| Research Article | ||
Open Vet. J.. 2025; 15(3): 1349-1357 Open Veterinary Journal, (2025), Vol. 15(3): 1349-1357 Research Article The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in ratsMaya Nurwartanti Yunita1*, Hani Plumeriastuti2, Bodhi Agustono3, Moch. Ilham Riza Fahlefi3, Aprilia Rizki Fadilla3, Ragil Ayu Pangastutie3, Shelly Oktania Nurvita Sari3, Muhammad Fikri Syamsuri3, Muhammad Alivio Putra Fajar3, Reina Puspita Rahmaniar4 and Md. Aliar Rahman51Veterinary Pathology Division, Faculty of Health, Medicine and Life Sciences, Universitas Airlangga, Surabaya, Indonesia 2Department of Veterinary Science, Division of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Veterinary Medicine, Faculty of Health, Medicine and Life Sciences, Universitas Airlangga, Indonesia 4Department of Microbiology, Faculty of Veterinary Medicine, Wijaya Kusuma University, Surabaya, Indonesia 5Faculty of Animal Husbandry, Department of Animal Nutrition, Bangladesh Agricultural University, Mymensingh, Bangladesh *Corresponding Author: Maya Nurwartanti Yunita. Veterinary Pathology Division, Faculty of Health, Medicine and Life Sciences, Universitas Airlangga, Surabaya, Indonesia. Email: mayanurwartanti [at] fkh.unair.ac.id Submitted: 20/12/2024 Accepted: 09/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
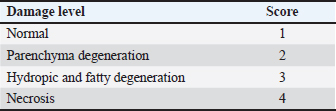
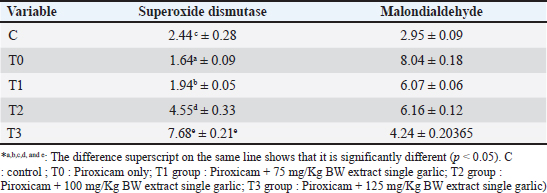
AbstractBackground: Piroxicam, a nonsteroidal antiinflammatory drug, is commonly used to induce inflammation in experimental models of inflammatory bowel disease (IBD). Piroxicam can modulate the immune system and disrupt the mucosal barrier, leading to increased production of reactive oxygen species. Aim: This study aimed to evaluate the effects of single-clove garlic (Allium sativum var. solo garlic) extract (SGE) on liver histopathological findings, superoxide dismutase (SOD), and malondialdehyde (MDA) levels in white rats (Rattus norvegicus) Wistar strain induced with piroxicam. Methods: The study used a completely randomized design with 30 male Wistar rats aged 2 months and weighing 150–200 g. The rats were divided into five groups: negative control (C), receiving CMC-Na 0,5% only; positive control (T0), receiving 20 mg/kgBW piroxicam; and three treatment groups, receiving 20 mg/kgBW piroxicam combined with 75 mg/kgBW (T1), 100 mg/kgBW (T2), or 125 mg/kgBW (T3) of SGE. Piroxicam was administered orally to induce inflammation, followed by SGE treatment for 14 days. Liver histopathological images were measured for damage repair using the scoring method. Superoxide dismutase and malondialdehyde levels were measured using enzyme-linked immunosorbent assay methods. Results: The results showed a significant increase in SOD levels (p < 0.05) in the treatment groups compared with the T0 (20 mg/kgBW piroxicam), with the T3 (125 mg/kgBW piroxicam) showing the most significant effect. Additionally, MDA levels were significantly decreased (p < 0.05) in all treatment groups, indicating a reduction in oxidative damage. Statistical analysis revealed a dose-dependent relationship between garlic extract administration and improvement in liver histopathological findings and oxidative stress markers. Conclusion: SGE is a potent antioxidant that reduces oxidative stress in a piroxicam-induced IBD model and contributes to good health. Further studies are needed to explore the molecular mechanisms underlying these effects and to optimize the therapeutic potential of IBD. Keywords: Good health, Liver histopathological, MDA, Piroxicam, Single garlic, SOD. IntroductionNonsteroidal anti-inflammatory drugs (NSAIDs) are one of the causes of inflammatory bowel disease (IBD). Piroxicam is an NSAID and a non-selective cyclooxygenase (COX) inhibitor (Nurahmanto et al., 2018). The ability of piroxicam to act as a nonselective COX inhibitor can inhibit prostaglandin synthesis by inhibiting the COX enzyme (Gunaydin and Bilge, 2018). In other words, piroxicam has a mechanism of action in inhibiting COX-1 and COX-2 but is more effective in inhibiting COX-1 (Mustaqim et al., 2018), where COX-1 plays a role in the protection of the gastrointestinal mucosa. The ability of piroxicam to inhibit prostaglandin synthesis can also cause a decrease in blood flow and ischemia (Bjarnason et al., 2018). Ischemia can cause mitochondrial leakage and trigger an increase in reactive oxygen species (ROS), which are harmful and involved in the pathogenesis of gastric ulceration (Nanayakkara et al., 2019). Piroxicam, which is a related NSAID class drug, can cause damage to the gastrointestinal tract. Gastrointestinal adverse effects result in inflammation and the risk of bleeding in the digestive tract (Srikandi et al., 2021; Aleem et al., 2023). Piroxicam affects the immune system and mucosal barrier by increasing ROS production through inflammatory reactions that lead to oxidative stress (Holgersen et al., 2014; Jomova et al., 2023; Nurkhasanah et al., 2023). Metabolic processes in the body are performed by the liver, which also plays a role in storing secreted and excreted substances as well as detoxifying various toxic compounds (Singh et al., 2013; Haris et al., 2016). It has been stated that long-term use of piroxicam and high doses can cause gastrointestinal bleeding because piroxicam has the highest toxicity to the gastrointestinal tract compared with other NSAIDs. Gastrointestinal bleeding can cause liver damage because toxins from the small intestine are absorbed by free radicals carried by the portal vein to the liver, causing changes in the composition of liver cell tissue. The increase in free radicals is initiated by lipid peroxidation with the final product malondialdehyde (MDA) resulting from the reaction between ROS, which induces proinflammatory cytokines [tumor necrosis factor-alpha (TNF-α), IL-1β, and interleukin-6 (IL-6)] with membrane phospholipids (Malino, 2016). One of the important parameters to measure the extent of oxidative stress is MDA levels, which are the end products of lipid peroxidation and serve as indicators of oxidative damage (Cordiano et al., 2023). On the other hand, body cells also develop a complex antioxidant system formed by enzymatic antioxidants as endogenous antioxidants, one of which is superoxide dismutase (SOD) (Halliwell, 2022). SOD effectively addresses cellular oxidative stress, regulates lipid metabolism, manages inflammation, and mitigates oxidation. It helps maintain a dynamic equilibrium between the production and removal of biological oxidants in the body, thereby protecting against the harmful effects of free radicals (Zheng et al., 2023). SOD helps the body eliminate superoxide radicals by converting them into hydrogen peroxide (H2O2) (Annamalay, 2018). A complex antioxidant defense system, including endogenous and exogenous antioxidants, protects cells from damage and toxicity via the induction of unstable molecules such as ROS (Jomova et al., 2023; Fadlilah and Lestari, 2023). Single garlic (Allium sativum Var. solo garlic) is an exogenous antioxidant plant with a higher content of the active compound allicin than regular garlic (Bae et al., 2012; Yunita et al.,2023). Allicin is the main bioactive compound with antioxidant ability obtained from the hydrolysis of alliin by the enzyme alliinase or by extracting it at low temperatures using ethanol solvent (Wahyudi et al., 2018). S-allyl-cysteine (SAC) in garlic also has antioxidant effects that can increase major antioxidants such as SOD, CAT, glutathione peroxidase (GPx), and GST (Ilmawati et al., 2019; Azhar, 2021). In addition, the presence of diallyl disulfide in garlic significantly enhances antioxidant enzyme activity and restores glutathione content in rats (Alrumaihi, 2020). This study aimed to explore the potential of SGE in inhibiting the negative effects of piroxicam on liver histopathological findings and the stress biomarkers SOD and MDA levels in white rats (Rattus norvegicus) as an animal model of IBD. The use of garlic as an alternative therapy for IBD is still considered to be limited, so the results of this study are expected to make an important contribution to the development of safer and more effective therapies for people with IBD by utilizing natural ingredients that are relatively safe and easy to acquire. Research Materials and MethodsPreparation of Allium sativum var. solo garlic extractThe production of SGE uses 2,000 g of a single garlic, which is sorted, peeled off, and cut into thin slices. Single garlic pieces were oven-dried for 24 hours at 50°C. The dried single garlic was then pulverized with a blender and sieved using a 60-mesh sieve until the results were obtained in the form of powdered simplicia. SGE is made by maceration through the addition of simplicia with 96% ethanol for 72 hours (Poernomo et al., 2022). The maceration results were then filtered three times using a Buchner funnel lined with filter paper and collected into an Erlenmeyer. The filtrate from the filtration was evaporated using a rotary vacuum evaporator and heated in a water bath at 40°C to separate the solvent and obtain a thick SGE. Research designThis study used 30 white rats (Rattus norvegicus) of the Wistar strain male sex and two months old, with a weight of 150–200 g (Aulanni’am et al., 2020). Given treatment for 14 days. The study was divided into five treatment groups: C : treatment with CMC-Na 0.5 % alone T0 : treatment with 20 mg/Kg BW piroxicam T1 : treatment with 20 mg/Kg BW piroxicam + 75 mg/Kg BW SGE T2 : treatment with 20 mg/Kg BW piroxicam + 100 mg/Kg BW SGE T3 : treatment with 20 mg/Kg BW piroxicam + 125 mg/Kg BW SGE The interval between piroxicam and SGE administration was six hours and was administered for 14 days. At the end of the treatment period, the experimental animals were anesthetized and blood serum samples were collected. The SOD and MDA levels were then measured using the Enzyme-Linked Immunosorbent Assay (ELISA) method. The liver organs were collected during necropsy and were fixed in a 10 % formalin buffer solution to facilitate histopathological examination. ELISA ExaminationThe levels of SOD and MDA were measured using the ELISA (Enzyme-Linked Immunosorbent Assay) technique with an ELISA Kit from MedikBio, INA. For SOD analysis, the ELISA plate was coated with specific antibodies that bind to SODs, followed by incubation with the sample to facilitate binding. A secondary antibody linked to the HRP (Horseradish Peroxidase) enzyme is then added, and the enzymatic reaction with the substrate produces a colored product, which is measured with an ELISA reader (Thermo Scientific) by spectrophotometer at a wavelength of approximately 450 nm. MDA analysis typically employs the capture assay principle, where MDA reacts with TBA (Thiobarbituric Acid) to form a detectable complex. This complex is recognized by specific antibodies, and absorbance is measured at 532 nm. The concentrations of SOD and MDA were determined by comparing the absorbance values with a standard curve. The ELISA technique is highly sensitive and ideal for research requiring precise measurements, such as studies on oxidative stress and inflammation (Christy Angelica, 2024). Liver histopathologyScoring was carried out referring to Muhartono (2020) and Lawung (2019), namely using the modified Manja Roenigk scoring method on 5 randomly selected visual fields, which were then averaged with the parameters of parenchymatous degeneration, fatty degeneration, hydropic degeneration, and necrosis. Statistical analysis Statistical analysis of SOD, MDA, and histopathology of liver data to evaluate significant differences between the tested sample groups, for example, between the control and treatment groups. The obtained data were then analyzed using the Shapiro–Wilk test to determine data normality. If the data distributed were not normally distributed, data processing was continued using the Kruskal–Wallis nonparametric test to determine whether or not there were differences between the treatment groups. If there was a significant difference (p < 0.05) between treatment groups in the Kruskal–Wallis test results, then the data analysis was continued by conducting the Mann–Whitney test to determine the differences between the treatment groups. If the data were distributed normally, data processing was continued using the ANOVA parametric test to show the differences between treatments, and data processing was continued using the post hoc Duncan test. The results of this statistical analysis will determine whether there is a significant difference between the groups, which could indicate the effect of the treatment on oxidative stress SOD and lipid damage MDA. In addition, correlation analysis can be performed to observe the relationship between SOD and MDA expression, which could provide insight into the mechanism of oxidative balance in the biological system under study. Statistically significant results are usually reported with a p-value indicating the level of confidence in the observed difference. Ethical approvalThis study was approved by the Health Research Ethical Clearance Commission of Universitas Airlangga, Surabaya, Indonesia (Approval No. 1/KEH.125.08.2023). (FODM/II/2022). ResultsSuperoxide dismutase levelsSOD levels in T0 tissues treated with only piroxicam treatment indicate low antioxidants in the body with 1.64 ± 0.09 µ/mg protein. Giving SGE can complement the reaction of SOD and GPx formation can improve natural antioxidants in the body. It was proven in the T3 treatment where the administration of piroxicam and antioxidant repair with SGE increased SOD levels to 7.64 ± 0.21 µ/mg protein. The results are shown in Table 2. The T0 group exhibited significantly lower SOD levels, indicating depletion of antioxidant defenses by piroxicam-induced oxidative stress. In contrast, the C group exhibited the highest SOD levels among the untreated rats. The treatment groups (T1, T2, T3) showed an increase in SOD levels with increasing doses of SGE, with T3 (125 mg/KgBW) showing the most significant elevation in SOD levels, exceeding those of the control group. ANOVA for SOD levels also revealed a significant difference between the groups (p < 0.001). The Duncan post hoc test revealed that T3 formed a distinct subset with the highest SOD levels, which was significantly different from the other groups. Malondialdehyde levelsThe average malondialdehyde level in group T0 treated with only piroxicam treatment has a score of 8.04 ± 0.18 µ/mg protein. After treatment in group T3 with piroxicam and treatment with a single garlic extract at a dose of 125 mg/KgBW, the MDA values of 4.24 ± 0.20 µ/mg protein as a result of SGE can reduce MDA levels. The results are shown in Table 2. The highest MDA levels were observed in the T0 group, indicating that piroxicam induces significant oxidative stress. In contrast, the C group had the lowest MDA levels, as expected for animals not exposed to oxidative stress. The treatment groups (T1, T2, T3) showed a dose-dependent reduction in MDA levels, with T3 (125 mg/KgBW garlic) showing the largest reduction, bringing MDA levels closer to those of the control group. The ANOVA test for MDA showed a significant difference between the groups (p < 0.001), and the Duncan post hoc test revealed that the T3 group formed a distinct subset with lower MDA levels compared to T1 and T2. This suggests that higher SGE doses are more effective in reducing piroxicam-induced lipid peroxidation. Table 1. Liver histopathology scoring scoring (Lawung et al., 2019).
Table 2. Superoxide dismutase and Malondialdehyde levels.
Table 3. Mean liver cell damage after piroxicam and SGE induction.
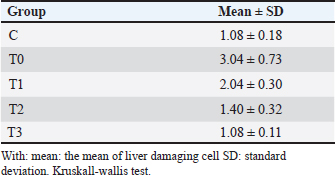
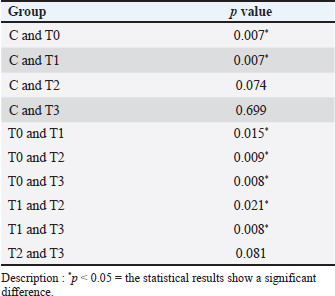
Histopathology of the liverThe results of the Shapiro–Wilk normality test showed a p-value < 0.05 in the C group (0.001) and the T3 group (0.026), so it can be concluded that the data is not normally distributed. Data that showed no normal distribution and were included in ordinal data were analyzed using the Kruskal–Wallis test (Table 3) and continued with the Mann–Whitney post Hoc test (Table 4). The results of the scoring method revealed varying liver damage. Group C, which was only given 0,5% CMC-Na solution, showed the lowest scoring value, indicating it had the lowest damage among all treatment groups. Group T0 receiving piroxicam at a dose of 20 mg/KgBW had the highest scoring value of 3.04. Group T0 showed a picture of hydropic degeneration commonly called Ballooning degeneration in hepatocyte cells. The cell size was extremely enlarged, and the cytoplasm appeared clear. However, the cell nucleus was still visible in the middle. Group T1 showed a scoring value of 2.04, which was the second-highest damage value after group T0. Group T1 experienced parenchymatous degeneration commonly called cloudy swelling hydropic degeneration in hepatocyte cells, the cell size was seen to be enlarged but not as extreme as in hydropic degeneration, with the cytoplasm appearing cloudy, and the cell nucleus remaining in the middle. Group T2 showed a scoring value of 1.40, which indicated a damage value approaching that of group C. Group T3 showed the same scoring value as group C, namely 1.08. Group T3 showed normal hepatocytes with a radial cell arrangement toward the central vein and polyhedral cell shape and hepatocyte plates. The decrease in damage value from group T1 to T3 compared with group T0 and C is shown in Figure 1.
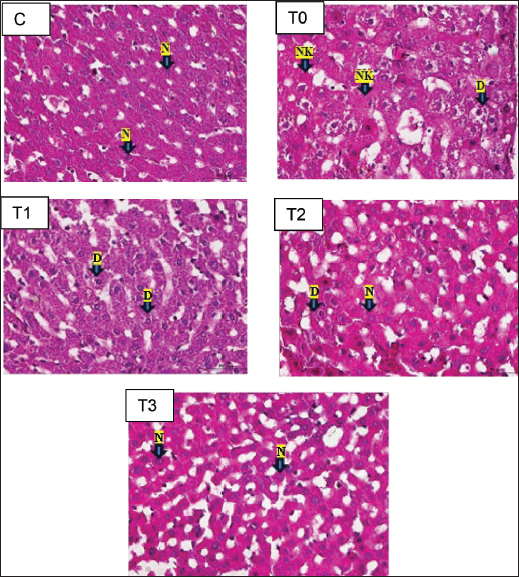
Fig. 1. Histopathological figures of the liver organ of white rat strain Wistar magnification 400x. Description: C (only given CMC-Na 0,5% solution); T0 (piroxicam 20 mg/KgBW); T1 (piroxicam 20 mg/KgBW and SGE 75 mg/KgBW); T2 (piroxicam 20 mg/KgBW and SGE 100 mg/KgBW); T3 (piroxicam 20 mg/KgBW and SGE 125 mg/KgBW). N: normal, DP: parenchymatous degeneration, DH: hydropic degeneration, NK: necrosis. Table 4. Post hoc result Mann–Whitney from the scoring of liver histopathology.
The results of the Kruskal–Wallis test obtained p=0.00, which means it is categorized as significant because p < 0.05 so that the hypothesis can be accepted, namely that single garlic extract (Allium sativum var. solo garlic) has the potential to reduce damage due to free radicals seen in the histopathology of the liver of white rats (Rattus norvegicus) induced by piroxicam. Based on the Mann–Whitney post hoc test in the statistical analysis in Table 3, the value of group T1 is significantly different (p < 0.05) from group T0, which is 0.015, and also significantly different from group C, which is 0.007. This shows that the dose of SGE in group T1, which was 75 mg/KgBW, provided a therapeutic effect, although it was still significantly different from group C. Group T2 showed a significant difference (p < 0.05) from group T0 with a value of 0.009, not significantly different (p > 0.05) from group C which showed a value of 0.074, and significantly different from group T1 with a value of 0.021. The results of the analysis showed that the SGE dose of group T2, namely 100 mg/KgBW, had the effect of reducing damage to liver tissue due to piroxicam induction because it was no longer significantly different from group C and the dose in this treatment group showed a better therapeutic effect compared to group T1. The results of the analysis of the T3 group with an SGE dose of 125 mg/Kg BW showed a significant difference (p < 0.05) with the T0 group with a value of 0.008 and the T1 group with a value of 0.008 and was not significantly different (p > 0.05) with the C group and the T2 group. This shows that the T3 group exerts a significant therapeutic effect, marked by a reduction in damaged liver tissue, and provides a better therapeutic effect than the T1 group. The histology of the liver tissue of white rats in group C was observed using a trinocular microscope with a magnification of 400x and the preparation was made with hematoxylin and eosin (HE) staining. Normal liver tissue was seen with intact hepatocytes without any damage such as degeneration or necrosis, and the tissue structure was still clearly visible (Eljafari et al., 2020). The T0 group, namely the group that was given 20 mg/KgBW piroxicam treatment alone, showed that hepatocytes experienced necrosis and degeneration. Based on the results of observations in group T1, with an average score of 2.04, hepatocytes experienced parenchymatous degeneration, indicating a significant difference with the T0 groups. Group T2 consisted of piroxicam induction treatment 20 mg/KgBW and SGE 100 mg/KgBW. The observation results obtained an average score of 1.40 and showed a histopathological picture, namely parenchymatous degeneration and normal hepatocytes. Group T3 in its analysis results showed an average score of 1.08, which showed the similarity of the average score in group C, namely 1.08, which showed that during the observation there was no hydropic degeneration or necrotic hepatocytes. The result is shown in Figure 1. DiscussionImpact of piroxicam on oxidative stressPiroxicam is an NSAID that is effective in managing inflammation and pain but is known to induce oxidative stress. This is primarily due to the inhibition of COX enzymes, which leads to an increased production of ROS (Sahu et al, 2016). In this study, the T0 group administered piroxicam without any antioxidant treatment exhibited significantly elevated MDA levels and decreased SOD levels, confirming the oxidative damage induced by piroxicam. These findings are consistent with previous research demonstrating that NSAIDs like piroxicam can disrupt the balance between pro-oxidants and antioxidants, leading to lipid peroxidation and depletion of endogenous antioxidant defenses garlic extract in modulating oxidative stress garlic, particularly single-clove garlic, has been extensively studied for its antioxidant properties, largely attributed to its organosulfur compounds, such as allicin, diallyl disulfide, and S-allyl cysteine. These compounds scavenge free radicals, enhance antioxidant enzyme activity, and inhibit lipid peroxidation (Nasr et al, 2014). In this study, MDA levels were significantly reduced and SOD levels were increased in a dose-dependent manner. The reduction in MDA levels in the T3 group (125 mg/KgBW SGE) suggests that garlic is effective in mitigating lipid peroxidation caused by piroxicam-induced oxidative stress. This result is consistent with previous studies that demonstrated the lipid-lowering effects of garlic. The increase in SOD levels indicates that garlic not only prevents oxidative damage and enhances the body’s endogenous antioxidant defenses (Savira et al., 2023). This is consistent with previous studies showing that garlic can upregulate the expression of antioxidant enzymes, including SOD. Impact of piroxicam on the liverPiroxicam has the potential to cause side effects on the liver (Pairul et al, 2018) because metabolic processes in the body are carried out by the liver, which also plays a role in the storage and excretion of substances and as a detoxifier of various toxic compounds (Singh et al., 2013). Haris et al., (2016) reported that long-term use of high-dose piroxicam can cause gastrointestinal bleeding because compared with other NSAIDs, piroxicam has the highest toxicity to the gastrointestinal tract. Gastrointestinal bleeding can cause hepatic damage because toxins from the small intestine are absorbed due to the presence of free radicals carried by the portal vein to the liver, causing changes in the preparation of hepatic cell tissue. The increase in free radicals is initiated by lipid peroxidation with the end product MDA resulting from the reaction between ROS-inducing proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and membrane phospholipids (Malino et al, 2016). Hepatic damage is characterized by high blood MDA levels (Kurniasari et al, 2021). According to Al-Hamdany et al., (2022), histopathological examination of piroxicam-induced hepatic tissue appears to show necrosis in hepatocytes and hydropic or ballooning degeneration. Mechanism of action of the single garlic extractThe effects of garlic extract have some complexity from the mechanism. First, the expression of SOD and MDA can be interpreted based on their concentration or activity in the sample. SOD is an endogenous antioxidant enzyme that regulates ROS levels (Landis and Tower, 2005; Younus et al, 2018). Antioxidant-active molecules play a crucial role in combating oxidative stress (Tan et al., 2018). High SOD expression indicates increased enzymatic activity to neutralize free radicals, usually in response to oxidative stress. However, low SOD expression may reflect the inability of cells to manage oxidative stress, potentially leading to cellular damage. High MDA expression generally indicates an increase in lipid peroxidation, reflecting cellular damage caused by oxidative stress. Free radicals exacerbate lipid peroxidation, resulting in the production of MDA in the blood, which serves as a marker of cellular damage due to free radicals (Latifa et al., 2015). Elevated MDA levels are often associated with inflammatory conditions or degenerative diseases. This analysis provides an overview of the oxidative status of the sample, where the balance between SOD and MDA expression can offer important insights into cellular health and the risk of oxidative stress-related diseases. SOD and MDA levels reflect oxidative stress and antioxidant activity. SOD is a key endogenous enzyme that neutralizes ROS, with high levels indicating enhanced antioxidant defense against oxidative stress. In contrast, low SOD indicates cellular vulnerability to damage. MDA is a marker of lipid peroxidation, is elevated in oxidative stress, and is often associated with inflammation or degenerative diseases. SGE, which is rich in SAC, reduces oxidative stress by inhibiting NF-κβ activation, preventing inflammatory molecule transcription, and lowering MDA levels. Additionally, SAC activates the NRF2 pathway, boosting SOD and other antioxidant enzymes, which counteract oxidative stress by reducing lipid peroxidation and neutralizing free radicals (Tocmo et al., 2015). Single garlic treatment increases SOD, catalase, and GPx in the endothelial cells of blood vessels. Single garlic acts as a chain-breaking antioxidant that scavenges free radicals (Ilmawati et al., 2019). First, its organosulfur compounds can directly scavenge free radicals, thereby reducing the burden of ROS (Tran et al., 2018). Second, garlic can enhance the activity of endogenous antioxidant enzymes, including SOD, CAT, and GPx (Asdaq et al, 2015). Third, garlic has been shown to inhibit lipid peroxidation by maintaining its integrity and preventing the formation of MDA (Al-Sahhaf et al., 2016). Additionally, garlic may modulate the inflammatory response by inhibiting the production of pro-inflammatory TNF-α and IL-6, which are known to contribute to oxidative stress (Tran et al., 2018). This antiinflammatory effect may further contribute to the reduction of oxidative damage observed in this study (Amer and El-Rahman et al, 2022). The clinical implications of this study have important clinical implications, especially in the context of long-term NSAID use. NSAIDs are widely prescribed for the management of chronic inflammatory conditions, but their potential to induce oxidative stress and cause tissue damage is a significant concern (Khoo et al., 2019). The results of this study suggest that single garlic extract could be used as an adjunct therapy to mitigate the oxidative damage associated with NSAIDs using the safety profile of NSAIDs and reduce the risk of adverse effects related to oxidative stress, such as gastrointestinal and cardiovascular complications (Al-Sahhaf et al, 2016; Tren et al., 2018; K et al., 2020). Higher doses of SGE may be more effective in counteracting oxidative stress induced by piroxicam. However, it is important to consider the potential for dose-dependent toxicity at very high doses (K et al., 2020; Dahiya et al., 2022). ConclusionIn conclusion, this study demonstrated the potential of single garlic extract in reducing piroxicam-induced oxidative stress in white rats. Malondialdehyde levels and increased SOD levels suggest that garlic is effective in preventing lipid peroxidation and enhancing antioxidant defenses. There was a reduction in damage observed in the histopathological features of the liver. The most significant effects were observed at the highest SGE dose (125 mg/KgBW), indicating that higher doses may offer greater protection against oxidative damage. These findings support the use of single garlic extract as a natural antioxidant therapy to mitigate the adverse effects of NSAID-induced oxidative stress. Further studies are required to explore the long-term effects and safety of single garlic extract in clinical settings. AcknowledgmentsWe thank the Rector and Dean of the Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga. Conflict interestThe authors declare that they have no conflicts of interest. Authors’ contributionsMNY: Conceptualization, methodology, data analysis, and writing of the original draft. HP, BA, MIRF, SONS, RAP, ARF, MAPF, MFS, RPR, and MAL: The authors contributed to the conceptualization and methodology, conducted the study, and revised the manuscript. All authors have read and approved the final version of the manuscript. Data availabilityAll references are open-access, so data can be obtained from the online website. ReferencesAleem, A., Aslam, B., Bilal Alim, M., Hussain, A., Naeem Faisal, M. and Sindhu Z. 2023. Phytochemical analysis and gastroprotective effect of Stellaria media (L.) Vill. methanolic extract on piroxicam-induced gastric ulcer in Wistar rats. Pak. J. Pharm. Sci. 36(5), 1425–1434. Al-Hamdany, M., Al-Tai, F. and Ismail, K.H. 2022. Histological changes induced by piroxicam in the hepatic and renal tissues of mice with and without peppermint oil. Pharm. Med. Sci. 25(4), 183–190. Alrumaihi, F. 2020. Garlic and its active compounds: a novel strategy to fight diseases through modulating biological activities. Phcog. J. 12(6), 1463–1474. Al-Sahhaf, Z.Y. 2016. Protective effects of garlic on methyl ethyl ketone-induced biochemical changes in male rabbits. J. Biosci. Appl. Res. 2(10), 695–699. Amer, S.A. and El Rahman, H.S. 2022. Anti-shigellosis activity of the aqueous extract of garlic, clove and fenugreek. J. Food Saf. 42(3). doi:10.1111/jfs.12978 Annamalay. 2018. Effects of antioxidants on oxidative stress: assessing MDA in urine samples. Int. J. Clin. Nutr. Diet. 4, 135. Asdaq SM. 2015. Antioxidant and hypolipidemic potential of aged garlic extract and its constituent, S-Allyl systeine, in rats. Evid. Based Complement. Alternat. Med. 2015, 328545. Aulanni’am, A., Anita, T.Z., Nahari, D.S., Aluka, I.A., Agustine, E.I., Novita, T. and Beltran, M.A.G. 2020. The potency of Polyalthia Longifolia from indonesia and the philippines as therapeutic agents for inflammatory bowel disease (IBD) in rats (Rattus Norvegicus) induced by Indomethacin. IOP Conf. Ser. Mater. Sci. Eng. 833(1). doi: 10.1088/1757-899X/833/1/012005 Azhar, S.F., Yuliawati, K.M. and Kodir, R.A. 2021. Pengaruh Waktu aging dan metode Ekstraksi terhadap Aktivitas Antioksidan Black Garlic yang Dibandingkan dengan Bawang Putih (Allium Sativum L.). J. Ris. Farm. 1(1), 16–23. Bae, S.E., Cho, S.Y., Won, Y.D., Lee, S.H. and Park, H.J. 2012. A comparative study of the different analytical methods for analysis of S-Allyl cysteine in black garlic by HPLC. LWT-Food Sci. Technol. 46(2), 532–535. Bjarnason, I., Scarpignato, C., Holmgren, E., Olszewski, M., Rainsford, K.D. and Lanas, A. 2018. Mechanisms of gastrointestinal tract damage from nonsteroidal anti-inflammatory drugs. Gastroenterology 154(3):500–514. Christy, A.P. 2024. Efek Sitotoksik Dan Selektivitas Ekstrak Heksana Umbi Rumput Teki (Cyperus Rotundus L.) Dari Provinsi Lampung Terhadap Sel Hela. Medicine Faculty, Lampung University. Lampung. Indonesia. 2024. Colín-González, A.L., Santana, R.A., Silva-Islas, C.A., Chánez-Cárdenas, M.E., Santamaría, A. and Maldonado, P.D. 2012. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid. Med. Cell. Longev. 2012, 907162. Cordiano, R., Di Gioacchino, M., Mangifesta, R., Panzera, C., Gangemi, S. and Minciullo, P.L. 2023. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: an update. Molecules 28(16), 5979. Dahiya, A., Prakash, A., Agrawala, P.K. and Dutta, A. 2022. Oral toxicity of diallyl sulfide as a principle organosulfur compound derived from allium Sativum garlic in mice. Defense Life Sci. J. 7(1), 3–10. Eljaafari, H., EL Mabrouk, Z., Mohamed, F., Tunsi, H., & Sasi, S. 2024. Carbon TetrachlorideInduced Testicular Toxicity and Histopathological Alteration in Male Swiss Albino Mice. AlQalam Journal of Medical and Applied Sciences, 36–43. https://doi.org/10.54361/ajmas.2471007 Fadlilah, A.R. and Lestari, K. 2023. Review : Peran Antioksidan, Dalam Imunitas Tubuh. Farmaka 21(2),171–178. Gunaydin, C. and Bilge, S.S. 2018. Effects of nonsteroidal anti-inflammatory drugs at the molecular level. Eurasian J. Med. 50(2), 116–121. Halliwell, B. 2022. Reactive oxygen species (ROS), oxygen radicals, and antioxidants: where are we now, where is the feld going, and where should we go?. Biochem. Biophys. Res. Commun. 633, 17–19. Haris, R.R., Mughni, A. and Margawati, A. 2016. Pengaruh Pemberian Injeksi Ketorolac Tromethamine Intraperitoneal Terhadap Gambaran Mikroskopis Gaster Tikus Wistar Dewasa Dengan Fraktur Kruris. Diponegoro Med. J. 5(4), 1642–1649. Holgersen, K., Kvist, P.H., Markholst, H., Hansen, A.K. and Holm, T.L. 2014. Characterization of Enterocolitis in the piroxicam-accelerated interleukin-10 knock-out mouse: a model mimicking inflammatory bowel disease. J. Crohn’s Colitis. 8(2), 147–160. Ilmawati, R.R., Gofur, A. and Lestari, S.R. 2019. Single bulb garlic oil improves interleukin-6 via decreased reactive oxygen species (ROS) in high-fat diet male mice. Universa. Med. 38(2), 100–107. Jomova, K., Raptova, R. and Alomar, S.Y. 2023. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 1997, 2499–2574. Pratap, R.K., Nagilla, B. and Varija, K. 2020. Ameliorative effects of garlic (Allium Sativum L.,) in streptozotocin induced oxidative stress in the diabetic brain of rat. Int. J. Res. Pharm. Sci. 11(4), 7950–7957. Khoo, B.L., Grenci, G., Lim, J.S.Y., Lim, Y.P., Fong, J., Yeap, W.H., Bin Lim, S., Chua, S.L., Wong, S.C., Yap, Y.S., Lee, S.C., Lim, C.T. and Han, J. 2019. Low-dose anti-inflammatory combinatorial therapy reduced cancer stem cell formation in patient-derived preclinical models for tumour relapse prevention. Br. J. Cancer 120(4), 407–423. Kurniasari, S. 2021. Efek Pemberian Jintan Hitam (Nigella Sativa) Terhadap Kadar Malondialdehyde Dan Superoksida Dismutase Pada Hepar Tikus (Rattus Norvegicus) yang Diinduksi Piroxicam. J. Fisika Flux: J. Ilmiah Fisika FMIPA Univ. Lambung Mangkurat 18(2), 133–138. Landis, G.N. and Tower, J. 2005. Corrigendum to “superoxide dismutase evolution and life span regulation”. Mech. Aging Dev. 126(8), 907–8. Latifa, K.I., Al. Profil Kadar MDA (Malondialdehid) Pada Tikus Yang Diberi Ekstrak Herba Thymi (Thymus Vulgaris L). Skripsi. Fakultas Farmasi, Universitas Muhammadiyah Surakarta, Indonesia. 2015. Lawung, K.T., Sasputra, I.N. and Liana, D.S. 2019. Efek pemberian minuman sopi dibandingkan alkohol jenis lainnya terhadap gambaran histopatologi hati tikus putih (Rattus Norvegicus) Galur Sprague-Dawley. CMJ. 7(1), 74–8. Malino, A. 2016. Pengaruh Terapi Air Alkali Terhadap Kadar Mda Dan Profil Protein Hepar Pada Tikus Putih (Rattus Norvegicus) model of inflammatory bowel disease (IBD) Hasil Induksi Indometasin. Doctoral Dissertation, Universitas Brawijaya. Malang. Indonesia Muhartono, M., Salsabila, N.A. and Ayu, P.R. 2020. The effect of green tea infusion (Camellia sinensis) on histopathology of white rat (Rattus Norvegicus) liver sprague–dawley strain induced by ethanol. J. Drug Deliv. Ther. 10(2-s), 90–3. Nanayakkara, G.K., Wang, H. and Yang, X. 2019. Proton leaks regulate mitochondrial reactive oxygen species generation in endothelial cell activation and inflammation. Biochem. Biophys. 662, 68–74. Nasr, A.Y. 2014. Protective effects of aged garlic extract against oxidative stress induced by cisplatin on blood cell parameters and hepatic antioxidant enzymes in rats. Toxicol. Rep. 1, 682–691. Nurahmanto, D., Sabrina, F.W. and Ameliana, L. 2018. Optimization of the preparation of polyvinylpyrrolidone and carbopol in solid dispersion patch preparation of piroxicam. Manuntung. Sci. J. 3(2), 197–206. Nurkhasanah, Bachri, M.S. and Yuliani, S. 2023. Antioksidan dan Stres Oksidatif. 1–3. UAD PRESS. ISBN: 6235635850 Pairul, P.P. 2018. Perbedaan Efek Anti-Inflamasi Jahe Merah (Zingiber Officinale Rosc. Var. Rubrum) dan Jahe Putih Besar (Zingiber Officinale Rosc. Var. Officinarum) Terhadap Ulkus Gaster Tikus Jantan Galur Sprague-Dawley Yang Diinduksi Piroksikam. Skripsi. Universitas Lampung, Bandar Lampung, Indonesia, 2018. Poernomo, H., Ma’ruf, M.T. and Dewi, L.N. 2022. Comparison of the effectiveness of maceration and soxhlet action methods on garlic extract to inhibit Staphylococcus Aureus growth. IJKG. 18(2), 86–92. Sahu, C.R. 2016. Mechanisms involved in the toxicity of the liver caused by piroxicam in mice and the protective effects of the leaf extract of Hibiscus Rosa-Sinensis L. clinical medicine insights. Arthritis Musculoskelet. Disord. 9(1), 9–13. Savira, M., Sari, D.K., Machrina, Y., Widjaja, S.S., Unitly, A.J.A., Ilyas, S., Siregar, J., Pandia, P., Rusda, M. and Amin, M.M. 2023. Effect of garlic ethanol extract administration on glutathione levels to prevent oxidative stress in a smoker rat model. Med. Arch. 77(6), 418–421. Singh, S., Fujii, L.L., Murad, M.H., Wang, Z., Asrani, S.K., Ehman, R.L. and Talwalkar, J.A. 2013. Liver stiffness is associated with decompensation risk, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 11(12), 1573–1584. Srikandi, A.E.P., Herda, A. and Hendera. 2021. Study literature efek penggunaan non-steroidal anti-inflammatory drugs (NSAID) pada sistem gastrointestinal. J. Curr. Pharm. Sci. 5, 418–428. Tan, B.L., Norhaizan, M.E., Liew, W.P. and Sulaiman Rahman, H. 2018. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front. Pharmacol. 16(9), 1162. Tocmo, R., Wang, R., Liang, D. and Huang, D. 2015. “Organosulfide profile and hydrogen sulfide-releasing capacity of garlic (Allium Sativum L.) scape oil: effects of PH and cooking”. J. Funct. Foods. 17, 410–421. Tran, G., Sao, D. and Le, N.T. 2018. Amelioration of single-cleve black garlic aqueous extract in dyslipidemia and hepatitis in chronic carbon tetrachloride-intoxicated swiss albino mice. Int. J. Hepatol. 2018, 9383950. Wahyudi, C.T., Wijayanti, S.D. and Harijono. 2018. Pengaruh konsentrasi media penyalut dan lama ultrasonikasi terhadap ukuran partikel dan aktivitas antioksidan nano ekstrak bawang putih tunggal (Allium Sativum L.). Jurnal. Pangan dan Agroindustri. 6(3), 8–17. Yunita, M.N., Fauzi, J.C., Rahmania, Z.D., Safinda, B., Sholikhah, T.I. and Agustono, B. 2023. Effects of single-bulb garlic (Allium sativum Var. solo garlic extract has a hematological profile in E-cigarette-induced male sprague-dawley rats. Pharmacogn. J. 15(3), 296–300. Younus, H. 2018. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 12(3), 88–93. Zheng, M., Liu, Y., Zhang, G., Yang, Z., Xu, W. and Chen, Q. 2023. The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxidants 12, 1675. | ||
| How to Cite this Article |
| Pubmed Style Yunita MN, Plumeriastuti H, Agustono B, Fahlefi MIR, Fadilla AR, Pangastutie RA, Sari SON, Syamsuri MF, Fajar MAP, Rahmaniar RP, Rahman MA. The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. Open Vet. J.. 2025; 15(3): 1349-1357. doi:10.5455/OVJ.2025.v15.i3.26 Web Style Yunita MN, Plumeriastuti H, Agustono B, Fahlefi MIR, Fadilla AR, Pangastutie RA, Sari SON, Syamsuri MF, Fajar MAP, Rahmaniar RP, Rahman MA. The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. https://www.openveterinaryjournal.com/?mno=233909 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.26 AMA (American Medical Association) Style Yunita MN, Plumeriastuti H, Agustono B, Fahlefi MIR, Fadilla AR, Pangastutie RA, Sari SON, Syamsuri MF, Fajar MAP, Rahmaniar RP, Rahman MA. The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. Open Vet. J.. 2025; 15(3): 1349-1357. doi:10.5455/OVJ.2025.v15.i3.26 Vancouver/ICMJE Style Yunita MN, Plumeriastuti H, Agustono B, Fahlefi MIR, Fadilla AR, Pangastutie RA, Sari SON, Syamsuri MF, Fajar MAP, Rahmaniar RP, Rahman MA. The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1349-1357. doi:10.5455/OVJ.2025.v15.i3.26 Harvard Style Yunita, M. N., Plumeriastuti, . H., Agustono, . B., Fahlefi, . M. I. R., Fadilla, . A. R., Pangastutie, . R. A., Sari, . S. O. N., Syamsuri, . M. F., Fajar, . M. A. P., Rahmaniar, . R. P. & Rahman, . M. A. (2025) The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. Open Vet. J., 15 (3), 1349-1357. doi:10.5455/OVJ.2025.v15.i3.26 Turabian Style Yunita, Maya Nurwartanti, Hani Plumeriastuti, Bodhi Agustono, Moch. Ilham Riza Fahlefi, Aprilia Rizki Fadilla, Ragil Ayu Pangastutie, Shelly Oktania Nurvita Sari, Muhammad Fikri Syamsuri, Muhammad Alivio Putra Fajar, Reina Puspita Rahmaniar, and Md. Aliar Rahman. 2025. The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. Open Veterinary Journal, 15 (3), 1349-1357. doi:10.5455/OVJ.2025.v15.i3.26 Chicago Style Yunita, Maya Nurwartanti, Hani Plumeriastuti, Bodhi Agustono, Moch. Ilham Riza Fahlefi, Aprilia Rizki Fadilla, Ragil Ayu Pangastutie, Shelly Oktania Nurvita Sari, Muhammad Fikri Syamsuri, Muhammad Alivio Putra Fajar, Reina Puspita Rahmaniar, and Md. Aliar Rahman. "The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats." Open Veterinary Journal 15 (2025), 1349-1357. doi:10.5455/OVJ.2025.v15.i3.26 MLA (The Modern Language Association) Style Yunita, Maya Nurwartanti, Hani Plumeriastuti, Bodhi Agustono, Moch. Ilham Riza Fahlefi, Aprilia Rizki Fadilla, Ragil Ayu Pangastutie, Shelly Oktania Nurvita Sari, Muhammad Fikri Syamsuri, Muhammad Alivio Putra Fajar, Reina Puspita Rahmaniar, and Md. Aliar Rahman. "The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats." Open Veterinary Journal 15.3 (2025), 1349-1357. Print. doi:10.5455/OVJ.2025.v15.i3.26 APA (American Psychological Association) Style Yunita, M. N., Plumeriastuti, . H., Agustono, . B., Fahlefi, . M. I. R., Fadilla, . A. R., Pangastutie, . R. A., Sari, . S. O. N., Syamsuri, . M. F., Fajar, . M. A. P., Rahmaniar, . R. P. & Rahman, . M. A. (2025) The protective effects of garlic (Allium sativum var. Solo Garlic) extract mitigates piroxicam-induced liver damage and oxidative stress in rats. Open Veterinary Journal, 15 (3), 1349-1357. doi:10.5455/OVJ.2025.v15.i3.26 |