| Review Article | ||
Open Vet. J.. 2025; 15(3): 1116-1139 Open Veterinary Journal, (2025), Vol. 15(3): 1116-1139 Review Article Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categoriesMohamed Tharwat* and Fahd Al-SobayilDepartment of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia *Corresponding Author: Mohamed Tharwat. Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia. Email: atieh [at] qu.edu.sa Submitted: 20/01/2025 Accepted: 22/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
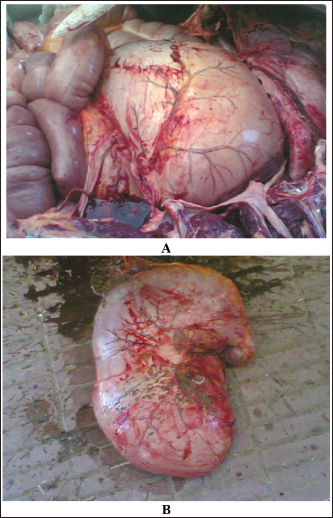
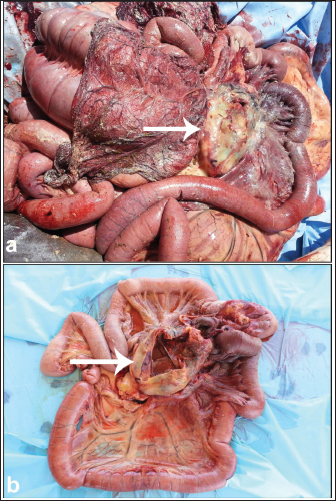
AbstractColic or acute abdominal pain is the most prevalent cause of emergency intervention in veterinary medicine, and it has been considered the principal reason for collapse and/or euthanasia in a wide range of studies. The condition may be initiated by a number of different disorders affecting the abdominal viscera, but acute gut disease is the most common etiology in equines showing colic symptoms. of the main goals of colic management is to distinguish between surgical and medical causes, as prompt surgical intervention can significantly improve outcomes for those requiring surgery. Despite the widespread use of diagnostics such as laboratory analyses and abdominal diagnostic imaging, the most common diagnostic indicators of the necessity for surgical intervention are the presence of either moderate or severe symptoms of pain reaction, pain recurrence after suitable therapy, and diminished intestinal sounds. Abdominal ultrasonography was performed in equines with signs of abdominal pain as a perfect tool for diagnosing small intestinal strangulation. The detection of unprecedented markers, which may help distinguish medical ailments from others that can be treated surgically, persists as an ongoing research area. This review was designed to highlight different categories of colic in equines with reference to sonographic assessment, diagnosis, and management. Abdominal pain can be divided into two major classes: gastrointestinal and non-gastrointestinal. The first class can be reasoned by different etiologies, starting from a harmless spasmodic colic to a life-threatening strangulating type of colic. Here, special emphasis will be given to several causes of gastrointestinal colic, including gastric impaction, gastroenteritis, flatulent colic, spasmodic colic, impaction colic, strangulating and obstructive colic, sand colic, verminous mesenteric arteritis, peritonitis, and hernias. This review will also discuss some important causes of non-gastrointestinal colic, including cystitis, urine retention, abdominal abscesses, and mesenteric abscesses. In conclusion, colic in equines is a fatal condition, and most cases do not recover if diagnosed late. Therefore, ancillary diagnostic tools should be implemented. Of these tools, abdominal ultrasound has been proven to be very effective in verifying equines with different causes of colic, such as flatulent colic, spasmodic colic, obstructive colic, impactive colic, strangulating colic, peritonitis, hernias, cystitis, urine retention, and abdominal abscesses. In addition, our estimation of serum biomarkers revealed potential diagnostic aids for patients with acute abdominal pain. Keywords: Abdominal pain, Colic, Diagnosis, Equines, Ultrasound. IntroductionThe expression “colic” is applied to verify abdominal suffering in horses (Mehdi and Mohammad, 2006). The term acute abdominal pain may also be used to describe colic. The condition can be attributed to a variety of different disorders influencing the abdominal viscera, but intense gastrointestinal affections are the common reasons for equines showing symptoms of colic (Curtis et al., 2015). In veterinary medicine, colic is the leading cause of emergency treatment (Bowden et al., 2017) and is a major risk factor for death and/or euthanasia across various studies (Archer et al., 2008). Research has shown that nearly 20% of colic cases presented to a primary clinic are critical and require intensive medical care or surgical intervention, while up to 16% of animals with colic either die or are euthanized (Curtis et al., 2015). One of the main goals of colic management is to distinguish between medical and surgical causes of abdominal pain, as early surgical intervention enhances outcomes in horses requiring surgery (Fikri et al., 2024). Despite the widespread availability of modern diagnostic tools such as laboratory tests and abdominal ultrasonography, the most reliable indicators for surgery include the onset of moderate to severe abdominal pain, recurrence of pain after appropriate analgesic treatment, and reduced intestinal sounds (Needles and Dubois, 2019). The search for new markers to help differentiate between medical conditions and those requiring surgical intervention remains an ongoing research area (Burke and Blikslager 2018). This review was designed to emphasize different categories of colic in equines with special reference to their sonographic assessment, diagnosis, and management. Initially, the importance of ultrasound and serum biomarkers in verifying colic in horses will be discussed. In the sequelae, special emphasis will be given to several causes of gastrointestinal colic, including gastric impaction, gastroenteritis, flatulent colic, spasmodic colic, impaction colic, strangulating and obstructive colic, sand colic, verminous mesenteric arteritis, peritonitis, and hernias. This review will also discuss some important causes of non-gastrointestinal colic, including cystitis, urine retention, abdominal abscesses, and mesenteric abscesses. Ultrasound and diagnosis of colicIn farm animals, ultrasonography has been reported to be a valuable tool for the early verification, diagnosis, and prognosis of cases with abdominal colic (Tharwat, 2011; Tharwat et al., 2012a,b; Tharwat and EL-Deeb, 2015; Tharwat and Al-Sobayil, 2016; Tharwat, 2019; Tharwat, 2020; Tharwat, 2021a,b; Almundarij and Tharwat, 2023; Tharwat, 2023; Tharwat et al., 2024a). The application of abdominal sonography in equines with symptoms of abdominal pain is a precise method for diagnosing small intestinal strangulation (Cribb and Arroyo, 2018). The procedure can be performed via a transcutaneous approach using a 3.5 MHz sector probe. In patients with large intestinal problems, transrectal sonography may be performed using 5.0–7.5 MHz linear transducers. The procedure revealed the small intestines with an average wall thickness and a diameter of 0.2–1.8 cm and 3.6–13.5 cm, respectively, without a proof of motility. On ultrasonography, equines with peritonitis showed proof of small intestinal motility with increased wall thickness and diameter (Le Jeune and Whitcomb, 2014). If small intestine motility is not detected by ultrasound, the specificity, sensitivity, negative predictive percent, and positive predictive percent for organ strangulation are 100% (Cribb and Arroyo, 2018). Transrectally, recognition of edematous or distended small intestine provides a specificity of 98%, a sensitivity of 50%, a positive predictive percent of 89%, and a negative predictive percent of 89% for small intestine obstructions (Le Jeune and Whitcomb, 2014). In an investigation conducted on 158 horses with acute abdominal pain, non-motile and distended small intestinal loops were linked with obstruction in 45 horses; elevated free abdominal fluid, absolutely distended small intestinal loops with differing motility, and thickened loops were also linked with eventual diagnosis, including small intestine, in 58 horses (Beccati et al., 2011). Biomarkers and diagnosis of colicIn equine medicine, biomarkers are usually used in animals with pathological and physiological conditions (Tharwat and Al-Sobayil, 2014a,b; Tharwat and Al-Sobayil, 2022a,b; Tharwat and Al-Sobayil, 2024b). With the clear difference between healthy horses and those with abdominal pain, serum amyloid A (SAA) and peritoneal fluid haptoglobin (Hp) values represent possible useful diagnostic biomarkers for inflammatory abdominal conditions in animals with acute abdominal pain (Pihl et al., 2013). In the latter report, horses with colic had significantly higher levels of serum SAA ( p < 0.0001) and peritoneal fluid ( p=0.0013). In addition, peritoneal fluid Hp concentrations were significantly higher in horses with colic. In addition, evaluation of SAA improves the capacity to distinguish between equines with inflammatory pain that require medical management and those with demanding surgery, as it permitted an additional 4% of animals to be correctly categorized into surgical and medical cases, thus minimizing the hazard of needless or late surgery (Pihl et al., 2016). In another study, it was found that horses with abdominal pain and eventually elevated SAA levels were more prone to surgical intervention, promoted thrombophlebitis, or euthanized due to poor outcomes despite therapy (Westerman et al., 2016). In the latter study, SAA concentration was significantly higher in the surgical group than in the control and medical groups. Horses with colic and an abnormally increased SAA concentration (> 5 μg/ml) were more likely to be treated surgically and not medically. However, in the same study, the Hp concentration did not differ significantly among the groups (Westerman et al., 2016). The Hp concentration may also be a valuable biomarker in horses presented to clinics a few days after the commencement of colic (Hajimohammadi et al., 2023). The average Hp concentration in the horses with colic was higher than that in the control group (p < 0.001) (Hajimohammadi et al., 2023). Although no single marker has been detected that is totally specific and sensitive for ischemia in the intestines, L-lactate has been found to be a very practical and greatly utilized diagnostic marker (Ludwig et al., 2023). Unfortunately, the preponderance of the available intestinal lesion marker tests are enzyme-linked immunosorbent assays, which must be carried out in laboratories with several hours to get results. In addition, most horse practitioners send samples to these laboratories and wait several days until the results are obtained (Radcliffe et al., 2022). At present, only SAA and L-lactate levels can be measured in situ using stall-side, point-of-care tests (Ludwig et al., 2023). Classification of colicThe coli can be classified into two principal classes: gastrointestinal and non-gastrointestinal. Non-gastrointestinal cases can usually be ruled out based on physical examination results, including symptoms of abdominal discomfort due to urinary calculi and disorders of respiratory, nervous, reproductive, or musculoskeletal systems (Tannahill et al., 2019). The etiologies of gastrointestinal colic include gut distension, ischemia, tension on the mesenteric root, peritoneal pain, and deep ulceration in the stomach or bowel walls (Smith, 2015). The judgment to manage colic either medically or surgically relies on 5 principal points: intensity of pain and whether it responded or not to analgesia, cardiovascular status, findings on per rectum palpation, the finding of nasogastric reflux, and results of peritoneal centesis (Singer and Smith, 2002). Most reasons of gastrointestinal colic can be treated medically and only 4%–10% require surgical intervention (Hillyer et al., 2001; Proudman et al., 2002). Generally, it is not easy to reach an eventual verification without exploration of the abdomen, but the veterinarian should try to supply the owner with all potential consequences and a prognosis (van der Linden et al., 2003). Non-gastrointestinal disorders can also cause recurrent fits of pain, and gut adhesions are popular reasons for colic after surgery of the abdomen (Mair and Sherlock, 2023). Gastrointestinal ColicGastric impactionGastric impaction, characterized by distension due to ingesta accumulation in the stomach, is an uncommon cause of abdominal pain in horses. Recurrent stomach impaction leads to gastric dilatation. In severe cases, the stomach may rupture (Vainio et al., 2011). Focal myositis, gross muscular thickening, and fibrosis of the stomach wall have been recognized in horses with gastric impaction, but the etiology and effect of relationship remain unknown. Some reports have categorized gastric impaction as either primary or secondary in etiology (Bird et al., 2012). Gastric impaction may occur secondary to a gastric polyp or phytobezoar (Kellam et al., 2000; Furness et al., 2013). In a study by Vainio et al. (2011), the common complaint of animals diagnosed with gastric impaction was decreased feed intake (50%) followed by acute pain (35%) and recurrent fits of colic (35%). Postmortem results included intense gastric impaction (Fig. 1) and thickened stomach wall muscle layers in addition to mucosal ulceration and gastric dilatation (Bird et al., 2012). In many cases of long-standing gastric impaction, the stomach may rupture (Winfield et al., 2015; Bergstrom et al., 2018). Treatment and outcome are dependent on the type of impaction. In the vast majority of cases, correction of abnormalities, such as dehydration and/or electrolyte imbalances, is indicated, and settlement of the impaction should be established by gastroscopic examination just before re-introducing normal ration (Smith, 2015). GastroenteritisThis problem is complicated by different agents of enteric disorders also being detected in the intestine of clinically healthy horses, which questions the authenticity of the clear detection of these agents in the gut (Uzal and Diab, 2015). Gasterophilus larvae are common parasites of the stomach in equines (Uzal et al., 2015).
Fig. 1. Postmortem findings of a horse with acute abdominal pain. The stomach in situ is severely enlarged and compresses the intestines A. Image B shows the large size of the stomach after removal.
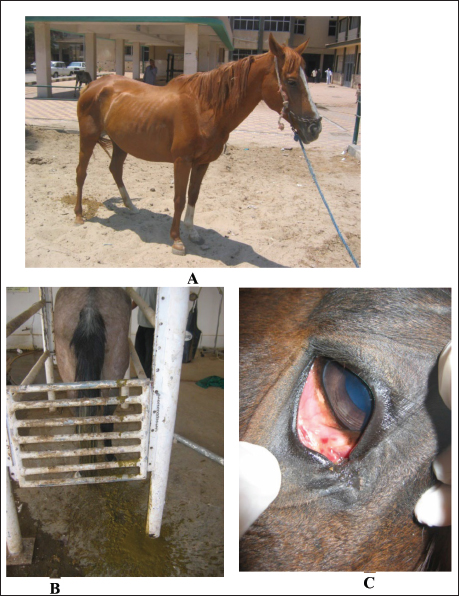
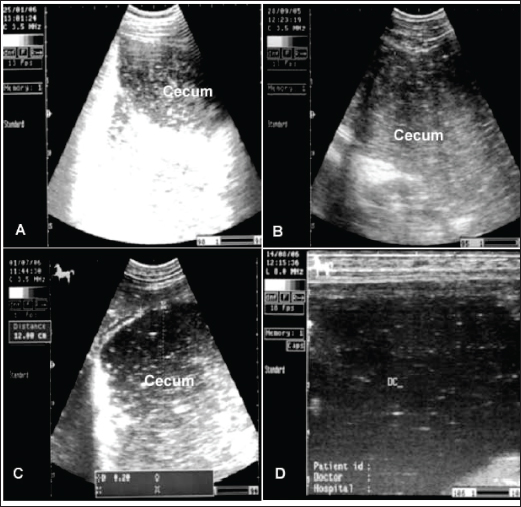
Fig. 2. Enteritis in 2 horses showing watery diarrhea (A and B). Image C shows the sunken eyes of the horse in image B due to long-standing diarrhea. In particular, horses with gastroenteritis often present with great depression and their heads are kept down. Mild pyrexia (38.6°C–39.5°C), marked tachycardia (80 to 100 beats/minute), tachypnea (30–40 beats/minute), dehydration, slow capillary refill time, bluish-purple discoloration of the mucus membranes, and oliguria are common findings (Constable et al., 2017). The diarrhea is watery and profuse, and in long-standing cases, the eyes are sunken (Fig. 2). Animals with mild affection, those that do not show systemic signs, usually recover with only symptomatic therapy. However, those with severe affection require a more specific therapy with supportive care, which is generally expensive and intensive (Hostetter and Uzal, 2022). The principles of management for equines with acute diarrhea are (1) re-establishment of normal hydration status of acid–base and electrolyte abnormalities, (2) analgesia, (3) treatment of endotoxemia, including correction of systemic inflammatory response, (4) limitation of toxin absorption, and (5) control of disseminated intravascular coagulation (Constable et al., 2017). Spasmodic colicSpasmodic colic is recognized in horses with short fits of pain and is associated with increased peristaltic sounds on auscultation of the small and large intestines (Abutarbush et al., 2005). Most episodes of spasmodic colic are recurrent. Equines admitted with a history of very frequent fits of pain are more likely to have grave diseases with a higher rate of mortality compared to animals with less frequent fits of pain. Horses with recurrent fits of elongated colic are likely to have partial obstruction of the intestines or motility problems (Mair and Sherlock, 2023). The general causes of spasmodic colic include excitement, such as during preparations for racing or showing, thunderstorms, and drinks of cold water when warm and sweating after work. In addition, the presence of heavy tapeworm infestation is associated with a high prevalence of spasmodic colic (Fikri et al., 2023). In addition, the submucosal migration and mucosal penetration of Strongylus vulgaris larvae is well-known to initiate changes in the ileal myoelectrical motion that could lead to the evolution of colic in equines. Hyperperistalsis of spasmodic colic in equines may arise due to an increase in the parasympathetic nerve tone under the effect of causative agents (Constable et al., 2017). Spasmodic colic is usually characterized in horses by brief intermittent attacks of abdominal pain, pawing, rolling, and kicking the belly for a few minutes, then shaking and standing in a normal way for a few minutes until the next fit of pain reoccurs. Therefore, the affected animal presented with multiple skin wounds (Fig. 3). The horse suffering from spasmodic colic had increased peristaltic activity, which might be attributed to inflammatory conditions such as enteritis and colitis (Fikri et al., 2023). The ultrasonographic appearance of the abdominal organ showed that the cecum appeared with an echogenic wall, and the contents were of variable echogenicity. The wall thickness increased, and the peristaltic activity of the cecum was hypermotile on real-time ultrasonography. The left ventral colon appeared with increased wall thickness, and sacculations were detected in some cases, whereas, in others, it appeared with normal wall thickness. The descending colon has a thin echogenic wall and contains isoechoic ingesta that appear in continuous motion, indicating hypermotility of the bowel (Tharwat et al., 2008).
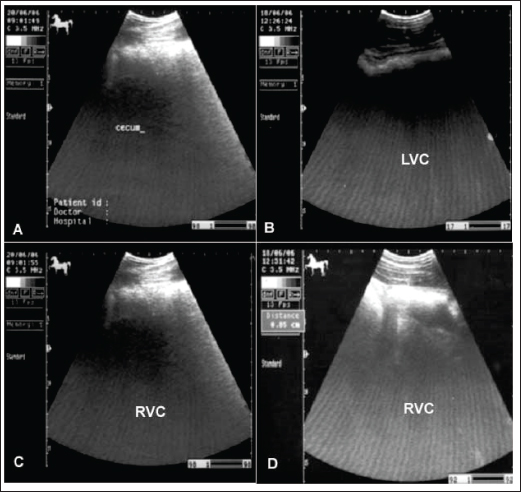
Fig. 3. Wounds and abrasions of a horse with recurrent spasmodic colic episodes. Intestinal ultrasonography generally shows increased intestinal peristalsis. The cecal wall appears echogenic, and the contents are of variable echogenicity. The wall thickness is increased, and the peristaltic activity of the cecum is detected as hypermotile on real-time ultrasonography (le Jeune and Whitcomb, 2014). The left ventral colon appears with increased wall thickness, and sacculations are detected in some cases, whereas, in others, it appears with normal wall thickness. The descending colon has a thin echogenic wall and contains isoechoic ingesta that appears in continuous motion (Fig. 4). Flatulent colicFlatulent colic (gastric tympany, wind colic, and tympanic colic) is caused by increases in fermentation or ineffectual gastrointestinal motility or may be secondary to partial luminal obstruction and aerophagia (wind cribbing) (Le Jeune and Whitcomb, 2014). Other causes include interruption in gastrointestinal motility resulting from stress, excitement, pain, or parasympatholytic medication, ileus secondary to uremia, liver disease, vascular compromise (thromboembolic colic), or surgical massive manipulation of the intestines, late pregnancy, impaction, displacements, constipation, and space-occupying masses (Worku et al., 2017). The cause of most cases of intestinal tympany is not well-known, although feeding on a highly fermentable green diet is considered to be a predisposing factor. In addition, the feeding of grains-rich diets is associated with changes in colonic inclusions that might initiate tympany. This condition may occur secondary to obstructive intestinal diseases that prevent the normal passage of gas and ingesta (Worku et al., 2017). In flatulent colic, temperature and respiration are elevated, the pulse rarely exceeds 70 beats /minute, and the mucous membrane is pale. Rectal examination revealed gas-filled portions of the intestines, and distended portions of the jejunum or ileum could be felt as thin-walled loops without sacculations and taeniae. Auscultation of the abdomen revealed decreased peristalsis but not totally absent. In addition, stomach distension may occur resulting in severe signs of pain, sweating, pawing, and hyperactivity (Worku et al., 2017). Horses with flatulent colic usually appear bloated, especially in the right flank, and show signs of severe pain, which may be intermittent or constant with particularly painful spasms. Auscultate pinging sounds are present over the right dorsal flank and midabdominal area (Beccati et al., 2011). The main diagnostic techniques for the tympanic colic in equines are radiography and sonography. Ultrasonographically, Tharwat et al., (2008) reported that flatulent colic bowel appeared distended with gas that prevented visualization of the ingesta inside, and the sacculations disappeared. The peristaltic activity of the gas-distended segment is decreased or absent (Tharwat et al., 2008). The cecum of horses with flatulent colic appears thin-walled due to gas distension; absence of sacculations; hypomotility on real-time ultrasonography, and the gases in the cecum prevent visualization of the ingesta inside the cecum (Le Jeune and Whitcomb, 2014). In comparison to healthy horses, the cecum is identified by its sacculations and contractions. The left ventral colon appears with a thin echogenic wall, the absence of sacculations, and the gases inside hinder the visualization of the ingesta. The right ventral colon compared to clinically normal horses shows the absence of sacculations and might be with thin or increased wall thickness, and this agrees well with Scharner et al., (2002), while in control horses it is characterized by the presence of sacculations, bright hyperechoic line, and the inability to identify the entire circumference of its wall (Fig. 5). Horses suffering from severe gas accumulation may collapse where postmortem examination confirms the diagnosis (Tharwat et al., 2008).
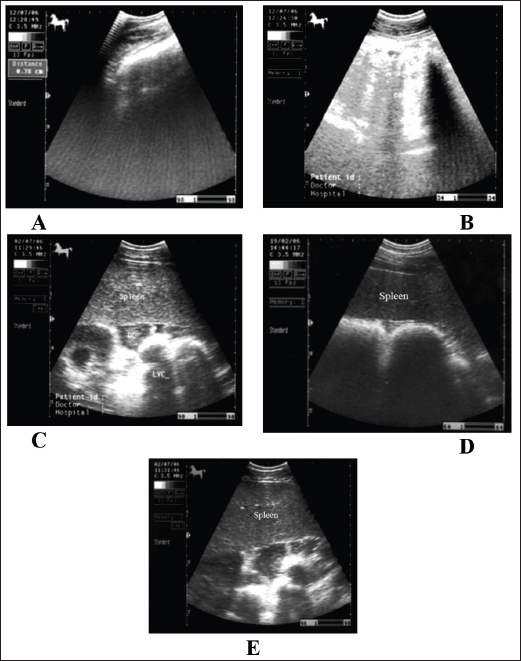
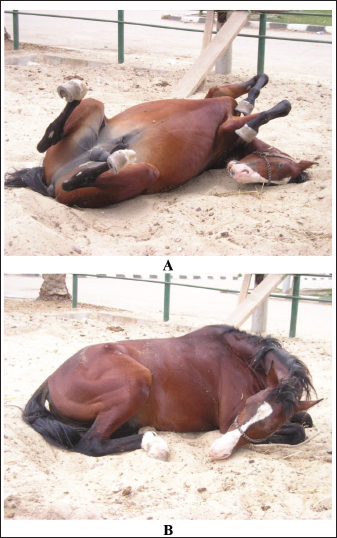
Fig. 4. Sonogram of 5 horses with spasmodic colic. Images A and B were taken at the right flank region and showed the echogenic cecal tissues with variable echogenicity and increased wall thickness. The peristaltic movement of the cecum was hypermotile during real-time scanning. Image C shows the spleen, descending colon, and left ventral colon obtained from the left 13th intercostal space. They appear normal, and only hyperperistalsis was detected. Image D shows the spleen and left ventral colon from the left 13 intercostal spaces. The spleen had a normal echotexture, the left ventral colon also had a normal echogenic wall, and only hypermotility of the left ventral colon was observed. Image E shows the spleen and descending colon. The spleen appears normal, and the descending colon appears as a circular echogenic wall with fluid and ingesta contents that appear with swirling motility more than expected. Impaction colicIt has been reported that dental disease, poor dentition, and mastication problems in aged horses; poor feeding regimens and diets too high in fiber, coarse and poor-quality hay; and when water is supplied infrequently are the main causes of the large intestine impaction. The etiology of the most large colon impacts is unknown (Constable et al., 2017). Poor feeding regimens, poor dentition particularly in aged horses, high-fiber diets, feeding hard diets after being fed soft grass, enteroliths, debility, fiber balls, meconium retention in foals, and parenteral injection of non-steroidal anti-inflammatories are possible contributing factors (Ryu et al., 2004). Equines admitted with impaction colic are depressed and usually take the posture of dorsal recumbency. Generally, intestinal impaction is detected on rectal examination. Horses suffering from severe long-standing impaction may collapse when postmortem examination confirms the diagnosis (Teodoro et al., 2023). Generally, horses with impactive colic ultrasonographically show a large circular hyperechoic mass in the cecum that casts an acoustic shadow consistent with a dense material or enteroliths (Fig. 6). Sacculation is absent, and wall thickness may decrease or increase according to the presence of intestinal compromise. Similar findings were reported by Reef et al. (2004). The descending colon appeared severely distended with isoechoic ingesta and reduced motility, consistent with previous reports. In addition, the right dorsal colon is more echogenic and casts an acoustic shadow; the wall also appears more echogenic and thicker than in healthy animals. Diagnosis is made based on postmortem examination in cases that have not been recovered (Tharwat et al., 2008).
Fig. 5. Ultrasonogram of the cecum, left ventral colon (LVC), and right ventral colon (RVC) in 4 horses with flatulent colic. In A, the cecal wall appears echogenic in the absence of sacculations. In B, the LVC shows an echogenic wall with no sacculations. In C, the RVC shows a thin wall that appears echogenic in the absence of sacculations. In D, RVC appears in the absence of sacculations and with increased wall thickness. These organs contain a large amount of gas, which hinders their visualization. The essential aspects of management include correction of fluid and electrolyte imbalances, pain control, and moistening of the ingesta to ease its passage. Magnesium sulfate, as a fecal softener, is associated with a relatively low risk of neurologic signs due to hypermagnesemia, whereas sodium sulfate causes mild hypokalemia and hypernatremia (Burke and Blikslager 2018). Alternatively, mineral oil is a lubricant, but it may not penetrate the impacted ingesta enough to soften it (Ruohoniemi et al. 2001). Surgical intervention may be needed in 15% of the cases, but it has been reported to have a poor outcome due to the risk of iatrogenic colonic rupture during trials to exteriorize it from the abdomen at surgery. Right dorsal colon impaction is more likely to require surgical intervention (Niinisto et al., 2018; Johnson et al., 2020). Obstructive and strangulating colicEquines admitted with obstructive colic have a history of loss of defecation. Strangulation of the small intestine is an important etiology of colic in horses for which surgical intervention is required (Giusto et al. 2024). Disorders of the small intestine account for 27%–64% of all presented colic cases, of which 55%–84% include strangulating disorders. In the latter group, up to 46% of strangulations are formed in the ileum (Loesch et al., 2002). Volvulus of the small and large intestines around the mesenteric root resulted in intense colic with comparatively rapid abdominal distension and circulatory failure. Intussusception is also reported in either the small or large intestine, but it is more frequently detected in the jejunum. The intussusceptum (oral portion of the gut) is usually propelled distally and engulfed by the peristaltic movement of the enveloping part (intussuscipiens) (Francoz and Guard, 2015). Volvulus of the large colon is also life-threatening when the organ twirls ≥360º, resulting in ischemia, cardiovascular compromise, and abdominal and colonic distension. Prompt surgical intervention, with or without colonic resection, followed by intensive medical management is vital for the survival of affected horses (Giusto et al., 2024). Even with immediate intervention, fatalities may extend from 10% to 50% due to colonic gangrene (Johnson et al., 2020). Increased wall thickness of the large colon (≥ 9mm) is an accurate and reliable preoperative test for torsion of the large colon in equines with surgical colic (Scharner et al., 2002; Pease et al., 2004). It was also reported that identification of the non-sacculated large colon in the left distal part of the abdomen was helpful in diagnosing large colon volvulus in horses (Abutarbush, 2006).
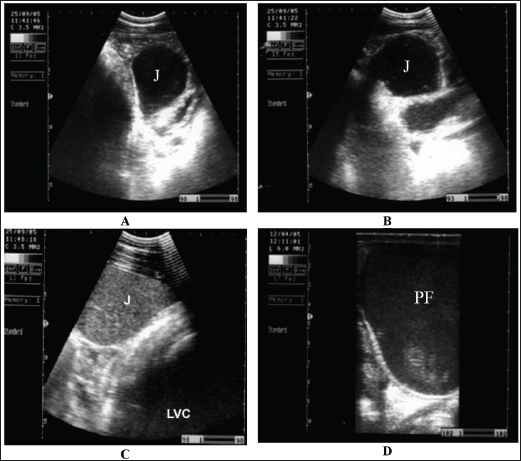
Fig. 6. Ultrasonogram of the cecum, left ventral colon (LVC), and right ventral colon (RVC) in 4 horses with flatulent colic. In A, the cecal wall appears echogenic in the absence of sacculations. In B, the LVC shows an echogenic wall with no sacculations. In C, the RVC shows a thin wall that appears echogenic in the absence of sacculations. In D, RVC appears in the absence of sacculations and with increased wall thickness. These organs contain a large amount of gas, which hinders their visualization. There are many causes of obstructive and strangulation colic. It may include obstruction by fecalith or enterolith, small colon impaction, atresia coli, retention of meconium, volvulus, strangulation by pedunculated lipoma, intussusception, and herniation through mesenteric rents (Abutarbush, 2006). Abdominal distension is usually mild initially, but it progresses as the disease intensity increases (Fig. 7). Non-strangulating lesions appear as mild to moderate colicky pain that may last without a difference of intensity for up to 36 hours (Fig. 8). Ultrasound can be helpful in recognizing the compromised intestine, allowing earlier verification. Elevated gut wall thickness reflects the stages of edema and hemorrhage and may predict prognosis (Fig. 9) (Tharwat et al., 2008). In horses with obstructive colic, the small intestine, especially the jejunum, appears distended with fluid-containing ingesta, which exhibits mixed echogenicity. The peristaltic activity of healthy equines (Giusto et al., 2024). The descending colon is distended with fluid and ingesta, and the left ventral colon appears without sacculations and detectable motility. Transrectal ultrasonographic examination showed that the pelvic flexure with gas, fluid, and ingesta with no detectable contractions (Tharwat et al., 2008). In advanced cases, intestinal perforation may occur because of either severe impaction or intestinal strangulation (Fig. 10) (Tharwat et al., 2008). Sand colicSwallowing sand can cause acute or recurrent fits of colic and/or diarrhea, poor performance, and weight loss (Korolainen and Ruohoniemi, 2002). Owing to the non-specificity of such changes, diagnosis based solely on clinical symptoms is difficult. To assist in sand detection, per rectum palpation of sand-filled organs, abdominal auscultation, sonography and radiography, and fecal sand sedimentation tests can be implemented (Keppie et al., 2008). Mild-to-severe fits of recurrent colic are associated with abdominal distension (80%), diarrhea (20%), and loss of appetite (10%). Signs of intense or persistent colicky pain are associated with an increased possibility of colon displacement or volvulus. The diarrhea is usually watery but not malodorous or profuse, and the affected animal is sometimes mildly pyrexic and frequently tachycardic (Kendall et al., 2008). Abdominal auscultation over the cranial ventral region revealed sounds that appeared to be heard when a paper bag was partially filled with sand and rotated (Kilcoyne et al., 2017). Radiography will detect sand in the dorsal and ventral colons and can be used to predict the efficacy of therapy. The intensity of sand accumulation can be estimated by radiography and grade assignment. However, abdominal sonography has good specificity and sensitivity (88%) versus the gold standard of radiography in the detection of sand in the ventral colon (Fig. 11). Conversely, ultrasound is not valuable in detecting sand in the transverse or right dorsal colon (Kendall et al., 2008). Management includes the re-establishment of electrolyte and fluid abnormalities, the administration of pain killers, the prevention of sand infection, and at the same time removal of the sand from the surroundings (Kaikkonen et al., 2016). In equids with severe colicky pain, surgical removal is indicated in cases with acute transverse or right dorsal obstruction by sand, displacement, or volvulus. Animals that require surgical intervention for sandy colic with other gastrointestinal disorders have a worse prognosis than those requiring only medical therapy (Hart et al., 2013). Oral magnesium sulfate (1 g/Kg) or mineral oil (1 ml/kg) can accelerate the removal of sand (Niinisto et al., 2018).
Fig. 7. Abdominal distension in a horse with impaction colic.
Fig. 8. Impaction colic attack in a horse. The animal usually takes the dorsal position during episode (A). Sternal recumbency position is usually noted between colic attacks (B). Verminous mesenteric arteritisStrongylus vulgaris is considered the ultimate pathogenic nematode of equines because of its intense arterial affections that develop in the mesenteric arteries as a result of larval migration. Symptoms of the disease vary depending on the intensity of the parasitic infestation (Domshy et al., 2024). It has been reported that mild and intermittent fits of colic that comply with short-term analgesic drugs and long-term anthelmintic drugs are caused by verminous arteritis (Nielsen et al., 2016). In some animals, diseased equines are often depressed and recumbent, with weight loss and inappetence. Intense forms of the disease are usually caused by infarction of the small and large intestines (Constable et al., 2017). Affected animals have an abrupt onset of intense abdominal pain with sweating, the heart is accelerated (over 100 beats/minute), and abdominal auscultation shows decreased intestinal sounds. Transrectal examination reveals mild enlargement of the small intestine or large colon, depending on the viscus of the bowel involved. The cranial mesenteric artery appears thickened and painful (Domshy et al., 2024). Death follows peritonitis due to gangrene of the intestines within 24 hours of the appearance of symptoms (Pihl et al., 2018). Acute colicky pain due to S. vulgaris is usually observed in young animals, as most equines acquire their relative immunity toward large strongyles as a result of natural infestation (Borji et al., 2014). Infarction of the large intestine is the most common postmortem lesion and is evident as either devitalization of a large section of the viscus or several mottled lesions that are edematous and red in color (Fig. 12). Diseased patients are managed with oral anthelmintics (ivermectin 200 µg/Kg once or fenbendazole 50 mg/Kg every 24 hours for 3 days), laxatives such as mineral oil, and analgesic drugs such as flunixin meglumine, intravenous fluid therapy, and supportive care (Nielsen et al., 2016). The severity of colicky pain usually requires prompt abdominal surgical intervention with resection of the lesions; however, most cases do not recover (Pihl et al., 2018). PeritonitisPeritonitis in equines is associated with long-term treatment with broad-spectrum antimicrobials and a poor prognosis. The condition is often reported secondary to abdominal surgery, traumatic abdominal pain, and bowel rupture. Despite this, cases of idiopathic etiology have been reported (Odelros et al., 2019). Affected animals often show symptoms of both colicky pain and systemic inflammation, but early detection is important for optimal therapy (Hedberg-Alm et al., 2022). The hypothesized etiologies of idiopathic peritonitis include the seepage of gut microorganisms from the digestive tract by emigration of foreign bodies or parasites, mucosal ulcerations in the large intestine initiated by remedy with non-steroidal anti-inflammatories (Watts et al., 2011), and non-strangulating intestinal infarctions (Pihl et al., 2018).
Fig. 9. Sonogram of horses with obstructive colic: (A) jejunum (J) imaged in the left ventral abdomen using a 3.5-MHz transducer. The greatly distended loop of the jejunum and its contents are of mixed echogenicity. (B) Image of jejunum in left ventral abdomen using a 3.5-MHz transducer. The greatly distended loop of the jejunum and its contents are of mixed echogenicity. (C) The jejunum and left ventral colon are obtained from the left flank region caudal to the costal arch. The jejunal contents were more echogenic. The left ventral colon shows no sacculations. (D) Pelvic flexure (PF) obtained from the transrectal window using a 6-MHz transducer. It appears that complete ileus was observed during real-time scanning. Principal clinical symptoms include pyrexia, colic, and, in more delayed cases, weight loss (Henderson et al., 2008). Confirmation of the diagnosis depends on the analysis of peritoneal exudation, with increased nucleated cells indicating inflammation of the peritoneum (Odelros et al., 2019). Severe primary or idiopathic cases with unknown etiology have very high recovery rates of 94%–100% (Odelros et al., 2019). Affected horses are usually seen standing. In addition, the forward extension of the forelimbs and backward extension of the hindlimbs (stretching) are frequently observed (Fig. 13). Cases detected following non-strangulating intestinal infarction are associated with a high outcome with medical therapy alone and a poor outcome even after surgical resection of the involved viscus (Pihl et al., 2018). Although several other causes of non-strangulating intestinal infarction have been suggested (Spanton et al., 2019), intestinal wall infarctions secondary to thrombosis of the mesenteric arterial due to S. vulgaris larval migration have been reported (Nielsen et al., 2016; Pihl et al., 2018). Abdominal ultrasonography shows peritoneal effusions with fibrin formation in prolonged cases (Figs. 14 and 15). The treatment of peritonitis should be directed toward reducing systemic shock, readjusting hypovolemia, correcting the imitating reason, administering antibiotics as well as anti-inflammatory therapy, and performing abdominal centesis and lavage. Prognosis is estimated by a quick response to intensive treatment (Davis et al., 2003). Daily intraperitoneal infusion of 25 mg/Kg ceftriaxone with supportive and parenteral antibiotic treatment was reported to be valuable in patients with septic peritonitis (Alonso et al., 2020).
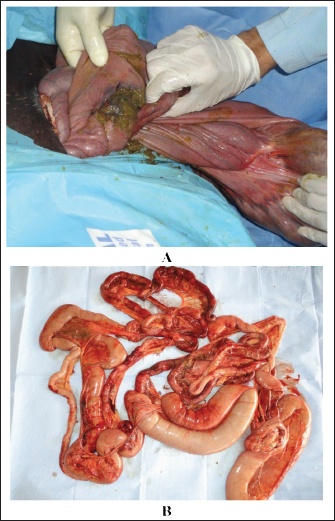
Fig. 10. Postmortem examination of a horse with severe colonic impaction and loss of defection. Image A shows intestinal perforation, whereas image B shows intestinal congestion and soling with feces following perforation.
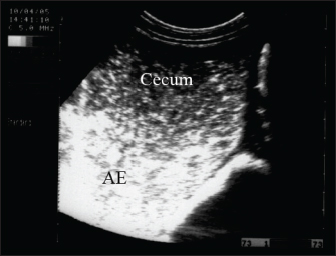
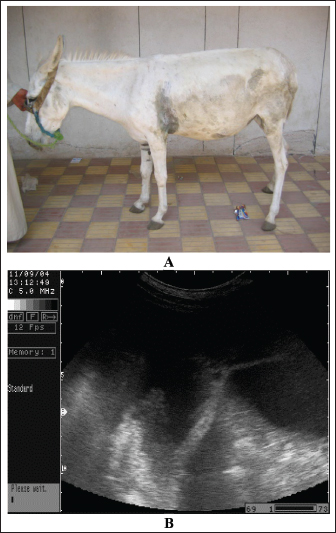
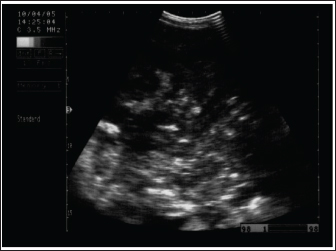
Fig. 11. Ultrasonography of a female donkey exhibiting sand colic. Case history included a recurrent episode of severe abdominal pain. Rectal examination revealed a large amount of sand in the feces. Abdominal ultrasonography shows cecal impaction with sand. Acoustic enhancement (AE) within the cecum was a characteristic sonographic finding.
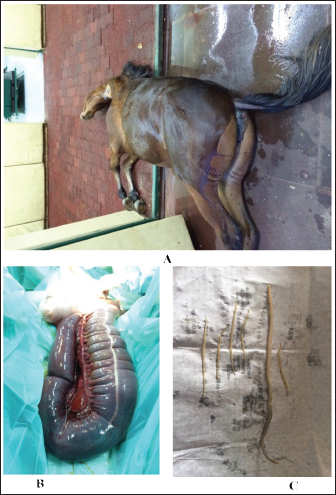
Fig. 12. Verminous mesenteric arteritis in a mare with acute abdominal pain. The animal collapsed immediately after abdominal surgery A. Image B shows bluish discoloration and gangrene of the large intestine. Image C shows Strongylus vulgaris nematodes were removed from the large intestine and intestine.
Fig. 13. Peritonitis in a stallion. The previous history included recurrent episodes of colic. The animal was usually seen standing with the forward extension of the forelimbs and the backward extension of the hindlimbs (stretching). The differential diagnosis for this posture includes laminitis and urethral calculi. HerniasA hernia is an abnormal slit in the abdomen that leads to the protrusion of a viscus or tissue from that opening. It is typically associated with swelling of the equine abdomen. Affected animals are usually admitted with a reaction of colicky. The degree of pain intensity differs according to the acuteness of the affection and its nature if strangulation occurred or not (Gough et al., 2022). It has 3 main types in equines as follows. Many types of hernias, such as umbilical, scrotal, and diaphragmatic, are reported in equines. There are several drawbacks linked with umbilical hernias, including enterocutaneous fistulae, colic, intestinal incarceration, and umbilical abscessation (Voermans et al., 2004). Inguinal hernias are very popular in Standardbred horses, which tend to have baggy inguinal canals. It may also be observed in neonatal foals, but compared with mature horses, they are typically non-strangulating. A pathognomonic sign of inguinal herniation is a cool and large unilateral scrotum enlargement indicating blood supply occlusion of the testicular tissue rather than a strangulated intestine (Ragle et al., 2013). Acquired hernias are assumed to be a result of chest trauma or an abrupt increase in intra-abdominal pressure due to parturition, abdominal distention, sudden drop, or arduous exercise (Pauwels et al., 2007; Hart and Brown, 2009; Romero and Rodgerson, 2010). Typical findings at the presentation of umbilical hernia include umbilical swelling, warmth, and firmness of the hernia sac. The intestines together with peritoneal effusions and fibrin formation in long-term cases may be imaged on abdominal ultrasonography (Figs. 16 and 17). The definitive treatment for traumatic or acquired hernia is surgical reconstruction of the viscus in the abdomen and repair of the opening (Smith, 2015). The prognosis for a horse presenting for surgical correction of a diaphragmatic hernia remains poor at 23% overall and 46% for those in whom surgical correction is attempted (Pauwels et al., 2007). As abdominal surgery advances and the prognosis for horses with intestinal ischemia improves, this topic should be reconsidered, as the prognosis seems to be influenced by factors beyond the diaphragmatic defect (Romero and Rodgerson, 2010). Non-Gastrointestinal ColicCystitisCystitis is defined as urinary bladder mucosa inflammation. Repeated urinary catheterization and vaginitis are predisposing factors for cystitis (Schumacher and Schmitz, 2002). In addition, cystitis commonly occurs following urinary flow disorders caused by bladder paralysis, urolithiasis, tumors, anatomical anomalies, endoscopic examination, and catheterization (Reed et al., 2004). Inflammation of the bladder may occur secondary to the ventral accumulation of urine sediment, mostly calcium carbonate crystals. The mechanical irritation caused by sediment and urea accretion results in mucosal inflammation and ulceration (Schott et al., 2018).
Fig. 14. Peritonitis in a 3-month-old pregnant female donkey. History of presentation indicated recurrent abdominal pain. Depression was defined as the initial clinical finding upon admission (A). Abdominal ultrasound showing peritoneal effusions with fibrin formation (B).
Fig. 15. Sonographic findings of a mule with chronic peritonitis. Previous history included inappetence, recurrent abdominal pain, and loss of defecation. Abdominal ultrasonography shows a massive fibrin network involving overall the intestines.
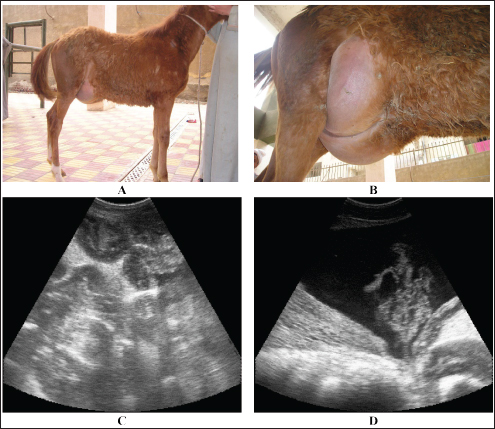
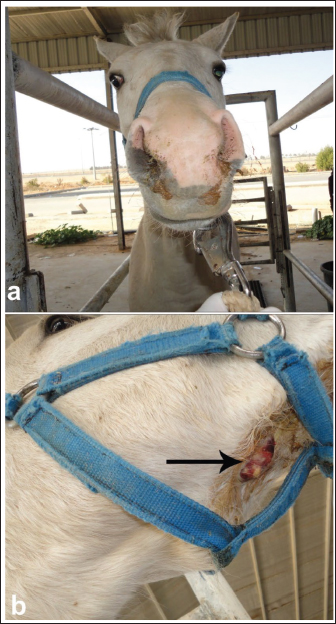
Fig. 16. Umbilical hernia in a 6-month-old foal A. Image B shows a close-up view of the hernia. Image C shows a sonographic image of the hernia in which the intestines are located. Image D also shows peritoneal effusion and fibrin formation in the same foal.
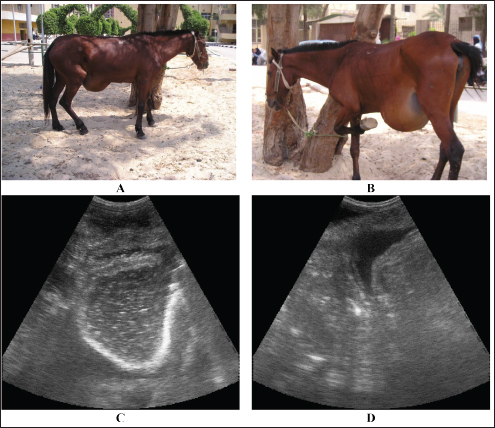
Fig. 17. Umbilical hernia in a mare A. Image B shows a close-up view of the hernia where the mare is frequently seen kicking the hernia swelling. Image C shows a sonographic image of the hernia in which the intestines are located. Image D also shows peritoneal effusion in the same mare. Horses with cystitis show signs of pollakiuria, hematuria, pyuria, stranguria, and urine scalding in the perineum in mares or in front of the hind legs of male equines (Smith, 2015). Usually, females are affected more frequently than males. In cases of cystitis, diffuse or focal thickening and irregularity of the bladder wall are the most common imaged abnormalities detected in horses with diseases, and the urine is usually echogenic to hyperechoic (Le Jeune and Whitcomb, 2014). Staining, arched back, and maiming urination posture for a long time are also observed. Case history and clinical signs are usually diagnostic in most cases. Pronounced thickening of the bladder wall is the most frequently observed finding on ultrasonography of the bladder, and the urine is more echogenic (Zakia et al., 2021). In addition, fibrin strands are imaged in equines with intense cystitis, whereas in healthy animals, the urinary bladder wall appears echogenic and the urine appears anechoic with hyperechoic particles (Fig. 18). Antimicrobials are prescribed to eliminate infections, and the verification of the antimicrobial sensitivity of the causative agent is crucial. Recurrence is common unless therapy is continued for a period of 7–14 days. Repeated urine culture at least once during treatment and within 7–10 days after treatment should be performed to estimate the success or failure of therapy (Reed et al., 2004). UrolithiasisIt has been reported that urinary calculi are principally a disease of adult equines, accounting for approximately 7.8% of urinary tract disease in equines. In addition, the urinary bladder is the common site of calculi, followed by the urethra, kidneys, and finally ureters (Duesterdieck-Zellmer, 2007). Smith (2015) reported that the disease could be unilateral or bilateral, mostly at the level of the renal pelvis, which might lead to hydronephrosis (Abu-Seida and Shamaa, 2020). Horses with incomplete urethral obstruction are usually associated with urinary incontinence, dysuria, and mild colicky pain. Absolute urethral obstruction results in moderate to intense pain symptoms (Reed et al., 2004). There are several symptoms in horses suffering from urinary calculi due to urinary calculi. It includes pollakiuria, dysuria, stranguria, hematuria, incontinence, urine scalding, painful urination, weight loss, and uroabdomen, which is an animal with a ruptured bladder (Smith, 2015).
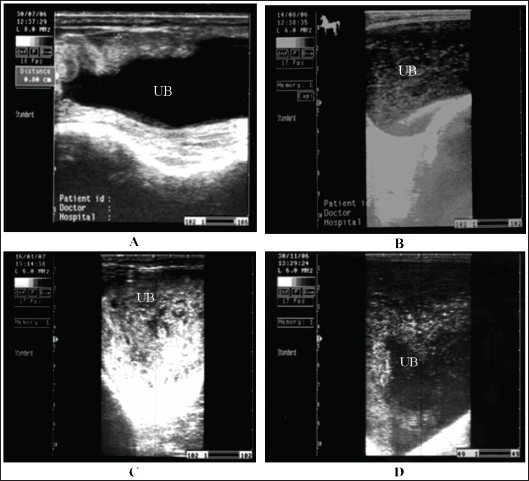
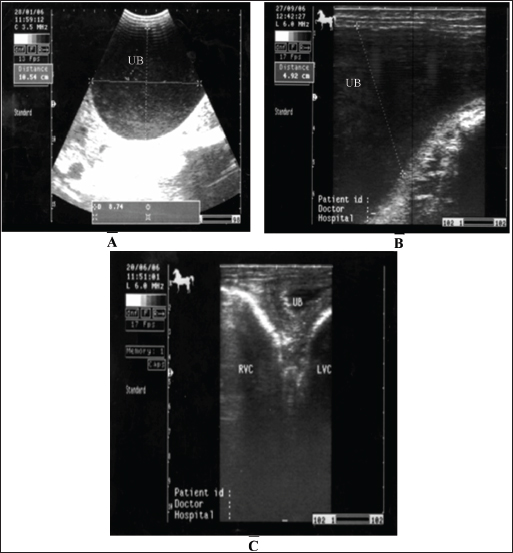
Fig. 18. A sonogram of horses suffering from cystitis showed. Images A and B were obtained from the transrectal window using an 8-MHz linear transducer. Corrugation of the bladder wall (A) and hyperechogenicity of the bladder contents with distal acoustic enhancement (B). Images C and D were obtained from the transrectal window using a 6-MHz transducer. Notice hyperechogenicity of the bladder contents, which leads to acoustic enhancement (C and D). UB=urinary bladder.
Fig. 19. Sonogram of horses with urine retention showed. In images A and B, the urinary bladder appears distended. In image C, the bladder collapses after catheter application. These sonograms were obtained from the transrectal window using a 6-MHz transducer. Image A was taken transcutaneously using a 3.5-MHz sector probe, while images B and C were taken transrectally using a 6.0-MHz linear transducer. Horses suffering from urine retention also showed severe restlessness with failure in urinate testing. History and clinical manifestations are usually diagnostic. The urinary bladder was imaged, revealing a marked distension with echogenic urine causing distal enhancement and hyperechoic sediment ventrally in the bladder. The bladder wall is normal unless concurrent cystitis is present (Abu-Seida and Shamaa, 2020). After catheter application, the urinary bladder returns to normal size, and the wall will also be normal (Fig. 19). Management of cystic calculi is performed by surgical removal of the calculi and correction of any bladder perforation, if present. Ultrasound-guided, transcutaneous right kidney nephrostomy has been reported in a horse with ureteral calculi for the purpose of short-term diversion of urine (Duesterdieck-Zellmer, 2007). Surgically, sub-ischial urethrotomy may be conducted under epidural anesthesia (Abu-Seida and Shamaa, 2020). Abdominal abscessesIntra-abdominal abscessation is not common in adult horses (Aleman et al., 2003; Recknagel et al., 2012) or in foals (Valdes and Johnson, 2005). In a 15-year-study, 40 horses with intra-abdominal masses were studied; 3 of them (7.5%) had mesenteric abscesses (Recknagel et al., 2012). The pathogenesis of abdominal abscessation in horses is presumably similar to that in ruminants (Aleman et al., 2003). It was not possible to determine the cause of the abdominal abscess in the present case. In addition, in equines, the abscess likely formed as a direct extension of a hematogenous infectious process, similar to the development of mesenteric abscess in a bull with chronic peritonitis (Elce, 2006). Several pathogens are considered causative agents of abdominal abscessation, such as Rhodococcus equi, Streptococcus equi subsp. equi, Corynebacterium pseudotuberculosis, Clostridium novyi type A, Blastomyces dermatitidis, and Fusobacterium necrophorum. Pseudomonas aeruginosa can also induce mesenteric abscesses in equines (Arnold et al., 2012; Tharwat et al., 2024c). Equines with abdominal abscesses are usually admitted with a history of fever, weight loss, colic, inappetence, and signs of depression of various durations (Tharwat et al., 2024c). Abdominal abscess was strongly suspected on the basis of ultrasonographic findings (Fig. 20). The differential diagnoses include abdominal neoplasia. Ultrasonography is also useful for percutaneous needle aspiration for bacteriologic evaluation and facilitates intralesional antibiotic treatment and drainage because the thick abscess capsule prevents the spread of antibiotics (Tharwat et al., 2024c) (Figs. 21 and 22). For large abscesses, transcutaneous aspiration of pus should be performed under ultrasound guidance, as reported in cattle (Mohamed et al., 2002; Mohamed et al., 2003a,b,c; Mohamed and Oikawa, 2008). The ultrasound-guided percutaneous drainage of abdominal abscesses has several advantages. First, the animal should not be anesthetized. Second, surgery is not required, and consequently postoperative drawbacks will not develop. Third, percutaneous drainage is typically cost-effective. Fourth, percutaneous ultrasound-guided drainage can be performed promptly after diagnostic ultrasound is performed because the methodology does not require any particular planning or preparation and can be completed quickly if the examiner is experienced. Complications associated with ultrasound-guided percutaneous aspiration of the abscess in the present case were not encountered (Tharwat et al., 2024c). Follow-up after pus aspiration confirmed the treatment response, reduction in abscess size, and recovery (Figs. 23 and 24).
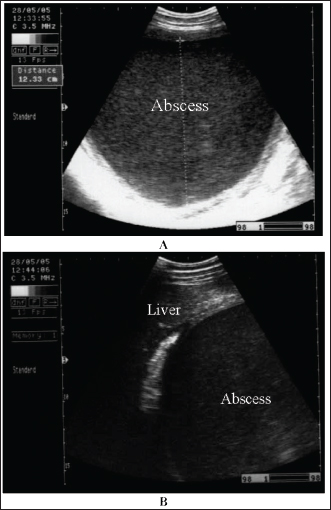
Fig. 20. Ultrasonograms of an 8-year-old Thoroughbred mare with abdominal abscess. Image A shows a sonogram obtained at admission (day 0) in which a mass with a thick echogenic capsule with homogenous and echogenic internal contents was identified. The mass was located close to the liver, deforming its right lobe margins B.
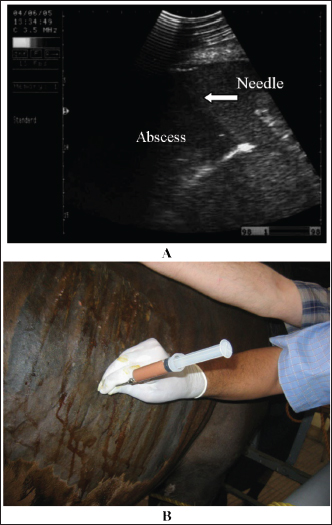
Fig. 21. Ultrasonograms of an 8-year-old Thoroughbred mare with abdominal abscess. Image A shows the fine-needle aspiration technique used to drain the content of the abscess. Image B an ultrasound-guided aspiration of pus from an abdominal abscess.
Fig. 22. An aspirated 10-l of thick, reddish-creamy, odorless pus was obtained from a mare with an abdominal abscess. After the culture of the pus samples, the causative agent was found to be Pseudomonas aeruginosa.
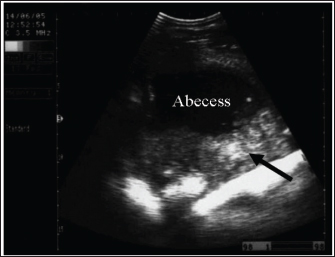
Fig. 23. Ultrasonogram images of an 8-year-old Thoroughbred mare with an abdominal abscess obtained 17 days after initial admission. The image shows that the mass appeared much smaller and had hypoechoic contents, with the capsule no longer seen and a fibrous tissue (arrow) surrounding the lesion. Mesenteric abscessesIn equines, strangles are a highly contagious bacterial disease of the respiratory system caused by infection by Streptococcus equi, and it is principally transmitted by direct contact with infected horses or indirectly through contact with contaminated utensils (Lindah et al., 2011; Gharieb et al., 2019). Numerous risk factors are associated with strangle outbreaks, such as mixing horses from different sources, exposure to bad weather, overcrowding, concomitant diseases, breeding season, parasitic infestations, and nutritional deficiencies (Moraes et al., 2009). The disease is epidemiologically manifested by elevated morbidity and low fatality, resulting in huge economic losses owing to treatment expenses and restriction of horse movement (Islam et al., 2014 Osman et al., 2021).
Fig. 24. The mare on day 30 from the initial day of examination appeared healthy and completely recovered.
Fig. 25. A strangle-infected horse shows (a) purulent nasal discharges and (b) abscess in the submandibular lymph nodes.
Fig. 26. Postmortem findings in a horse with bastard strangles showing (a) a perforated mesenteric abscess and (b) a closeup view of the abscess. Clinically, the infected equines show 2 forms of the disease: typical and bastard forms. Clinical manifestations include depression, high fever, soft non-productive cough, anorexia, bilateral muco-purulent nasal discharge, and abscessation and enlargement of the submandibular lymph nodes (Osman et al., 2021) (Fig. 25). In some cases, metastatic infection and abdominal abscessation are observed. Postmortem findings revealed punctured mesenteric abscesses that may have led to peritoneal effusions, severe peritonitis, and intestinal adhesions (Osman et al., 2021) (Fig. 26). Gram staining revealed Gram-positive cocci (Fig. 27). The outbreak was controlled through parenteral administration of penicillin–streptomycin once daily for 7 successive days (8 mg procaine penicillin and 10 mg dihydro-streptomycin sulfate per kg BW). Moreover, some measures must be taken, such as avoiding contact between healthy and diseased horses and avoiding overcrowding (Sweeney et al., 2005 and Firew and Pal 2015; Osman et al., 2021). In conclusion, colic in equines is a fatal condition, and most cases do not recover if diagnosed late. Therefore, ancillary diagnostic tools should be implemented. This review showed that abdominal ultrasound is very useful in verifying equines with different causes of colic, such as flatulent colic, spasmodic colic, obstructive colic, impactive colic, strangulating colic, peritonitis, hernias, cystitis, urine retention, and abdominal abscesses. Promising estimation of serum biomarkers revealed potential diagnostic aid in equines suffering from acute abdominal pain. Evaluation of some biomarkers, such as SAA, Hp, and L-lactate, enhanced the ability of horses with intense inflammatory colic requiring medical therapy from those with colic requesting surgical intervention.
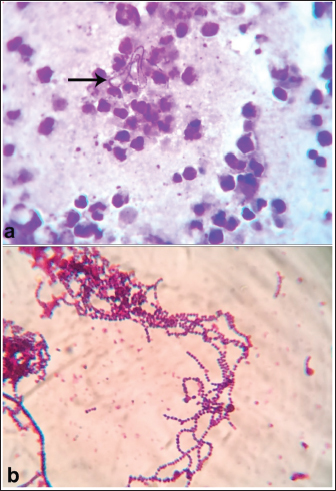
Fig. 27. Gram-stained smears showing (a) Gram-positive cocci arranged in short chains from the solid medium and (b) long chains from the liquid medium. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Authors contributionMT: conceptualization, data collection, and writing of the original manuscript draft and Figures. FA: review and editing. The authors have approved the final manuscript for publication. Data availabilityAll data supporting the findings of this study are available in the manuscript, and no additional data sources are required. ReferencesAbu-Seida, A.M. and Shamaa, A.A. 2020. Ultrasonography and surgical treatment of an unusual case of urethral calculus in an Arabian horse. J. Equine Vet. Sci. 92, 103150. Abutarbush, S.M. 2006. Use of ultrasonography to diagnose large colon volvulus in horses. J. Am. Vet. Med. Assoc. 228, 409–413. Abutarbush, S.M., Carmalt, J.L. and Shoemaker RW. 2005. Causes of gastrointestinal colic in horses in western Canada: 604 cases (1992 to 2002). Can. Vet. J. 46, 800–805. Aleman, M., Watson, J.L. and Jang, S.S. 2003. Clostridium novyi Type A intra-abdominal abscess in a horse. J. Vet. Intern. Med. 17, 934–936. Almundarij, T. and Tharwat, M. 2023. Impact of intestinal and urinary tracts obstruction on oxidative stress biomarkers in dromedary camels. Int. J. Vet. Sci. 12, 422–427. Alonso, J.M., Rosa, G.D.S., Santos, B., Guerra, S., Ribeiro, M., Watanabe, M.J., Alves, A., Rodrigues, C., Takahira, R.K. and Hussni, C.A. 2020. Adjuvant intraperitoneal ceftriaxone in the treatment of septic peritonitis in horses. Vet. Rec. 187, e29. Archer, D.C., Pinchbeck, G.L., French, N.P. and Proudman, C.J. 2008. Risk factors for epiploic foramen entrapment colic: an international study. Equine Vet. J. 40, 224–230. Arnold, C.E. and Chaffin, M.K. 2012. Abdominal abscesses in adult horses: 61 cases (1993-2008). J. Am. Vet. Med. Assoc. 241, 1659–1665. Beccati, F., Pepe, M., Gialletti, R., Cercone, M., Bazzica, C. and Nannarone, S. 2011. Is there a statistical correlation between ultrasonographic findings and definitive diagnosis in horses with acute abdominal pain? Equine Vet. J. Suppl. 39, 98–105. Beccati, F., Pepe, M., Gialletti, R., Cercone, M., Bazzica, C. and Nannarone, S. 2011. Is there a statistical correlation between ultrasonographic findings and definitive diagnosis in horses with acute abdominal pain? Equine Vet. J. Suppl. 39, 98–105. Beccati, F., Pepe, M., Gialletti, R., Cercone, M., Bazzica, C. and Nannarone, S. 2011. Is there a statistical correlation between ultrasonographic findings and definitive diagnosis in horses with acute abdominal pain? Equine Vet. J. Suppl. 39, 98–105. Bergstrom, T.C., Sakai, R.R. and Nieto, J.E. 2018. Catastrophic gastric rupture in a horse secondary to psyllium pharmacobezoars. Can. Vet. J. 59, 249–253. Bird, A.R., Knowles, E.J., Sherlock, C.E., Pearson, G.R. and Mair, T.S. 2012. The clinical and pathological features of gastric impaction in twelve horses. Equine Vet. J. Suppl 43, 105–110. Borji, H., Moosavi, Z. and Ahmadi, F. 2014. Cranial mesenteric arterial obstruction due to Strongylus vulgaris larvae in a donkey (Equus asinus). Iran. J. Parasitol. 9, 441–444. Bowden, A., England, G.C.W., Burford J.H., Mair T. S., Furness, W. and Freeman, S.L. 2017. Prevalence and outcome of conditions seen ‘out of hours’ by first opinion equine clinicians at two practices over a three-year period (2011–2013). Equine Vet. J. 49, 11. Burke, M. and Blikslager, A. 2018. Advances in diagnostics and treatments in horses with acute colic and postoperative ileus. Vet. Clin. North Am. Equine Pract. 34, 81–96. Constable, P.D., Hinchcliff, K.W., Done, S.H. and Gruenberg, W. 2017. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th ed. Saunders, St. Louis, Missouri, USA. Cribb, N.C. and Arroyo, L.G. 2018. Techniques and accuracy of abdominal ultrasound in gastrointestinal diseases of horses and foals. Vet. Clin. North Am. Equine Pract. 34, 25–38. Curtis, L., Burford, J.H., Thomas, J.S., Curran, M.L., Bayes, T.C., England, G.C. and Freeman, S.L. 2015. Prospective study of the primary evaluation of 1016 horses with clinical signs of abdominal pain by veterinary practitioners, and the differentiation of critical and non-critical cases. Acta Vet. Scand. 57, 69. Davis, J.L. 2003. Treatment of peritonitis. Vet. Clin. North Am. Equine Pract. 19, 765–778. Domshy, K.A., Whitehead, A.E., Poissant, J., Goldsmith, D.A., Legge, C., Knight, C.G., Zachar, E.K., Loch, S.S. and Davies, J.L. 2024. A retrospective study of the prevalence in equine postmortems of cranial mesenteric arteritis caused by Strongylus vulgaris in Alberta (2010 to 2022). Can. Vet. J. 65, 587–593. Duesterdieck-Zellmer, K.F. 2007. Equine urolithiasis. Vet. Clin. North Am. Equine Pract. 23, 613–629. Elce YA. 2006. Infections in the equine abdomen and pelvis: perirectal abscesses, umbilical infections, and peritonitis. Vet. Clin. North Am. Equine Pract. 22, 419–36. Fikri, F., Hendrawan, D., Wicaksono, A.P., Purnomo, A., Khairani, S., Chhetri, S., Purnama, M.T.E. and Çalışkan, H. 2024. Colic incidence, risk factors, and therapeutic management in a working horse population in Tuban, Indonesia. Vet. World. 17, 963–972. Fikri, F., Hendrawan, D., Wicaksono, A.P., Purnomo, A., Khairani, S., Chhetri, S., Maslamama, S.T. and Purnama, M.T.E. 2023. Incidence, risk factors, and therapeutic management of equine colic in Lamongan, Indonesia. Vet. World. 16, 1408–1414. Firew. S. and Pal, M. 2015. Clinical and microbiological observations on strangles in donkey. Haryana Vet. 54, 64–66. Francoz, D. and Guard, C.L. 2015. In Smith, B.P., editor: Large animal internal medicine. 5th ed. St. Louis, MI: Mosby, pp. 1233–1236. Furness, M.C., Snyman, H.N., Abrahams, M., Moore, A., Vince, A. and Anderson, M.E. 2013. Severe gastric impaction secondary to a gastric polyp in a horse. Can. Vet. J. 54, 979–982. Gharieb, N.M., Ali, W.S., Tartor, Y.H., EL-Naenaeey, E.S.Y. and Ammar, A.M., 2019. Rapid and precise diagnostic tests for S. equi: an etiologic agent of equine strangles. Zagazig Vet. J. 47, 146–159. Giusto, G., Cerullo, A. and Gandini, M. 2024. Anastomotic techniques for small intestinal obstruction in horses. A scoping review. Equine Vet. J. 56, 1103–1114. Gough, R., McGovern, K., Bladon, B. and De Oliveira, F. 2022. Umbilical cord herniation with small intestinal evisceration in two thoroughbred neonates. J. Equine Vet. Sci. 114, 103959. Hajimohammadi, A., Ghane, M., Ghari Tehrani, M., Paravar, B., Mirzaei, A., Razavi, S. and Nikzad, M. 2023. Association of the severity of colic in horses with oxidative stress biomarkers, acute-phase proteins, and certain trace elements. J. Equine Sci. 34, 73–81. Hart, K.A., Linnenkohl, W., Mayer, J.R., House, A.M., Gold, J.R. and Giguère, S. 2013. Medical management of sand enteropathy in 62 horses. Equine Vet. J. 45, 465–469. Hart, S.K. and Brown, J.A. 2009. Diaphragmatic hernia in horses: 44 cases (1986-2006). J. Vet. Emerg. Crit. Care 19, 357–362. Hedberg-Alm, Y., Tydén, E., Tamminen, L.M., Lindström, L., Anlén, K., Svensson, M. and Riihimäki, M. 2022. Clinical features and treatment response to differentiate idiopathic peritonitis from non-strangulating intestinal infarction of the pelvic flexure associated with Strongylus vulgaris infection in the horse. BMC Vet. Res. 18, 149. Henderson, I.S.F., Mair, T.S., Keen, J.A., Shaw. D.J. and McGorum, B.C. 2008. Study of the short- and long-term outcomes of 65 horses with peritonitis. Vet. Rec. 163, 293–297. Hillyer, M.H., Taylor, F.G. and French, N.P. 2001. A cross-sectional study of colic in horses on thoroughbred training premises in the British Isles in 1997. Equine Vet. J. 33, 380–385. Hostetter, J.M. and Uzal, F.A. 2022. Gastrointestinal biopsy in the horse: overview of collection, interpretation, and applications. J. Vet. Diagn. Invest. 34, 376–388. Islam, S.M.S., Purnat, T.D., Phuong, N.T.A., Mwingira, U., Schacht, K. and Fröschl, G. 2014. Non-communicable diseases (NCDs) in developing countries: a symposium report. Glob. Health 10, 81. Johnson, L.M., Holcombe, S.J., Shearer, T.R., Watson, V., Gandy, J., Southwood, L.L., Lynch, T.M., Schroeder, E.L., Fogle, CA. and Sordillo, L.M. 2020. Multicenter placebo-controlled randomized study of ethyl pyruvate in horses following surgical treatment for ≥ 360° large colon volvulus. Front. Vet. Sci. 7, 204. Kaikkonen, R., Niinistö, K., Lindholm, T. and Raekallio, M. 2016. Comparison of psyllium feeding at home and nasogastric intubation of psyllium and magnesium sulfate in the hospital as a treatment for naturally occurring colonic sand (geosediment) accumulations in horses: a retrospective study. Acta Vet. Scand. 58, 73. Kellam, L.L., Johnson, P.J., Kramer, J. and Keegan, K.G. 2000. Gastric impaction and obstruction of the small intestine associated with persimmon phytobezoar in a horse. J. Am. Vet. Med. Assoc. 216, 1279–1281. Kendall, A., Ley, C., Egenvall, A. and Bröjer, J. 2008. Radiographic parameters for diagnosing sand colic in horses. Acta Vet. Scand. 50, 17. Keppie, N.J., Rosenstein, D.S., Holcombe, S.J. and Schott, H.C. 2008. Objective radiographic assessment of abdominal sand accumulation in horses. Vet. Radiol. Ultrasound 49, 122–128. Kilcoyne, I., Dechant, J.E., Spier, S.J., Spriet, M. and Nieto, J.E. 2017. Clinical findings and management of 153 horses with large colon sand accumulations. Vet. Surg. 46, 860–867. Korolainen, R. and Ruohoniemi, M. 2002. Reliability of ultrasonography compared to radiography in revealing intestinal sand accumulations in horses. Equine Vet. J. 34, 499–504. Le Jeune, S. and Whitcomb, M.B. 2014. Ultrasound of the equine acute abdomen. Vet. Clin. North Am. Equine Pract. 30, 353–381. Lindah, S., Söderlund, R., Frosth, S., Pringle, J., Båverud, V. and Aspán, A. 2011. Tracing out- breaks of Streptococcus equi infection (strangles) in horses using sequence variation in the SeM gene and pulse-field gel electrophoresis. Vet. Microbiol. 153, 144–149. Loesch, D.A., Rodgerson, D.H., Haines, G.R. and Watt, B.C. 2002. Jejunoileal anastomosis following small intestinal resection in horses: seven cases (1999-2001). J. Am. Vet. Med. Assoc. 221,541–545. Ludwig, E.K., Hobbs, K.J., McKinney-Aguirre, C.A. and Gonzalez, L.M. 2023. Biomarkers of intestinal injury in colic. Animals 13, 227. Mair, T. and Sherlock, C. 2023. Recurrent colic: diagnosis, management, and expectations. Vet. Clin. North Am. Equine Pract. 39, 399–417. Mehdi, S. and Mohammad, V. 2006. A farm-based prospective study of equine colic incidence and associated risk factors. J. Equine Vet. Sci. 26, 171–174. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2002. Echo-guided studies on portal and hepatic blood in cattle. J. Vet. Med. Sci. 64, 23–28. Mohamed, T., Oikawa, S., Nakada, K., Kurwasawa, T., Sawamukai, Y. and Sato, H. 2003a. Percutaneous ultrasound-guided over-the-wire catheterization of the portal and hepatic vessels in cattle. J. Vet. Med. Sci. 65, 813–816. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2003b. Transcutaneous ultrasound-guided pancreatic biopsy in cattle and its safety: a preliminary report. Vet. J. 166, 188–193. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2003c. Ultrasound-guided biopsy of the pancreas of cattle. Vet. Rec. 153, 467. Mohamed, T. and Oikawa, S. 2008. Efficacy and safety of ultrasound-guided percutaneous biopsy of the right kidney in cattle. J. Vet. Med. Sci. 70, 175–179. Moraes, C.M., Vargas, A.P.C., Leite, F.P.L., Nogueira, C.E.W. and Turnes, C.G. 2009. Strangles: etiology, diagnosis and control. Ciência Rural 39,1944–1952. Needles, R.K. and Dubois, M.S. 2019. Surgical treatment of persistent colic in a horse caused by an anomalous vascularized fibrous band. Can Vet J. 60, 991–994. Nielsen, M.K., Jacobsen, S. and Olsen, S.N. 2016. Nonstrangulating intestinal infarction associated with Strongylus vulgaris in referred Danish equine cases. Equine Vet. J. 48, 376–379. Nielsen, M.K., Jacobsen, S., Olsen, S.N., Bousquet, E. and Pihl, T. 2016. Nonstrangulating intestinal infarction associated with Strongylus vulgaris in referred Danish equine cases. Equine Vet J. 48, 376–379. Niinisto, K.E., Ruohoniemi, M.O., Freccero, F. and Raekallio, M.R. 2018. Investigation of the treatment of sand accumulations in the equine large colon with psyllium and magnesium sulphate. Vet. J. 238, 22–26. Odelros, E., Kendall, A., Hedberg-Alm, Y. and Pringle, J. 2019. Idiopathic peritonitis in horses: a retrospective study of 130 cases in Sweden (2002-2017). Acta Vet Scand. 61(1), 18. Osman, S.A., Tharwat, M. and Saeed, E.M.A. 2021. An outbreak of strangles in Arabian horses in Saudi Arabia: epidemiology, clinical signs and treatment outcomes. Int. J. Vet. Sci. 10, 323–328. Pauwels, F.F., Hawkins, J.F., MacHarg, M.A., Rothenbuhler, R.D., Baird, D.K. and Moulton, J.S. 2007. Congenital retrosternal (Morgagni) diaphragmatic hernias in three horses. J. Am. Vet. Med. Assoc. 231, 427–432. Pease, A.P., Scrivani, P.V., Erb, H.N. and Cook, V.L. 2004. Accuracy of increased large-intestinal wall thickness during ultrasonography for diagnosing large-colon torsion in 42 horses. Vet. Radiol. Ultrasound 45, 220–224. Pihl, T.H., Andersen, P.H., Kjelgaard-Hansen, M., Mørck, N.B. and Jacobsen, S. 2013. Serum amyloid A and haptoglobin concentrations in serum and peritoneal fluid of healthy horses and horses with acute abdominal pain. Vet. Clin. Pathol. 42, 177–183. Pihl, T.H., Nielsen, M.K., Olsen, S.N., Leifsson, P.S. and Jacobsen, S. 2018. Nonstrangulating intestinal infarctions associated with Strongylus vulgaris: clinical presentation and treatment outcomes of 30 horses (2008-2016). Equine Vet. J. 50, 474–480. Pihl, T.H., Scheepers, E., Sanz, M., Goddard, A., Page, P., Toft, N., Kjelgaard-Hansen, M., Andersen, P.H. and Jacobsen, S. 2016. Acute-phase proteins as diagnostic markers in horses with colic. J. Vet. Emerg. Crit. Care 26, 664–674. Proudman, C.J., Smith, J.E., Edwards, G.B. and French, N.P. 2002. Long-term survival of equine surgical cases. Part 1: patterns of mortality and morbidity. Equine Vet. J. 34, 432–437. Radcliffe, R.M., Liu, S.Y., Cook, V.L., Hurcombe, S.D.A. and Divers, T.J. 2022. Interpreting abdominal fluid in colic horses: understanding and applying peritoneal fluid evidence. J. Vet. Emerg. Crit. Care 32, 81–96. Ragle, C.A., Yiannikouris, S., Tibary, A.A. and Fransson, B.A. Use 2013. of a barbed suture for laparoscopic closure of the internal inguinal rings in a horse. J. Am. Vet. Med. Assoc. 242, 249–253. Recknagel, S., Nicke, M. and Schusser, G.F. 2012. Diagnostic assessment of peritoneal fluid cytology in horses with abdominal neoplasia. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 40, 85–93. Reed, S.M., Bayly, W.M. and Selon, D. 2004. Equine internal medicine. Saunders, Philadelphia, pp: 1253–1289. Reef, V.B., Whittier, M. and Allam, L.G. 2004. Sonographic evaluation of the adult abdomen. Clin. Tech. Equine. Pract. 3, 294–307. Romero, A.E. and Rodgerson, D.H. 2010. Diaphragmatic herniation in the horse: 31 cases from 2001-2006. Can. Vet. J. 51, 1247–1250. Ruohoniemi, M., Kaikkonen, R., Raekallio, M. and Luukkanen, L. 2001. Abdominal radiography in monitoring the resolution of sand accumulations from the large colon of horses treated medically. Equine Vet. J. 33, 59–64. Ryu, S.H., Jang, J.D., Bak, U.B., Lee, C., Youn, H.J. and Lee, Y.L. 2004. Gastrointestinal impaction by parascaris equorum in a thoroughbred foal in Jeju, Korea. J. Vet. Sci. 5, 181–182. Scharner, D., Rotting, A., Gerlach, K., Rasch, K. and Freeman, D.E. 2002. Ultrasonography of the abdomen in a horse with colic. Clin. Tech. Equine Pract. 1, 118–124. Schott, H.C., Waldridge, B. and Bayly, W.M. 2018. Disorders of the urinary system. In: Reed SM, Bayly WM, Sellon DC, editors. Equine internal medicine. St. Louis, MI: Elsevier; pp: 888–889. Schumacher, J. and Schmitz, D. 2002. Macroscopic hematuria of horses. Equine Vet. Educ. 14, 201–210. Singer, E.R. and Smith, M.A., 2002. Examination of the horse with colic: is it medical or surgical? Equine Vet. Educ. 34, 87–96. Smith, B.P. 2015. Large Animal Internal Medicine. 5th ed. Mosby, St. Louis, Missouri, USA. Spanton, J.A., Mair, T.S. and Sherlock, C.E. 2019. Non-strangulating intestinal infarction in horses in the UK: a review of 15 cases. Equine Vet. Educ. 32, 603–610. Sweeney, C.R., Timoney, J.F., Newton, J.R. and Hines, M.T. 2005. Streptococcus equi infections in horses: guidelines for the treatment, control, and prevention of strangles. J. Vet. Intern. Med. 19, 123–134. Tannahill, V.J., Cardwell, J.M. and Witte, T.H., 2019. Colic in the British military working horse population: a retrospective analysis. Vet. Rec. 184, 24. Teodoro, T.G.W., Uzal, F.A., Streitenberger, N., Samol, M.A., Henderson, E.E. and Asin J. 2023. Colonic sand impaction with cecal rupture and peritonitis in an adult African savanna elephant: a review of the noninfectious causes of gastrointestinal disease in elephants. J. Vet. Diagn. Invest. 35, 47–52. Tharwat, M., El-Magawry South, Abdel Raouf, M. and Shety, T. 2008. Gastrointestinal colic in horses: clinical, laboratory, ultrasonographic, and postmortem findings. Zagazig Vet. J. 36, 107–121. Tharwat, M. 2011. Diagnostic ultrasonography in cattle and buffaloes with intestinal obstruction. J. Agri. Vet. Sci. 4, 67–80. Tharwat, M., Ahmed, A.F. and El-Tookhy, O. 2012a. Chronic peritonitis in buffaloes and cattle: clinical, hematological, ultrasonographic findings, and treatment. J. Anim. Vet. Adv. 15, 2775–2781. Tharwat, M., Al-Sobayil, F., Ali, A. and Buczinski, S. 2012b. Ultrasonographic evaluation of abdominal distension in 52 camels (Camelus dromedarius). Res. Vet. Sci. 93, 448–456. Tharwat, M. and Al-Sobayil, F. 2014a. Influence of cardiac glycoside digoxin on cardiac troponin I, acid–base and electrolyte balance, and hematobiochemical profiles in healthy donkeys (Equus asinus). BVC Vet. Res. 10, 64. Tharwat, M. and Al-Sobayil, F. 2014b. Influence of transportation on serum concentrations of the cardiac biomarkers troponin I and creatine kinase myocardial band in horses. J. Equine Vet. Sci. 34, 662–667. Tharwat, M. and EL-Deeb, W. 2015. Clinical, biochemical, and ultrasonographic findings in buffalo calves with obstructive urolithiasis. Glob. Vet. 14, 118–123. Tharwat, M. and Al-Sobayil, F. 2016. Ultrasonographic findings in camels (Camelus dromedarius) with different urinary affections. J. Camel Pract. Res. 23, 301–8. Tharwat, M. 2019. Chronic peritonitis in dromedary camels: clinical, ultrasonographic, and pathologic findings. J. Camel Pract. Res. 26, 169–172. Tharwat, M. 2020. Ultrasonography of the gastrointestinal tract in healthy and diseased camels (Camelus dromedarius). Camel Health 2, 10–15. Tharwat, M. 2021a. Clinical, ultrasonographic, and postmortem findings in sheep and goats with different urinary tract disorders. Vet. World 14, 1879–1887. Tharwat, M. 2021b. Obstructive urolithiasis in dromedary camels: clinical, ultrasonographic, and postmortem findings. J. Camel Pract. Res 28, 85–93. Tharwat, M. and Al-Sobayil, F. 2022a. Effect of a long road transport journey on serum biomarkers of bone formation and resorption in athletic horses. Int. J. Vet. Sci. 11, 268–271. Tharwat, M. and Al-Sobayil, F. 2022b. Effects of acute blood loss on inflammatory and bone biomarkers, acid base balance, blood gases, and hemato-biochemical profiles in sedated donkeys (Equus asinus). Int. J. Vet. Sci. 11, 479–485. Tharwat, M. 2023. Changes in acid-base balance, blood gases, and hemato-biochemical parameters in Arabian camels with different urinary tract disorders. Int. J. Vet. Sci. 12, 724–729. Tharwat, M., Alkhedhairi, and South Marzok, M. 2024a. Intestinal obstruction in dromedary camels: clinical and ultrasonographic findings as well as variations in acid-base balance, blood gases, and hematobiochemical profiles. Int. J. Agri. Bios. 13, 500–504. Tharwat, M., Al-Sobayil, F., Haytham, and Ali H. 2024b. Changes in the hematobiochemical, acid–base, and blood gas elements, as well as biomarkers of inflammation and bone metabolism, in donkeys (Equus asinus) with acute bleeding. Open Vet. J. 14, 1146–1153. Tharwat, M., El-Magawry, S., Kandeel, A. and Alkheraif, A.A. 2024c. Mesenteric abscessation caused by Pseudomonas aeruginosa in a thoroughbred mare: clinical, etiological, hematobiochemical, sonographic and treatment follow-up. Int. J. Vet. Sci.13, 1017–1022. Uzal, F.A. and Diab, S.S. 2015. Gastritis, enteritis, and colitis in horses. Vet. Clin. North Am. Equine Pract. 31, 337–358. Uzal, F.A., Plattner, B.L. and Hostetter, J.M. 2015. Alimentary system. In: Maxie M.G., editor. Jubb, Kennedy, and Palmer’s pathology of domestic animals. 6th edition. St Louis, MI: Elsevier; pp. 1–257. Vainio, K., Sykes, B.W. and Blikslager, A.T. 2011. Primary gastric impaction in horses: a retrospective study of 20 cases (2005–2008). Equine Vet. Educ. 23, 186–190. Valdes, A. and Johnson, J.R. 2005. Septic pleuritis and abdominal abscess formation caused by Rhodococcus equi in a foal. J. Am. Vet. Med. Assoc. 227, 960–963. van der Linden, M.A., Laffont, C.M. and van Oldruitenborgh-Oosterbaan, M.M.S. 2003. Prognosis in equine medical and surgical colic. J. Vet. Intern. Med. 17, 343–348. Voermans, M., Butler, C.M., van der Velden, M.A. and Sloet van Oldruitenborgh-Oosterbaan, M.M. 2004. Hernia umbilicalis incarcerata bij het paard, een literatuuroverzicht aan de hand van een casus [Incarcerated umbilical hernia in the horse: a case with a review of the literature]. Tijdschr. Diergeneeskd. 129, 142–149. Article in German. Watts, A.E., Johnson, A.L., Felippe, M.J. and Divers, T.J. 2011. Recurrent actinobacillus peritonitis in an otherwise healthy thoroughbred horse. Aust. Vet. J. 89, 143–146. Westerman, T.L., Foster, C.M., Tornquist, S.J. and Poulsen, K.P. 2016. Evaluation of serum amyloid A and haptoglobin concentrations as prognostic indicators for horses with colic. J. Am. Vet. Med. Assoc. 248, 935–940. Winfield, L.S. and Dechant, J.E. 2015. Primary gastric rupture in 47 horses (1995-2011). Can. Vet. J. 56, 953–958. Worku, Y., Wondimagegn, W., Aklilu, N., Assefa, Z. and Gizachew, A. 2017. Equine colic: clinical epidemiology and associated risk factors in and around Debre Zeit. Trop. Anim. Health Prod. 49. 959–965. Zakia, L.S., Gomez, D.E., Kenney, D.G. and Arroyo, L.G. 2021. Sabulous cystitis in the horse: 13 cases (2013-2020). Can. Vet. J. 62, 743–750. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Al-sobayil F. Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories. Open Vet. J.. 2025; 15(3): 1116-1139. doi:10.5455/OVJ.2025.v15.i3.5 Web Style Tharwat M, Al-sobayil F. Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories. https://www.openveterinaryjournal.com/?mno=238827 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.5 AMA (American Medical Association) Style Tharwat M, Al-sobayil F. Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories. Open Vet. J.. 2025; 15(3): 1116-1139. doi:10.5455/OVJ.2025.v15.i3.5 Vancouver/ICMJE Style Tharwat M, Al-sobayil F. Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1116-1139. doi:10.5455/OVJ.2025.v15.i3.5 Harvard Style Tharwat, M. & Al-sobayil, . F. (2025) Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories. Open Vet. J., 15 (3), 1116-1139. doi:10.5455/OVJ.2025.v15.i3.5 Turabian Style Tharwat, Mohamed, and Fahd Al-sobayil. 2025. Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories. Open Veterinary Journal, 15 (3), 1116-1139. doi:10.5455/OVJ.2025.v15.i3.5 Chicago Style Tharwat, Mohamed, and Fahd Al-sobayil. "Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories." Open Veterinary Journal 15 (2025), 1116-1139. doi:10.5455/OVJ.2025.v15.i3.5 MLA (The Modern Language Association) Style Tharwat, Mohamed, and Fahd Al-sobayil. "Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories." Open Veterinary Journal 15.3 (2025), 1116-1139. Print. doi:10.5455/OVJ.2025.v15.i3.5 APA (American Psychological Association) Style Tharwat, M. & Al-sobayil, . F. (2025) Equine colic: A comprehensive overview of the sonographic evaluation, diagnostic criteria, and management of different categories. Open Veterinary Journal, 15 (3), 1116-1139. doi:10.5455/OVJ.2025.v15.i3.5 |