| Research Article | ||
Open Vet. J.. 2025; 15(3): 1322-1330 Open Veterinary Journal, (2025), Vol. 15(3): 1322-1330 Research Article Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roostersJaswandi Jaswandi*, Rusfidra Rusfidra, Ananda Ananda, Agusti Azones Abimanyu and Harif GusdinalDepartment of Livestock Production Technology, Faculty of Animal Science, Universitas Andalas, Padang, Indonesia *Corresponding Author: Jaswandi. Department of Livestock Production Technology, Faculty of Animal Science, Universitas Andalas, Padang, Indonesia. Email: jaswandij [at] ansci.unand.ac.id Submitted: 14/12/2024 Accepted: 14/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
AbstractBackground: The Kokok Balenggek rooster (KBR), with its distinct phenotypes—Biriang, Balang, Kinantan, and Kuriak—offers a unique opportunity to study variations in semen characteristics and protein profiles. Understanding these variations can aid in the development of better management strategies for poultry breeding programs. Aim: This study aimed to characterize the spermatozoa and seminal plasma protein profiles based on molecular weight (MW) across four phenotypes of KBRs, focusing on semen parameters, such as motility, viability, and protein concentration. Methods: Semen samples were collected from the KBR of four phenotypes: Biriang, Balang, Kinantan, and Kuriak. The parameters analyzed included semen volume, color, smell, consistency, sperm concentration, motility, viability, abnormality, plasma membrane integrity, and protein concentration. Protein profiles of spermatozoa and seminal plasma were analyzed using MW markers via gel electrophoresis. Results: The results revealed significant variations in semen volume (p < 0.05) and protein concentration (p < 0.01), with the Kinantan phenotype exhibiting the highest protein concentration (2.23 mg/ml). Sperm motility (p < 0.05) and viability (p > 0.05) were highest in the Biriang, Balang, and Kinantan phenotypes, whereas the Kuriak phenotype showed lower motility (64%, p < 0.01). Protein profile analysis indicated the presence of proteins in sperm with MWs of 10, 25–35, 35–45, 45–65, and 100 kDa and in seminal plasma with MWs of 10, 20–25, 25–35, 45, 65, 75, 140, and 180–245 kDa, respectively, across all phenotypes. Conclusion: This study highlighted variations in sperm characteristics and protein profiles among KBR phenotypes, with the Kuriak phenotype showing lower motility, providing insights for improving genetic resource management and semen preservation. Keywords: Kokok Balenggek roosters, Protein, Seminal plasm, Sperm. IntroductionSpermatozoa and seminal plasma are critical components of male fertility and reproduction, and their protein profiles offer valuable insights into the physiological and functional characteristics of male gametes (Zylbersztejn et al., 2013; Selvam and Agarwal, 2021). Understanding the protein composition of spermatozoa and seminal plasma can enhance our knowledge of sperm function, maturation, and fertilizing potential (Mogielnicka-Brzozowska et al., 2015; Apic et al., 2016). This study investigated the protein profile of spermatozoa and seminal plasma in Kokok Balenggek roosters (KBR), an Indonesian indigenous breed, focusing on the molecular weight (MW) of proteins in different phenotypes. Seminal plasma, a complex biological fluid, comprises lipids, carbohydrates, peptides, and proteins (Zylbersztejn et al., 2013). The high protein content in seminal plasma (35–55 g/l) makes it an ideal source for proteomic analysis (Zylbersztejn et al., 2013). These proteins, which are secreted by various parts of the male reproductive tract, including the seminal vesicles, vas deferens, periurethral glands, epididymis, and prostate gland, play vital roles in sperm function and fertilization (Apic et al., 2016). Proteomic studies of seminal plasma have been used to identify biomarkers for male infertility and to explore the molecular mechanisms underlying sperm dysfunction (Feugang et al., 2018). In avian species, the proteome of seminal plasma has been studied in various species, including chickens, with findings revealing proteins related to energy metabolism, antioxidant defense, and sperm function regulation (Lin et al., 2019; Li et al., 2020a). However, little is known about the protein profile of spermatozoa and seminal plasma in KBRs, a unique breed of chicken indigenous to Indonesia. KBRs are native to the Tigo Lurah District in Solok Regency, West Sumatra. Known for their distinctive multilevel crowing, KBR roosters are considered a cultural icon in the region (Masfi and Mafardi, 2022). In addition to its unique vocalization, the KBR is a breed with the potential to breed superior local broilers (Husmaini et al., 2023). Despite its significance, the population of the KBRR is dwindling, with only 1,960 individuals reported in 2021, leading to concerns about genetic diversity and sustainability (Husmaini et al., 2022). Conservation efforts, such as artificial insemination (AI), have been initiated to improve fertility and preserve this breed, with reported fertility rates of 50.87% in KBR spermatozoa (Jaswandi et al., 2023). However, the potential influence of KBR phenotypic variation on reproductive quality requires further investigation. At the Faculty of Animal Science, Universitas Andalas, four KBR phenotypes have been identified, each exhibiting distinct coat colors and characteristics: Balang (white fur with black spots), Biriang (red neck, back, and loin), Kinantan (white fur, legs, beak, and neck), and Kuriak (spotted plumage) (Muryanto and Pramono, 2014). These phenotypic differences may influence spermatozoa quality, as observed in other species, where traits such as feather color and comb size are linked to sperm quality (Rahimpoor et al., 2016; Talebi et al., 2018; Ananda et al., 2023, 2024). Furthermore, the role of phenotypic variation in sperm quality among KBR roosters has not been fully explored. AI has revolutionized poultry breeding by improving reproductive management and enabling the rapid spread of superior genetic traits (Getachew, 2016; Mohan et al., 2018). This technology has the potential to enhance genetic quality and control venereal diseases in poultry populations. Despite its success in other breeds, AI in the KBR has not yet been widely applied, highlighting the need for a deeper understanding of sperm quality across different phenotypes. Proteomic analysis has been increasingly applied to identify sperm proteins and explore their roles in fertility, spermatogenesis, and fertilization (Fuentes-Albero et al., 2021). By profiling the protein content of spermatozoa and seminal plasma, researchers can uncover potential biomarkers for semen quality and fertility prediction (Parvin et al., 2024). This study aimed to characterize the protein profile of spermatozoa and seminal plasma from four phenotypes of KBRs based on MW, with the goal of providing insights into the reproductive biology of this important Indonesian breed. Materials and MethodsAnimal and semen collectionA total of 11 KBRs were selected for this study, consisting of four Kinantan, three Biriang, two Kuriak, and two Balang phenotypes. These numbers reflect the limited availability of KBRs with specific phenotypes in the population. The roosters are maintained at the Faculty of Animal Science, Universitas Andalas, in collaboration with conservation and research efforts to preserve this unique genetic resource. All roosters aged 1–2 years were in good health. Prior to semen collection, the roosters were acclimatized for one week in a controlled environment with a 12/12-hour light/dark cycle and free access to clean water and feed. Semen was collected using the abdominal massage technique, as described by Arifiantini (2012). Semen was collected twice a week from each rooster for 4 weeks. After each collection, the semen samples were immediately transported to the laboratory on ice for further processing. Sperm concentration and motility were assessed immediately using a hemocytometer and phase-contrast microscope at 400× magnification. Semen evaluationThe characteristics of sperm were assessed using light microscopy (Imager 2, Zeiss). The total motility and vigor were evaluated at 10× magnification. Sperm concentration was determined using a Neubauer chamber and a 10 ml aliquot of semen diluted in 2 ml of a saline solution containing 1% formalin (1,000×). The percentage of live sperm was evaluated using eosin-nigrosin and counting 100 cells/ejaculate under a light microscope at 400× magnification. Wet smears stained with eosin-nigrosin were prepared to evaluate the percentage of live sperm and normal morphology. Live and dead sperm were identified as colorless and red colored. Sperm with deformed heads, broken tails, or severed tails were considered abnormal. To assess the percentage of sperm with an intact plasma membrane, 50 μl of each ejaculate was incubated at 38°C for 45 minutes in 450 μl of a hyposmotic solution (composed of 7.35 g sodium citrate and 13.5 g fructose dissolved in 1l of distilled water). For each sample, 100 sperm were examined using light microscopy at 400× magnification, and those with a swollen tail were considered to have a functional membrane. Separation of spermatozoa and seminal plasmaCollected semen was processed to separate spermatozoa from the seminal plasma. Semen samples were centrifuged at 33,000 rpm for 45 minutes at 4°C to pellet the spermatozoa. The supernatant, which contained the seminal plasma, was carefully aspirated and stored at –20°C until protein analysis. The spermatozoa pellet was resuspended in 1 ml of phosphate-buffered saline (PBS) and washed three times by centrifugation at 3,000 rpm for 5 minutes. After washing, the sperm was promptly processed for extraction. Protein extraction from seminal plasma and spermProtein was extracted from seminal plasma and spermatozoa using a modified version of the method described by Selvam and Agarwal (2021). For seminal plasma, the supernatant was thawed and centrifuged at 13,000 rpm for 15 minutes to remove residual cellular debris. For spermatozoa, the cell suspension was extracted with PRO-PREP™ Protein Extraction Solution (iNtRON Biotechnology). The suspension was incubated on ice for 30 minutes with intermittent vortexing. The lysate was then centrifuged at 13,000 rpm for 5 minutes at 4°C, and the supernatant containing sperm proteins was collected and stored at –20°C until further analysis. Protein concentrations in the seminal plasma and sperm were determined using the Bradford assay (Bradford Protein Colorimetric Assay Kit, E-BC-K168-M). The working solution was prepared by diluting Reagent 1 with distilled water (1:4), and the standard solution was prepared by dissolving 0.563 mg of bovine serum albumin with PBS. Blank materials (3,000 µl working solution and 50 µl PBS), standards (3,000 µl working solution and 50 µl standard solution), and samples (3,000 µl working solution and 50 µl sample) were each incubated at room temperature for 10 minutes. The optical density (OD) was measured at a wavelength of 595 nm using a spectrophotometer (Shimadzu, UV-1800). The total dissolved protein concentration can be calculated using the following formula:
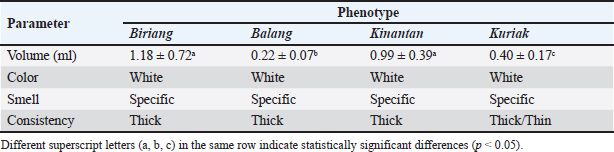
ΔA1: OD sample–OD blank ΔA2: OD standard–OD blank c: Standard concentration (0.563 mg/ml) f: Sample dilution factor Protein profiling by SDS-PAGEProtein profiles of the spermatozoa and seminal plasma from each phenotype were analyzed using Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE). A total of 20 µg of protein from each sample was mixed with NuPAGE™ LDS Sample Buffer, boiled for 10 minutes at 75°C, and loaded onto a 10% acrylamide gel (Q-PAGE™ TGN Precast Gel, QP4210). Electrophoresis was performed at 110 V for approximately 90 minutes, followed by staining with Fast Coomassie Blue Staining Solution (Elabscience®) overnight. The remaining stain was removed by soaking the gel in ddH2O in a closed container and washing it 3 times. Protein bands on the gel were observed using Gel Doc (iBright 1500, Invitrogen, Thermo Fisher Sci). The read sample protein bands were compared with the marker band (Excell band 3-color Broad Range Protein Marker 3.5-245 kDA, SMOBIO® Technology, Inc. Taiwan) to determine the protein MW. Data analysisSperm characteristics are presented as averages and standard deviations. The normality of the data was checked using the Shapiro–Wilk test in IBM SPSS. The data were statistically analyzed using a randomized block design, followed by the Duncan multiple range test in IBM SPSS to determine differences between phenotypes. For percentage data, arcsine transformation was applied before analysis using the appropriate statistical tests in IBM SPSS. Protein data were presented descriptively. Table 1. Microscopic characteristics of Kokok Balenggek roosters semen across different phenotypes.
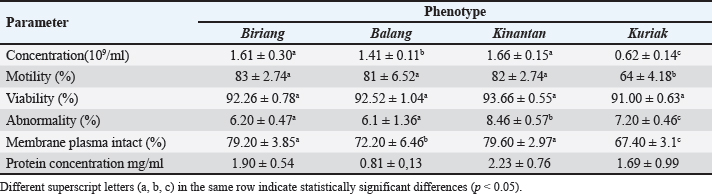
Table 2. Microscopic characteristics of Kokok Balenggek roosters semen across different phenotypes.
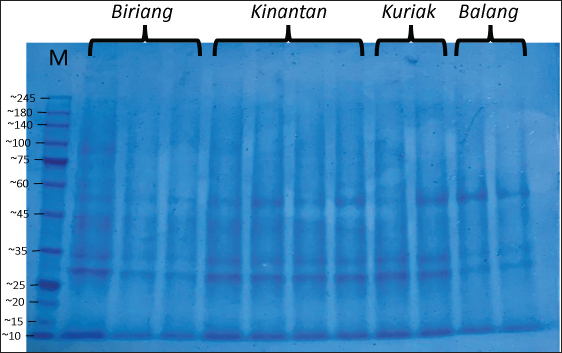
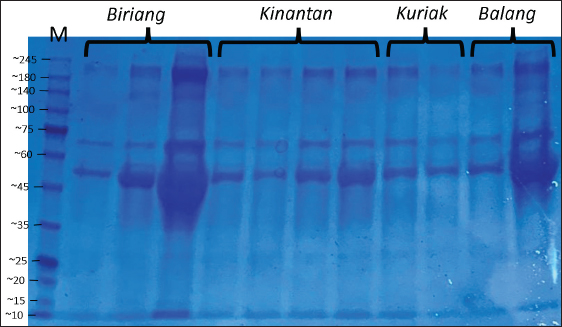
Fig. 1. SDS-PAGE profile of spermatozoa proteins in Kokok Balenggek roosters. Ethical approvalThe Faculty of Medicine, Universitas Andalas, Indonesia’s ethical clearance committee helped secure consent for the use of animals in research (Ethics no: 268/UN.16.2/KEP-FK/2024). ResultThe macroscopic characteristics of the semen of the four Kokok Balenggek (AKB) rooster phenotypes are presented in Table 1. The semen volume varied significantly across phenotypes (p < 0.05), with the highest volume observed in the Biriang phenotype (1.18 ± 0.72 ml), followed by Kinantan (0.99 ± 0.39 ml), Kuriak (0.40 ± 0.17 ml), and the lowest in Balang (0.22 ± 0.07 ml). All phenotypes had white-colored semen with a specific smell and a thick consistency, except for the Kuriak phenotype, which had a more fluid consistency. Microscopic examination of sperm characteristics is shown in Table 2. The highest sperm concentration was found in the Kinantan phenotype (1.66 ± 0.15 × 109/ml), followed by Biriang (1.61 ± 0.30 × 109/ml) and Balang (1.41 ± 0.11 × 109/ml), with the Kuriak phenotype having the lowest concentration (0.62 ± 0.14 × 109/ml) (p < 0.05). Sperm motility was highest in the Biriang, Balang, and Kinantan phenotypes (83%, 81%, and 82%, respectively), whereas the Kuriak phenotype exhibited significantly lower motility (64%) (p < 0.05). Viability percentages were similar across all phenotypes, with no significant differences (ranging from 91% to 93.66%) (p > 0.05). The percentages of abnormalities were lowest in the Biriang and Balang phenotypes (6.20% and 6.1%, respectively), whereas the Kinantan and Kuriak phenotypes had slightly higher abnormalities (8.46% and 7.20%, respectively) (p > 0.05). Plasma membrane integrity was highest in the Kinantan and Biriang phenotypes (79.60% and 79.20%, respectively), whereas the Kuriak phenotype had the lowest membrane integrity (67.40%) (p < 0.05). The protein concentration was highest in the Kinantan phenotype (2.23 ± 0.76 mg/ml), followed by Biriang (1.90 ± 0.54 mg/ml), Kuriak (1.69 ± 0.99 mg/ml), and Balang (0.81 ± 0.13 mg/ml) (p < 0.05). Protein profiles in both spermatozoa and seminal plasma from four Kokok Balenggek (AKB) rooster phenotypes were analyzed according to MW. As shown in Figure 1, sperm proteins with MWs of 10, 25–35, 35–45, 45–65, and 100 kDa were identified in all phenotypes (Kinantan, Biriang, Jalak, and Balang). These proteins were consistently present across the different phenotypes, indicating a common protein profile in AKB sperm. In seminal plasma, as presented in Figure 2, proteins with MWs of 10, 20–25, 25–35, 45, 65, 75, 140, and 180–245 kDa were detected in all phenotypes. Similar to sperm proteins, these seminal plasma proteins were found consistently across the Kinantan, Biriang, Jalak, and Balang phenotypes, further suggesting a shared protein profile among the different phenotypes. DiscussionThe semen characteristics of Kokok Balenggek chickens observed in this study were slightly different from those of previous research (Ananda et al., 2023, 2024; Jaswandi et al., 2023; Husmaini et al., 2024). Specifically, the sperm concentration was higher in our study, and the percentage of abnormalities was also higher. In contrast, the percentages of motility and viability were relatively similar. These differences can be attributed to variations in the methodology used or to potential differences in environmental conditions. Compared with Thai chickens, our results indicated a lower sperm concentration and slightly lower viability (Lewchalermvong et al., 2022). Despite these differences, sperm quality in the four Kokok Balenggek phenotypes studied remained within the reference range for chickens. Similar findings were reported by Shanmugam et al. (2012), whose research showed that the volume of adult chickens ranged from 0.36 ± 0.05 to 0.48 ± 0.06 ml, with a viability range of 91.21% ± 1.49% to 92.12% ± 1.90%, abnormality percentages from 5.58% ± 2.82% to 6.13% ± 2.76%, and intact plasma membrane percentages ranging from 87.54% ± 1.75% to 89.65% ± 1.60%.
Fig. 2. SDS-PAGE profile of seminal plasma proteins in Kokok Balenggek roosters. The variations in sperm quality among the phenotypes in this study are likely due to genetic differences, as noted in several previous studies. Shanmugam et al. (2012) and Tesfay et al. (2020) reported significant differences in semen characteristics, such as volume, appearance, motility, sperm concentration, and the percentage of live and dead sperm, across different genetic lines. Similarly, (Tarif et al., 2013) found notable differences in ejaculate volume, sperm motility, sperm concentration, and the proportion of live sperm between male chickens of different lines. According to (Rui et al ., 2017), low sperm motility could be associated with lipid peroxidation, with chickens with low motility having higher concentrations of malondialdehyde than those with high motility. These findings suggest that both genetic factors and environmental factors play significant roles in determining semen quality in Kokok Balenggek chicken. Further research is required to explore the underlying genetic mechanisms and environmental factors contributing to these differences. This study identified several proteins with varying MWs that may play critical roles in sperm quality and fertility, particularly in KBRs. One such protein is Complement C3 (180 kDa), which has been linked to spermatozoa membrane modulation and capacitation. Complement C3 can interact with Apolipoprotein A1 (APOA1), a component of high-density lipoprotein. APOA1 can interact with proteins in the flagella and acrosomes of spermatozoa, influencing their function (Jha et al ., 2008; Boe-Hansen et al ., 2015). Another protein of interest is arylsulfatase-a (MW 54-87 kDa), which plays a significant role in sperm membrane stability and permeability (Diansyah et al ., 2022). This protein, located on the sperm head, functions as a lectin or hydrolase during the binding and penetration process (Tumova et al ., 2021). Aryl-sulfatase-a has been suggested as a useful marker for sperm capacitation, with proteins in the MW range of 180–140 kDa, such as Insulin-like Growth Factor 1 (IGF-1) (150 kDa), being involved in sperm characteristics. IGF-1 is known to regulate cell growth, proliferation, and differentiation, including the modulation of reproductive performance (Tantibhedhyangkul et al., 2002; Gómez-Torres et al., 2021). The protein N-acetyl β-glucosaminidase (MW 62–44 kDa), a glucose hydrolysis enzyme, has been identified as essential during sperm maturation in the epididymis (Moura et al., 2018). It plays a role in sperm–oocyte interactions (Sakaguchi et al., 2009) and is associated with sperm morphology and viability (Selvam and Agarwal, 2018; Baharun et al., 2023). These proteins may contribute to the unique reproductive characteristics of this breed. Astacin-like metalloendopeptidase (MW 35–45 kDa) is another important protein involved in oocyte activation during fertilization. This protein, along with Phospholipase C Zeta 1 (PLCZ1), prevents polyspermy by breaking the bond between the sperm and the zona pellucida after fertilization (Sachdev et al., 2012). While research on the role of Astacin-like Metalloendopeptidase (ASTL) in sperm is limited, it is known to be involved in the digestion of the pre-vitamin membrane in poultry (Li et al., 2020b). This protein’s function may also be relevant to KBRs, where the prevention of polyspermy and fertilization mechanisms are critical for reproductive success. Additionally, proteins in the MW range of 25–35 kDa, such as matrix metalloproteinases, are important for maintaining sperm viability by protecting membrane integrity(Cabral-Pacheco et al., 2020). Clusterin, also found in sufficient quantities in seminal plasma, serves as an inhibitor of oxidative damage and sperm lysis, thus maintaining sperm viability (Moura and Memili, 2016; Janiszewska et al., 2022; Modiba et al., 2022). Antioxidant proteins like glutathione peroxidase also contribute to sperm longevity by reducing oxidative stress (Pei et al., 2023). The presence of these proteins in KBR semen may play a significant role in enhancing sperm survival and fertility outcomes. The superoxide dismutase protein (MW 20–25 kDa) is involved in oxidative phosphorylation, a critical process in Adenosine Triphosphate (ATP) synthesis following the Krebs cycle. Proteins such as succinate dehydrogenase and cytochrome c oxidase (COX) are involved in this process and were found exclusively in the single-comb sperm group in this study. COX proteins play a crucial role in the mitochondrial electron transport chain, which is essential for energy production in sperm cells (Čunátová et al., 2020; Fujisawa et al., 2024). Additionally, cytochrome c is involved in various metabolic pathways, apoptosis, and cell signaling processes, making it an essential protein for cellular energy production and sperm function (Choi et al., 2008). Finally, proteins in the 10–15 kDa MW range, such as Spermadhesin-1 and binding of sperm protein 1 (BSP1), are critical for sperm quality and fertility. BSP1 plays a significant role in semen cryopreservation by binding to sperm, although its levels decrease during cryopreservation (Iskandar et al., 2023). However, the impact of cryopreservation and sperm viability may vary depending on the diluent composition, as noted by Zong et al. (2023). In KBRs, understanding the dynamics of these proteins during semen storage and cryopreservation may improve fertility outcomes. These findings highlight the diverse range of proteins involved in sperm quality, capacitation, and fertilization, particularly in KBRs. Understanding the roles of these species could provide valuable insights into improving fertility management and semen preservation techniques for this unique breed of poultry (Shanmugam et al., 2014; Zhang et al., 1999). ConclusionThis study examined the semen characteristics and protein profiles of four KBR phenotypes, revealing significant differences in semen volume, sperm concentration, motility, and protein concentration. The Kinantan and Biriang phenotypes exhibited the highest sperm quality. Protein profiles across all phenotypes showed consistent MW patterns, with five protein bands in sperm and eight in seminal plasma, with no variation between genotypes. Key proteins identified, such as Complement C3, arylsulfatase-a, and IGF-1, may play critical roles in sperm capacitation and fertilization. These findings contribute to our understanding of the reproductive biology of KBRs, with implications for breeding, semen preservation, and fertility management. AcknowledgmentsThe authors would like to express their sincere gratitude to Djon Hok Tjong for providing laboratory facilities and to Nana Trigustina for her assistance with SDS-PAGE analysis. Conflict of interestThe authors declare no conflict of interest. FundingThe authors would like to thank the Directorate General of Higher Education, Ministry of Education, Research, and Technology, for funding our research under contract number 041/E5/PG.02.00.PL/2024, dated June 11, 2024. Author’s contributionsThe authors contributed to this study as follows: J. conceptualized the study, designed the methodology, performed data analysis, and wrote the manuscript. R was responsible for data collection, analysis, and manuscript revision. A researcher supervised the study, interpreted the data, and reviewed the manuscript. AA conducted the laboratory work and assisted in data collection. HG contributed to data analysis and manuscript editing. Data availabilityAll data are available in the manuscript. ReferencesAnanda, Jaswandi, Rusfidra and Gusdinal, H. 2023. Sperm longevity and motility in Ringer’s lactate solution with the addition of egg yolk among five phenotypes of Kokok Balenggek chicken. Bul. Peternak. 47, 127–135; doi:10.21059/buletinpeternak.v47i3.83647. Ananda, Jaswandi, Rusfidra, Gusdinal H., Abimanyu, G.A., Anggraini, L. and Arlina, F. 2024. Sperm quality and daily fecal testosterone among six phenotypes of Kokok Balenggek rooster. Int. J. Vet. Sci. 13, 527–536; doi:10.47278/journal.ijvs/2024.145. Apic, J., Stancic, I., Vakanjac, S., Radovic, I., Kanacki, Z., Jotanovic, S. and Stankovic, B. 2016. Motility of boar spermatozoa supplemented with homologous seminal plasma of high or low protein content after storage for three days. Vet. Med. (Praha). 61, 72–79; doi:10.17221/8720-VETMED. Arifiantini, R.I. 2012. Teknik koleksi dan evaluasi semen pada hewan. Bogor, Indonesia: Penerbit IPB Press. Baharun, A., Rahmi, A., Kardaya, D., Said, S., Fahrudin, M., Arifiantini, R. and Karja, N. 2023. Profiling of seminal plasma proteins to identify the fertility of Simmental bull with low semen quality. J. Adv. Vet. Anim. Res. 10, 1; doi:10.5455/javar.2023.j689. Boe-Hansen, G.B., Rego, J.P.A., Crisp, J.M., Moura, A.A., Nouwens, A.S., Li, Y., Venus, B., Burns, B.M. and McGowan, M.R. 2015. Seminal plasma proteins and their relationship with percentage of morphologically normal sperm in 2-year-old Brahman (Bos indicus) bulls. Anim. Reprod. Sci. 162, 20–30; doi:10.1016/j.anireprosci.2015.09.003. Cabral-Pacheco, G.A., Garza-Veloz, I., Castruita-De la Rosa, C., Ramirez-Acuña, J.M., Perez-Romero, B.A., Guerrero-Rodriguez, J.F., Martinez-Avila, N. and Martinez-Fierro, M.L. 2020. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21, 9739; doi:10.3390/ijms21249739. Choi, Y.J., Uhm, S.J., Song, S.J., Song, H., Park, J.K., Kim, T., Park, C. and Kim, J.H. 2008. Cytochrome c upregulation during capacitation and spontaneous acrosome reaction determines the fate of pig sperm cells: linking proteome analysis. J. Reprod. Dev. 54, 68–83; doi:10.1262/jrd.19116. Čunátová, K., Pajuelo Reguera, D., Houštěk, J., Mráček, T. and Pecina, P. 2020. Role of cytochrome c oxidase nuclear-encoded subunits in health and disease. Physiol. Res. 69, 947–965; doi:10.33549/physiolres.934446. Diansyah, A.M., Yusuf, M., Toleng, A.L., Dagong, M.I.A. and Maulana, T. 2022. The expression of plasma protein in Bali-polled bulls using 1D-SDS-PAGE. World Vet. J. 12, 316–322; doi:10.54203/scil.2022.wvj40. Feugang, J.M., Liao, S.F., Willard, S.T. and Ryan, P.L. 2018. In-depth proteomic analysis of boar spermatozoa through shotgun and gel-based methods. BMC Genomics. 19, 62; doi:10.1186/s12864-018-4442-2. Fuentes-Albero, M.C., González-Brusi, L., Cots, P., Luongo, C., Abril-Sánchez, S., Ros-Santaella, J.L., Pintus, E., Ruiz-Díaz, S., Barros-García, C., Sánchez-Calabuig, M.J., García-Párraga, D., Avilés, M., Rico, J.I. and García-Vázquez, F.A. 2021. Protein identification of spermatozoa and seminal plasma in bottlenose dolphin (Tursiops truncatus). Front. Cell Dev. Biol. 9, 673961; doi:10.3389/fcell.2021.673961. Fujisawa, Y., Kikuchi, S., Kuba, F., Oishi, K., Murayama, S., Sugiyama, T., Tokito, R., Ueno, H., Kashiwabara, S., Yumura, Y. and Kurihara, Y. 2024. Ectopic expression of the mitochondrial protein COXFA4L3 in human sperm acrosome and its potential application in the selection of male infertility treatments. Reprod. Med. Biol. 23, 12602; doi:10.1002/rmb2.12602. Getachew, T. 2016. A review article of artificial insemination in poultry. World Vet. J. 6, 25; doi:10.5455/wvj.20160263. Gómez-Torres, M.J., Huerta-Retamal, N., Robles-Gómez, L., Sáez-Espinosa, P., Aizpurua, J., Avilés, M. and Romero, A. 2021. Arylsulfatase A remodeling during human sperm in vitro capacitation using field emission scanning electron microscopy (FE-SEM). Cells 10, 222; doi:10.3390/cells10020222. Husmaini, Putra, R.A., Juliyarsi, I., Edwin, T., Suhartati, L., Alianta, A.A. and Harmaini. 2022. Population structure of Kokok Balenggek chicken in in-situ area as indigenous chicken of Indonesia. Adv. Anim. Vet. Sci. 10, 993–998; doi:10.17582/journal.aavs/2022/10.5.993.998. Husmaini, Suhartati, L. and Rusfidra. 2023. Qualitative and quantitative characteristics of G0 Kokok Balenggek chicken: the formation of superior local meat-type chicken. Int. J. Vet. Sci. 12, 554–558; doi:10.47278/journal.ijvs/2023.001. Husmaini, Suhartati, L., Rusfidra, Rachman, F.A. and Ananda. 2024. Hatching performance of Kokok Balenggek chicken (G1): formation of superior local chicken in West Sumatra. Int. J. Vet. Sci. 13, 661–666; doi:10.47278/journal.ijvs/2024.152. Iskandar, H., Andersson, G., Sonjaya, H., Arifiantini, R.I., Said, S., Hasbi, Maulana, T. and Baharun, A. 2023. Protein identification of seminal plasma in Bali bull (Bos javanicus). Animals 13, 514; doi:10.3390/ani13030514. Janiszewska, E., Kokot, I., Kmieciak, A., Stelmasiak, Z., Gilowska, I., Faundez, R. and Kratz, E.M. 2022. The association between clusterin sialylation degree and levels of oxidative–antioxidant balance markers in seminal plasmas and blood sera of male partners with abnormal sperm parameters. Int. J. Mol. Sci. 23, 10598; doi:10.3390/ijms231810598. Jaswandi, Ananda, Rusfidra, Subekti, K., Gusdinal, H., Wahyuni, R.S. and Caniago, F.A. 2023. Fertility rate, fertility period, and DOC sex ratio of Kokok Balenggek chicken after artificial insemination. Adv. Anim. Vet. Sci. 11, 795–801; doi:10.17582/journal.aavs/2023/11.5.795.801. Jha, K.N., Shumilin, I.A., Digilio, L.C., Chertihin, O., Zheng, H., Schmitz, G., Visconti, P.E., Flickinger, C.J., Minor, W. and Herr, J.C. 2008. Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation. Endocrinology 149, 2108–2120; doi:10.1210/en.2007-0582. Karaca, A., Parker, H. and McDaniel, C. 2002. Elevated body temperature directly contributes to heat stress infertility of broiler breeder males. Poult. Sci. 81, 1892–1897; doi:10.1093/ps/81.12.1892. Lewchalermvong, K., Kulnanan, P., Boonchay, K., Thaikoed, S., Phetcharat, Y., Yiengvisavakul, V., Damrongwatanapokin, T., Lavilla, C. and Sumretprasong, J. 2022. Semen characterization of Dang Surat Thai native chicken. Vet. Integr. Sci. 21, 175–185; doi:10.12982/VIS.2023.014. Li, Y., Sun, Y., Ni, A., Shi, L., Wang, P., Isa, A.M., Ge, P., Jiang, L., Fan, J., Ma, H., Yang, G. and Chen, J. 2020. Seminal plasma proteome as an indicator of sperm dysfunction and low sperm motility in chickens. Mol. Cell. Proteomics. 19, 1035–1046; doi:10.1074/mcp.RA120.002017. Lin, Y., Liang, A., He, Y., Li, Z.Z., Li, Z.H., Wang, G. and Sun, F. 2019. Proteomic analysis of seminal extracellular vesicle proteins involved in asthenozoospermia by iTRAQ. Mol. Reprod. Dev. 86, 1094–1105; doi:10.1002/mrd.23224. Masfi, R. and Mafardi. 2022. The symbolization of the Kukuak Balenggek chicken statue, Solok Regency, West Sumatra Province: analysis of cultural mythology. Int. J. Sci. Soc. 4, 251–264; doi:10.54783/ijsoc.v4i3.517. Modiba, M.C., Nephawe, K.A., Mdladla, K.H., Lu, W. and Mtileni, B. 2022. Candidate genes in bull semen production traits: an information approach review. Vet. Sci. 9, 155; doi:10.3390/vetsci9040155. Mogielnicka-Brzozowska, M., Strzeżek, R., Wasilewska, K. and Kordan, W. 2015. Prostasomes of canine seminal plasma – Zinc-binding ability and effects on motility characteristics and plasma membrane integrity of spermatozoa. Reprod. Domest. Anim. 50, 484–491; doi:10.1111/rda.12516. Mohan, J., Sharma, S.K., Kolluri, G. and Dhama, K. 2018. History of artificial insemination in poultry, its components and significance. Worlds Poult. Sci. J. 74, 475–488; doi:10.1017/S0043933918000430. Moura, A.A. and Memili, E. 2016. Functional aspects of seminal plasma and sperm proteins and their potential as molecular markers of fertility. Anim. Reprod. 13, 191–199; doi:10.21451/1984-3143-AR884. Moura, A.A., Memili, E., Portela, A.M.R., Viana, A.G., Velho, A.L.C., Bezerra, M.J.B. and Vasconselos, F.R. 2018. Seminal plasma proteins and metabolites: effects on sperm function and potential as fertility markers. Anim. Reprod. 15(Suppl. 1), 691–702; doi:10.21451/1984-3143-AR2018-0029. Muryanto and Pramono, D. 2014. Sukses budi daya ayam kampung, Penebar Swadaya, Ungaran. Eds., Sudaryanto, B. and Winarti, Y. Ungaran, Indonesia. Parvin, A., Erabi, G., Alemi, A., Rezanezhad, A., Maleksabet, A., Sadeghpour, S., Taheri-Anganeh, M. and Ghasemnejad-Berenji, H. 2024. Seminal plasma proteomics as putative biomarkers for male infertility diagnosis. Clin. Chim. Acta. 561, 119757; doi:10.1016/j.cca.2024.119757. Pei, J., Pan, X., Wei, G. and Hua, Y. 2023. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 14, 1147414; doi:10.3389/fphar.2023.1147414. Rahimpoor, M., Khosravinia, H. and Khaldari, M. 2016. Rooster phenotypic traits influence on the reproductive performance of the broiler parents. Indian J. Fundam. Appl. Life Sci. 6, 7–12. Rui, B.R., Shibuya, F.Y., Kawaoku, A.J.T., Losano, J.D.A., Angrimani, D.S.R., Dalmazzo, A., Nichi, M. and Pereira, R.J.G. 2017. Impact of induced levels of specific free radicals and malondialdehyde on chicken semen quality and fertility. Theriogenology 90, 11–19; doi:10.1016/j.theriogenology.2016.11.001. Sachdev, M., Mandal, A., Mulders, S., Digilio, L.C., Panneerdoss, S., Suryavathi, V., Pires, E., Klotz, K.L., Hermens, L., Herrero, M.B. and Flickinger, C.J. 2012. Oocyte-specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization. Dev. Biol. 363, 40–51; doi:10.1016/j.ydbio.2011.12.021. Sakaguchi, Y., Iwata, H., Kuwayama, T. and Monji, Y. 2009. Effect of N-Acetyl-D-Glucosamine on bovine sperm-oocyte interactions. J. Reprod. Dev. 55, 676–684; doi:10.1262/jrd.09-59H. Selvam, M.K.P. and Agarwal, A. 2018. Update on the proteomics of male infertility: a systematic review. Arab J. Urol. 16, 103–112; doi:10.1016/j.aju.2017.11.016. Selvam, M.K.P. and Agarwal, A. 2021. Proteomic profiling of seminal plasma proteins in varicocele patients. World J. Mens Health. 39, 90; doi:10.5534/wjmh.180118. Shanmugam, M., Rajkumar, U., Reddy, M.R. and Rao, S.V.R. 2012. Effect of age on semen quality in naked neck and dwarf chicken under tropical climatic conditions. Anim. Prod. Sci. 52, 964–968; doi:10.1071/AN12033. Shanmugam, M., Vinoth, A., Rajaravindra, K.S. and Rajkumar, U. 2014. Evaluation of semen quality in roosters of different ages during hot climatic conditions. Anim. Reprod. Sci. 145, 81–85; doi:10.1016/j.anireprosci.2013.12.015. Talebi, A., Alimehr, M., Alavi, M.H., Najafi, G. and Simaei, N. 2018. Comparative study of semen traits and histomorphometric features of testes of broiler breeder males with different phenotypic traits. Vet. Res. Forum. 9, 1–6. Tantibhedhyangkul, J., Weerachatyanukul, W., Carmona, E., Xu, H., Anupriwan, A., Michaud, D. and Tanphaichitr, N. 2002. Role of sperm surface arylsulfatase A in mouse sperm-zona pellucida binding. Biol. Reprod. 67, 212–219; doi:10.1095/biolreprod67.1.212. Tarif, A.M.M., Bhuiyan, M.M.U., Ferdousy, R.N., Juyena, N.S. and Mollah, M.B.R. 2013. Evaluation of semen quality among four chicken lines. IOSR J Agric Vet Sci. 6(5), 7–13; doi:10.9790/2380-0650713. Tesfay, H.H., Sun, Y., Li, Y., Shi, L., Fan, J., Wang, P., Zong, Y., Ni, A., Ma, H., Mani, A.I. and Chen, J. 2020. Comparative studies of semen quality traits and sperm kinematic parameters in relation to fertility rate between 2 genetic groups of breed lines. Poult. Sci. 99, 6139–6146; doi:10.1016/j.psj.2020.06.088. Tumova, L., Zigo, M., Sutovsky, P., Sedmikova, M. and Postlerova, P. 2021. Ligands and receptors involved in the sperm-zona pellucida interactions in mammals. Cells 10, 133; doi:10.3390/cells10010133. Zhang, X., Berry, W., McDaniel, G., Roland, D., Liu, P., Calvert, C. and Wilhite, R. 1999. Body weight and semen production of broiler breeder males as influenced by crude protein levels and feeding regimens during rearing. Poult Sci. 78(2), 190–196; doi:10.1093/ps/78.2.190. Zong, Y., Li, Y., Sun, Y., Mehaisen, G.M.K., Ma, T. and Chen, J. 2023. Chicken sperm cryopreservation: review of techniques, freezing damage, and freezability mechanisms. Agriculture 13, 445; doi:10.3390/agriculture13020445. Zylbersztejn, D.S., Andreoni, C., Del-Giudice, P.T., Spaine, D.M., Borsari, L., Souza, G.H.M.F., Bertolla, R.P. and Fraietta, R. 2013. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil. Steril. 99(1), 92–98; doi:10.1016/j.fertnstert.2012.08.048. | ||
| How to Cite this Article |
| Pubmed Style Jaswandi J, Rusfidra R, Ananda A, Abimanyu AA, Gusdinal H. Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters. Open Vet. J.. 2025; 15(3): 1322-1330. doi:10.5455/OVJ.2025.v15.i3.23 Web Style Jaswandi J, Rusfidra R, Ananda A, Abimanyu AA, Gusdinal H. Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters. https://www.openveterinaryjournal.com/?mno=232932 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.23 AMA (American Medical Association) Style Jaswandi J, Rusfidra R, Ananda A, Abimanyu AA, Gusdinal H. Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters. Open Vet. J.. 2025; 15(3): 1322-1330. doi:10.5455/OVJ.2025.v15.i3.23 Vancouver/ICMJE Style Jaswandi J, Rusfidra R, Ananda A, Abimanyu AA, Gusdinal H. Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1322-1330. doi:10.5455/OVJ.2025.v15.i3.23 Harvard Style Jaswandi, J., Rusfidra, . R., Ananda, . A., Abimanyu, . A. A. & Gusdinal, . H. (2025) Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters. Open Vet. J., 15 (3), 1322-1330. doi:10.5455/OVJ.2025.v15.i3.23 Turabian Style Jaswandi, Jaswandi, Rusfidra Rusfidra, Ananda Ananda, Agusti Azones Abimanyu, and Harif Gusdinal. 2025. Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters. Open Veterinary Journal, 15 (3), 1322-1330. doi:10.5455/OVJ.2025.v15.i3.23 Chicago Style Jaswandi, Jaswandi, Rusfidra Rusfidra, Ananda Ananda, Agusti Azones Abimanyu, and Harif Gusdinal. "Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters." Open Veterinary Journal 15 (2025), 1322-1330. doi:10.5455/OVJ.2025.v15.i3.23 MLA (The Modern Language Association) Style Jaswandi, Jaswandi, Rusfidra Rusfidra, Ananda Ananda, Agusti Azones Abimanyu, and Harif Gusdinal. "Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters." Open Veterinary Journal 15.3 (2025), 1322-1330. Print. doi:10.5455/OVJ.2025.v15.i3.23 APA (American Psychological Association) Style Jaswandi, J., Rusfidra, . R., Ananda, . A., Abimanyu, . A. A. & Gusdinal, . H. (2025) Protein profile of spermatozoa and seminal plasma based on molecular weight in four phenotypes of Kokok Balenggek roosters. Open Veterinary Journal, 15 (3), 1322-1330. doi:10.5455/OVJ.2025.v15.i3.23 |