| Research Article | ||
Open Vet. J.. 2025; 15(3): 1407-1423 Open Veterinary Journal, (2025), Vol. 15(3): 1407-1423 Research Article Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, PakistanAyesha Haleem Shah1, Asma Saeed1 Sanaullah Khan2, Abdul Haleem Shah1* and Jawad Ullah Shah11Institute of Biological Sciences, Gomal University, Dera Ismail Khan, Pakistan 2Institute of Zoological Sciences, University of Peshawar, Peshawar, Pakistan *Corresponding Author: Abdul Haleem Shah. Institute of Biological Sciences, Gomal University, Dera Ismail Khan, Pakistan. Email: haleemgud [at] yahoo.com Submitted: 22/12/2024 Accepted: 25/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
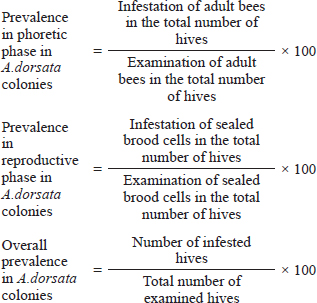
AbstractBackground: Apis dorsata is one of the most economically important bees. The A. dorsata colonies are rapidly declining in Pakistan due to multiple factors. Investigation regarding Varroa destructor in A. dorsata was completely ignored in the past. Aim: This study aimed to investigate the morphological identification, prevalence, and infestation rate of V. destructor in the phoretic phase and reproductive phase of A. dorsata in Pakistan. Methods: This research was performed season-wise from December 2022 to November 2023 in the selected districts of Khyber Pakhtunkhwa, Pakistan. Season-wise (one month of each season) examination of A. dorsata hives, samples collection, and examination of adult and sealed brood cells of A. dorsata for V. destructor collection, morphological identification, prevalence, and infestation rate was carried out. The data were analyzed by ANOVA and t-test. Results: The different life stages of V. destructor were identified based on morphological characters. Sizes of protonymph, deutonymph, and adult female V. destructor were 631–682 × 735–784 μm, 1,011–1,197× 1,080–1,667 μm, and 1,168–1,205 × 1,741–1,785 μm, respectively, while male V. destructor were 598–626 × 605–661 μm, 739–787 × 706–741 μm and 801–870 × 803–874 μm, respectively. In winter, spring, summer, and autumn, the prevalence of V. destructor in the phoretic phase of A. dorsata was 36%, 60%, 44%, and 32%, respectively, whereas in the reproductive phase was 4%, 32%, 60%, and 48%, respectively. Seasonal prevalence of V. destructor showed a statistically significant effect (p < 0.05) in the phoretic and reproductive phases of A. dorsata. A comparative prevalence of V. destructor between phoretic and reproductive phases of A. dorsata colonies was found highly significant (p < 0.05) in winter and spring. The overall prevalence of V. destructor in A. dorsata colonies in winter, spring, summer, and autumn was 36%, 60%, 60%, and 48%, respectively. The comparative infestation rate of V. destructor between phoretic and reproductive phases was found highly significant (p < 0.05) in winter, summer, and autumn. Season-wise overall infestation rate of V. destructor showed significant results (p < 0.05) in the phoretic and reproductive phases. Conclusion: The infestation of V. destructor was first time recorded in A. dorsata. This parasite continuously declines the A. dorsata in Pakistan. Keywords: Honeybee, Invasion, Mite, Pathogen, Varroosis. IntroductionHoneybees play an important role in food production, maintaining biodiversity, economy, and apicultural industries (Warner et al., 2024). Humans depend on honeybees both directly to produce honey, royal jelly, beeswax, and propolis and indirectly for the pollination of different crops. Of all the general pollinators, the honeybee is the most important pollinator because from flowers they collect the nectar for honey formation and also collect the pollens that are the main source to feed the larvae. This pollen spreads from their body and increases pollination. Therefore, to obtain better quality and quantity of food production, improvement in bee management is required (Ruiz, 2016). Honeybees are social insects that live in colonies. The process of producing more colonies due to reproduction is called swarming, which occurs mostly in spring due to the availability of more nectar in flowers as food (Mortensen et al., 2013). In each colony, different developmental stages such as eggs, larvae, and pupae are collectively known as brood (Gupta et al., 2010). Recently, the number of honeybee hives has been decreasing in many countries of the world due to multiple factors such as pesticides, environmental factors, and pathogens. In many cases, one of the major factors is the honey bee parasite, Varroa destructor. Varroa destructor is attached to the adult and brood stage of honeybees and has caused the death of millions of colonies throughout the world over the last fifty years (Ruiz, 2016; Schurischuster et al., 2016; Morgan et al., 2020). Varroa mites are the major cause of honeybees declining worldwide (Warner et al., 2024). In 1904 initially, V. destructor was discovered by A. C. Oudemans as Varroa jacobsoni and then rediscovered in 2000 by Anderson and Trueman as V. destructor the most significant parasite of honeybee (Oudemans, 1904; Anderson and Trueman, 2000). The phenotype difference between these two mites is that the body size of V. destructor is large as compared to V. jacobsoni (Anderson and Trueman, 2000). Four different species of the new genus Varroa were identified in many countries that affect honey bee species in most regions of the world (Dietemann et al., 2013). If the population of varroa mites goes unnoticed and untreated the parasitized honeybee colonies may be destroyed in one year from late autumn to early spring from varroosis, diseases of varroa mites cause major economic losses (Gregorc and Sampson, 2019; Mahmoud et al., 2019). Varroosis increases the risk of honeybees collapse. Colony collapse disorder is a major problem for agriculture and beekeepers because it mostly affects honey production and pollination. Climate change spreads the varroa mite and impacts bee colonies. Temperature, humidity, location of the hives, and hygienic behavior of bees affect the natural fall varroa mites (Korena et al., 2022). The V. destructor greatly impacts the pollination and beekeeping industries as it spreads from Apis cerana to Apis mellifera which is used for honey production and pollination worldwide. The weak and highly infested bee colonies spread the varroosis to the healthier strong colonies (Traynor et al., 2020). The worker bees transport the female varroa mite in the brood cells of the hive. The female varroa mite lays unfertilized eggs (males) and after 30 hours lays fertilized eggs (females). About 6–7 eggs of varroa mites may be present in one brood (Morgan et al., 2020). The eggs hatch into nymphs and feed from the single opening in the cuticle in the 5th abdominal sclerite of the honeybee pupae (Donze and Guerin, 1994). The hole in bee pupae is continuously open for feeding which facilitates bacterial and viral transmission that increases the risk of infections (Kanbar and Engels, 2003). The V. destructor saliva proteins are harmful to the honeybee immune system and spread viral diseases. These proteins also affect the metabolism, brain, and behavior of the bees (Morfin et al., 2023). The reproduction period of varroa mites depends upon the time of metamorphosis of the honey bee. When the adult bee is raised from the cocoon, at the same time varroa mother mites and immature and mature daughter mites also arise from that cell, jump on the adult bee’s body and start the phoretic stage (FAO, 2018). Varroa mite causes hemolymph reduction in honeybees which leads to the protein deficiency required for the development of hypopharyngeal gland in infested pupae. The destruction of the hypopharyngeal gland directly affects the production and value of royal jelly which leads to abnormal growth (Ayoub, 2020). The continuous decrease in the honeybee population adversely affects the structure and function of ecosystem (Ayoub, 2020). The loss of honeybees leads to the loss of plants, insectivorous birds, biodiversity, and food production (Ruiz, 2016). In 1977–1978 A. mellifera (European honey bee) was transported to Pakistan by the Honey Bee Research Institute NARC, Islamabad, and varroa mites spread as a severe pest of these new honey bees (Mahmood et al., 2021). In Pakistan, the three naturally distributed honeybee species are A. cerana, A. florea, and Apis dorsata (Anjum, 2015). Apis dorsata are also called rock bees because they build open single large combs on the branches of trees, rocks, and buildings and are called giant honeybees due to their largest size. This honeybee produces a large quantity of honey (Mahindru, 2007; Shukla and Upadhyay, 2016; Vasantharaj and Ramamurthy, 2016). Recently, A. dorsata species rapidly disappeared. Proper conservation, protection, and management are required for the existence of A. dorsata (Anjum, 2015). This study focuses on the morphological identification, seasonal prevalence, and infestation rate of V. destructor in the phoretic phase and reproductive phase of A. dorsata. Materials and MethodsThis study is designed to investigate the current status of V. destructor infesting A. dorsata. Study areasThe present research was performed season-wise from December 2022 to November 2023 in the selected districts of Khyber Pakhtunkhwa (KP) Pakistan (Bannu, Dera Ismail Khan, Kohat, Tank, and Karak) (Supplementary Fig. 1). All these districts are collectively known as southern districts of KP. These are the honey-producing areas and possess diverse varieties of honey bee flora for the collection of nectars and pollens. The season-wise temperature in the study areas is 15°C–22°C in winter, 25°C–33°C in spring, 35°C–48°C in summer, and 28°C–34°C in autumn. (Khan, 2013). Measurement of the selected A. dorsata hivesIn each season, five colonies/hives of A. dorsata that were present naturally under the roof of buildings and trees were selected and measured in order to collect the V. destructor in each district. These colonies remained nesting at that site from 1–2 months to one year. Samples collection from adults A. dorsataThe method of Dietemann et al. (2013) was slightly modified. In the selected locations of each district, the adult A. dorsata were collected in 4 seasons by using an insect net. The insects from the net were immediately put into a plastic jar with a wide mouth closed with a lid. The central large part of the lid was replaced by about 2 to 3 mm mesh. At one side of the jar, a hole was present closed with a lid. The hole size was about the size of the bees. The jar was transferred to the laboratory. Samples collection from sealed brood cells of A. dorsataThe method of Koeniger et al. (2002) was slightly modified. In each district, in the presence of smoke, the samples of sealed brood cells were collected from each selected hive by cutting a piece of comb from the lower part and were transferred to the Laboratory. Examination of adult A. dorsata for V. destructor collectionThe method of Dietemann et al. (2013) was slightly modified. Through the mesh, one teaspoon (7–8 g) of powdered sugar was poured into the plastic jar. The jar was shaken for about 1 minute in such a way that sugar-coated with the bees, inspired the grooming behavior of bees, and removed the mites from bees. Then the jar was stood for 1 minute and then shaken for 1–2 minutes over a white paper. The collected mites with sugar were placed in a sieve, then normal saline was poured into a sieve to separate the sugar from the mites. The isolated mites were collected over a white sheet of paper and were counted. On the other hand, the hole of the jar was open, and the A. dorsata passed through these holes was counted. Examination of sealed brood cells of A. dorsata for V. destructor collectionThe method of Dietemann et al. (2013) was slightly modified, in the laboratory each sealed brood cell was opened by fork, and carefully examined the cell wall, pupa, and larva by using the source of light for mite detection. The total opened cells and the collected mites were counted. It may be at the time of harvesting honey; a large number of mites were collected with the opening of more brood cells and forced the mites by knocking (up and down movement over a white paper) or by washing. Morphological identification of V. destructor male and female (protonymph, deutonymph, and adult stage)The morphological identification of V. destructor was carried out with the help of a microscope. For the morphological identification of V. destructor, Deosi (2017); De-Guzman et al. (1999); and Fernandez and Coineau (2007) standard keys were used which are as follows: 1. The protonymph and deutonymph of V. destructor differ based on the number of sternal setae. In protonymph three pairs and in deutonymph 5–6 pairs of sternal setae are present. 2. The nymph stages (protonymph and deutonymph) of V. destructor male and female are different based on shape, size, and arrangement of ventral side setae. The male is oblong and the female is globose in shape, the male is small as compared to the female. In males setae are arranged close to the anal pore and in females, setae are present on the whole body. 3, The adult males and females of V. destructor are different based on body size, shape, legs, and color. The body size of females is larger than males. The legs of males are larger than females. The color of the female is reddish brown and the male is yellow-white (Ifantidis, 1983; Deosi, 2017). 4. Peritreme size. 5. Number of pores on the sternal shield. 6. Number of setae on the foreleg, palp, mat apodal shield, epigynal shield, and marginal setae (De-Guzman et al., 1999; Fernandez and Coineau, 2007; Rehman et al., 2018). 7. The approximate size of V. destructor (Length × Width) Female protonymph (660.52 × 749.96 μm) Male protonymph (598.32 × 630.00 μm) Female deutonymph (913.25–1172.81 × 1043.26–1620.41 μm) Male deutonymph (769.79 × 722.48 μm) Female adult (1169.98 × 1751.53 μm) Male adult (847.57 × 818.91 μm) (Deosi, 2017). Measurement of V. destructor prevalence rateThe prevalence of V. destructor in the phoretic phase, reproductive phase, and overall prevalence in A. dorsata colonies/hives were calculated as:
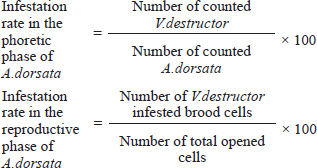
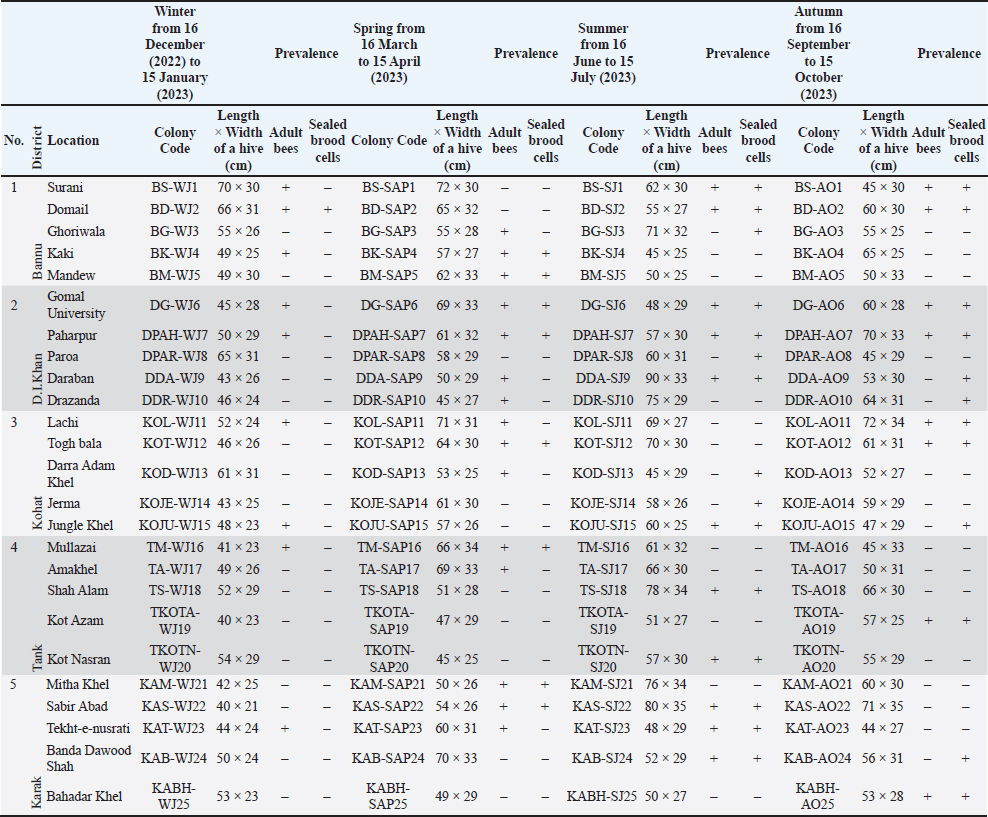
Measurement of V. destructor infestation rateThe infestation rate of V. destructor in the phoretic phase and reproductive phase of A. dorsata was calculated as:
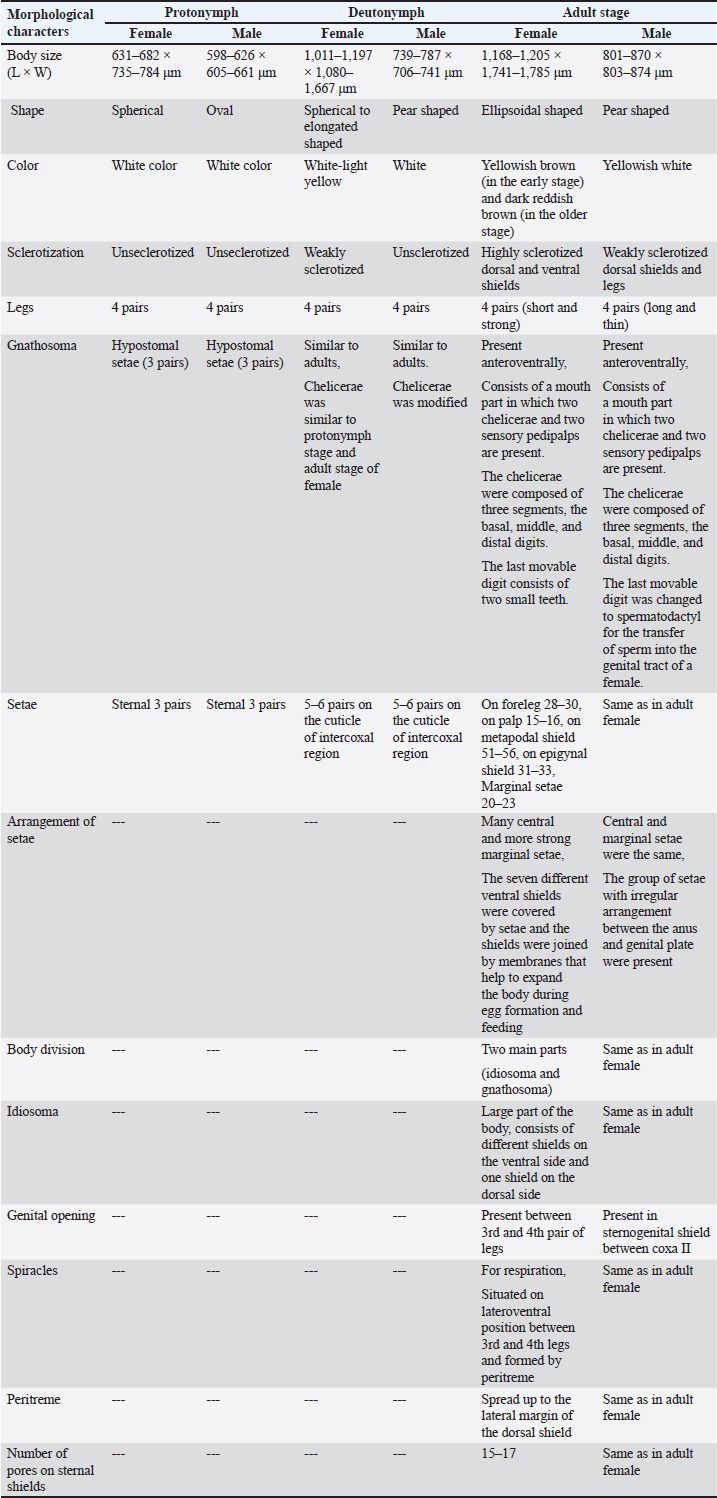
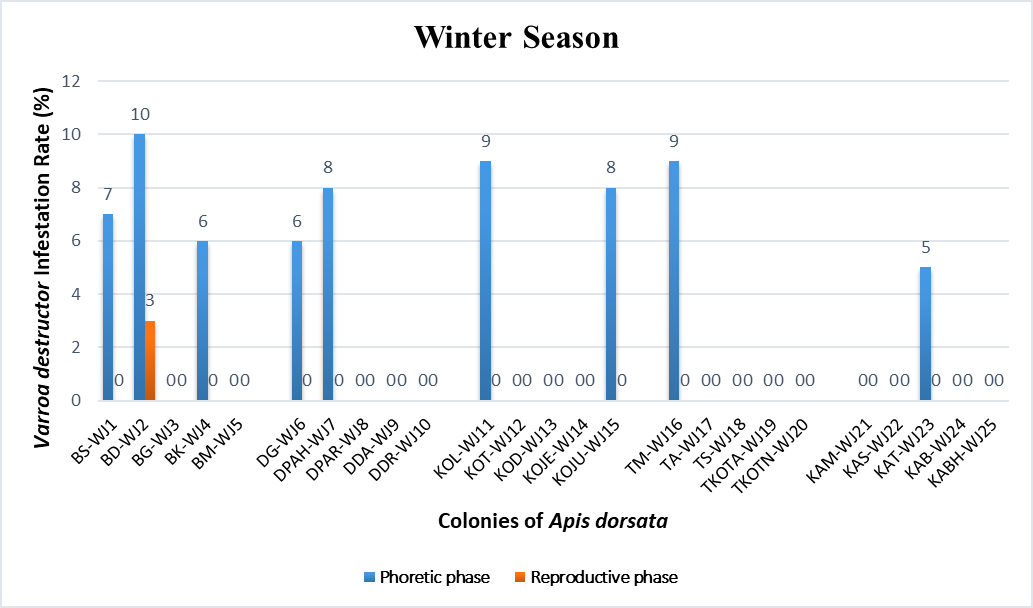
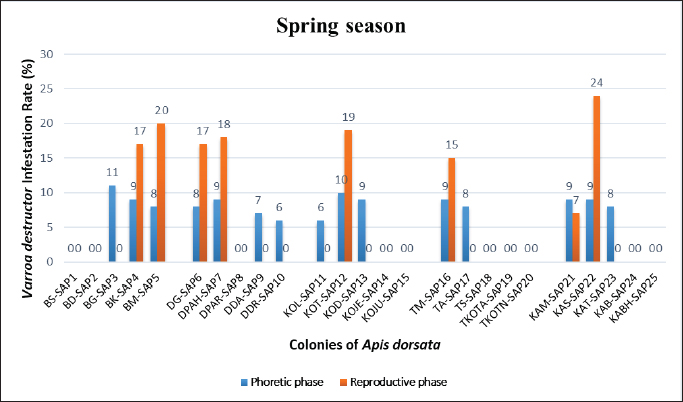
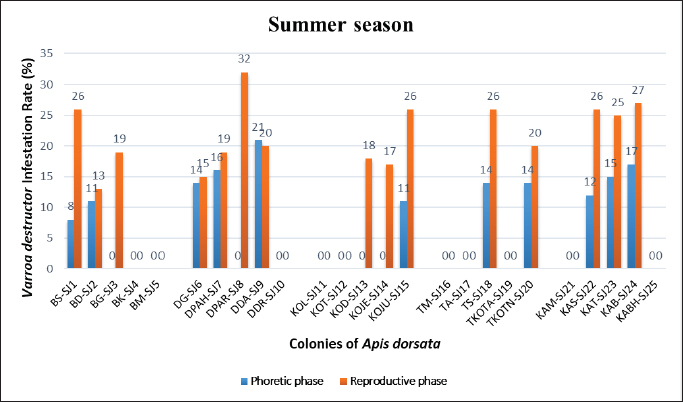
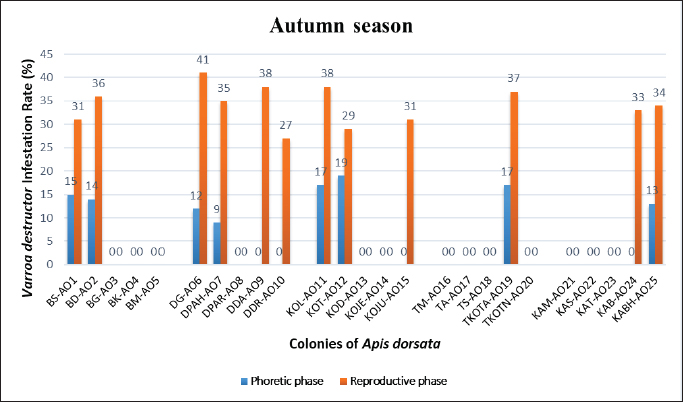
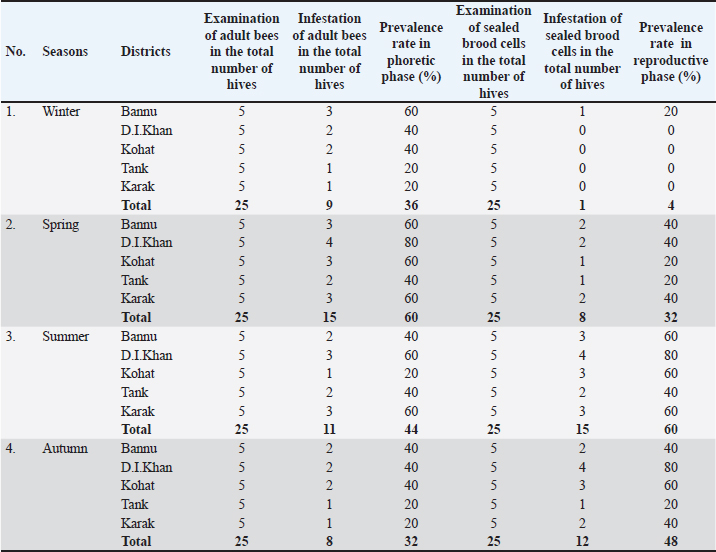
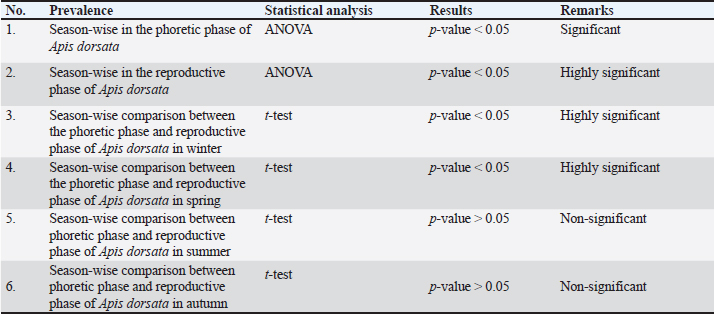
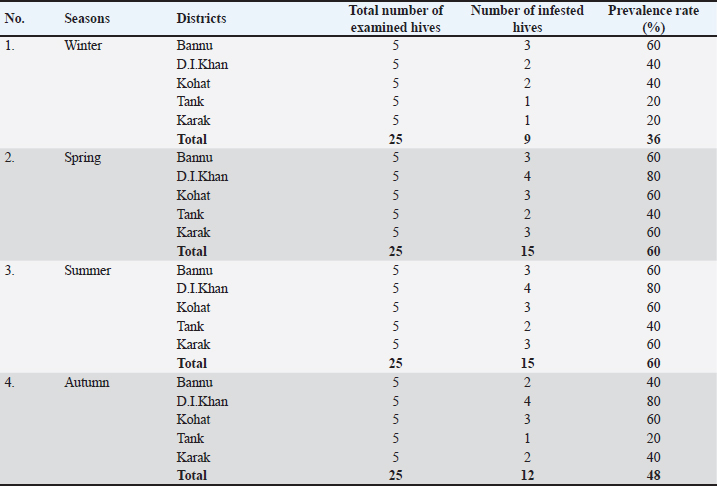
Statistical analysisThe analysis was carried out via MS Excel and SPSS. The normality of the measured data was tested using the Shapiro-Wilk test. The Shapiro-Wilk test significance value for all variables was >0.05 which shows that the data is normally distributed. The parametric tests ANOVA and t-test were used to analyze the data. ResultsIn this study, the morphological identification, season-wise prevalence, and infestation rate of V. destructor in the phoretic phase (adult stage) and reproductive phase (brood) of A. dorsata were investigated in the selected districts of KP, Pakistan. The research was performed season-wise in winter (16 December 2022–15 January 2023), spring (16 March–15 April 2023), summer (16 June–15 July 2023) and autumn (16 September–15 October 2023). In each season, five colonies/hives of A. dorsata were selected from different locations of each selected district (Bannu, D. I. Khan, Kohat, Tank, and Karak) of KP Pakistan. In each season, each colony was given a special code. The length and width of each hive were measured (Supplementary Table S1). Morphological identification of protonymph, deutonymph, and adult stages of male and female V. destructorThe varroa mites collected from A. dorsata in different regions of the selected districts of KP, Pakistan were different stages of V. destructor (Anderson and Trueman) species. The morphological identification of V. destructor male and female (protonymph, deutonymph, and adult stage) was carried out with the help of a microscope. The nymph stages (protonymph and deutonymph) of V. destructor were observed in the sealed brood cells (larvae and pupae) of A. dorsata, while the adult stage of V. destructor was found in the sealed brood cells as well as in the adult stage of A. dorsata. The protonymph, deutonymph, and adult stages of male and female V. destructor were identified because of morphological characteristics. The morphological characters of different stages of male and female V. destructor are shown in Table 1. Season-wise comparative prevalence of V. destructor in phoretic and reproductive phases in A. dorsata colonies/hivesThe seasonal comparative prevalence of V. destructor between the phoretic phase and reproductive phase in A. dorsata hives was recorded. In winter, nine colonies were found positive for V. destructor prevalence in the phoretic phase of A. dorsata (prevalence rate 36%), while in the reproductive phase, one colony was found positive (prevalence rate 4%). In spring, 15 colonies were recorded positive for V. destructor prevalence in the phoretic phase (prevalence rate 60%), whereas in the reproductive phase, eight colonies were found positive (prevalence rate 32%). In summer, 11 colonies were found positive for V. destructor prevalence in the phoretic phase (prevalence rate 44%), however in the reproductive phase 15 colonies were found positive (prevalence rate 60%). In autumn, eight colonies were recorded positive for V. destructor prevalence in the phoretic phase (prevalence rate 32%), while in the reproductive phase, 12 colonies were found positive (prevalence rate 48%) (Supplementary Table S2a). Prevalence of V. destructor in phoretic and reproductive phases of A. dorsata colonies/hives for different seasons was analyzed using ANOVA. Prevalence in the phoretic phase of A. dorsata was found significant with a p-value < 0.05 and in the reproductive phase was highly significant with a p-value < 0.05, revealing that for different seasons average prevalence of V. destructor in phoretic and reproductive phases of A. dorsata colonies was significantly different (Supplementary Table. S2b). Seasonal comparative prevalence of V. destructor between phoretic and reproductive phases of A. dorsata colonies was analyzed using a t-test. In winter and spring the p-value < 0.05, showed the prevalence of V. destructor was highly significant between phoretic and reproductive phases of A. dorsata, while in summer and autumn the p-value > 0.05, indicating that there was no significant difference (Supplementary Table. S2b). Overall seasonal prevalence of V. destructor in A. dorsata colonies/hivesThe V. destructor-infested colonies in winter, spring, summer, and autumn were 9, 15, 15, and 12, respectively. The overall prevalence rate of V. destructor in winter, spring, summer, and autumn was 36%, 60%, 60%, and 48%, respectively (Supplementary Table S3a). The overall prevalence of V. destructor in A. dorsata colonies was found insignificant using ANOVA with p-value > 0.05, revealing that the average prevalence of V. destructor in A. dorsata colonies was not different in different seasons (Supplementary Table. S3b). Seasonal infestation rate of V. destructor in phoretic phase and reproductive phase of A. dorsataThe five colonies in each selected district were examined season-wise for V. destructor infestation rate in the phoretic phase and reproductive phase of A. dorsata. In winter high infestation rate was found in the phoretic phase of A. dorsata in BD-WJ2 (10%), followed by KOL-WJ11 (9%), TM-WJ16 (9%), DPAH-WJ7 (8%), KOJU-WJ15 (8%), BS-WJ1 (7%), DG-WJ6 (6%), DG-WJ6 (6%), and KAT-WJ23 (5%), while in the reproductive phase of A. dorsata, the infestation rate was only found in BD-WJ2 (3%) (Fig. 1). In spring, the infestation rate was higher in reproductive phase of A. dorsata in KAS-SAP22 (24%), followed by BM-SAP5 (20%) and KOT-SAP12 (19%). In the phoretic phase of A. dorsata, the infestation rate was high in BG-SAP3 (11%) and KOT-SAP12 (10%) (Fig. 2). In summer, the infestation rate was higher in the reproductive phase of A. dorsata in DPAR-SJ8 (32%), followed by KAB-SJ24 (27%), BS-SJ1 (26%), KOJU-SJ15 (26%), TS-SJ18 (26%) and KAT-SJ23 (25%); while in the phoretic phase of A. dorsata, the infestation rate was high in DDA-SJ9 (21%), followed by KAB-SJ24 (17%) and KAT-SJ23 (15%) (Fig. 3). In autumn, the infestation rate was highest in reproductive phase of A. dorsata in DG-AO6 (41%), followed by DDA-AO9 (38%), KOL-AO11 (38%), and TKOTA-AO19 (37%). In the phoretic phase of A. dorsata, the infestation rate was higher in KOT-AO12 (19%), followed by KOL-AO11 (17%), TKOTA-AO19 (17%), and BS-AO1 (15%) (Fig. 4). Table 1. Morphological characters of protonymph, deutonymph, and adult stage of female and male Varroa destructor in Apis dorsata in the selected districts of KP, Pakistan.
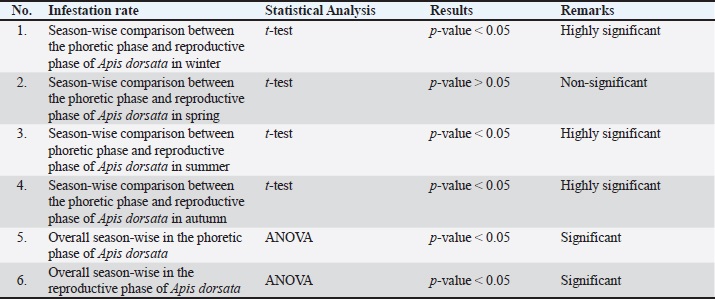
Comparative infestation rate of V. destructor between phoretic and reproductive phases of A. dorsata in winter, summer, and autumn showed a highly significant effect as p-value < 0.05, whereas in spring, revealed non-significant difference with p-value > 0.05 using t-test (Supplementary Table. S4). The overall infestation rate of V. destructor in phoretic and reproductive phases of A. dorsata was found significant using ANOVA with p-value < 0.05, revealing that for different seasons the average infestation rate of V. destructor in phoretic and reproductive phases of A. dorsata was significantly different (Supplementary Table. S4).
Fig. 1. Varroa destructor infestation rate in phoretic phase and reproductive phase of Apis dorsata in winter season in KP, Pakistan.
Fig. 2. Varroa destructor infestation rate in phoretic phase and reproductive phase of Apis dorsata in spring season in KP Pakistan.
Fig. 3. Varroa destructor infestation rate in phoretic phase and reproductive phase of Apis dorsata in summer season in KP Pakistan.
Fig. 4. Varroa destructor infestation rate in phoretic phase and reproductive phase of Apis dorsata in autumn season in KP Pakistan. DiscussionA. dorsata is an economically important pollinator all over the world and plays a vital role in the production of honey, wax, royal jelly, and propolis. Nowadays, A. dorsata is facing many challenges such as natural enemies (parasites and pathogens), pesticides, and habitat loss. The parasite V. destructor is the most destructive pest of honeybees that directly attacks the adult stage and brood. V. destructor also acts as a vector and indirectly spreads viral, bacterial, and fungal diseases. It plays a fundamental role in declining the honey bee population. The V. destructor is responsible for the major losses of the colonies of honey bees and is the main risk for the apiculture industry. In Pakistan, there is no published data regarding V. destructor infestation in A. dorsata population to date. Sammataro et al. (2000) reported that honeybee hives were found as suitable habitats for non-parasitic, pollen-feeding, omnivorous, and parasitic mites. This association leads to damage to honeybee colonies. Only a few reports are presented on mites of A. dorsata (Laigo and Morse, 1968; Delfinado-Baker et al., 1985, 1989; Buchler et al., 1993; Basavarajappa et al., 2009). According to Koeniger et al. (1983), there are no records of varroa mites on adult and brood cells of A. dorsata. In the present study, the first report of V. destructor in A. dorsata was recorded. The varroa mites collected from different regions of the selected districts of KP Pakistan were different stages of V. destructor species. The protonymph, deutonymph, and adult stages of male and female V. destructor were identified based on morphological characteristics. The female protonymph was spherical, large, and body size was about 631–682 × 735–784 μm, while the male protonymph was oval, small, and body size was about 598–626 × 605–661 μm. The female deutonymph was white-light yellow, large, and body size was about 1,011–1,197 × 1,080–1,667 μm, while the male deutonymph was white, small, and body size was about 739–787 × 706–741 μm. The female V. destructor in the adult stage was ellipsoidal in shape, yellowish-brown (in the early stage) and dark reddish brown (in the older stage), highly sclerotized dorsal and ventral shields, large (width is greater than length) and body size was about 1,168–1,205 × 1,741–1,785 μm. The male V. destructor in the adult stage was pear-shaped, yellowish-white, weakly sclerotized dorsal shields and legs, small (resembling the nymph stage of female) and body size was about 801–870 × 803–874 μm. Deosi (2017) also identified V. destructor in India because of its morphological character. They recorded that the size of male protonymph was about 598.32 ± 6.78 × 630.00 ± 8.02 μm, and the size of female protonymph was about 660.52 ± 7.13 × 749.96 ± 8.91 μm. The size of male deutonymph was about 769.79 ± 7.63 × 722.48 ± 5.75 μm, and the size of female deutonymph was about 913.25 ± 7.18 – 1172.81 ± 24.21 × 1043.26 ± 3.96 – 1620.41 ± 6.17 μm. The size of an adult male was about 847.57 ± 9.22 × 818.91 ± 9.51 μm, and the size of an adult female was about 1169.98 ± 2.74 × 1751.53 ± 5.71 μm. Rehman et al. (2018) collected V. destructor from A. mellifera in KP and was identified based on different morphological characters. The body size of V. destructor in A. mellifera was about 1.5–1.6 μm (length and width ratio). The location of the peritreme did not extend beyond the lateral margin of the dorsal shield. The setae of epigynal shield, foreleg, marginal, and metapodal shield were about 30, 28–30, 19–23, and 40–60, respectively in numbers. The sternal shield pores were about 14–18 in numbers. According to Delfinado and Baker (1974), the adult female varroa mite is dark reddish brown in color as the mite gets older, ellipsoidal in shape, length is smaller than width, the dorsal shield is highly sclerotized, many feather-like central setae and healthier marginal setae. On the ventral side, different shields are present covered by protective sensory setae. The respiratory spiracles are present in the lateroventral position, between the 3rd and 4th legs. Delfinado-Baker (1984) and Colin et al. (1999) found that the adult male varroa mite is smaller than the female, yellowish white in color, pear-shaped, different from the female by the occurrence of genital opening, present in the sternogenital shield between coxa II and the presence of moveable digit on chelicerae which change in tubular structure for the transfer of sperm. The body of the male varroa mite was weakly sclerotized and the dorsal shield was covered with setae. The legs of male varroa mites were long and thin. The above-mentioned finding regarding morphology also strengthened this study. In the current study, the morphological characteristics of V. destructor are helpful in distinguishing the V. destructor from other mites within the genus on the evolutionary position and for evolving the control procedures for the same but different life stages (protonymph, deutonymph, and adult) of V. destructor make the identification harder and need for molecular conformation of the species in future research. In this study, the highest prevalence of V. destructor in A. dorsata hives in the phoretic phase was found in spring and the low prevalence was found in autumn. In the reproductive phase, the highest prevalence was recorded in summer and low prevalence was found in winter. Season-wise prevalence of V. destructor was highly significant (p < 0.05) in the reproductive phase of A. dosata, and in the phoretic phase also showed significant (p < 0.05). Yenew and Sisay (2025) reported a 66% overall prevalence of V. destructor in A. mellifera honeybee colonies in the Ankasha district, Ethiopia. Arega (2020) reported a varroa mite prevalence rate of 69% in adult honeybees in East Wollega, Ethiopia. Robi et al. (2023) reported a 43% prevalence in Southwest Ethiopia. Shegaw et al. (2022) reported a 48.4% prevalence in South-western, Ethiopia. Keshlaf et al. (2023) reported a 53.8% prevalence rate of V. destructor in A. mellifera in Libya. Salkova and Gurgulova (2022) reported a 32.4% prevalence in Bulgaria. Gela et al. (2023) reported a 95.8% prevalence in the Oromia region. Abban et al. (2024) reported an 85% prevalence in the United States. Koeniger et al. (2002) reported the mites in sealed brood cells of A. dorsata colonies in Sabah, Malaysia. Tropilaelaps koenigerum and Tropilaelaps Clareae together and their offspring were detected in 1673 brood cells in two colonies of A. dorsata but varroa mites were not found in the brood cells of A. dorsata. According to Koeniger et al. (1983), adult bees and brood cells were inspected in three bee species such as A. cerana, A. dorsata, and A. florea. The V. jacobsoni was found in A. cerana, T. koenigerum was found in A. dorsata and Euvarroa sinhai was found in A. florea. Koeniger (1990) found that the mite species that are closely related can parasite the closely related Apis species. In the current study, season-wise overall prevalence of V. destructor in A. dorsata hives was carried out. The highest percentage was found in spring and summer, while the low percentage was found in winter. Hussain et al. (2018) investigated the V. destructor in A. mellifera species in the Karak district of KP from November 2016 to May 2017. The prevalence of V. destructor in A. mellifera hives was 0.64%. The lowest prevalence was found in December (3.3%) and February (1.2%). The highest number was found in November (5%) and April (7.5%). The higher infection was found in larvae and pupae than in adults. Delfinado-Baker et al. (1985) and Atwal and Goyal (1971) reported Tropilaelaps mites in the hive of A. dorsata as well as in other honey bee hives. Eickwort (1988) and Robaux (1986) recorded 100 harmless mite species that infesting the bees. The variations in the prevalence of varroa mites in honeybee colonies at different times and places might be due to differences in environmental factors, availability of bee flora, seasons, beekeeping practices, sample type (brood and adult), and honeybee species (Yenew and Sisay, 2025). In this study, the season-wise comparative infestation rate of V. destructor between the phoretic phase and reproductive phase of A. dorsata was highly significant (p < 0.05) in winter, summer, and autumn, while in spring showed insignificant (p > 0.05). The season-wise overall infestation rate of V. destructor was significant (p < 0.05) in phoretic and reproductive phases. Abban et al. (2024) reported that in the USA varroa mite infestation level significantly increased from 2015 to 2018 (1–4%), stable from 2018 to 2021 (4%), and decreased in 2022 (3%). The varroa mite infestation level is significantly different among climate regions. According to Morfin et al. (2024) in honeybee colonies, more than 1% varroa mite infestation in autumn leads to a higher mortality rate of honeybee in spring. Medina-Flores et al. (2024) found a significant effect (p < 0.05) on the population of mites in the phoretic phase, reproductive phase, total mites, and natural fallen mites per day. When the brood increases in summer, the mite infestation level decreases in adult bees because the mite migrates to the brood. When brood decreases in winter, the mite infestation increases in adult bees because larvae are not available for the reproduction of mites. According to Bava et al. (2023), 54% of apiaries in 2020 and 50% of apiaries in 2021 had V. destructor in Italy. In another study conducted by Koeniger et al. (1993), the T. clareae infestation rate in the sealed brood cells of A. dorsata was about 63% in Malaysia. In Thailand, the infestation rate of T. clareae in sealed brood cells of A. dorsata was observed similar to that found in Malaysia comb. Basavarajappa et al. (2010) reported the mite infestation in A. dorsata season-wise in Mysore, Karnataka, from 2008 to 2010. The infestation rate fluctuates in different seasons in 2008 and 2009. The high infestation rate was found during post-monsoon season followed by monsoon and the low infestation rate was found during winter. The infestation rate was 30.6%, 8.6%, and 2.5% during post-monsoon, monsoon, and winter, respectively, in 2008. The infestation rate was 13.6%, 3.5%, and 1.7% during post-monsoon, monsoon, and winter, respectively, in 2009. In another study in Northern Iraq, Duhok region V. destructor mite infestation level is high in about all apiaries of A. mellifera (Ayoub et al., 2015). According to Morgan et al. (2020), the number of varroa mites varies depending on the season and region. Generally, 1–2 mites on a single bee are observed mostly, or unusually 3–4 per bee are present. Ayoub (2020) investigated that infestation levels are different in different colonies, due to the population of colonies, the experience of beekeepers by using different pesticides, temperature, availability of food and humidity, etc. Varroosis in A. dorsata colonies was found in Pakistan. In the future, the V. destructor may be a great challenge for beekeeping and beekeepers if untreated. Similar studies are needed in other regions and between the seasons for the development of effective mite control strategies. This will pave the way for the elimination of V. destructor from the honeybee population and will increase the yield of honey. Monitoring of honeybee colonies for mite infestation levels, treatment of colonies before reaching harmful levels, the need for special care for weak colonies, and the use of natural products for treatments that lead to stronger honeybee colonies are recommended. ConclusionThe mites collected from A. dorsata colonies from different regions of the selected districts of KP Pakistan were identified as V. destructor based on morphological characteristics. This V. destructor is the most destructive pest of A. dorsata colonies in Pakistan. The prevalence of V. destructor in A. dorsata colonies varies season-wise. The prevalence of V. destructor in the phoretic phase of A. dorsata was found high in spring and low was found in autumn. The prevalence in the reproductive phase of A. dorsata was found high in summer, and low was found in winter. The overall seasonal prevalence rate of V. destructor in A. dorsata colonies was found highest in summer and spring, and low prevalence was found in winter. The highest infestation rate of V. destructor in the phoretic phase of A. dorsata colonies was found in summer, while in reproductive phase was found in autumn. V. destructor leads to great threats to beekeeping industries and needs further research for better control methods to improve the growth of A. dorsata. AcknowledgmentsAuthors would like to acknowledge the Gomal University. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Authors’ contributionsAyesha Haleem Shah: Principal investigator. Asma Saeed: Supervisor. Sanaullah Khan: Co-supervisor. Abdul Haleem Shah: Fieldwork management. Jawad Ullah Shah: Technical support. All authors approved the final version of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbban, S., Smith, B., Corona, M., Cook, S.C., Evans, J. D., Chen, Y. and Alburaki, M. 2024. Prevalence and distribution of Varroa destructor and Nosema spp. In symptomatic honey bee colonies across the USA from 2015 to 2022. Sci. Rep. 14, 1726. Anderson, D.L. and Trueman, J.W. 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24, 165–189. Anjum, S.I. 2015. Identification and characterization of honey bee (A. mellifera) gut bacterial flora in Khyber Pakhtunkhwa, Pakistan. Ph.D. Thesis, Gomal University, Dera Ismail Khan, Pakistan. Arega, A. 2020. The prevalence of honeybee diseases and Varroa mite in selected districts of East Wollega Zone, Oromia National Regional State, Ethiopia. IJARBS. 7(4), 46–59. Atwal, A.S. and Goyal, N.P. 1971. Infestation of honey bee colonies with Tropilaelaps and its control. J. Apic. Res. 10, 137–142. Ayoub, Z.N. 2020. Virulence of V. destructor in Colonies of Honey Bee A. mellifera. Duhok, Iraq: University of Duhok. IntechOpen. Ayoub, Z.N., Ahmed, D.S., Abdulla, M. and Mosa, M.H. 2015. Impact of Varroa mite infestation on the mandibular and hypopharyngeal glands of honey bee workers. Acarina 23(1), 92–97. Basavarajappa, S., Raghunandan, K.S. and Hegde, S.N. New record of ectoparasite, Acarine mite on rock bee, A. dorsata Fabr. in Chamundi Hills, Tirupati, India, ISEPEHH, 2009, pp 131–132. Basavarajappa, S., Raghunandan, K.S. and Hegde, S.N. 2010. Seasonal incidence of the parasitic mite (arachnida: acarinidae) on A. dorsata f. (hymenoptera: apidae) in Mysore, Karnataka. Hexapoda 17(2), 166–173. Bava, R., Castagna, F., Palma, E., Ceniti, C., Millea, M., Lupia, C., Britti, D. and Musella, V. 2023. Prevalence of Varroa destructor in honeybee (Apis mellifera) farms and varroosis control practices in Southern Italy. Microorganisms 11(5), 1228. Buchler, R., Drescher, W. and Tornier, I. 1993. Grooming behaviour of A. cerana, A. mellifera and A. dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Exp. Appl. Acarol. 16, 313–319. Colin, M.E., Fernandez, P.C. and Hamida, T.B. (1999). Varoosis. Pp 121–142. In: Bee disease diagnosis. Eds., Colin, M.E., Ball, B.Y. and Kilani, M. Paris, France: CIHEAM, p: 182. De-Guzman, L.I., Rinderer, T.E. and Stelzer, J.A. 1999. Occurrence of two genotypes of Varroa jacobsoni Oud. In North America. Apido 30, 31–36. Delfinado, M.D. and Baker, E.W. 1974. Varroidae, a new family of mites on honey bees (Mesostigmata: Acarina). J. Wash. Acad. Sci. 64, 4–10. Delfinado-Baker, M. D. 1984. The nymphal stages and male of Varroa jacobsoni a parasite of the honey bee. Int. J. Acar. 10, 75–80. Delfinado-Baker, M., Baker, E.W. and Phoon, A.C.G. 1989. Mites (Acari) associated with bees (Apidae) in Asia, with description of a new species. Am. Bee J. 122, 416–417. Delfinado-Baker, M., Underwood, B.A. and Baker, E.W. 1985. The occurrence of Tropilaelaps mites in brood nests of A. dorsata and A. laboriosa in Nepal, with description of the nymphal stages. Am. Bee J. 125, 703–206. Deosi, H.K. 2017. Varroa destructor Anderson and Truman in A. mellifera Linnaeus colonies – morphometry of the mite and its developmental stages. J. Exp. Zool India 20(1), 223–226. Dietemann, V., Francesco, N., Stephen, J.M., Anderson, D.L., Barbara, L., Keith, S.D., Quentin, W., Cindy, T., Eva, F., Bettina, Z., Peter, R. and James, D.E. 2013. Standard methods for varroa research. J. Apic. Res. 52(1), 1–54. Donze, G. and Guerin, P.M. 1994. Behavioral attributes and parental care of Varroa mites parasitizing honeybee brood. Behav. Ecol. Sociobiol. 34, 305–319. Eickwort, G.C. 1988. The origins of mites associated with honey bees. In: Africanized Honey Bees and Bee Mites. Eds., Needham, G.R., Page, R.E., Delfinado-Baker, Jr M. and Bowman, C. Chichester, England: Ellis-Horwood, Ltd., Pp: 327–338. FAO (Food and Agriculture Organization of the United Nations). 2018. Thematic catalogue for smallholder farmers to promote innovation main bee diseases: good beekeeping practices. Rome, Italy: FAO. Fernandez, N. and Coineau, Y. 2007. Varroa, the serial bee killer mite. Atlantica, France. pp: 264. Gela, A., Atickem, A., Bezabeh, A., Woldehawariat, Y. and Gebresilassie, A. 2023. Insights into Varroa mite (Varroa destructor) infestation levels in local honeybee (Apis mellifera) colonies of Ethiopia. J. Appl. Entomol. 147(9), 798–808. Gregorc, A. and Sampson, B. 2019. Diagnosis of Varroa mite (Varroa destructor) and sustainable control in honey bee (A. mellifera) colonies—a review. Diversity 11(12), 243. Gupta, J.K., Belavadi, V.V. and Singh, S.M. 2010. Apiculture. India, AgriMoon. Hussain, R., Sidra, F., Maira, K. and Hameed, U.R. 2018. Prevalence of Varroa destructor on honey bees hives in district Karak, Khyber Pakhtunkhwa, Pakistan. J. Entomol. Zool. Stud. 6(1), 169–171. Ifantidis, M.D. 1983. Ontogenesis of the mite Varroa jacobsoni in worker and drone honey bee brood cells. J. Apic. Res. 22(3), 200–206. Kanbar, G. and Engels, W. 2003. Ultrastructure and bacterial infection of wounds in honey bee (A. mellifera) pupae punctured by Varroa mites. Parasitol. Res. 90, 349–354. Keshlaf, M.M., Mirwan, H.B., Ghana, S., Mubrok, S. and Shaibi, T. 2023. Prevalence of Varroa mites (Varroa destructor Anderson&Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. Open Vet J. 13(7), 834–838. Khan, M.A. 2013. Epidemiology, zoonotic potential, haematological effect, in vivo and in vitro control of Sarcoptes scabiei (LAM) in animals and humans in Khyber Pakhtunkhwa (Pakistan). Ph.D. Thesis, Gomal University, Dera Ismail Khan, Pakistan. Koeniger, G., Koeniger, N., Anderson, D., Lekprayoon, C. and Tingek, S. 2002. Mites from debris and sealed brood cells of A. dorsata colonies in Sabah (Borneo) Malaysia, including a new haplotype of Varroa jacobsoni. Apidologie 33(1), 15–24. Koeniger, N. Co-evolution of the Asian honeybee and their parasitic mites. In Proceedings of the 11th International Conference, New Delhi, India, 1990, IUSSI, pp 130–131. Koeniger, N., Koeniger, G. and Delfinado-Baker, M. 1983. Observations on mites of the Asian honeybee species (A. cerana, A. dorsata, A. florea). Apidologie 14, 197–204. Koeniger, N., Koeniger, G., Mardan, M. and Wongsiri, S. 1993. Possible effects of regular treatments of Varroatosis on the host-parasite relationship between A. mellifera and Varroa jacobsoni in Asian Apiculture. In Proceedings of the International Conference on Asian honey bees and bee mites Eds., Connor, L.J., Rinderer, T., Sylvester, H.A. and Wongsiri, S. Kalamazoo, MI: Wicwas Press, pp: 541–550. Korena Hillayova, M., Korený, L. and Skvarenina, J. 2022. The local environmental factors impact the infestation of bee colonies by mite Varroa destructor. Ecol. Indic. 141(4), 109104. Laigo, F.M. and Morse, R.A. 1968. The mite Tropilaelaps clareae in A. dorsata colonies in the Philippines. Bee World 49, 116–118. Mahindru, S.N. 2007. Beekeeping. Delhi, India: APH Publishing Corporation. Mahmood, R., Muhammad, A.B., Muhammad, F.R., Ziyad, A.Q. and Muhammad, Y. 2021. Effcacy of naturally occurring chemicals for the integrated control of Varroa destructor (Anderson and Trueman) in honeybee colonies. Pakistan J. Zool. 53(3), 1173–1176. Mahmoud, S.O.M., Mohamed, S.H., Mohamed, S.Y. and Wael, M.M. 2019. Efficiency assessment of modified defined chemical compounds for controlling varroa mite, Varroa destructor (Parasitiformes: Varroidae) in Egyptian apiaries. Egypt. J. Plant Prot. Res. Inst. 2(1), 123–133. Medina-Flores, C.A., Saucedo Rojas, A., Guzman-Novoa, E. and Alaniz Gutiérrez, L. 2024. Population dynamics of the mite Varroa destructor in honey bee (Apis mellifera) colonies in a temperate semi-arid climate. Insects, 15, 696. Morfin, N., Foster, L.J., Guzman-Novoa, E., Van Westendorp, P., Currie, R.W. and Higo, H. 2024. Varroa destructor economic injury levels and pathogens associated with colony losses in Western Canada. Front. Bee Sci. 2, 1355401. Morfin, N., Goodwin, P.H. and Guzman-Novoa, E. 2023. Varroa destructor and its impacts on honey bee biology. Front. Bee Sci. 1, 1272937. Morgan, A.R., James, M.W., Keith, R.T. and Aaron, D.G. 2020. Biology and management of Varroa destructor (Mesostigmata: Varroidae) in A. mellifera (Hymenoptera: Apidae) colonies. J. Integr. Pest Manag. 11(1), 1–8. Mortensen, A., Schmehl, D. and Ellis, J. 2013. Featured creatures: European honey bee. Gainesville, FL: University of Florida. Oudemans, A.C. 1904. On a new genus and species of parasitic acari. Notes Leyden Mus. 24(4), 216–222. Rehman, M., Sajjad, A., Maqsood, S. and Inamullah, K. 2018. Identification of Varroa mite species of honeybee, A. mellifera in Khyber Pukhtunkhawa Peshawar, Pakistan. SJA. 34(2), 414–417. Robaux, P. 1986. Varroa et varroasis. Paris, France: Opida, pp: 238. Robi, D.T., Temteme, S., Aleme, M., Bogale, A., Getachew, A. and Mendesil, E. 2023. Epidemiology, factors influencing prevalence and level of varroosis infestation (Varroa destructor) in honeybee (Apis mellifera) colonies in different agroecologies of Southwest Ethiopia. Parasite Epidemiol. Control. 23, 00325. Ruiz, C.M. (2016). Importance and health of honey bee (A. mellifera): a review. Scientific writing and presentations in English. Scientific review article; doi:10.13140/RG.2.2.24378.49603. Salkova, D. and Gurgulova, K. 2022. Detection of Varroa destructor mite and Nosema spp. in bee samples from Bulgaria. Bee Stud. 14(1), 21–26. Sammataro, D., Gerson, U. and Needham. G. 2000. Parasitic mites of honey bees: life history, implications and impact. Ann. Review of Entomol. 45, 519–548. Schurischuster, S., Zambanini, S., Kampel, M. and Lamp, B. 2016. Sensor study for monitoring Varroa mites on honey bees (Apis mellifera). In Proceedings of visual observation and analysis of vertebrate and insect behavior workshop. pp: 4. Shegaw, T., Arke, A., Belay, N. and Giorgis, D.H. 2022. Diagnostic survey on Varroa mite (Varroa distractor) prevalence in South-Western Ethiopia. Cogent Food Agric. 8(1), 2143610. Shukla, G.S. and Upadhyay, V.B. 2016. Economic zoology. Meerut, India: Rastogi Publications. Traynor, K.S., Mondet, F., de Miranda, J.R., Techer, M., Kowallik, V., Oddie, M.A., Chantawannakul, P. and McAfee, A. 2020. Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol. 36(7), 592–606. Vasantharaj, D.B. and Ramamurthy V.V. 2016. Elements of economic entomology. New Delhi, India, Brillion Publishing. Warner, S., Pokhrel, L.R., Akula, S.M., Ubah, C.S., Richards, S.L., Jensen, H. and Kearney, G.D. 2024. A scoping review on the effects of Varroa mite (Varroa destructor) on global honey bee decline. Sci. Total Environ. 906, 167492. Yenew, A.S. and Sisay, Y.A. 2025. Prevalence of Varroa destructor infestations in Apis mellifera honeybee colonies and associated risk factors in Ankasha District, Northwest Ethiopia. Vet. Med. Sci. 11(1), 70140. Supplementary Material
Supplementary Fig 1. Map of the Study Areas of Khyber Pakhtunkhwa Pakistan. Supplementary Table 1. Season wise prevalence of Varroa destructor in phoretic phase (adult stage) and reproductive phase (sealed brood cells) of Apis dorsata in KP Pakistan.
Supplementary Table 2a. Comparative prevalence of Varroa destructor in phoretic phase and reproductive phase in Apis dorsata hives in the selected districts of KP Pakistan.
Supplementary Table 2b. Statistical analysis of Varroa destructor prevalence in Apis dorsata hives in the selected districts of KP Pakistan.
Supplementary Table 3a. Overall prevalence of Varroa destructor in Apis dorsata hives in the selected districts of KP Pakistan.
Supplementary Table 3b. Statistical analysis of overall Varroa destructor prevalence in Apis dorsata hives in the selected districts of KP, Pakistan.
Supplementary Table 4. Statistical analysis of Varroa destructor infestation rate in Apis dorsata hives in the selected districts of KP, Pakistan.
| ||
| How to Cite this Article |
| Pubmed Style Shah AH, Saeed A, Khan S, Shah AH, Shah JU. Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan. Open Vet. J.. 2025; 15(3): 1407-1423. doi:10.5455/OVJ.2025.v15.i3.32 Web Style Shah AH, Saeed A, Khan S, Shah AH, Shah JU. Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan. https://www.openveterinaryjournal.com/?mno=234155 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.32 AMA (American Medical Association) Style Shah AH, Saeed A, Khan S, Shah AH, Shah JU. Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan. Open Vet. J.. 2025; 15(3): 1407-1423. doi:10.5455/OVJ.2025.v15.i3.32 Vancouver/ICMJE Style Shah AH, Saeed A, Khan S, Shah AH, Shah JU. Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1407-1423. doi:10.5455/OVJ.2025.v15.i3.32 Harvard Style Shah, A. H., Saeed, . A., Khan, . S., Shah, . A. H. & Shah, . J. U. (2025) Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan. Open Vet. J., 15 (3), 1407-1423. doi:10.5455/OVJ.2025.v15.i3.32 Turabian Style Shah, Ayesha Haleem, Asma Saeed, Sanaullah Khan, Abdul Haleem Shah, and Jawad Ullah Shah. 2025. Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan. Open Veterinary Journal, 15 (3), 1407-1423. doi:10.5455/OVJ.2025.v15.i3.32 Chicago Style Shah, Ayesha Haleem, Asma Saeed, Sanaullah Khan, Abdul Haleem Shah, and Jawad Ullah Shah. "Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan." Open Veterinary Journal 15 (2025), 1407-1423. doi:10.5455/OVJ.2025.v15.i3.32 MLA (The Modern Language Association) Style Shah, Ayesha Haleem, Asma Saeed, Sanaullah Khan, Abdul Haleem Shah, and Jawad Ullah Shah. "Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan." Open Veterinary Journal 15.3 (2025), 1407-1423. Print. doi:10.5455/OVJ.2025.v15.i3.32 APA (American Psychological Association) Style Shah, A. H., Saeed, . A., Khan, . S., Shah, . A. H. & Shah, . J. U. (2025) Morphological identification, prevalence, and infestation rate of Varroa destructor in Apis dorsata in Khyber Pakhtunkhwa, Pakistan. Open Veterinary Journal, 15 (3), 1407-1423. doi:10.5455/OVJ.2025.v15.i3.32 |