematic review and meta-analysis. Iran J. Public Health 49(10), 1839–1847. Brunetti, E. and White, A.C. 2012. Cestode infestations: hydatid disease and cysticercosis. Infect. Dis. Clin. North Am. 26(2), 421–435. Bulaya, C., Mwape, K.E., Michelo, C., Sikasunge, C.S., Makungu, C., Gabriel, S., Dorny, P. and Phiri, I.K. 2015. Preliminary evaluation of Community-Led Total Sanitation for the control of Taenia solium cysticercosis in Katete District of Zambia. Vet. Parasitol. 207(3–4), 241–248. Bustos, J.A., Arroyo, G., Del Brutto, O.H., Gonzales, I., Saavedra, H., Guzman, C., Sanchez-Boluarte, S.S., Thakur, K.T., Coyle, C., O’Neal, S.E., Garcia, H.H. and Cysticercosis Working Group in Peru (CWGP). 2023. Calcified neurocysticercosis: demographic, clinical, and radiological characteristics of a large hospital-based patient cohort. Pathogens 13(1), 26. Bustos, J., Gonzales, I., Saavedra, H., Handali, S., Garcia, H.H. and Cysticercosis Working Group in Peru. 2021. Neurocysticercosis. A frequent cause of seizures, epilepsy, and other neurological morbidity in most of the world. J. Neurol. Sci. 427(1), 117527. Butala, C., Brook, T.M., Majekodunmi, A.O. and Welburn, S.C. 2021. Neurocysticercosis: current perspectives on diagnosis and management. Front. Vet. Sci. 8(1), 615703. Carabin, H. and Traoré, A.A. 2014. Taenia solium taeniasis and cysticercosis control and elimination through community-based interventions. Curr. Trop. Med. Rep. 1(4), 181–193. Carpio, A. and Roma, M.L. 2014. The relationship between neurocysticercosis and epilepsy: an endless debate. Arq. Neuro-Psiquiatr. 72(5), 383–390. Chege, B., Ndambuki, G., Owiny, M., Kiyong’a, A., Fèvre, E.M. and Cook, E.A.J. 2023. Improved latrine coverage may reduce porcine cysticercosis: a comparative cross-sectional study, Busia County, Kenya 2021. Front. Vet. Sci. 10(1), 1155467. Chen, W., Mook, R.A. Jr, Premont, R.T. and Wang, J. 2018. Niclosamide: beyond an antihelminthic drug. Cell Signal 41(1), 89–96. Christensen, N.M., Trevisan, C., Leifsson, P.S. and Johansen, M.V. 2016. The association between seizures and deposition of collagen in the brain in porcine Taenia solium neurocysticercosis Vet. Parasitol. 228(1), 180–182. Coyle, C.M. and Tanowitz, H.B. 2009. Diagnosis and treatment of neurocysticercosis. Interdiscip. Perspect. Infect. Dis. 2009(1), 180742. Coyle, C.M., Bustos, J.A. and Garcia, H.H. 2024. Current challenges in neurocysticercosis: recent data and where we are heading. Curr. Opin. Infect. Dis. 37(5), 313–319. Croker, C. 2015. Challenges and opportunities in detecting Taenia solium tapeworm carriers in Los Angeles County California, 2009-2014. J. Epidemiol. Glob. Health 5(4), 359–363. Del Brutto, O.H. 2012. Neurocysticercosis: a review. Sci. World J. 2012(1), 159821. Del Brutto, O.H. 2013. Human cysticercosis (Taenia solium). Trop. Parasitol. 3(2), 100–103. Del Brutto, O.H. 2014. Neurocysticercosis. Neurohospitalist 4(4), 205–212. Del Brutto, O.H. and García, H.H. 2015. Taenia solium cysticercosis--the lessons of history. J. Neurol. Sci. 359(1–2), 392–395. Del Brutto, O.H., Nash, T.E., White, A.C. Jr, Rajshekhar, V., Wilkins, P.P., Singh, G., Vasquez, C.M., Salgado, P., Gilman, R.H. and Garcia, H.H. 2017. Revised diagnostic criteria for neurocysticercosis. J. Neurol. Sci. 372(1), 202–210. DeMaio, A., Mehrotra, S., Sambamurti, K. and Husain, S. 2022. The role of the adaptive immune system and T cell dysfunction in neurodegenerative diseases. J. Neuroinflammation 19(1), 251. Dhesi, B., Karia, S.J., Adab, N. and Nair, S. 2015. Imaging in neurocysticercosis. Pract. Neurol. 15(2), 135–137. Dhiman, R., Devi, S., Duraipandi, K., Chandra, P., Vanathi, M., Tandon, R. and Sen, S. 2017. Cysticercosis of the eye. Int. J. Ophthalmol. 10(8), 1319–1324. Dorny, P., Brandt, J., Zoli, A. and Geerts, S. 2003. Immunodiagnostic tools for human and porcine cysticercosis. Acta Trop. 87(1), 79–86. Edia-Asuke, A.U., Inabo, H.I., Umoh, V.J., Whong, C.M., Asuke, S. and Edeh, R.E. 2014. Assessment of sanitary conditions of unregistered pig slaughter slabs and post mortem examination of pigs for Taenia solium metacestodes in Kaduna metropolis, Nigeria. Infect. Dis. Poverty. 3(1), 45. El-Kady, A.M., Allemailem, K.S., Almatroudi, A., Abler, B. and Elsayed, M. 2021. Psychiatric disorders of neurocysticercosis: narrative review. Neuropsychiatr. Dis. Treat. 17(1), 1599–1610. Ezike, K.N., Okwudire-Ejeh, I.A., Dallang, B.C. and Ndaiya, R. 2023. Cysticercosis mimicking fibroadenoma of the breast in a young female: a case report from North Central Nigeria. Cureus 15(4), e38141. Fan, X., Zhang, Y., Ouyang, R., Luo, B., Li, L., He, W., Liu, M., Jiang, N., Yang, F., Wang, L. and Zhou, B. 2021. Cysticercus cellulosae regulates T-cell responses and interacts with the host immune system by excreting and secreting antigens. Front. Cell. Infect. Microbiol. 11(1), 728222. Fleury, A., Sciutto, E. and Larralde, C. 2012. Neurocysticercosis is still prevalent in Mexico. Salud. Publica Mex. 54(6), 632–636. Flisser, A., Espinoza, B., Tovar, A., Plancarte, A. and Correa, D. 1986. Host--parasite relationship in cysticercosis: immunologic study in different compartments of the host. Vet. Parasitol. 20(1–3), 95–102. Fogang, Y.F., Savadogo, A.A., Camara, M., Toffa, D.H., Basse, A., Sow, A.D. and Ndiaye, M.M. 2015. Managing neurocysticercosis: challenges and solutions. Int. J. Gen. Med. 8(1), 333–344. Friedman, J.F., Olveda, R.M., Mirochnick, M.H., Bustinduy, A.L. and Elliott, A.M. 2018. Praziquantel for the treatment of schistosomiasis during human pregnancy. Bull. World Health Organ. 96(1), 59–65. Galán-Puchades, M.T. and Fuentes, M.V. 2013. Lights and shadows of the Taenia asiatica life cycle and pathogenicity. Trop. Parasitol. 3(2), 114–119. Garcia, H.H., Gonzalez, A.E. and Gilman, R.H. 2011. Cysticercosis of the central nervous system: how should it be managed? Curr. Opin. Infect. Dis. 24(5), 423–427. Garcia, H.H., Gonzalez, A.E. and Gilman, R.H. 2020. Taenia solium cysticercosis and its impact in neurological disease. Clin. Microbiol. Rev. 33(3), e00085-19. García, H.H., Gonzalez, A.E., Evans, C.A., Gilman, R.H. and Cysticercosis Working Group in Peru. 2003. Taenia solium cysticercosis. Lancet 362(9383), 547–556. García, H.H., Gonzalez, A.E., Rodriguez, S., Tsang, V.C., Pretell, E.J., Gonzales, I., Gilman, R.H. and Cysticercosis Working Group in Peru. 2010. Neurocysticercosis: unraveling the nature of the single cysticercal granuloma. Neurology 75(7), 654–658. Garcia, H.H., Nash, T.E. and Del Brutto, O.H. 2014a. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet. Neurol. 13(12), 1202–1215. Garcia, H.H., Gonzales, I., Lescano, A.G., Bustos, J.A., Zimic, M., Escalante, D., Saavedra, H., Gavidia, M., Rodriguez, L., Najar, E., Umeres, H., Pretell, E.J. and Cysticercosis Working Group in Peru. 2014b. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect. Dis. 14(8), 687–695. Gauci, C., Jayashi, C. and Lightowlers, M.W. 2013. Vaccine development against the Taenia solium parasite: the role of recombinant protein expression in Escherichia coli. Bioengineered 4(5), 343–347. Gonzalez, A.E., Lopez-Urbina, T., Tsang, B., Gavidia, C., Garcia, H.H., Silva, M.E., Ramos, D.D., Manzanedo, R., Sanchez-Hidalgo, L., Gilman, R.H., Tsang, V.C. and cysticercosis working group in Peru. 2006. Transmission dynamics of Taenia solium and potential for pig-to-pig transmission. Parasitol. Int. 55(Suppl), S131–S135. Gonzalez-Alcaide, G., Sosa, N., Shevy, L., Belinchon-Romero, I. and Ramos-Rincon, J.M. 2023. Global research on cysticercosis and neurocysticercosis: a bibliometric analysis. Front. Vet. Sci. 10(1), 1156834. Gulelat, Y., Eguale, T., Kebede, N., Aleme, H., Fèvre, E.M. and Cook, E.A.J. 2022. Epidemiology of porcine cysticercosis in Eastern and Southern Africa: systematic review and meta-analysis. Front. Public Health 10(1), 836177. Gupta, S., Gupta, S., Mittal, A., Mahendra, A., Aggarwal, A., Batra, R. and Jindal, N. 2014. A rare manifestation of cysticercosis infestation. Acta Med. Indones. 46(1), 54–57. Gyorkos, T.W. and St-Denis, K. 2019. Systematic review of exposure to albendazole or mebendazole during pregnancy and effects on maternal and child outcomes, with particular reference to exposure in the first trimester. Int. J. Parasitol. 49(7), 541–554. Hong, S.T. 2018. Albendazole and praziquantel: review and safety monitoring in Korea. Infect. Chemother. 50(1), 1–10. Hossain, M.S., Shabir, S., Toye, P., Thomas, L.F. and Falcone, F.H. 2023. Insights into the diagnosis, vaccines, and control of Taenia solium, a zoonotic, neglected parasite. Parasites Vectors, 16(1), 380. Hotez, P.J. 2008. Neglected infections of poverty in the United States of America. PLoS Negl. Trop. Dis. 2(6), e256. Hunter, E., Burton, K., Iqbal, A., Birchall, D., Jackson, M., Rogathe, J., Jusabani, A., Gray, W., Aris, E., Kamuyu, G., Wilkins, P.P., Newton, C.R. and Walker, R. 2015. Cysticercosis and epilepsy in rural Tanzania: a community-based case-control and imaging study. Trop. Med. Int. Health 20(9), 1171–1179. Hurła, M., Pikor, D., Kościelecka, K., Drelichowska, A., Banaszek, N. and Paul, M. 2024. Neurocysticercosis—diagnostic mystery: current status for Europe. BioMed 4(3), 302–313. Ito, A., Saito, M., Donadeu, M. and Lightowlers, M.W. 2020. Kozen Yoshino’s experimental infections with Taenia solium tapeworms: An experiment never to be repeated. Acta Trop. 205(1), 105378. Ito, A., Yanagida, T. and Nakao, M. 2016. Recent advances and perspectives in molecular epidemiology of Taenia solium cysticercosis. Infect. Genet. Evol. 40(1), 357–367. Jansen, F., Dorny, P., Gabriël, S., Dermauw, V., Johansen, M.V. and Trevisan, C. 2021. The survival and dispersal of Taenia eggs in the environment: what are the implications for transmission? a systematic review. Parasit Vectors 14(1), 88. Jayashi, C.M., Kyngdon, C.T., Gauci, C.G., Gonzalez, A.E. and Lightowlers, M.W. 2012. Successful immunization of naturally reared pigs against porcine cysticercosis with a recombinant oncosphere antigen vaccine. Vet. Parasitol. 188(3–4), 261–267. Kabululu, M.L., Johansen, M.V., Lightowlers, M., Trevisan, C., Braae, U.C. and Ngowi, H.A. 2023. Aggregation of Taenia solium cysticerci in pigs: implications for transmission and control. Parasite Epidemiol. Control 22(1), e00307. Kabululu, M.L., Johansen, M.V., Mlangwa, J.E.D., Mkupasi, E.M., Braae, U.C., Trevisan, C., Colston, A., Cordel, C., Lightowlers, M.W. and Ngowi, H.A. 2020. Performance of Ag-ELISA in the diagnosis of Taenia solium cysticercosis in naturally infected pigs in Tanzania. Parasites Vectors 13(1), 534. Kajuna, F., Mwang’onde, B.J., Holst, C., Ngowi, B., Sukums, F., Noll, J., Winkler, A.S. and Ngowi, H. 2022. Porcine cysticercosis sero-prevalence and factors associated with its occurrence in Southern Highlands, Tanzania. Sci. Afr. 17(1), e01382. Kala, P. and Khare, P. 2014. Fine-needle aspiration cytology as a diagnostic modality for cysticercosis: a clinicocytological study of 137 cases. J. Cytol. 31(2), 68–72. Kassa, S.T. and Zekarias, T. 2023. Review on the prevalence and associated risk factors of parasitic zoonosis in Ethiopia: taeniasis. J. Nutr. Food Process. 6(7), 1–7. Kraft, R. 2007. Cysticercosis: an emerging parasitic disease. Am. Fam. Physician. 76(1), 91–96. Kungu, J.M., Dione, M.M., Ejobi, F., Ocaido, M. and Grace, D. 2017. Risk factors, perceptions and practices associated with Taenia solium cysticercosis and its control in the smallholder pig production systems in Uganda: a cross-sectional survey. BMC Infect. Dis. 17(1), 1. Kungu, J.M., Dione, M.M., Ocaido, M. and Ejobi, F. 2015. Status of Taenia solium cysticercosis and predisposing factors in developing countries involved in pig farming. Int. J. One Health 1(1), 6–13. Lescano, A.G. and Zunt, J. 2013. Other cestodes: sparganosis, coenurosis and Taenia crassiceps cysticercosis. Handb. Clin. Neurol. 114(1), 335–345. Lightowlers, M.W., Garcia, H.H., Gauci, C.G., Donadeu, M. and Abela-Ridder, B. 2016. Monitoring the outcomes of interventions against Taenia solium: options and suggestions. Parasite Immunol. 38(3), 158–169. Lobo, F.D., Vatsala, K.B., Adiga, A.S.D. and Rai, S. 2021. Cysts that still persist: a case series of cysticercosis on histopathological evaluation. Turk. Patoloji Derg. 37(3), 254–257. Mahale, R.R., Mehta, A. and Rangasetty, S. 2015. Extraparenchymal (Racemose) neurocysticercosis and its multitude manifestations: a comprehensive review. J. Clin. Neurol. 11(3), 203–211. Mahanty, S., Orrego, M.A., Mayta, H., Marzal, M., Cangalaya, C., Paredes, A., Gonzales-Gustavson, E., Arroyo, G., Gonzalez, A.E., Guerra-Giraldez, C., García, H.H., Nash, T.E. and Cysticercosis Working Group in Peru. 2015. Post-treatment vascular leakage and inflammatory responses around brain cysts in porcine neurocysticercosis. PLoS Negl. Trop. Dis. 9(3), e0003577. Mansour, M.A., Tahir, M. and Ahmadi, Z. 2023. Neurocysticercosis presenting as a locked-in lateral ventricle: A case report and evidence-based review. IDCases 32(1), e01778. Matthaiou, D.K., Panos, G., Adamidi, E.S. and Falagas, M.E. 2008. Albendazole versus praziquantel in the treatment of neurocysticercosis: a meta-analysis of comparative trials. PLoS Negl. Trop. Dis. 2(3), e194. Meena, D., Gupta, M., Jain, V.K. and Arya, R.K. 2016. Isolated intramuscular cysticercosis: clinicopathological features, diagnosis and management—a review. J. Clin. Orthop. Trauma 7(Suppl 2), 243–249. Melki, J., Koffi, E., Boka, M., Touré, A., Soumahoro, M.K. and Jambou, R. 2018. Taenia solium cysticercosis in West Africa: status update. Parasite 25(1), 49. Moss, D.M., Handali, S., Chard, A.N., Trinies, V., Bullard, S., Wiegand, R.E., Doumbia, S., Freeman, M.C. and Lammie, P.J. 2018. Detection of immunoglobulin G antibodies to Taenia solium cysticercosis antigen glutathione-S-transferase-rT24H in malian children using multiplex bead assay. Am. J. Trop. Med. Hyg. 98(5), 1408–1412. Nash, T.E. and Garcia, H.H. 2011. Diagnosis and treatment of neurocysticercosis. Nat. Rev. Neurol. 7(10), 584–594. Nash, T.E., O’Connell, E.M., Hammoud, D.A., Wetzler, L., Ware, J.M. and Mahanty, S. 2020. Natural history of treated subarachnoid neurocysticercosis. Am. J. Trop. Med. Hyg. 102(1), 78–89. Neethu, K.C., Jain, A. and Haritha, S. 2019. Cysticercosis cellulosae cutis: a forgotten entity. Indian Dermatol. Online J. 10(5), 574–576. Ngowi, H., Ozbolt, I., Millogo, A., Dermauw, V., Somé, T., Spicer, P., Jervis, L.L., Ganaba, R., Gabriel, S., Dorny, P. and Carabin, H. 2017. Development of a health education intervention strategy using an implementation research method to control taeniasis and cysticercosis in Burkina Faso. Infect. Dis. Poverty 6(1), 95. Nkwengulila, G. 2014. A review of human cysticercosis and diagnostic challenges in endemic resource poor countries. Adv. Infect. Dis. 04(04), 207–213. Ojo, O.A., Onyia, C.U., Lawal, B.O. and Awolola, N.A. 2024. Neurocysticercosis in a Nigerian woman -missed diagnosis of a neglected disease and surgical management. World Neurosurg. X 23(1), 100380. Okello, A.L. and Thomas, L.F. 2017. Human taeniasis: current insights into prevention and management strategies in endemic countries. Risk Manag. Healthc. Policy 10(1), 107–116. Ouma, E., Dione, M., Mtimet, N., Lule, P., Colston, A., Adediran, S. and Grace, D. 2021. Demand for Taenia solium cysticercosis vaccine: lessons and insights from the pig production and trading nodes of the uganda pig value chain. Front. Vet. Sci. 8(1), 611166. Owolabi, L.F., Adamu, B., Jibo, A.M., Owolabi, S.D., Imam, A.I. and Alhaji, I.D. 2020. Neurocysticercosis in people with epilepsy in Sub-Saharan Africa: a systematic review and meta-analysis of the prevalence and strength of association. Seizure 76(1), 1–11. Pawlowski, Z.S. 2006. Role of chemotherapy of taeniasis in prevention of neurocysticercosis. Parasitol. Int. 55(Suppl), S105-S109. Pondja, A., Neves, L., Mlangwa, J., Afonso, S., Fafetine, J., Willingham, A.L. 3rd, Thamsborg, S.M. and Johansen, M.V. 2010. Prevalence and risk factors of porcine cysticercosis in Angónia District, Mozambique. PLoS Negl. Trop. Dis. 4(2), e594. Praet, N., Speybroeck, N., Manzanedo, R., Berkvens, D., Nforninwe, D.N., Zoli, A., Quet, F., Preux, P.M., Carabin, H. and Geerts, S. 2009. The disease burden of Taenia solium cysticercosis in Cameroon. PLoS Negl. Trop. Dis. 3(3), e406. Pray, I.W., Ayvar, V., Gamboa, R., Muro, C., Moyano, L.M., Benavides, V., Flecker, R.H., Garcia, H.H. and O’Neal, S.E. 2017. Spatial relationship between Taenia solium tapeworm carriers and necropsy cyst burden in pigs. PLoS Negl. Trop. Dis. 11(4), e0005536. Pray, I.W., Wakeland, W., Pan, W., Lambert, W.E., Garcia, H.H., Gonzalez, A.E., O’Neal, S.E. and Cysticercosis Working Group in Peru. 2020. Understanding transmission and control of the pork tapeworm with CystiAgent: a spatially explicit agent-based model. Parasit Vectors 13(1), 372. Prodjinotho, U.F., Lema, J., Lacorcia, M., Schmidt, V., Vejzagic, N., Sikasunge, C., Ngowi, B., Winkler, A.S. and da Costa, C.P. 2020. Host immune responses during Taenia solium Neurocysticercosis infection and treatment. PLoS Negl. Trop. Dis. 14(4), e0008005. Rodriguez, S., Dorny, P., Tsang, V.C., Pretell, E.J., Brandt, J., Lescano, A.G., Gonzalez, A.E., Gilman, R.H., Garcia, H.H. and Cysticercosis Working Group in Peru. 2009. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J. Infect. Dis. 199(9), 1345–1352. Rodriguez, S., Wilkins, P. and Dorny, P. 2012. Immunological and molecular diagnosis of cysticercosis. Pathog. Glob. Health 106(5), 286–298. Sinha, S. and Sharma, B.S. 2012. Intraventricular neurocysticercosis: a review of current status and management issues. Br. J. Neurosurg. 26(3), 305–309. Qian, M.B., Xiao, N., Li, S.Z., Abela-Ridder, B., Carabin, H., Fahrion, A.S., Engels, D. and Zhou, X.N. 2020. Control of taeniasis and cysticercosis in China. Adv. Parasitol. 110(1), 289–317. Qiu, N., Jiang, Y., Sun, Z. and Du, M. 2023. The impact of disability-related deprivation on employment opportunity at the neighborhood level: does family socioeconomic status matter? Front. Public Health 11(1), 1232829. Raccurt, C.P., Agnamey, P., Boncy, J., Henrys, J.H. and Totet, A. 2009. Seroprevalence of human Taenia solium cysticercosis in Haiti. J. Helminthol. 83(2), 113–116. Rajshekhar, V., Joshi, D.D., Doanh, N.Q., van De, N. and Xiaonong, Z. 2003. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop. 87(1), 53–60. Rama, K., Jahagirdar, V., Ginnaram, A.R.R., Pottabathini, R. and Mandapalli, V. 2023. Worm in the eye: a case report of ocular neurocysticercosis with adherent retinal cyst. Cureus 15(12), e50194. Rodríguez-Rivas, R., Flisser, A., Norcia, L.F., Filho, P.T.H., Bonilla-Aldana, D.K., Rodriguez-Morales, A.J., Carpio, A., Romo, M.L. and Fleury, A. 2022. Neurocysticercosis in Latin America: current epidemiological situation based on official statistics from four countries. PLoS Negl. Trop. Dis. 16(8), e0010652. Samorek-Pieróg, M., Karamon, J. and Cencek, T. 2018. Identification and control of sources of Taenia Solium infection—the attempts to eradicate the parasite. J. Vet. Res. 62(1), 27–34. Satya, S.M.N., Mayilvaganan, K.R., Amogh, V.N., Balakrishna, B.V., Gautam, M.S. and Prathyusha, I.S. 2016. A classic case of subcutaneous cysticercosis: a rare case with sonological findings and review of literature. Pol. J. Radiol. 81(1), 478–482. Schantz, P.M., Moore, A.C., Muñoz, J.L., Hartman, B.J., Schaefer, J.A., Aron, A.M., Persaud, D., Sarti, E., Wilson, M. and Flisser, A. 1992. Neurocysticercosis in an orthodox jewish community in New York City. N. Engl. J. Med. 327(10), 692–695. Sciutto, E., Fragoso, G., Fleury, A., Laclette, J.P., Sotelo, J., Aluja, A., Vargas, L. and Larralde, C. 2000. Taenia solium disease in humans and pigs: an ancient parasitosis disease rooted in developing countries and emerging as a major health problem of global dimensions. Microbes Infect. 2(15), 1875–1890. Shyaka, A., Rujeni, N., Kanyamibwa, E.I., Kagabo, G., Fèvre, E.M. and Quinnell, R.J. 2024. High prevalence of porcine cysticercosis in slaughtered pigs in Rwanda: an abattoir survey. PLoS Negl. Trop. Dis. 18(10), e0012598. Sikasunge, C.S., Johansen, M.V., Phiri, I.K., Willingham, A.L. 3rd and Leifsson, P.S. 2009. The immune response in Taenia solium neurocysticercosis in pigs is associated with astrogliosis, axonal degeneration and altered blood-brain barrier permeability. Vet. Parasitol. 160(3–4), 242–250. Singhi, P. and Saini, A.G. 2016. Pediatric neurocysticercosis: current challenges and future prospects. Pediatric Health Med. Ther. 7(1), 5–16. Sithole, M.I., Bekker, J.L., Tsotetsi-Khambule, A.M. and Mukaratirwa, S. 2019. Ineffectiveness of meat inspection in the detection of Taenia solium cysticerci in pigs slaughtered at two abattoirs in the Eastern Cape Province of South Africa. Vet. Parasitol. Reg. Stud. Rep. 17(1), 100299. Sorvillo, F., Wilkins, P., Shafir, S. and Eberhard, M. 2011. Public health implications of cysticercosis acquired in the United States. Emerg. Infect. Dis. 17(1), 1–6. Soto, L.A., Parker, L.A., Irisarri-Gutiérrez, M.J., Bustos, J.A., Castillo, Y., Perez, E., Muñoz-Antoli, C., Esteban, J.G., García, H.H. and Bornay-Llinares, F.J. 2021. Evidence for transmission of Taenia solium taeniasis/cysticercosis in a rural area of Northern Rwanda. Front. Vet. Sci. 8(1), 645076. Sugianto, P., MacHin, A., Islamiyah, W. and Cecilia, C. 2023. Long-term albendazole therapy for diffuse parenchymal neurocysticercosis in an immunocompetent patient: a case report. Asian Pac. J. Trop. Med. 16(2), 89–91. Symeonidou, I., Arsenopoulos, K., Tzilves, D., Soba, B., Gabriël, S. and Papadopoulos, E. 2018. Human taeniasis/cysticercosis: a potentially emerging parasitic disease in Europe. Ann. Gastroenterol. 31(4), 406–412. Takayanagui, O.M. and Haes, T.M. 2022. Update on the diagnosis and management of neurocysticercosis. Arq. Neuropsiquiatr. 80(5 Suppl 1), 296–306. Thomas, L.F., de Glanville, W.A., Cook, E.A., Bronsvoort, B.M., Handel, I., Wamae, C.N., Kariuki, S. and Fèvre, E.M. 2017. Modelling the risk of Taenia solium exposure from pork produced in western Kenya. PLoS Negl. Trop. Dis. 11(2), e0005371. Toledo, A., Larralde, C., Fragoso, G., Gevorkian, G., Manoutcharian, K., Hernández, M., Acero, G., Rosas, G., López-Casillas, F., Garfias, C.K., Vázquez, R., Terrazas, I. and Sciutto, E. 1999. Towards a Taenia solium cysticercosis vaccine: an epitope shared by Taenia crassiceps and Taenia solium protects mice against experimental cysticercosis. Infect. Immun. 67(5), 2522–2530. Toribio, L.M., Bustos, J.A. and Garcia, H.H. 2024. From laboratory to clinical practice: an update of the immunological and molecular tools for neurocysticercosis diagnosis. Front. Parasitol. 3(1), 1394089. Venkat, B., Aggarwal, N., Makhaik, S. and Sood, R. 2016. A comprehensive review of imaging findings in human cysticercosis. Jpn. J. Radiol. 34(4), 241–257. Verma, S., Das, A.K. and Pan, S. 2016. Unusual presentations of extraocular cysticercosis: a clinical challenge to the ophthalmologists. Med. J. Armed. Forces India 72(3), 293–296. Wandra, T., Ito, A., Swastika, K., Dharmawan, N.S., Sako, Y. and Okamoto, M. 2013. Taeniases and cysticercosis in Indonesia: past and present situations. Parasitology 140(13), 1608–1616. White, A.C. Jr, Coyle, C.M., Rajshekhar, V., Singh, G., Hauser, W.A., Mohanty, A., Garcia, H.H. and Nash, T.E. 2018. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 98(4), 945–966. Wilson, C., Mdegela, R.H., Nonga, H.E., Makingi, G., Churi, A.J., Stelzle, D., Mkupasi, E.M., Schmidt, V., Carabin, H., Winkler, A.S. and Ngowi, H.A. 2023. Seroprevalence and risk factors for Taenia spp infection in pigs in Kongwa and Songwe districts, Tanzania: a cross-sectional study. Food Waterb. Parasitol. 33(1), e00215. Wu, H.W., Ito, A., Ai, L., Zhou, X.N., Acosta, L.P. and Willingham, A.L. 2017. Cysticercosis/taeniasis endemicity in Southeast Asia: current status and control measures. Acta Trop. 165(1), 121–132. Yajima, A., Lin, Z., Mohamed, A.J., Dash, A.P. and Rijal, S. 2023. Finishing the task of eliminating neglected tropical diseases (NTDs) in WHO South-East Asia Region: promises kept, challenges, and the way forward. Lancet Reg. Health Southeast Asia 18(1), 100302. Zammarchi, L., Strohmeyer, M., Bartalesi, F., Bruno, E., Muñoz, J., Buonfrate, D., Nicoletti, A., García, H.H., Pozio, E., Bartoloni, A. and COHEMI Project Study Group. 2013. Epidemiology and management of cysticercosis and Taenia solium taeniasis in Europe, systematic review 1990–2011. PLoS One 8(7), e69537. Zoli, A., Shey-Njila, O., Assana, E., Nguekam, J.P., Dorny, P., Brandt, J. and Geerts, S. 2003. Regional status, epidemiology and impact of Taenia solium cysticercosis in Western and Central Africa. Acta Trop. 87(1), 35–42. Zumaeta, J., Contreras, C., Tapia, P., Morales, D., Rea, N.S. and Valerio, J. 2024. Giant neurocysticercosis: a rare medical condition. Cureus 16(10), e71090. | ||
| How to Cite this Article |
| Pubmed Style Kusnoto K, Khairullah AR, Sunarso A, Suprihati E, Aryaloka S, Sawitri DH, Moses IB, Kurniasih DAA, Wibowo S, Wardhani BWK, Wasito W, Ahmad RZ, Fauziah I, Kusala MKJ, Yanestria SM, Julaeha J, Fauzia KA, Ekawasti F. The hidden threat of cysticercosis: A neglected public health problem. Open Vet. J.. 2025; 15(3): 1101-1115. doi:10.5455/OVJ.2025.v15.i3.4 Web Style Kusnoto K, Khairullah AR, Sunarso A, Suprihati E, Aryaloka S, Sawitri DH, Moses IB, Kurniasih DAA, Wibowo S, Wardhani BWK, Wasito W, Ahmad RZ, Fauziah I, Kusala MKJ, Yanestria SM, Julaeha J, Fauzia KA, Ekawasti F. The hidden threat of cysticercosis: A neglected public health problem. https://www.openveterinaryjournal.com/?mno=232568 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.4 AMA (American Medical Association) Style Kusnoto K, Khairullah AR, Sunarso A, Suprihati E, Aryaloka S, Sawitri DH, Moses IB, Kurniasih DAA, Wibowo S, Wardhani BWK, Wasito W, Ahmad RZ, Fauziah I, Kusala MKJ, Yanestria SM, Julaeha J, Fauzia KA, Ekawasti F. The hidden threat of cysticercosis: A neglected public health problem. Open Vet. J.. 2025; 15(3): 1101-1115. doi:10.5455/OVJ.2025.v15.i3.4 Vancouver/ICMJE Style Kusnoto K, Khairullah AR, Sunarso A, Suprihati E, Aryaloka S, Sawitri DH, Moses IB, Kurniasih DAA, Wibowo S, Wardhani BWK, Wasito W, Ahmad RZ, Fauziah I, Kusala MKJ, Yanestria SM, Julaeha J, Fauzia KA, Ekawasti F. The hidden threat of cysticercosis: A neglected public health problem. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1101-1115. doi:10.5455/OVJ.2025.v15.i3.4 Harvard Style Kusnoto, K., Khairullah, . A. R., Sunarso, . A., Suprihati, . E., Aryaloka, . S., Sawitri, . D. H., Moses, . I. B., Kurniasih, . D. A. A., Wibowo, . S., Wardhani, . B. W. K., Wasito, . W., Ahmad, . R. Z., Fauziah, . I., Kusala, . M. K. J., Yanestria, . S. M., Julaeha, . J., Fauzia, . K. A. & Ekawasti, . F. (2025) The hidden threat of cysticercosis: A neglected public health problem. Open Vet. J., 15 (3), 1101-1115. doi:10.5455/OVJ.2025.v15.i3.4 Turabian Style Kusnoto, Kusnoto, Aswin Rafif Khairullah, Agus Sunarso, Endang Suprihati, Suhita Aryaloka, Dyah Haryuningtyas Sawitri, Ikechukwu Benjamin Moses, Dea Anita Ariani Kurniasih, Syahputra Wibowo, Bantari Wisynu Kusuma Wardhani, Wasito Wasito, Riza Zainuddin Ahmad, Ima Fauziah, Muhammad Khaliim Jati Kusala, Sheila Marty Yanestria, Julaeha Julaeha, Kartika Afrida Fauzia, and Fitrine Ekawasti. 2025. The hidden threat of cysticercosis: A neglected public health problem. Open Veterinary Journal, 15 (3), 1101-1115. doi:10.5455/OVJ.2025.v15.i3.4 Chicago Style Kusnoto, Kusnoto, Aswin Rafif Khairullah, Agus Sunarso, Endang Suprihati, Suhita Aryaloka, Dyah Haryuningtyas Sawitri, Ikechukwu Benjamin Moses, Dea Anita Ariani Kurniasih, Syahputra Wibowo, Bantari Wisynu Kusuma Wardhani, Wasito Wasito, Riza Zainuddin Ahmad, Ima Fauziah, Muhammad Khaliim Jati Kusala, Sheila Marty Yanestria, Julaeha Julaeha, Kartika Afrida Fauzia, and Fitrine Ekawasti. "The hidden threat of cysticercosis: A neglected public health problem." Open Veterinary Journal 15 (2025), 1101-1115. doi:10.5455/OVJ.2025.v15.i3.4 MLA (The Modern Language Association) Style Kusnoto, Kusnoto, Aswin Rafif Khairullah, Agus Sunarso, Endang Suprihati, Suhita Aryaloka, Dyah Haryuningtyas Sawitri, Ikechukwu Benjamin Moses, Dea Anita Ariani Kurniasih, Syahputra Wibowo, Bantari Wisynu Kusuma Wardhani, Wasito Wasito, Riza Zainuddin Ahmad, Ima Fauziah, Muhammad Khaliim Jati Kusala, Sheila Marty Yanestria, Julaeha Julaeha, Kartika Afrida Fauzia, and Fitrine Ekawasti. "The hidden threat of cysticercosis: A neglected public health problem." Open Veterinary Journal 15.3 (2025), 1101-1115. Print. doi:10.5455/OVJ.2025.v15.i3.4 APA (American Psychological Association) Style Kusnoto, K., Khairullah, . A. R., Sunarso, . A., Suprihati, . E., Aryaloka, . S., Sawitri, . D. H., Moses, . I. B., Kurniasih, . D. A. A., Wibowo, . S., Wardhani, . B. W. K., Wasito, . W., Ahmad, . R. Z., Fauziah, . I., Kusala, . M. K. J., Yanestria, . S. M., Julaeha, . J., Fauzia, . K. A. & Ekawasti, . F. (2025) The hidden threat of cysticercosis: A neglected public health problem. Open Veterinary Journal, 15 (3), 1101-1115. doi:10.5455/OVJ.2025.v15.i3.4 |