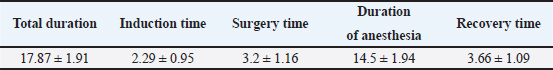| Research Article | ||
Open Vet. J.. 2025; 15(3): 1370-1378 Open Veterinary Journal, (2025), Vol. 15(3): 1370-1378 Research Article Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical proceduresLuca Pennasilico, Federica Serino, Margherita Galosi, Angela Palumbo Piccionello, Alessio Angorini, Fabrizio Dini, Caterina Di Bella*School of Bioscience and Veterinary Medicine, University of Camerino, Camerino, Italy *Corresponding Author: Caterina Di Bella. School of Bioscience and Veterinary Medicine, University of Camerino, Camerino, Italy. Email: caterina.dibella [at] unicam.it Submitted: 23/12/2024 Accepted: 10/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
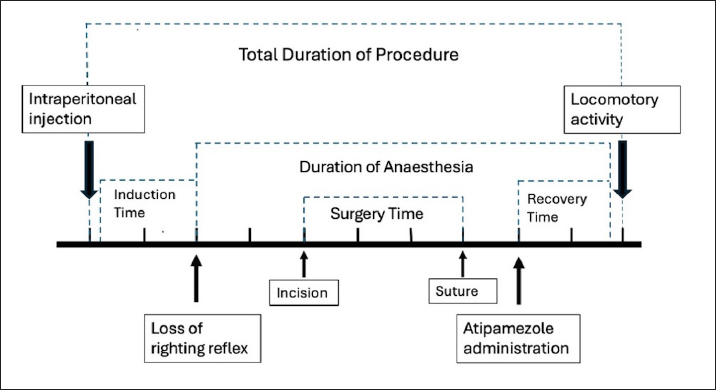
Abstract Background: Rodents are commonly used as models in experimental procedures, and researchers often need to perform rapid manipulations involving sedation and analgesia. Aim: The aim of this study was to evaluate the validity of the combination of xylazine and ketamine in association with buprenorphine in experimental rats undergoing short-term surgical procedures. Methods: Twenty-six male rats were enrolled in experiments. Thirty minutes before the start of the procedure, buprenorphine (0.05 mg/Kg) was administered subcutaneously. The sedative protocol included intraperitoneal (IP) administration of 70 mg/Kg ketamine and 10 mg/Kg xylazine. Additionally, at the end of the procedure, all rats received 0.1 mg/Kg of atipamezole IP. Immediately before sedation and at 5, 10, 15, and 20 minutes after atipamezole administration, the main cardiorespiratory parameters were recorded. In addition, induction time, depth of anesthesia, duration of the procedure, recovery time, and pain score were recorded. Results: The mean induction time was 2.29 ± 0.95 minutes. At the time of surgery, all subjects showed a deep anesthetic plane (score ≥ 3), and no response to skin incision was observed (score=0). The time to recovery from the righting reflex after atipamezole administration was 3.66 ± 1.09 minutes. No rats showed signs of pain based on the rat Grimace scale. Conclusion: Our results suggested that the association of opioids with the xylazine/ketamine protocol ensures rapid induction and good analgesia during short procedures with mild/moderate painful stimulation. Furthermore, the administration of atipamezole facilitates rapid recovery and resumption of motor activity. Keywords: Rats, Xylazine/ketamine, Buprenorphine, Short-term procedures, Atipamezole. IntroductionRats are commonly used in scientific research, and experimental procedures often involve surgery in which anesthesia and analgesia are required and recommended (Cicero et al., 2018). In recent years, various anesthetic protocols have been described in experimental rodents, and the choice of a specific protocol is influenced by several aspects, such as age, health status, type and duration of the procedure, and the desired recovery time (Dittman et al., 2004; Stokes et al., 2009; Salice et al., 2013). In addition, it is necessary to consider some peculiar aspects of rodents, including their small size, accelerated metabolism, and easy development of hypothermia, which make anesthesiologic management complex and worthy of attention (Gargiulo et al., 2012; Albrecht et al., 2014A; Alemán-Laporte et al., 2020). Short-term manipulations and procedures on large numbers of rats are performed daily, and the application of anesthetic protocols that include minimal stress, rapid sedation, and awakening with immediate recovery of major organ functions should be the gold standard Alemán-Laporte et al., 2020. Furthermore, the addition of analgesic drugs reduces pain during procedures, representing a key point of the “3R” principle (Roughan et al., 2000; Roughan et al., 2001; Roughan et al., 2003). The main methods of induction and maintenance of anesthesia in rats include the use of halogenated gases and/or injectable anesthetics. The administration of halogens involves the use of an induction chamber or face mask. Typically, isoflurane and sevoflurane are used, although isoflurane, despite its pungent smell, is cheaper and ensures faster induction (Buitrago et al., 2008; Cicero et al., 2018). The advantages of this approach include: 1) greater operator control over the depth of anesthesia; 2) rapid absorption and elimination; 3) direct action on GABA (gamma-aminobutyric acid) and glycine receptors, resulting in immobility, hypnosis, and muscle relaxation (Rislin et al., 2012; Oh and Narver, 2024). On the other hand, there are several disadvantages to consider, such as respiratory and blood pressure depression, the requirement of heavy and expensive equipment (e.g., properly calibrated flowmeters and vaporizers), potential environmental pollution, and the high risk of anesthetic gas inhalation by the operator (Oh and Narver, 2024). Furthermore, considering the small size of the rats, the equipment can be uncomfortable during surgical procedures, especially when performing procedures involving the head and neck (e.g., tumor or neuroscience studies) (Buitrago et al., 2008). Injectable anesthetics are particularly useful when short surgical procedures need to be performed on a large group of animals (Oh et al., 2024). For these reasons, over the years, numerous anesthesiologic protocols have been studied for use in experimental rodents. Among these, the most frequently used drugs are: α2-agonists, benzodiazepines, ketamine, and opioids (Buitrago et al., 2008; Kawai et al., 2011; Tsukamoto et al., 2018; Oh and Narver, 2024). Ketamine is a noncompetitive NMDA receptor antagonist that is widely used to induce anesthesia and analgesia. It ensures the maintenance of good vascular tone and heart rate (Annetta et al., 2005). However, ketamine can have adverse effects, including agitation upon awakening and dysphoric events (Zhou et al., 2021). For these reasons, it is commonly associated with an α2-agonist such as xylazine (XK) (Albrecht et al., 2014A; Cicero et al., 2018; Hohlbaum et al., 2018). Among the various combinations proposed in the literature, it has been shown that the addition of acepromazine to a protocol with XK causes hypotension and prolongs the duration of anesthesia (up to approximately 70 minutes) (Alemán-Laporte et al., 2020). Alternatively, the use of midazolam, medetomidine, and fentanyl seems to ensure good analgesia and an anesthesiologic plan, but causes greater hypertension and bradycardia compared with XK, leading to a greater risk of intraoperative bleeding (Albrecht et al., 2014A; Fleischmann et al., 2016). Albrecht et al. (2014AB) reported that although the XK protocol ensures greater hemodynamic stability, long recovery times require prolonged monitoring. Contextually, the authors suggested that the addition of an antagonist could accelerate recovery times (Albrecht et al., 2014A). Regarding the analgesic effect, during surgical procedures, several studies suggested the addition of an opioid. Among the opioids described, buprenorphine is the most commonly used in experimental rats, considering its good analgesic effect, long duration of action, and few respiratory side effects (Goldkuhl et al., 2010; Guarnieri et al., 2012; Houston et al., 2021). Based on the information gathered from the extensive literature presented in previous years, our considerations are as follows: 1) the XK protocol can be used for short-term procedures, but a quicker recovery and better quality of awakening is obtained if the α2-agonist is antagonized; 2) the addition of an opioid such as buprenorphine during painful procedures ensures good intra- and postoperative analgesia. Considering this, the aim of this study was to set up and evaluate a valid anesthesiologic protocol to be applied in experimental rats subjected to short-term surgical procedures, which guarantees rapid sedation, quick recovery, and good analgesia throughout the perioperative period. To this end, the subjects included in the study were monitored during the pre-, intra-, and post-operative periods, considering physiological parameters, anesthesia, and surgery times, and a post-operative pain assessment score (Rat Grimace scale) (Sotocina et al., 2011; Leung et al., 2016). Materials and MethodsAnimal housingTwenty-six male Wistar rats weighing 300–400 g and aged 4–5 months were enrolled to perform a translational experimental project. The subjects were obtained from Charles River Laboratories Italia S.r.l. (Calco, Italy) and acclimated to the University of Camerino animal facility for 7 days before carrying out the experimental procedures. Rats were divided into groups of two to ensure physiological socialization of the species and placed in Plexiglas cages at room temperature (20°C–22°C, 45%–55% humidity) and a 12-hour light-dark cycle. All subjects were fed specific food pellets (4RF Mucedola, Settimo Milanese, Italy) and were provided water ad libitum. Subjects with respiratory symptoms, loss of appetite, weight loss, and skin or hair changes were excluded from the study a priori. Anesthetic protocolAll rats received the same anesthetic protocol, and they were adequately weighed to ensure proper drug administration. Thirty minutes before the start of the procedures, buprenorphine (0.05 mg/Kg; Buprefelican 0.3 mg/ml, Dechra S.r.l, Italy) was administered subcutaneously (Oh and Narver, 2024). Considering the small size of the subjects and to ensure greater precision of the injected volume, 0.1 ml of buprenorphine was diluted with 0.9 ml of NaCl 0.9% (Sodium Chloride 0.9%, BBraun, Italy), resulting in a drug concentration of 0.03 mg/ml in a total volume of 1 ml (1:10 ratio). The sedative protocol included intraperitoneal (IP) administration of 70 mg/Kg of ketamine (Lobotor 100 mg/ml, Acme S.r.l., Italy) and 10 mg/Kg of xylazine (Nerfasin 20 mg/ml, P.H. Farmaceutici S.r.l., Italy) (Oh and Narver, 2024). These two drugs were mixed in a sterile 1 ml syringe with a 25-gauge hypodermic needle and injected into the right caudal quadrant of the ventral abdomen to avoid iatrogenic damage to the cecum. At the end of the surgery (after the last suture was applied), all rats received intraperitoneal administration of 0.1 mg/Kg atipamezole (Antisedan 5 mg/ml, Zoetis S.r.l., Roma, Italy) and were placed on a heating pad (Oh and Narver, 2024). To obtain a volume of atipamezole suitable for administration, 0.1 ml of the drug was diluted with 9.9 ml of NaCl 0.9% (Sodium Chloride 0.9%, BBraun, Italy), resulting in a final concentration of 0.05 mg/ml (1:100 ratio). Then, rats were handled gently with a towel to minimize the containment stress and were placed in a single cage until the loss of the right reflex. Furthermore, during the sedation and recovery phases, supplemental pure oxygen was administered via a face mask. Pre- and intraoperative assessmentsImmediately before IP injection (Tpre), body temperature (Tbody; C°), heart rate (HR; beats/minutes), and respiratory rate (RR; breaths/minutes) were recorded. Tbody was assessed using a rectal temperature probe. HR was calculated using a stethoscope by quadrupling the number of contractions detected in 15 seconds. Similarly, the RR was calculated by quadrupling the number of thoracic excursions observed in 15 seconds. Following the administration of sedative drugs, a 5-point score (0–4) was used to assess the depth of anesthesia based on reflex responses. Specifically, the thoracic and pelvic limb withdrawal, eyelid, and tail jerk reflexes were studied (0=presence of all 4 reflexes; 1=loss 1 of 4 reflexes; 2=loss 2 of 4 reflexes; 3=loss 3 of 4 reflexes; 4=loss of all reflexes) (Tsukamoto et al., 2018). The withdrawal reflex was elicited by applying pressure with forceps to the forelimb and hindlimb, the eyelid reflex by touching the medial canthus of the eye with the finger, and the tail reflex by pinching the tail with the same forceps. Nociceptive stimuli were applied by the same operator using atraumatic forceps to reduce pressure variability. A score ≥ 3 indicated a sufficient depth of anesthetic to begin the surgical procedure, whereas a score < 3 indicated an excessively low level of anesthetic. Monitoring was performed starting 60 seconds after IP puncture and then every minute until a score of 3 or higher was achieved. Subsequently, the duration of surgery (from the surgical incision to the placement of the last suture) was recorded (Surgery Time). The reaction to surgical stimulation was assessed according to the reflex in response to the skin incision (0=no response; 1=mild response; 2=massive response) (Tsukamoto et al., 2018). Pure oxygen was administered via a face mask during the entire procedure, and partial oxygen saturation (SpO2; %) was continuously monitored by placing a pulse oximetry probe (VE-H100B Edan, Alcyon Italy S.p.a, Cherasco, Italy) on the toes of the hind limb. Surgery procedureThe primary objective of the present study was to evaluate the biocompatibility of the human acellular dermal matrix in an animal model. Hence, rats were positioned in the sternal recumbency region, and the hair of the infrascapular region was clipped. The skin of the surgical site was disinfected with 10% povidone-iodine 10% and ethyl alcohol 95%. Aseptically, an infrascapular skin incision (about 2 cm) was made, and the biomaterial was implanted in the hypodermal layer. Subsequently, the skin was sutured using USP 4/0 polydioxanone thread (Ethicon, Somerville, NJ). Postoperative assessmentsAt the end of the surgery, atipamezole was administered as previously described, and the main physiological parameters (Spo2, HR, RR, and Tbody) were concurrently recorded. The RR and Tbody were also monitored every 5 minutes after atipamezole administration for 20 minutes (T5, T10, T15, and T20, respectively). The quality and time of recovery were assessed using a five-point Likert-type scale. The score includes the assessment of the ability to maintain the quadrupedal station, the presence of ataxia (identified as the tendency to fall to the right or left during walking), and the presence/absence of the righting reflex, palpebral reflex, and limb withdrawal reflex (Hohlbaum et al., 2018). The latter was assessed by applying pressure with a forceps to the extremity of the right hind limb. Specifically, a score ≤ 1 suggested a good quality of recovery characterized by the return of locomotory activity in the absence of ataxia and falls (Table 1) (Lee et al., 1998). The duration of anesthesia was defined as the time between loss of righting reflex and return of locomotor activity (recovery score < 2), while recovery time was defined as the period from subcutaneous injection of atipamezole (T0) to return of locomotor activity. In addition, the total duration of the procedure (time between IP injection and return of locomotor activity) was recorded. All study times are shown in Figure 1. Postoperative pain was assessed using the Rat Grimace Scale 30 minutes after atipamezole injection (TGrim0) and 3 (TGrim1) and 6 hours (TGrim2) after TGrim0 (Sotocina et al., 2011). In cases with a score indicative of pain (score > 1), rescue analgesia (50 µg/Kg of buprenorphine SC) was administered. Table 1. Five-point Likert-type scale used to evaluate the quality of recovery after surgery.
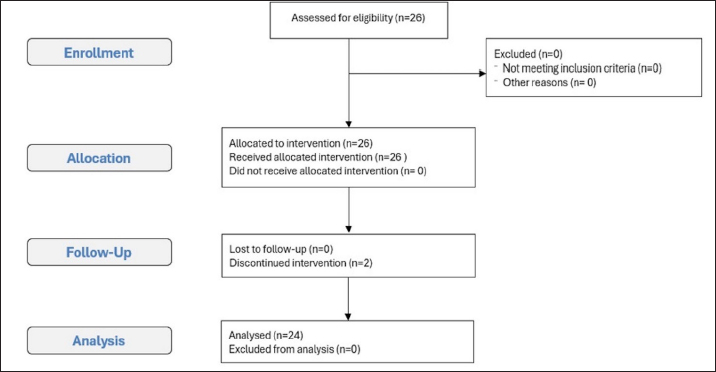
Statistical analysisStatistical analysis was performed using MedCalc software 9.0 (MedCalc version 9.2.10). All data resulted normally distributed based on the Shapiro–Wilk test. Physiological parameters were evaluated using the one-way ANOVA test to perform a comparison between times. All results are reported as mean ± standard deviation. Differences with a p value < 0.05 were considered statistically significant. Power calculations and the primary study aimThe aim of the primary study was to evaluate the inflammatory response, immunological properties, and integrative capacity of decellularized membranes obtained through tissue engineering from human donor tissue. The sample size calculation was performed using Prudente et al. 2016. To obtain the correct sample unit, G*Power Version 3.0.10 software was used at the University of Düsseldorf, Germany (Faul et al., 2009). The analysis was performed using two different parameters to obtain greater reliability in the sample selection process. From the evaluation of interleukin 1, the sample power calculation indicated a “power” of 0.95, an “effect size” of 1.85, and an alpha error of 0.05. The extrapolated results from the analysis suggest that a minimum number of 8 rats per study group is sufficient to obtain statistical significance. From the evaluation of tumor necrosis factor alpha, the calculation of the sampling power indicated a “power” of 0.95, an “effect size” of 1.36, and an alpha error of 0.05. The extrapolated results from the analysis suggest that a minimum of 13 rats per study group are sufficient to obtain sampling significance. The two parameters were compared 4 and 30 days after tissue implantation, as described in the literature. The one-tailed t-test was used. An a priori analysis was used to evaluate the differences between the two independent means. It was, therefore, decided to use 2 groups of 13 animals (a total of 26) because the number of the two groups would have enough power to guarantee a sample significance for the different inflammatory factors to be analyzed. Ethical approvalThis study was approved by the Italian Ministry of Health (protocol number 424/2021-PR) in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. ResultsThe mean weight of the animals on the day of surgery was 349.69 ± 93.46 g. Two rats were excluded due to incorrect intraperitoneal administration of the anesthetic drugs. The remaining 24 subjects survived the surgical procedures and completed the study. This study conforms to the Consolidated Standards of Reporting Trials (CONSORT) Statement 2010 for reporting randomized trials (Moher et al., 2010) (Fig. 2). Reflexes and times recordedThe mean total procedure duration was 17.87 ± 1.91 minutes, while the surgery time was 3.2 ± 1.16 minutes. The mean time from intraperitoneal injection to loss of righting reflex (Induction Time) was 2.29 ± 0.95 minutes. At the time of surgery, all subjects showed a deep anesthetic plane (score ≥ 3), and no response to skin incision was observed (score=0). The mean time from loss of righting reflex to return of locomotor activity (Duration of Anesthesia) was 14.5 ± 1.94 minutes. The period from atipamezole injection to return of locomotor activity (Recovery Time) was 3.66 ± 1.09 minutes. At T0, all rats showed an absence of spontaneous activity and loss of righting reflex (Recovery Score ≥ 2), whereas at T5, they returned to spontaneous activity (Recovery Score < 2) (Table 2).
Fig. 1. Schematic representation of the times recorded during the experimental study.
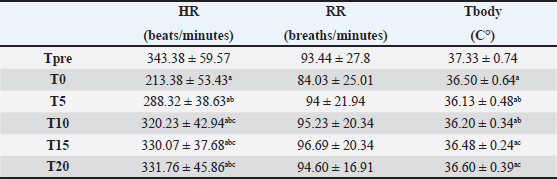
Fig. 2. Consolidated standards of reporting trials flow diagram of the rats included in the experimental study. Physiological parametersHR decreased significantly at T0 (p < 0.05) compared with baseline (Tpre). After 5 minutes from atipamezole administration (T5), the heart rate increased significantly compared with T0, reaching values comparable to Tpre and remaining stable at subsequent times T10, T15, and T20. Similarly, RR was lower at T0 than at Tpre; however, no statistical differences were observed between the various study times (p > 0.05). Body temperature decreased significantly at all study times compared with Tpre (p < 0.05). Subsequently, at T15 and T20, it increased significantly compared with T5, but without reaching baseline values. Regarding SpO2 values, the mean was 99% ± 0.32 % and 98% ± 0.45 % at Tsurg and T0, respectively. All of the above parameters are reported as mean ± standard deviation in Table 3. No subject received rescue analgesia, and the Rat Grimace Scale showed score=0 at TGrim0, TGrim1, and TGrim2. DiscussionIn this study, the application of the anesthesiologic protocol consisting of xylazine/ketamine/buprenorphine in short-term surgical procedures in experimental rats was described. From the data obtained, we can state that the above-mentioned protocol guarantees the following: 1) rapid sedation after intraperitoneal inoculation; 2) suitable anesthetic depth during short-term procedures that involve mild/moderate nociceptive stimulation; 3) rapid awakening, thanks to the antagonization of the α2-agonist by administration of atipamezole. Regarding the induction phase, all subjects achieved a sedation score ≥ to 3 within 3 minutes of drug administration. The X/K association has been described in several studies; however, there are conflicting opinions regarding its efficacy (Stokes et al., 2009). Albrecht et al. (2024AB) highlighted an induction time of about 4 minutes; however, tolerance to surgery was evidenced only after 10 minutes from drug administration (Albrecht et al., 2014A). It is the opinion of the authors that the addition of buprenorphine in our protocol has induced a synergism of potentiation with the other drugs favoring a deeper anesthetic level in faster times and good analgesia during nociceptive stimulations (Curtin et al., 2009). The absence of an analgesic in an anesthetic protocol could, therefore, lead to a greater response to the induced painful stimulations (Hedenqvist et al., 2000; Oh and Narver 2024). It should be noted, however, that the nature of buprenorphine (partial µ agonist) makes it an excellent analgesic for the management of mild/moderate pain but may not be sufficient for the management of severe nociceptive stimuli, in which the administration of pure µ agonists is more recommended (e.g., fentanyl and methadone) (Arenillas et Gomez de Segura, 2018; Alemán-Laporte et al., 2020). Table 2. Mean ± SD of different times (minutes) registered during the experimental procedure.
Table 3. Mean ± SD of physiological parameters. ap < 0.05 differences compared with Tpre. bp < 0.05 differences compared with T0. cp < 0.05 differences compared with T5.
Another aspect that we highlight is that the respiratory rate did not show statistically significant differences in the study phases. Hedenquivist et al (2000) reported two deaths following the administration of buprenorphine in experimental rats. However, they did not show significant differences in the respiratory rate, but they were associated with mortality in severe hypoxic states, recommending always administering supplemental oxygen after opioid administration (Hedenquivist et al., 2000). In this study, we did not highlight similar problems; however, the subjects were oxygenated throughout the procedure, and SpO2 was monitored. The authors, therefore, recommend that supplemental oxygen be always provided to patients receiving anesthetic drugs, especially if the protocol includes opioids. The HR decreased significantly at T0 and then gradually increased until it returned to baseline. The reasons for this decrease are probably: 1) the bradycardic effect of the α2-agonist (the baroreceptor reflex triggered by the marked elevation of blood pressure) and 2) the achievement of an adequately deep anesthetic plane (Sano et al., 2013). Unfortunately, due to the very short surgical times and the need to respect the times required by the primary experimental study, we could not adequately monitor arterial blood pressure (ABP). This is a limitation of our study. Albrecht et al. (2024AB) did not find alterations in HR using the XK protocol; however, they used lower (half) xylazine doses than us, which could have influenced the result (Albrecht et al., 2014B). On the other hand, in confirmation of this, other authors, using our xylazine dosage (10 mg/Kg) in experimental rats, showed significant bradycardia (Picollo et al., 2012; Sano et al., 2013; Navarro et al., 2021). Another consideration is that the addition of opioids may have further favored the decline in heart rate (Mahinda et al., 2004). Body temperature significantly decreased after the administration of the anesthetic protocol. Maintaining a good body temperature is extremely important in these animals, and unfortunately, many anesthetics cause hypothermia by inhibiting central and peripheral thermoregulatory mechanisms; consequently, loss of thermal homeostasis could directly contribute to an increase in mortality in laboratory rodents (Wixson et al., 1987). The use of the X/K protocol may directly influence the decrease in body temperature for the aforementioned reasons (Redfors et al., 2014). The authors did not highlight temperatures below 36°C in the present study; however, it should be considered that the total duration of the procedure was very short. Based on these evaluations, it is our opinion that during short procedures, the X/K protocol does not have a high impact on body temperature. However, longer procedures could involve greater risks, and it is, therefore, recommended to always use thermal supports during experimental procedures. All subjects regained walking ability approximately 4 minutes after the administration of atipamezole. Rapid recovery from general anesthesia ensures less stress and rapid resumption of feeding and, in general, of major organic functions. In addition, it guarantees faster work in less time. The use of atipamezole in rats is still debated because it has been shown that, due to its cytochrome P450 metabolite, its pharmacokinetics are nonlinear, unlike that in humans and other species. On the other hand, clinical and experimental studies have defined the effective dose range in rats of 0.1–1 mg/Kg administered SC or IP (Navarro et al., 2021; Bennett et Lewis, 2022; Oh and Narver, 2024). Furthermore, several studies have shown that during the recovery phase from general anesthesia, atipamezole antagonizes the action of xylazine better than yohimbine, inducing a rapid return of the righting reflex and a normal heart rate (Janssen et al., 2017). These aspects are extremely important in experimental rats because they reduce the risk of peri-anesthetic mortality (Brodbelt et al., 2008). Furthermore, another advantage to consider is that the administration of atipamezole does not alter the analgesia induced by opioids (e.g., buprenorphine) (Izer et al., 2014). For these reasons, unless there are reasons that induce the operator to maintain a longer recovery phase, the authors recommend antagonizing α2-agonists at the end of the anesthetic procedures in experimental rodents using previously described doses of atipamezole. As previously mentioned, this study has some limitations. ABP was not measured during the perioperative period. It would have been interesting to evaluate the arterial pressure changes induced by the anesthetic protocol used and to relate them to the HRs detected at the different study times. Another aspect to consider is that HR and RR were calculated by an operator through cardiac auscultation and observation of thoracic excursions, respectively. This could lead to operator-dependent alterations with consequent underestimation of the recorded values (considering for example the high physiological HR of rats). The use of instrumental methods (such as electrocardiogram) would have made the recorded data more reliable. Another limitation to consider is the absence of a control group to include in our study design. Unfortunately, having to comply with the protocols defined in the ministerial authorization, we could not use other drugs; however, the study would have been more complete if our protocol had been compared with a control group or with different protocols. ConclusionThe X/K anesthesia protocol with the addition of buprenorphine appears to be optimal for the execution of short and rapid procedures in experimental rats. Moreover, the addition of opioids guarantees good analgesia during mild/moderate painful stimulations. Furthermore, the use of atipamezole as an antagonist of the α2-agonist at the end of the procedure guarantees rapid awakening and rapid resumption of motor activity. Conflicts of interestThe authors declare no conflicts of interest. Author contributionsLuca Pennasilico: study execution, data analysis, and writing of the first draft of the manuscript. Federica Serino and Margherita Galosi: study execution, data collection. Angela Palumbo Piccionello, Alessio Angorini, and Fabrizio Dini: analysis and interpretation of the data, reviewing, and editing. Caterina Di Bella: study design, data interpretation, review, and editing. All authors have read and approved the final version of the manuscript. FundingThe author(s) received no financial support for this research, authorship, or publication of this article. Data availability statementThe corresponding author (email: caterina.dibella [at] unicam.it) is available to be contacted to request additional material and raw data relating to the presented study. ReferencesAlbrecht, M., Henke, J., Tacke , S. Markert, M. and Guth, B. 2014A. Effects of isoflurane, ketamine-xylazine, and a combination of medetomidine, midazolam, and fentanyl on physiological variables continuously measured by telemetry in Wistar rats. BMC Vet. Res. 10, 1–14 (A). Albrecht, M., Henke, J., Tacke, S., Markert, M. and Guth, B. 2014B. Influence of repeated anesthesia on physiological parameters in male Wistar rats: a telemetric study about isoflurane, ketamine-xylazine and a combination of medetomidine, midazolam and fentanyl. BMC Vet. Res. 10, 1-–15 (B). Alemán-Laporte, J., Bandini, L.A., Garcia-Gomes, M.S., Zanatto, D.A., Fantoni, D.T., Amador Pereira, M.A., Navas-Suàrez, P.E., Kirsten, T.B., Jimenez, R.R., Alvarado, G. and Mori, C.C. 2020. Combination of ketamine and xylazine with opioids and acepromazine in rats: physiological changes and their analgesic effect analyzed by ultrasonic vocalization. Lab. Anim. 54, 171–182. Annetta, M.G., Iemma, D., Garisto, C., Tafani, C. and Proietti, R. 2005. Ketamine: new indications for an old drug. Curr. Drug. Targets. 6, 789–794. Arenillas, M. and Gomez De Segura IA. 2018. Anesthetic effects of alfaxalone administered intraperitoneally alone or combined with dexmedetomidine and fentanyl in the rat. Lab. Anim. 52, 588–598. Bennett, K. and Lewis, K. 2022. Sedation and anesthesia in rodents. Vet. Clin. North Am. Exot. Anim. Pract. 25, 211–255. Brodbelt, D.C., Blissitt, K.J., Hammond, R.A., Neath, P.J., Young, L.E., Pfeiffer, D.U. and Wood, J.L.N. 2008. Risk of death: Confidential enquiry into perioperative small animal fatalities. Anaesth. Analg. 35, 365–373. Buitrago, S., Martin, T.E., Tetens-Woodring, J., Belicha-Villanueva, A. and Wilding, G.E. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J. Am. Assoc. Lab. Anim. Sci. 47, 11–17. Cicero, L., Fazzotta, S., Palumbo, V.D., Cassata, G. and Lo Monte, I.A., 2018. Anesthesia protocols in laboratory animals used for scientific purposes. Acta Biomed. Atenei Parmensis. 89, 337. Curtin, L.I., Grakowsky, J.A., Suarez, M., Thompson, A.C., Di Pirro, J.M., Martin, L.B.E. and Kristal, M.B. 2009. Evaluation of buprenorphine in a postoperative pain model in rats. Comp. Med. 59, 60–71. Dittmar, M.M.S., Fehm, N.N.P., Vatankhah, B. and Horn, M. 2004. Ketamine/xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp. Med. 54, 652–655. Fleischmann, T., Jirkof, P., Henke, J., Arras, M. and Cesarovic, N. 2016. Injection anesthesia with fentanyl–midazolam–medetomidine in adult female mice: importance of antagonization and perioperative care. Lab. Anim. 50, 264–274. Gargiulo, South, Greco, A., Gramanzini, M., Esposito, South, Affuso, A., Brunetti, A. and Vesce, G. 2012. Mice anesthesia, analgesia and care, part I: anesthetic considerations in preclinical research. Ilar. J. 53, 55–69. Goldkuhl, R., Jacobsen, K.R., Kalliokoski, O., Hau, J. and Abelson, K.S.P. 2010. Plasma concentrations of corticosterone and buprenorphine in rats subjected to jugular vein catheterization. Lab. Anim. 44, 337–343. Guarnieri, M., Brayton, C., DeTolla, L., Forbes-McBean, N., Sarabia-Estrada, R. and Zadnik, P., 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab. Anim. 41, 337–343. Hedenqvist, P., Roughan, J.V. and Flecknell, P.A. 2000. Effects of repeated anesthesia with ketamine/medetomidine and of pre-anesthetic administration of buprenorphine in rats. Lab Anim. 34, 207–211. Hohlbaum, K., Bert, B., Dietze, S., Palme, R., Fink, H. and Thone-Reineke, C. 2018. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS oOe. 13.9, e0203559. Houston, E.R., Tan, S.M., Thomas, S.M., Stasula, U.L., Burton, M.K., Knych, H.K. and Kendall, L.V. 2021. Pharmacokinetics and efficacy of a long-lasting, highly concentrated buprenorphine solution in rats. J. Am. Assoc. Lab. Anim. Sci. 60, 667–674. Izer, J.M., Whitcomb, T.L. and Wilson, R.P. 2014. Atipamezole reverses ketamine–dexmedetomidine anesthesia without altering the antinociceptive effects of butorphanol and buprenorphine in female C57BL/6J mice. J. Am. Assoc. Lab. Anim. Sci. 53, 675–683. Janssen, C.F., Maiello, P., Wright Jr, M.J., Kracinovsky, K.B. and Newsome, J.T. 2017. Comparison of atipamezole with yohimbine for antagonism of xylazine in mice anesthetized with ketamine and xylazine. J Am. Assoc. Lab. Anim. Sci. 56, 142–147. Kawai, S., Takagi, Y., Kaneko, South and Kurosawa, T. 2011. Effects of three types of mixed anesthetic agents alternated with ketamine in mice. Exp. Anim. 60, 481– 487. Lee, V.C., Moscicki, J.C. and Di Fazio, C.A. 1998. Propofol sedation produces dose-dependent suppression of lidocaine-induced seizures in rats. Anesth. Analg. 86, 652–657. Leung, V., Zhang, E. and Pang, D. 2016. Real-time application of the Rat Grimace Scale as a welfare refinement in laboratory rats. Sci. Rep. 6, 31667. Mahinda, TT.B. Lovell, B.M. and Taylor, B.K. 2004. Morphine-induced analgesia, hypotension, and bradycardia are enhanced in hypertensive rats. Anesth. Analg. 98, 1698–1704. Moher, D., Hopewell, S., Schulz, K., Montori, V., Gotzsche, P.C., Devereaux, P.J., Elbourne, D., Egger, M. and Altman, D.G. 2010. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. BMJ. 23, 340. Navarro, K.L., Huss, M., Smith, J.C., Sharp, P., Marx, J.O. and Pacharinsak, C. 2021. Mouse anesthesia: the art and science. ILAR J. 62, 238–273. Oh, S.S. and Narver, H.L. 2024. Mouse and rat anesthesia and analgesia. Curr. Protoc. 4, e995. Picollo, C., Serra, A.J., Levy, R.F., dos Santos, A.L. and Tucci, P.J.F. 2012. Hemodynamic and thermoregulatory effects of xylazine-ketamine mixture persist even after the anesthetic stage in rats. Arq. Bras. Med. Vet. Zootec. 64, 860–864. Prudente, A., Fávaro, W.J., Filho, P.L. and Zanettini, Riccetto C.L. 2016. Host inflammatory response to polypropylene implants: quantitative immunohistochemical and birefringence insights analysis in a rat subcutaneous model. Int. Braz. J. Urol. 42, 585–593. Redfors, B., Shao, Y. and Omerovic, E. 2014. Influence of anesthetic agent, depth of anesthesia, and body temperature on cardiovascular functional parameters in the rat. Lab. Anim. 48, 6–14. Risling, TT.E., Caulkett, N.A. and Florence, D. 2012. Open-drop anesthesia for small laboratory animals. Can. Vet. J. 53, 299. Roughan, J.V., Flecknell. P.A. 2001. Behavioral effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain. 90, 65–74. Roughan, J.V. and Flecknell, P.A. 2000. Effects of surgery and analgesic administration on spontaneous behavior in singly housed rats. Res. Vet. Sci. 69, 283–288. Roughan, J.V. and Flecknell, P.A. 2003. Evaluation of a short duration behavior-based postoperative pain scoring system in rats. Eur. J. Pain. 7, 397–406. Salice, V.S., Valenza, F.V., Pizzocri, M.P., Valenti, L.V., Chevallard, G.C., Umbrello, M.U., Gatti, S.G., Fargion, S.F., Iapichno, G.I. and Gattinoni, L.G. 2013. Benzodiazepines induce hyperglycemia in rats by affecting peripheral disposal of glucose. Crit. Care. 17, 1–200. Sano Y., Ito, S., Yoneda, M., Nagasawa, K., Matsuura, N., Yamada, Y., Uchinaka, A., Bando, Y.K., Murohara, T. and Nagata, K. 2016. Effects of various types of anesthesia on hemodynamics, cardiac function, and glucose and lipid metabolism in rats. Am. Jf. Physiol. Heart Circ. Physiol. 311, H1360–H1366. Sotocinal, S.G., Sorge, R.E., Zaloum, A., Tuttle, A.H., Martin, L.J., Wieskopf, J.S., Mapplebeck, J.C.S., Wei, P., Zhan, S., Zhang, S., McDougall, J.J., King, O.D. and Mogil, J.S. 2011 The rat grimace scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 7, 55. Stokes, E.L., Flecknell, P.A. and Richardson, C.A. 2009. Reported analgesic and anesthetic administration to rodents undergoing experimental surgical procedures. Lab. Anim. 43, 149–154. Tsukamoto, A., Niino, O., Sakamoto, M., Ohtani, R. and Inomata, T. 2018. Validity of anesthetic protocols for the surgical procedure of castration in rats. Exp. Anim. 67, 329–336. Wixson, S.K., White, W.J., Hughes Jr, H.C., Lang, C.M. and Marshall, W.K. 1987. A comparison of pentobarbital, fentanyl-droperidol, ketamine-xylazine, and ketamine-diazepam anesthesia in adult male rats. Lab. Anim. Sci. 37, 726–730. Zhou, X., Li, West, Wang, H., Li, C. and Jiang, H. 2021. Safety and efficacy of ketamine-fentanyl-dexmedetomidine-induced anesthesia and analgesia in neonatal and aged rats. Dose-Response. 19(4), 15593258211063987. | ||
| How to Cite this Article |
| Pubmed Style Pennasilico L, Serino F, Galosi M, Piccionello AP, Angorini A, Dini F, Bella CD. Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures. Open Vet. J.. 2025; 15(3): 1370-1378. doi:10.5455/OVJ.2025.v15.i3.28 Web Style Pennasilico L, Serino F, Galosi M, Piccionello AP, Angorini A, Dini F, Bella CD. Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures. https://www.openveterinaryjournal.com/?mno=234285 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.28 AMA (American Medical Association) Style Pennasilico L, Serino F, Galosi M, Piccionello AP, Angorini A, Dini F, Bella CD. Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures. Open Vet. J.. 2025; 15(3): 1370-1378. doi:10.5455/OVJ.2025.v15.i3.28 Vancouver/ICMJE Style Pennasilico L, Serino F, Galosi M, Piccionello AP, Angorini A, Dini F, Bella CD. Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1370-1378. doi:10.5455/OVJ.2025.v15.i3.28 Harvard Style Pennasilico, L., Serino, . F., Galosi, . M., Piccionello, . A. P., Angorini, . A., Dini, . F. & Bella, . C. D. (2025) Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures. Open Vet. J., 15 (3), 1370-1378. doi:10.5455/OVJ.2025.v15.i3.28 Turabian Style Pennasilico, Luca, Federica Serino, Margherita Galosi, Angela Palumbo Piccionello, Alessio Angorini, Fabrizio Dini, and Caterina Di Bella. 2025. Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures. Open Veterinary Journal, 15 (3), 1370-1378. doi:10.5455/OVJ.2025.v15.i3.28 Chicago Style Pennasilico, Luca, Federica Serino, Margherita Galosi, Angela Palumbo Piccionello, Alessio Angorini, Fabrizio Dini, and Caterina Di Bella. "Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures." Open Veterinary Journal 15 (2025), 1370-1378. doi:10.5455/OVJ.2025.v15.i3.28 MLA (The Modern Language Association) Style Pennasilico, Luca, Federica Serino, Margherita Galosi, Angela Palumbo Piccionello, Alessio Angorini, Fabrizio Dini, and Caterina Di Bella. "Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures." Open Veterinary Journal 15.3 (2025), 1370-1378. Print. doi:10.5455/OVJ.2025.v15.i3.28 APA (American Psychological Association) Style Pennasilico, L., Serino, . F., Galosi, . M., Piccionello, . A. P., Angorini, . A., Dini, . F. & Bella, . C. D. (2025) Anesthetic effects of a mixture of xylazine, ketamine, and buprenorphine in laboratory rats subjected to short surgical procedures. Open Veterinary Journal, 15 (3), 1370-1378. doi:10.5455/OVJ.2025.v15.i3.28 |