| Research Article | ||
Open Vet. J.. 2025; 15(3): 1446-1467 Open Veterinary Journal, (2025), Vol. 15(3): 1446-1467 Research Article Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impactReham M. ElBakrey1, Amal A.M. Eid1*, Ahmed A. ElKholy2, Mohamed R. Mousa3, Mohamed A. El-Morsy4, Walaa S. Abdelaziz11Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Veterinarian, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 4Department of Poultry and Rabbit Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt *Corresponding Author: Amal A. M. Eid, Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: amalaeidvet [at] gmail.com Submitted: 02/02/2025 Accepted: 13/03/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
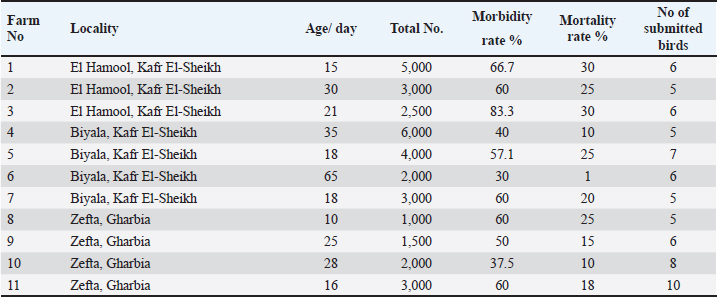
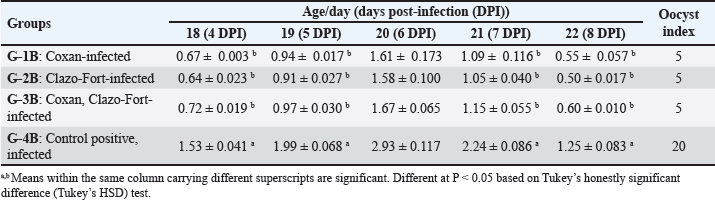
AbstractBackground: Among the serious quail diseases is coccidiosis, caused by several Eimeria species, particularly cecal coccidiosis, with considerable economic losses. Aim: First, 11 quail flocks showing brownish diarrhea tinged with blood and bloody cecal core were submitted for Eimeria spp. detection. A selected E. tsunodai was used to evaluate the comparative anticoccidial efficacy of a commercial herbal product containing oregano and garlic essential oils (EOs) as a prophylactic supplement via drinking water taking into consideration that it had never been applied in quails’ coccidiosis before. Methods: A total of 300 Japanese quails were equally assigned to four groups. One of the groups was given basal drinking water and served as the control (G-4). The remaining groups (G-1, 2, and 3) received drinking water containing herbal (Coxan), chemical (Clazo-Fort), and herbal interchangeably with chemical products, respectively. At 14 days of age, each group was subdivided into two subgroups. The subgroups, 1B–4B were infected with sporulated oocysts of E. tsunodai (4.1 × 104). Results: The infected group showed a typical cecal lesion of E. tsunodai which was confirmed histopathologically by the presence of different developmental stages in both intracellular lining and cecal content. The alternative herbal product had the highest anticoccidial index with a value of 154.88. Anticoccidial sensitivity test and reduction of lesion score were 77.3, which indicated both herbal and chemical products were sensitive against E. tsunodai. Additionally, quails supplemented with Coxan had the highest BWG (p < 0.05). Additionally, a high HI antibody titer against NDV was obtained in the Coxan group with a significant increase (7.66 ± 0.67 log2; p=0.039) at 21 days of age and a high CD4 antigen value (1762 ± 87.5 pg/ml) in sera at 14 days of age. In the jejunum of the Coxan group, a significant increase in villi length was associated with a reduction in the crypt depth, and the highest villi length and crypt depth ratio were represented, accompanied by a higher count of intestinal lactobacillus ( p=0.0236) compared to the infected group. Conclusion: The prophylactic supply of alternative herbal products containing oregano and garlic EOs could be a safe, potent anticoccidial in quails that is consistent with the world’s transition to a green economy besides its immune-stimulant properties. Keywords: Japanese quails, E. tsunodai, Essential oils, Anticoccidial index, Intestinal morphology. IntroductionQuails are mid-sized birds, reared for the production of meat and eggs, and used as laboratory animals (Tsutsumi, 1972; Sreeranjini et al., 2010; Bahar et al., 2014). Coccidiosis is one of the most significant diseases affecting poultry, including quails and is recognized as one of the limiting factors for this industry development (Seok et al., 2003; Bashtar et al., 2010; Gesek et al., 2014). Coccidiosis is caused by intracellular parasites that belong to different Eimeria species (Rose and Hesketh, 1979; Kemp et al., 2013). Quails are affected by different Eimeria species: E. bateri and E. conturnicis from grey quails (Tsutsumi, 1972); E. okanaganensis and E. lophortygis from California quails; E. crusti and E. oreortygis from mountain quails (Duszynski and Gutievrez, 1981); E. colini and E. lettyae from Bobwhite quails (Ruff, 1985); E. tahamensis from Arabian quails (Amoudi, 1987); E. tsunodai, E. uzura, E. bateri, and E. taldykurganica from Japanese quails (Ruff et al., 1984; Teixeira et al., 2004). Furthermore, E. minima (Teixeira and Lopes, 2000), E. bahli, and E. colini (Ramadan et al., 2021) were recovered from the Japanese quail. In the Japanese quail, E. bateri and E. uzura species were detected in the small intestine, while E. tsunodai in the ceca was considered more pathogenic (Teixeira et al., 2004). Eimeria species commonly cause the gastrointestinal tract and different degrees of enteritis, such as diarrhea, dehydration, depression, anorexia, weight loss, and poor reproductive performance (Duszynski, 2001; Teixeira et al., 2004; Gesek et al., 2014; El-Morsy et al., 2016). Furthermore, the subclinical infection results in a loss of performance due to a disorder in the function of the intestine (Marien and Gussem, 2007). The imbalance of the gut microbiota caused by coccidiosis reduces food intake, impairs digestion and absorption, and makes the birds more vulnerable to secondary infections with negative impact on bird performance (Peek and Landmanab, 2011; Lu et al., 2021). The mainstream strategy in poultry for coccidiosis control is using anticoccidial chemicals with rotation programs or vaccination with live Eimeria oocytes (Lillehoj et al., 2005). The increased resistance of Eimeria species to chemotherapeutic agents has been reported worldwide (Abbas et al., 2012; Habibi et al., 2016). The laborious manufacturing process, the high expense of creating new vaccines, and the hazard of pathogen spread could be limitations (Witcombe and Smith, 2014; Khater et al., 2020). As a consequence, research is now focusing on the development of antimicrobial alternatives, and the animal agricultural sector has made them a top priority in order to maintain the health and growth performance of animals (Peek and Landmanab, 2011; Landers et al., 2012; Gadde et al., 2017; Lillehoj et al., 2018). Botanicals and natural identical compounds are well renowned for their antimicrobial and antiparasitic activity, so they can represent a valuable tool against Eimeria (Cobaxin-Cardenas, 2018). There are many plant-derived drugs with anticoccidial effects, such as plant extracts and essential oils (EOs) (Nweze and Obiwulu, 2009). Compared with chemotherapeutic drugs, anticoccidial herbal medicines usually exhibit fewer drug residues and less drug resistance, naturally stimulate the immune system, and enhance growth performance (Quiroz-Castaneda and Dantan-Gonzalez, 2015; Habibi et al., 2016). Among potential sources of plant-based products that have been effective in treating coccidiosis are oregano and garlic (Giannenas et al., 2003; Pourali et al., 2014). Oregano (Origanum Vulgare sp.) is an aromatic-medicinal plant that is used as a natural growth enhancer with potent anticoccidial properties and has a significant impact on intestinal microbiota and intestinal cell functionality as its EOs improve weight gain and feed conversion ratio (Giannenas et al., 2003; Paraskeuas et al., 2017; Tzora et al., 2017; Giannenas et al., 2018). Carvacrol and thymol compounds are the main components of oregano EOs and are thought to provide anticoccidial activity by maintaining intestinal integrity (Economou et al., 1991; Giannenas et al., 2003; Greathead and Kamel, 2006; da Silva et al., 2009). Garlic (Allium Sativum) has antiparasitic effects in addition to anti-bacterial, anti-viral, and antioxidant effects (Gebreyohannes and Gebreyohannes, 2013). Garlic has high levels of organosulfur with their precursors (allicin, diallyl disulfide, and diallyl trisulfide) that play key roles in anti-inflammatory and antioxidant pathways with probable anticoccidial effects (Dkhil et al., 2011; Pourali et al., 2014; Ali et al., 2019). In view of the potentially harmful effects of chemotherapy on human health, with the outlawing of the European Union from using antibiotic growth promoters in animal diets in 2006 (Franz et al., 2010), the use of phytocompounds has become a necessary strategy. Subsequently, the objectives of this study were to investigate coccidiosis among quail flocks suffering from diarrhea via the clinical diagnosis, and identification of Eimeria species as well as to evaluate the comparative efficacy of a commercial herbal EOs compound with one of the widely used anticoccidial chemicals as prophylaxis against cecal coccidiosis in Japanese quails in terms of their potential effects on body weight, blood in feces, fecal oocyst shedding, survival rate, lesion scoring, intestinal histomorphometry, and the immune status of the birds. Materials and MethodsStudy areas, sample collection, and identification of coccidia from quailDuring 2022, 11 commercial quail farms allocated in Kafr El-Sheikh and Gharbia provinces in Egypt were examined. The birds exhibited diarrhea, ruffled feathers, anorexia, recumbence, and decreased body weight. The size of the examined Japanese quail flocks ranged from 1,000 to 6,000 birds; the ages were 10 to 65 days. Based on the aforementioned signs, sixty-nine quails were selected (5–10 birds/ farm) and submitted to the Avian and Rabbit Medicine Department, Zagazig University, Egypt, for laboratory diagnosis (Table 1). The submitted quails were euthanized according to the guidelines for the care and use of laboratory animals. The post-mortem examination was carried out. In addition, samples of scraped mucosa were taken from the small and large intestines and examined microscopically for the detection of developmental stages of Eimeria spp. The coccidial oocysts were examined using the floatation technique under a light microscope (Duszynski and Wilber, 1997). The species of oocysts were identified based on the site of lesions and the morphological characteristics as described by Teixeira et al. (2004). Oocyst-positive samples were harvested using the modified Wisconsin (sugar flotation) technique. Table 1. Details of collected samples from commercial Japanese quail farms in Egypt during 2022.
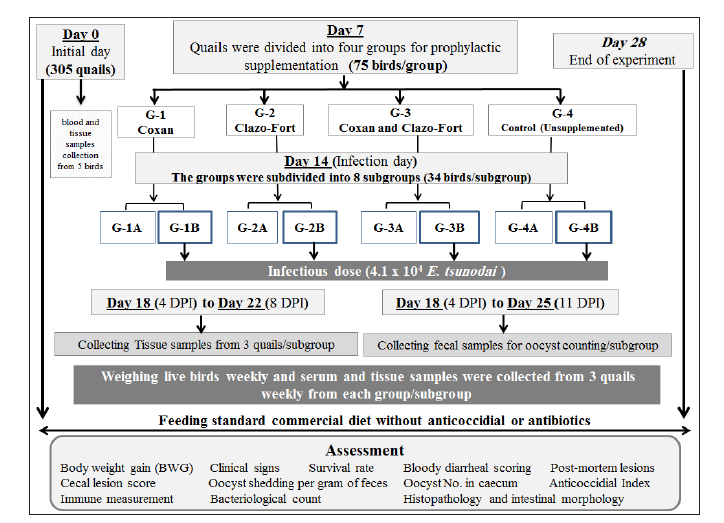
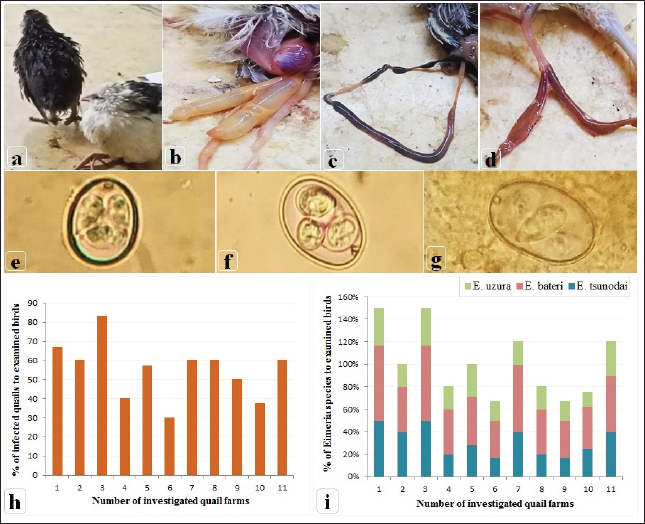
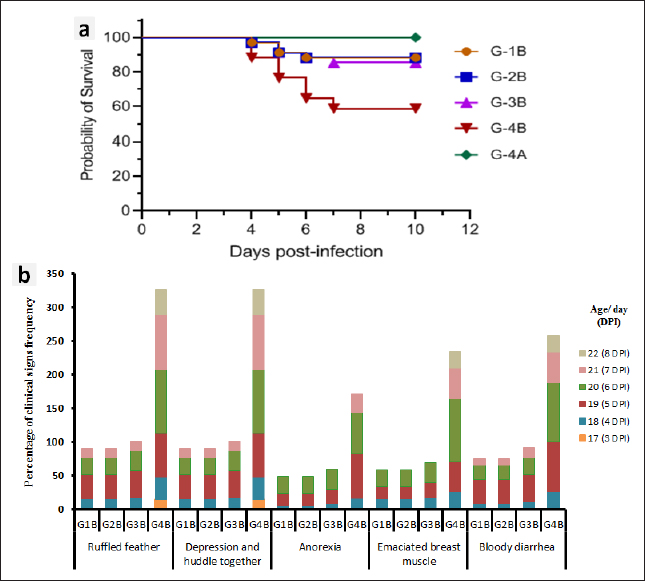
Fig. 1. Experimental design for studying the anticoccidial effects of phytotherapy and chemotherapy supplementation in Japanese quails. Experimental efficacy of phytotherapyPreparation of Eimeria tsunodai Positive samples of coccidial oocysts of E. tsunodai that were obtained from the ceca of naturally infected quails (Flock No. 11) were collected and sporulated using freshly prepared 2.5 % (w/v) potassium dichromate (K2Cr2O7) and then incubated at room temperature (28°C) for 4 days. After sporulation, oocysts were recovered by centrifugation with saturated sugar solution (Duszynski and Wilber, 1997) and then preserved in 2.5% potassium dichromate solution at 4°C. The total number of oocysts was determined using the McMaster technique as described by Hodgson (1970). The sporulated oocysts were diluted using normal saline and each bird was orally infected with 1 ml of 4.1 × 104 sporulated oocysts of E. tsunodai via the crop-route using a syringe with a fine rubber tube at 14 days of age (El-Morsy et al., 2016). Herbal and chemotherapy productsTwo commercial products are used: one is an herbal product “Coxan” obtained from the phytotherapic Solutions, S.L. company, Spain, which is registered as an animal health product with a License number: Reg.: aESP08200489 and Egyptian license number: 7194 /Date: 17/5/2018; batch #5EG003_04. Its composition per 100 ml contains: 80 g oregano oil (45 g carvacrol and 2 g thymol) and 25 g garlic oil (10 g diallyl disulfide and 3 g diallyl trisulfide). The second was chemotherapy “Clazo-Fort” obtained from the 2M group Company and contained diclazuril in a water-soluble formulation of 2%. Both herbal and chemotherapy were used in this study as prophylactic supplements in drinking water with a dose of 1 ml/ liter as recommended by the manufacturer’s structure and 0.5 ml /10 liter as a concentration of 1 ppm (El-Banna et al., 2005), respectively. Experimental animalsA total of 305 1-day-old clinically healthy Japanese quails (Coturnix japonica) were purchased from a commercial hatchery. Five birds were selected and euthanized for blood and tissue sample collection to measure antibodies and pathogenic bacterial and protozoa detection, respectively, in which the free bacterial and protozoan birds (n=300) were allocated to the experimental units in battery system rearing with optimum 24 hours lightening program under strict hygienic conditions and reared as a single group from day 1 until day 6. The quails were fed a standard commercial diet based on corn and soybean meal and were formulated without anticoccidial or antibiotic growth promoters at the starter and grower phases. Feeding and watering were given ad libitum. Strict sanitation practices were maintained in the house before and during the experiment. Experimental DesignOn day 7, the quail chicks were randomly assigned to four groups (75 birds/ group) and housed in batteries of identical size. The first and second bird groups were prophylactically supplemented with a commercial herbal product (G-1: Coxan) and a commercial chemotherapy product (G-2: Clazo-Fort). In the third group, both products were supplemented interchangeably for 12 hours daily for each (G-3: Coxan and Clazo-Fort). While the fourth group was an unsupplemented control group (G-4: Control). The supplementation of Coxan and Clazo-Fort was added to the drinking water and lasted for the duration of the experiment. At 14 days of age, the four bird groups were equally subdivided into eight subgroups. One subgroup from each group was selected and infected with a suspension of fresh sporulated oocysts of 4.1 × 104 E. tsunodai (El-Morsy et al., 2016). All quails were routinely vaccinated against Newcastle disease virus (NDV) at 6 and 13 days of age using MEVACTM ND HB1 and MEVACTM ND Elite (both vaccines are live attenuated Newcastle disease vaccines) via eye dropping, respectively. The design of the experiment is summarized in Figure 1. Three quails from each subgroup were randomly selected and euthanized at 7, 14, and 18 to 22 days of age. At the end of the experiment, five quails from each subgroup were euthanized for the collection of blood for serological examination. Additionally, tissue samples (intestinal segments such as jejunum and caecum) were submitted for pathological and histopathological examination. The prophylactic anticoccidial efficacy was assessed on the basis of the recording and calculation of body weight gain (BWG), survival rate, clinical symptoms, blood in feces, oocysts shedding per gram of feces (OPG), cecal lesion score, anticoccidial index (ACI), histopathology and intestinal morphology, enumeration of intestinal microflora, the relative weight of immune organs, and immune status measurement of the birds. Clinical symptoms and BWGAll experimental quails were observed and examined twice daily to record the survival rate and clinical signs. The live birds of each group or subgroup were weighed at zero, 7, 14, 21, and 28 days of age to determine the live body weight (BW) and BWG. Fecal blood scoringThe extent of blood in feces was observed daily post-infection and assigned from 0 to 4 according to Youn and Noh (2011). A score of 0 indicated normal feces without hemorrhage; a score of 1 indicated 1%–25% hemorrhage in the feces; a score of 2 indicated 25%–50% hemorrhage in the feces; a score of 3 indicated 51%–75% hemorrhage in the feces; and a score of 4 indicated 76%–100% hemorrhage in the feces. Cecal lesion scoreThree quails from each subgroup were randomly selected and euthanized at 4–8 DPI, in addition to the dead birds. The gross lesions in the ceca of each bird were examined. The lesions included ballooning, hemorrhages, thickening of the cecum wall, and mucoid to mass consistency of cecal content. The lesion scores ranged from 0 to +3 according to Elmorsy et al. (2021a). Oocyst counts (oocysts shedded per gram: OPG) of fecesFecal droppings were collected daily from each subgroup from 4 to 11 days post-infection (DPI), and the number of oocysts was counted microscopically using a McMaster chamber technique as described by Hodgson (1970). Concisely, the collected feces from each subgroup were thoroughly mixed. One gram of homogenized feces was uniformly dissolved in a 30-ml saturated salt solution with vigorous churning, and then filtration was performed. For oocyst counting, the filtrate was put onto a McMaster Egg Slide that has two chambers with two identical 10-, 10-, and 1.5-mm grids (0.15 ml). Under both grids, the total number of oocysts was counted. The total number of oocysts in the two chambers was multiplied by 100 to determine the oocyst counts (oocysts per gram). Oocyst count in cecum contentsThe counting of oocysts in the cecum contents was determined as described above (OPG). The score of the oocysts index was calculated at 18 to 22 days old (4–8 DPI) and ranged from 0 to 40 as described by Song et al. (2020): a score of 0 revealed the oocyst counts ranged from 0 to 0.1 × 106; a score of five revealed the oocyst counts were 0.11 × 106–1 × 106; a score of 10 revealed the oocyst counts were 1.1 × 106−1.9 × 106; a score of 20 revealed the oocyst counts were 2.0 × 106−5.9 × 106; a score of 30 revealed the oocyst counts were 6.0 × 106−10.9 × 106; and a score of 40 revealed the oocyst counts were more than 1.1 × 107. Anticoccidial sensitivity and protectionACI, AST, Reduction lesion score (RLS), Protection rate against lesion scores, and oocyst outputs were conducted to evaluate the efficiency of prophylactic treatment. ACI was calculated using the formula: ACI=(Relative body weight gain rate (RBWG %) + Survival rate (S %)) − (lesions score index (LSI) + Oocyst index) according to De Pablos et al. (2010). The RBWG% was detected as a ratio of the average BWG rate of the infected-unsupplemented or supplemented groups to the ABWG of the uninfected-unsupplemented group × 100. Briefly, ACI values usually range from 0 to 200. Therefore, ACI values less than 120 were considered as having an inactive anticoccidial effect. ACI values between 120 to 140, 140 to 160, 160 and 180, or above 180 were categorized as mild, moderate, marked, and excellent, respectively (Shetshak et al., 2021). The formula of AST according to McDougald et al. (2015) was applied to calculate the values of AST and Reduction of lesion scores (RLS). The formula=(average lesion score in unsupplemented-infected group – average lesion score in prophylactic supplemented group) / average lesion score in unsupplemented-infected group ×100. The AST and RLS ≥ 50% were judged to be sensitive and < 50% were resistant. The protection percentage against lesion score was detected using the formula described by Singh and Gill (1976) after modification according to the lesion score to E. tsunodai in quails. Protection %=3 – mean lesion score /3 (maximum expected score) × 100 Protection rate (%) against oocyst output=(oocyst output of unsupplemented-infected group–oocyst output of healthy control or supplemented groups) ÷ (oocyst output of unsupplemented-infected group) × 100 (Zhang et al., 2012, Kaingu et al., 2017, Qaid et al., 2021). The total number of oocysts produced by each bird in its feces and cecum was used to calculate the protection rate. Histopathology, microscopic lesion score, and intestinal histomorphometryOn dissection of birds, intestinal segments collected from 4 to 8 DPI from different subgroups were kept in neutral buffered formalin (10%) for fixation, followed by routine tissue processing in different grades of alcohols, changes of xylenes, and finally embedded in melted paraffin wax. Five-micrometer sections were cut on glass slides to be stained with hematoxylin and eosin (H&E) for light microscopy (Bancroft and Gamble, 2008). An Olympus CX43 (Olympus, Japan) light microscope equipped with an XCAM digital camera (ToupTek, China) was used to examine the stained tissue sections and capture photomicrographs. A microscopic lesion scoring system was followed to evaluate the severity of infestation in the examined intestinal segments. Briefly, a score from 0 to 4 was given describing the presence of the parasite per each day of examination (4–8 DPI): 0=absence of the parasite; 1=slight amounts of parasite in less than 10 villi or crypts; 2=moderate amounts of parasites in less than 10 villi or crypts; 3=moderate to large amounts of parasites in more than 10 villi or crypts; 4=heavy infestation in 80% to 100% of villi or crypts (Balestrin et al., 2022). Mean values for intestinal villi length, crypt depth, and villi-to-crypt ratio were estimated in each experimental group (Ali et al., 2020). NDV-specific antibody assayWhole-blood samples were collected from the euthanized quails at zero, 7, 14, 21, and 28 days of age to separate sera and evaluate the immune status of the quails under the supplementation of different products and infection with coccidia. The antibody titers against NDV were determined by a hemagglutination inhibition (HI) assay using 4 hemagglutination (HA) units for the LaSota vaccine strain of NDV (Pestikal® LA SOTA SPF) and 1% washed chicken RBCs, in accordance with the WOAH Terrestrial Manual (2021). All sera samples were heat inactivated at 56°C for 30 minutes and then treated with 1% chicken RBCs. The HI titer was expressed as the log2 of the reciprocal of the highest serum dilution, which inhibited the HA activity. Determination of CD4/CD8 molecules by ELISAThe separated serum samples collected weekly were used for the detection of the avian cluster of differentiations. To determine the concentrations of CD4 and CD8 molecules, we used the Avian Cluster of Differentiation 4 and 8 (CD4 and CD8) ELISA Kit (SunLong Biotech Co., LTD), based on the manufacturer’s instructions. Enumeration of intestinal microfloraThe digesta and scraping of the mucosal surface of the ilium and caecum of five euthanized quails from each subgroup at 28 days old (the end of the experiment) were collected aseptically into sterile tubes. A quantity of 1 g of the homogenized ileocecal content was transferred into a sterile test tube containing 9 ml of sterile nutrient broth and then diluted by tenfold serial dilution using standard 96-well plates for microdilutions. Then, 10 μl of each mixed diluted sample was plated using the drop plate technique described by Herigstad et al. (2001) into the following media: Standard plate count agar, De Man-Rogosa-Sharpe agar (MRS), and MacConkey agar (HiMedia Laboratories Pvt. Ltd., India) for total aerobic, lactobacillus, and coliform bacterial counts, respectively. Total aerobic bacteria and coliform were cultivated at 37°C for 24 to 48 hours. Lactobacilli were incubated in a 3% CO2 atmosphere at 37°C for 48 to 72 hours. The results were expressed as arithmetical means ± SME in log10 colony-forming units per gram of ileocecal content (log10 CFU/g). Relative weight of lymphoid organsFive birds from each group were humanly decapitated for determine the relative weight of spleen and bursa of Fabricius, and the values were adjusted for body weight according to Verma et al. (2004). Statistical analysisThe data were reported as mean ± SEM (standard error of mean). The differences between groups were analyzed using one-way ANOVA measures followed by Tukey’s honestly significant difference (Tukey’s HSD) test as a post hoc test. A value of p < 0.05 was used to indicate statistical significance. In addition, the generalized linear mixed model (GLMM) was used to analyze the OPG results, in which the model included samples of supplemented groups and time as a fixed effect. The charts were done using Statistical Package for Social Sciences version 24.0 (SPSS, IBM Corp., Armonk, NY) and Graph Pad prism 9.5.1 (GraphPad Software, Inc.). Ethical approvalAnimal welfare and experimental procedures were performed by adopting applicable guidelines for the care and use of laboratory animals. They were approved by the IACUC Institutional Animal Care and Use Committee, Zagazig University, Egypt (approval No.: ZU-IACUC/2/F/75/2021). ResultsClinical signs, postmortem findings of Eimeria infection among quail farmsThe examined quails exhibited ruffled feathers, anorexia, recumbence, decreased body weight, anemia, brownish diarrhea tinged with blood, and up to 30% mortality. The autopsied birds revealed protruded keel bone, ballooning of the small intestine, and two ceca, with thickening in the mucosa with bloody content in the small intestine and/or a bloody cecal core (Fig. 2a–d). The scraping from the small intestine and ceca revealed numerous coccidial oocysts of Eimeria species. The mixed species infection of Eimeria was detected in the 11 examined farms (100%); based on the collected birds from each farm, the infection ranged from 30% to 83.3% (Fig. 2h), and the total number of collected quails was 55.1% (38/69). Morphological characterization of Eimeria oocystsMorphologically, three Eimeria species were identified as intestinal coccidiosis (E. bateri and E. uzura) and cecal coccidiosis (E. tsunodai). After the sporulation of Eimeria oocysts, E. bateri oocysts were subspherical to ellipsoidal with a double-layered and smooth wall. Sporocysts were ovoid with a nipple-like Stieda body. While E. tsunodai oocysts were subspherical to oval with a double-layered and smooth wall, sporocysts were ovoid with a pyriform to triangular Stieda body. As well, E. uzura oocysts were ovoid to pear-shaped with a double-layered and smooth wall. Sporocysts were ovoid with a crescent- or half-moon-like Stieda body (Fig. 2e–g). On the level of farms, the incidence of infection with E. bateri was 33.33% to 66.67%, whereas that of E. tsunodai and E. uzura was 16.67% to 50% and 12.5% to 33.33%, respectively (Fig. 2i). Regarding collected quails, E. bateri was in 46.4% (32/69), E. tsunodai in 31.9% (22/69), and E. uzura in 23.2% (16/69). The anticoccidial efficacy in the experimental quailsSurvival rate, clinical signs, and body weight gain (BWG) The results demonstrated that the survival rate was 88.24% in each of the Coxan and Clazo-Fort-infected groups (G-1B and G-2B). However, in the group of Coxan interchangeably with Clazo-Fort (G-3B), 85.29% of quails survived. Meanwhile, the survival rate in the unsupplemented-infected group (control positive; G-4B) was 58.82% (Fig. 3a). The clinical signs observed in the infected groups with oocysts of E. tsunodai either supplemented with Coxan and Clazo-Fort or unsupplemented varied in severity and frequency (Fig. 3b). The signs began on the 3rd day post-infection (DPI) in unsupplemented-infected group (G-4B) with substantial evidence and severity. They included ruffled feathers, depression, huddling together, anorexia, emaciation, and bloody diarrhea. These symptoms gradually worsened and then subsided from the 7th DPI until the end of the experiment. The three supplemented quail groups showed low evidence, frequency, and severity of signs that appeared on the 4th DPI, in which the Coxan and Clazo-Fort groups were the lowest followed by the Coxan interchangeably with Clazo-Fort. Bloody diarrhea appeared on the 4th DPI with 26.67% in the unsupplemented-infected group (G-4B), 12.12% in Coxan interchangeably with the Clazo-Fort-infected group (G-3B), and 9.09% in each of the Coxan (G-1B) and Clazo-Fort (G-2B) groups; later, it disappeared in the three supplemented-infected groups on the 8th DPI. The validity of the experiment was confirmed by the absence of any clinical signs or mortalities in the negative control group (G-4A; unsupplemented-uninfected) and prophylactic supplemented-uninfected groups (G-1A, 2A, and 3A). The body weight gain (BWG) of the supplemented groups significantly improved (p < 0.05) in comparison with the unsupplemented groups (Table 2). The Coxan group had the highest BWG among the other prophylactic-supplemented and unsupplemented groups at the experiment’s most time intervals, either before or after infection. However, quails supplemented with Coxan interchangeably with Clazo-Fort had an elevation of BWG at 15 to 21 days compared with other infected groups. Bloody diarrheal score and oocyst counts (OPG) of feces
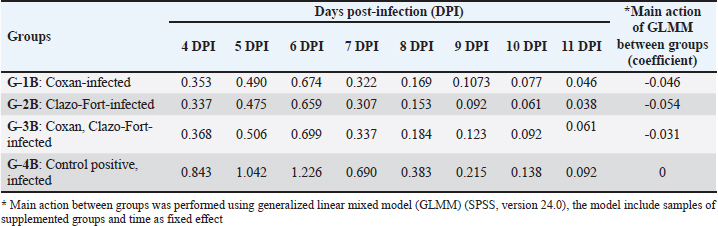
Fig. 2. Clinical signs, post-mortem lesions, Oocyst morphologies of Eimeria species, and incidence of Eimeria infection among the examined quail farms. (a) Ruffled feathers, weakness, and anemic quail. (b) Ballooning of two ceca. (c) Ballooning of the small intestine and tinged with blood. (d) Bloody cecal content. (e) Oocyst of Eimeria bateri. (f) Oocyst of Eimeria tsunodai. (g) Oocyst of Eimeria uzura. (h) Percentage of infected quails with Eimeria among the examined quail farms. (i) Percentage of Eimeria species among the infected quail in the examined farms. The scoring of bloody diarrhea from 4 to 8 DPI was recorded (Table 3). The three prophylactic-supplemented groups exhibited markedly decreased bloody diarrhea scores compared to the unsupplemented-infected group. Thus, both Coxan and Clazo-Fort have the ability to reduce the bloody diarrheal score when used as prophylactics. The total number of OPG of feces from 4 to 11 DPI (Table 4) was measured. When hemorrhagic feces were observed in the infected groups at 4 DPI, the oocyst shedding in the feces was detected. The high number of oocysts shed was measured in the feces at six DPI once the high bloody cecal score (score=2) was seen. The number of oocyst counts in feces decreased in the three supplemented groups compared to the unsupplemented one. The lowest number of oocysts was detected in the group of quails supplemented with Clazo-Fort and ranged from 0.659–0.038×106 with GLMM main action −0.054, followed by quails supplemented with Coxan which the oocyst count was 0.674–0.046×106 (GLMM main action −0.046) and then Coxan interchangeably with Clazo-Fort with 0.699–0.061×106 oocyst count (GLMM main action −0.031). While in the unsupplemented-infected group, the oocyst of E. tsunodai was 1.226–0.092×106.
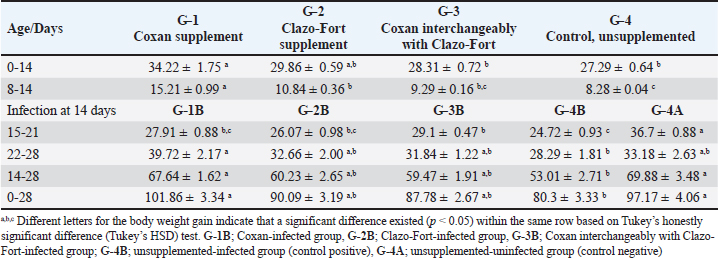
Fig. 3. Survival rates (a), and frequency of clinical signs (b) among the Coxan (G-1B), Clazo-Fort (G-2B), and Coxan interchangeably with Clazo-Fort (G-3B) prophylactic supplemented and unsupplemented (G-4B) quail groups postinfected with the oocyst of E. tsunodai. Table 2. Body weight gain (BWG; g) of quail groups after supplement of Coxan and Clazo-Fort at 7 days of age either alone or interchangeably, and infection with an inoculum containing 4.1 × 104 sporulated oocysts of E. tsunodai at 14 days of age.
Table 3. The bloody diarrheal score from 4 to 8 day post-infection with oocysts of E. tsunodai.
Table 4. The number of oocysts shedding per gram of feces post E. Tsunodai infection (X106).
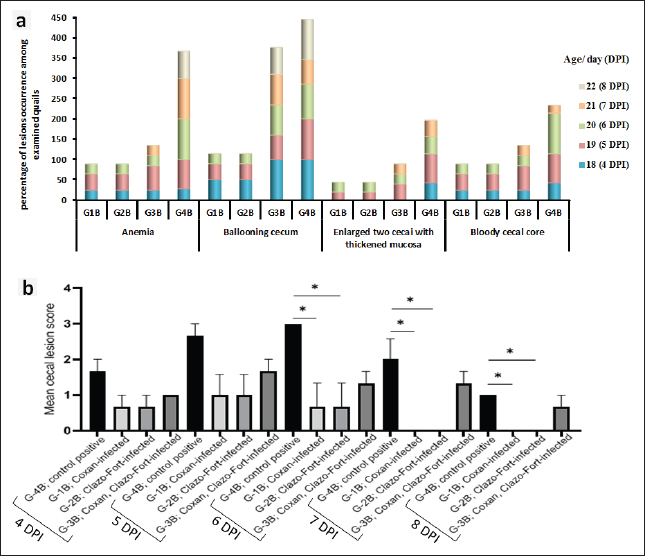
Cecal lesion examination, scoring, and oocyst countOn autopsy, all euthanized and dead quails from all groups at 4 to 8 days post-infection (DPI) were examined. Typical lesions in the ceca were noticed in the unsupplemented-infected group (G-4B) in the form of anemia, enlargement, and ballooning of two ceca with or without thickening mucosa, as well as the bloody cecal core. When comparing the three prophylactic supplemented-infected groups to the infected one, it was found that Coxan and Clazo-Fort as preventive supplements have the ability to reduce the intensity and occurrence of lesions. It is noticeable that both Coxan and Clazo-Fort, when supplemented alone (G-1B and G-2B), could fade the lesions from the 7th DPI (Fig. 4a). On the other hand, the gross lesions score of the cecum was lower in the prophylactically supplemented quail groups than in the unsupplemented-infected group (Fig. 4b). A significantly lower lesion score was recorded and analogous in the quails supplemented with Coxan (G-1B) and Clazo-Fort (G-2B) at 6, 7, and 8 DPI with p values of 0.0436, 0.0121, and 0.0121 at each DPI, respectively, where the lesion scores became zero from the 7th DPI. In the quails supplemented with Coxan interchangeably with Clazo-Fort (G-3B), the lesion score showed a non-significant reduction. The significant reduction of the oocyst counting numbers in the cecum of the three protective supplemented groups was recorded with an oocyst index of 5 when compared with the unsupplemented group, which had an oocyst index of 20 (Table 5). Protective impact of prophylactic phytochemotherapy against E. tsunodai in experimental quailsThe AST, RLS, ACI, and protection rates against infection in quails are displayed in Table 6. The preventive supplementation of Coxan (G-1B), Clazo-Fort (G-2B), and Coxan interchangeably with Clazo-Fort (G-3B) achieved protection against lesion scores of 84.3%, 84.3%, and 60%, as well as against oocyst outputs of 51.4%, 53.2%, and 49%, respectively. Concerning the results of the AST and RLS, E. tsunodai was sensitive to Coxan and Clazo-Fort supplementation (77.3; ≥50%), while it was resistant to using Coxan interchangeably with Clazo-Fort (42.03; < 50%). According to the ACI, the three prophylactic supplementation groups had a moderate effect on E. tsunodai infection, whereas the G-1B supplemented with Coxan showed the highest anticoccidial activity with an ACI value of 154.88.
Fig. 4. Frequency occurrence of cecal lesions (a), and cecal lesion scores (b) among the prophylactic supplemented and unsupplemented quail groups post-infected with the oocyst of E. tsunodai. The figure “b” displays the mean ± SEM (bar), p <0.05, which represents a significant difference using Tukey’s multiple comparisons test. Table 5. The number of oocysts counted in the cecal content per gram (X106) post-E. Tsunodai infection.
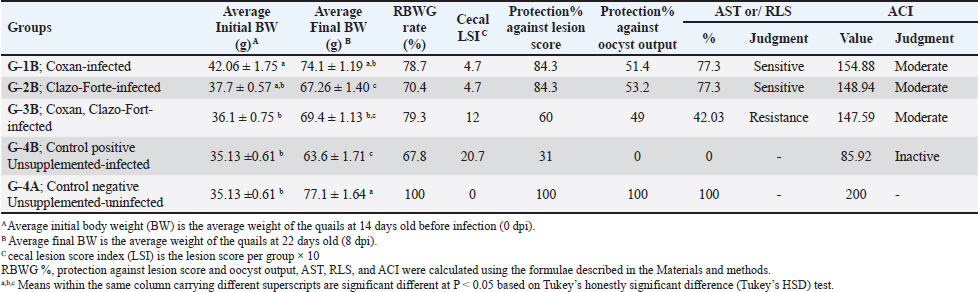
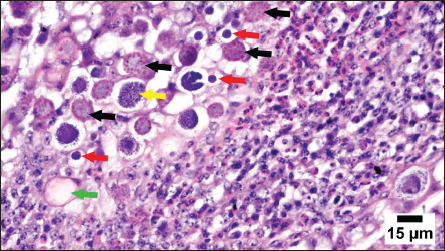
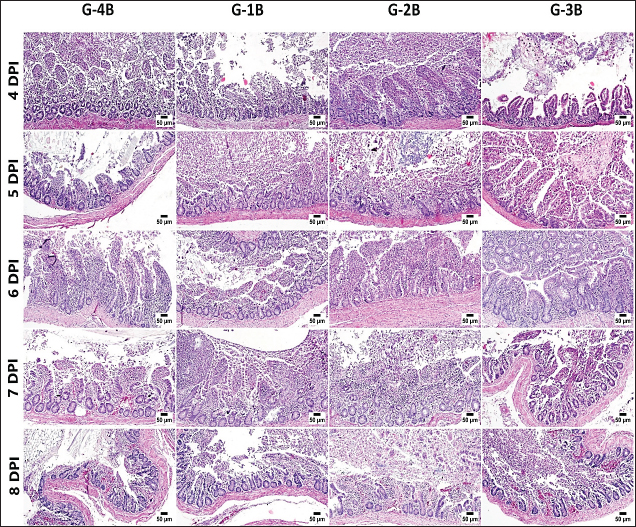
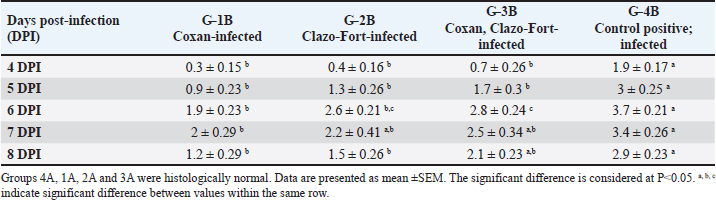
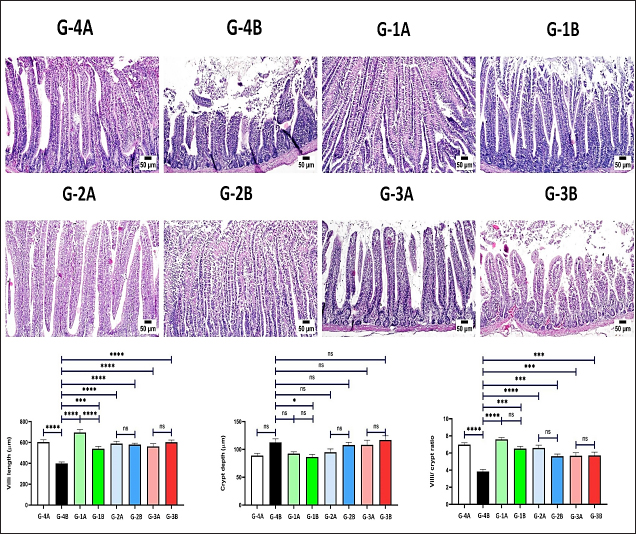
Histopathology, lesion score, and intestinal histomorphometryMicroscopic examination of cecal tissue sections from control-negative (G-4A) and all supplemented-uninfected groups (G-1A–3A) revealed the absence of histopathological changes. Meanwhile, the positive control group (G-4B) showed marked alterations represented by the presence of varying parasite loads in different developmental stages, either intracellularly in the cecal lining epithelium or the cecal contents (Fig. 5). Macrogamonts or zygotes were found intracellular in the cecal epithelium; they appeared rounded with multiple peripherally located eosinophilic granules and a single central granule; their average diameter was 12 µm and the average diameter of eosinophilic granules was 1.9 µm. Microgamonts of 13 µm diameter were also observed with their characteristic multiple basophilic nuclei. Schizonts revealed an average of 18.6 µm in diameter and contained numerous crescent-shaped merozoites. Oocytes were detected in the enterocytes or the cecal content; they exhibited an average diameter of 12.2 µm, appeared oval with a thick wall, and were filled with granular eosinophilic material. Moreover, the cecal mucosa showed multifocal areas of epithelial sloughing that were covered by fibrinous exudate admixed with increasing amounts of necrotic cell debris, hemorrhage, and inflammatory cell infiltrations. The propria and submucosa were expanded by edema and intense mononuclear and heterophilic cell infiltrations. As shown in Figure 6 and Table 7. The severity of coccidia-induced cecal lesions varied in the different supplemented groups at different time intervals. Generally, a significantly higher lesion score was always observed in the unsupplemented-infected group (G-4B) at all-time intervals when compared to other supplemented-infected groups. The lowest lesion score was detected in the group supplemented with Coxan (G-1B), followed by that observed in the group supplemented with Clazo-Fort (G-2B). The least improvement was detected in the group supplemented with Coxan interchangeably with Clazo-Fort (G-3B) when compared to other supplemented groups. Regarding the results on intestinal histomorphometry (Fig. 7), in comparison to the control positive group, all supplemented groups exhibited a significant increase in the estimated villi length of jejunum. On the contrary, a non-significant change was observed in the crypt depth between G-4B and other supplemented groups except for G-1B, which showed a significant reduction in the crypt depth. Meanwhile, a significant increase in the ratio between villi length and crypt depth was observed in all supplemented groups when compared with G-4B. The highest ratio was detected in G-1B, followed by that calculated in G-2B and G-3B. Table 6. Protection rate against lesion scores and oocyst outputs, AST, RLS, ACI, as well as judging the effect of using Coxan and/or Clazo-Fort on quails infected with E. tsunodai at 8 days post-infection.
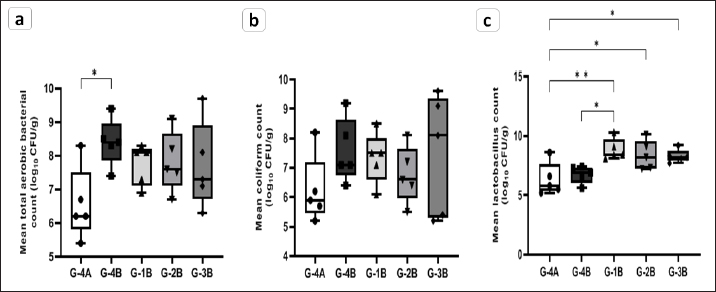
Intestinal microbiotaTotal aerobic, coliforms, and lactic acid bacterial populations of quail’s ileocecal at 28 days of age were summarized in Figure 8. The supplemented groups showed a significant elevation of the lactobacillus population (p < 0.05) compared to the unsupplemented groups. Which in The Coxan group was greater than both control negative (G-4A) ( p=0.0059) and positive (G-4B) ( p=0.0236) groups. Moreover, the Clazo-Fort (G-2B) and Coxan interchangeably with Clazo-Fort (G-3B) groups were higher than control negative with p value 0.0309 and 0.0403, respectively. The coliform bacterial count revealed no significant difference among all groups, supplemented or unsupplemented. For the total aerobic bacteria, the control positive group had a significantly higher count (p < 0.05) than the control negative group, with no significant difference between the three supplemented groups and the control groups. However, there was a tendency toward a decrease in the coliform and total aerobic bacterial populations in the supplemented groups comparable to the control positive.
Fig. 5. Photomicrograph of cecum from a Coccidia-infected group showing different developmental stages of coccidia, including macrogamonts containing eosinophilic granules (black arrows), microgamonts with basophilic nuclei (red arrows), schizonts (yellow arrows), and oocysts (green arrow). Note that intense heterophilic and mononuclear inflammatory cell infiltration.
Fig. 6. Photomicrographs of the cecum, H&E stained; show coccidia-induced lesions, including mucosal sloughing, hemorrhage, and typhlitis, in the supplemented and unsupplemented infected groups at different time intervals. Table 7. Microscopic lesion score in different experimental groups post-infection with E. tsunodai.
Fig. 7. Photomicrographs of jejunum H&E stained showing villi and crypt in different experimental groups. Charts represent villi length (μm), crypt depth (μm), and villi-to-crypt ratio. Data are presented as mean ±SEM. A significant difference is considered at P˂0.05.
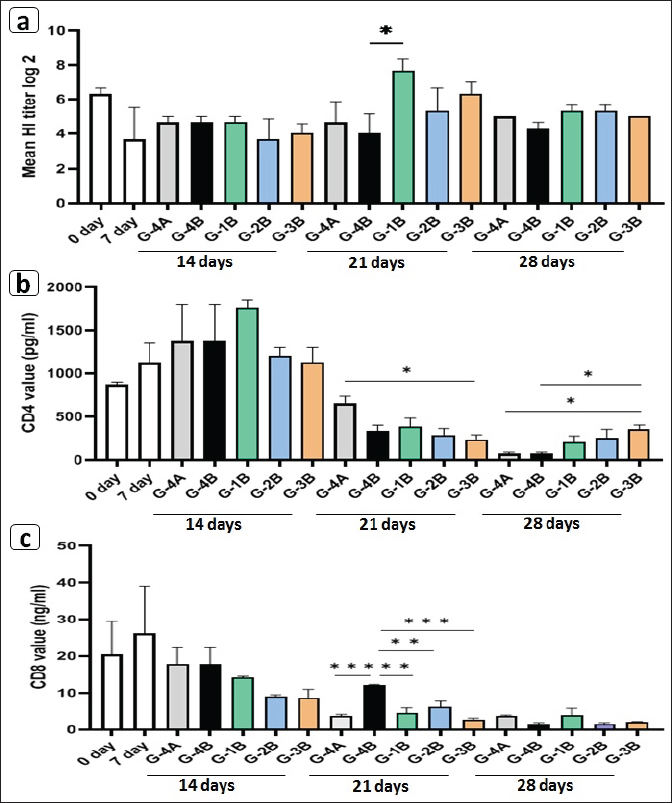
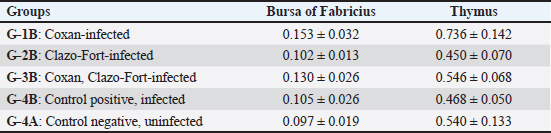
Fig. 8. Enumeration of intestinal microbiota in the supplemented (G-1B, G-2B, G-3B) and unsupplemented (G-3B) groups postinfection with the oocyst of E. tsunodai, in addition to control negative group (G-4A). (a) Total aerobic bacteria, (b) Coliforms bacteria, and (c) Lactobacillus bacteria. Specific antibody response to Newcastle disease virus vaccineThe mean antibody titers detected by HI for NDV in different groups of quails are represented in Figure 9a, in which the maternal-derived antibody (MDA) was 6.33 ± 0.33 log2. At 21 days of age (2 weeks after 1st vaccination and a week after 2nd vaccination), the three supplemented-infected groups had an elevation of antibody responses compared to the control groups either uninfected or infected. The higher antibody response (7.66 ± 0.67 log2) was recorded in the Coxan group (G-1B) which was significantly increased (p=0.039) when compared to control positive, followed by Coxan interchangeably with Clazo-Fort (G-3B) and then the Clazo-Fort (G-2B) group with mean HI antibody titers 6.33 ± 0.67 and 5.33 ± 1.33 log2, respectively. The HI antibody titers of the control positive and negative groups were 4 ± 1.15 and 4.66 ± 1.20 log2, respectively. CD4/CD8 measurementThe determination of the value of CD4 and CD8 T-cell molecules using the ELISA is shown in Figure 9b and c. Among the control groups either uninfected or infected (G-4A and G-4B) and the supplemented-infected groups (G-1B, G-2B, and G-3B), the value of CD4 was increased (1762 ± 87.5 pg/ml) in the quails supplemented with Coxan (G-1B) at 14 days of age (the initial of infection), although it was non-significant. At 21 days of age, the CD4 value decreased in all infected groups, with a significantly lower value in the group supplemented with Coxan interchangeably with Clazo-Fort (G-3B) compared with the control negative group (G-4A). On the other hand, the CD8 value of the three supplemented-infected groups was significantly reduced at 21 days of age compared to the control positive group (G-4B), and the lower value showed in the group supplemented with Coxan interchangeably with Clazo-Fort (G-3B). Relative weight of lymphoid organsThe effect of different supplementation substances on lymphoid organs (bursa of Fabricius and thymus) weights as percentages of body weight revealed that no significant effects were observed on organ weight during the experimental period (Table 8). However, in the birds receiving the Coxan supplement, the relative weight of lymphoid organs tended to be the highest among other groups. Discussion The economic losses caused by coccidiosis can be destructive and constitute a prior challenge for the poultry industry. This disease is one of the factors that constrain quail aviculture development in Egypt. The extensive use of anticoccidials caused the eventuality of drug residues in meat and eggs (Clarke et al., 2014), as well as the development of anticoccidial resistance among Eimeria species (Blake and Tomley, 2014). Therefore, in the last few years, there has been a move toward herbal medicine as a new alternative to traditional anticoccidial drugs (Masood et al., 2013; Gholami-Ahangaran et al., 2022). Herein, eleven commercial quail farms were examined and mainly suffered from diarrhea, anorexia, decreased body weight, and mortality up to 30%, with lesions including ballooning of the small intestine and two ceca, thickening in the mucosa with bloody content in the small intestine, and a bloody cecal core. These findings were comparably detected by Umar et al. (2014); Anbarasi et al. (2016); and Elmorsy et al. (2020) accompanying quail’s coccidiosis. Microscopically, the examined intestinal samples of submitted quails (n=69) revealed mixed Eimeria infection in 100% of the birds. Several studies recorded a high incidence of Eimeria infection in Egyptian quail farms and ranged from 70.59% to 89.8% (El-Madawy, 2001; Arafat and Abbas, 2018). According to the morphological character of oocysts, especially the shape of Stieda bodies, the Eimeria spp. were discriminated into E. bateri and E. uzura as intestinal coccidiosis and E. tsunodai as cecal coccidiosis, as previously described (Teixeira et al., 2004; Bashtar et al., 2010; Umar et al., 2014).
Fig. 9. Effects of Coxan and/or Clazo-Fort supplementation on HI antibody titers using HI test (a) and on the value of the CD4 (b) and CD8 (c) T-cell molecules in the serum using ELISA. Table 8. Relative weight of lymphoid organs (% BW) of prophylactic supplemented and unsupplemented quails infected with E. tsunodai at age 28 days old.
Diclazuril is the best among the anticoccidial drugs, decreasing oocyst shedding, mortality rate, and cecal lesion score (El-Gaos, 2014; El-Morsy et al., 2016). Subsequently, the identified E. tsunodai oocysts were used for in vivo evaluation of the prophylaxis effectiveness of commercial herbal products “Coxan” on coccidial infection and the health status of Japanese quails and compared with the commonly used anticoccidial chemicals “Clazo-Fort” that contain 2% diclazuril to complete the adopted approach. An interesting finding of this study was that the group supplemented with Coxan had body weight gain (BWG) (p < 0.05) higher than that of the control (infected and uninfected), and other groups supplemented with Clazo-Fort in most time intervals of the experiment, either before or after infection. There is evidence to suggest that the EOs in Coxan from oregano and garlic have the ability to enhance intestinal functions (Gopi et al., 2014; Ghazi et al., 2015; Al-Hijazeen et al., 2016), as well as its ability to reduce the adverse impact of coccidial infection in the intestine. During the coccidial challenge, the control positive group suffered from a significant reduction in BWG, and this was a result of reduced absorptive surface area, malabsorption of nutrients, and inflammation. Abou-elkhair et al. (2014); Ali et al. (2019); Sidiropoulou et al. (2020); and Chang et al. (2021) proved that dietary supplementation of oregano and/or garlic EOs improved the growth performance of broiler chickens infected with Eimeria oocysts and was higher than those supplemented with amprolium sulfate (Abou-elkhair et al., 2014). Regarding quails, these EOs have been used only as growth promoters (Masood et al., 2020; El garhy et al., 2015; El-sayed et al., 2017), but until now, the effectiveness of these oils has not been applied during coccidiosis infection in quails. Both Coxan and Clazo-Fort decrease the frequency and severity of clinical signs besides the score of bloody diarrhea after E. tsunodai infection with an 88.24% survival rate, followed by Coxan interchangeably with Clazo-Fort with a survival rate of 85.29%. However, the control positive group exhibited severe clinical signs with a high bloody diarrheal score and 41.18% mortality. This is due to severe inflammation, a remarkable decrease in extracellular fluid, electrolyte disorder, and acute hypoproteinemia (Hein, 1971). The clinical signs shown due to infection with E. tsunodai are in accordance with the findings of El-Morsy et al. (2016); Elmorsy et al. (2020, 2021b) in quails. It was worth mentioning that the three supplemented quail groups had a significantly lower oocyst value in the feces and cecum, with an oocyst index of 5 in the cecum compared to 20 in the control positive group. The highest protection level against total oocyst outputs (˃50%) was recorded in the Coxan and Clazo-Fort groups. Moreover, a significantly lowered cecal lesion score with 84.3% protection was detected in both groups (Coxan and Clazo-Fort). In contrast, no significant reduction was detected in the quails supplemented with Coxan interchangeably with Clazo-Fort compared to the control positive group. Through that, Coxan proved that it was able to match the effect of the Clazo-Fort in reducing the oocyst output and cecal lesion score. da Silva et al. (2009); Abou-elkhair et al. (2014) evidenced that the anticoccidial effects of oregano, thyme, and garlic oils were similar and might be better than the anticoccidial drugs. Plant bioactives such as oregano and garlic have antioxidant properties that could reduce the negative impacts of oxidation and thus might be useful in controlling coccidiosis (Bozkurt et al., 2016; Giannenas et al., 2003; Tsinas et al., 2011; Khan et al., 2012). In addition, the phenolic compounds in garlic oil have the ability to decrease the number of oocysts as a result of an alteration in the permeability of the cytoplasmic membrane of Eimeria, which finally leads to its death (Tanweer et al., 2014). The results of using Coxan interchangeably with Clazo-Fort aiming to minimize the negative impact of chemical product were relatively inferior among the other supplemented groups in decreasing the side effects of E. tsunodai infection in quails and enhancing the health status, although it outperformed the infected group. At the same pace, Bozkurt et al. (2016) confirmed that the combination of monensin sodium and oregano EO failed to create a synergism to lower fecal oocyst output. In this study, three anticoccidial efficacy indexes; AST, RLS, and ACI were applied. Based on the results of AST and RLS, E. tsunodai was sensitive (77.3%) to the prophylaxis supplementation of Coxan and Clazo-Fort but was resistant (<50%) to Coxan interchangeably with Clazo-Fort. Meanwhile, the ACI, a commonly employed index was the highest (154.88) in the Coxan group. These results suggested that Coxan, with its oregano and garlic oil content, is a potent and effective anticoccidial drug comparable to or higher than that of Clazo-Fort in quails. Another notable finding among histopathological examinations was the lowest cecal lesion score and parasitic loads in the Coxan group. Besides, the birds had the highest villi length, decreased crypt depth, and a high ratio of villi length to crypt depth in the jejunum. Obviously, the improvement in these geometry parameters of the jejunum villi and crypts leads to a positive impact on intestinal morphology and subsequently enhances nutrient absorption (Awad et al., 2008; Heydarian et al., 2020; Su et al., 2021). This could be associated with the augmentations observed in BWG in the Coxan group and alleviates the noxious effect of coccidiosis on the intestine (Fernando and McCraw, 1973; Ruff and Edgar, 1982; Adedokun and Olojede, 2019). Consistent with our results, Masood et al. (2020) detected the height of villus in the jejunum of Japanese quails supplemented with a mix of EOs that contained garlic and oregano oils, and it was significantly different from the control group. Intestine morphology may be mediated by an alteration of the intestinal microbiota which improves the absorption of nutrients, develops the immune system of the intestine, and protects from pathogens (Lu et al., 2021). Following the coccidial infection, dysbiosis of the gut microbiota occurs with marked intestinal inflammation, resulting in increased colonization of pathogenic bacteria such as aerobic bacteria, especially Enterobacteriaceae (Lupp et al., 2007), as well as directly antagonizing commensal bacteria (Lu et al., 2021). Based on the results of this study, the intestinal histomorphometry improvement in the quails supplemented with Coxan had a correlation with the ileocecal microbiota of lactic acid bacteria (Lactobacillus), which was significantly increased (p < 0.05) compared with the unsupplemented groups. Lactobacillus bacteria have the ability to improve the function of the intestinal barrier and increase the resistance of the body to coccidial infection (Schlee et al., 2008). Likewise, Cetin et al. (2016); Elbaz et al. (2022) confirmed that the diet containing the aforementioned EOs increased Lactobacillus spp. and reduced coliform bacteria counts in broiler chickens. Adaptive immunity includes humoral and cell-mediated immune responses, and both of them play essential roles against extracellular and intracellular pathogens (Lee et al., 2022). The cellular and humoral constituents are involved in the particular immune response of coccidiosis (Hughes et al., 1985). It has been reported that plant EOs have immunomodulatory properties that are crucial for treating infectious diseases (Awaad et al., 2010; Dhama et al., 2015). In our trials, quails supplemented with Coxan showed potent elevation in the humoral antibody titers against NDV using the HI test (7.66 ± 0.67 log2; p=0.039) at 21 days of age. There were several statements proving that garlic (Rahimi et al., 2011; Elbaz et al., 2022) and oregano (Franciosini et al., 2016; Mohiti-Asli and Ghanaatparast-Rashti, 2017) as extracts or oils added to broiler diets resulted in improving the humoral antibody response against the NDV. Besides this, comparable CD4 values were detected in the sera of examined groups with relatively higher level in the Coxan group at 14 days of age. This suggested a protective function of these T-cell subsets in the innate immune response against E. tsunodai infection in quails. The differential role of CD4+ and CD8+ T lymphocytes was concerned with resistance to primary and secondary coccidial infection (Rose et al., 1992; Rothwell et al., 1995), in which the CD4+ T cells help to eliminate pathogens (Alvarez et al., 2020). Elevation of CD4+ cells has a crucial role in producing the proinflammatory cytokine interferon (IFN-γ) in response to antigen challenge (McSorley et al., 2000), as well as inhibition of the intracellular development of the coccidia (Lillehoj and Choi, 1998; Choi et al., 1999). Furthermore, Lestari et al. (2023) confirmed that the chickens with high ND antibody titers exhibited increased lymphocyte, CD4+, and CD8+ cell populations. Although the CD8 value of supplemented groups in this study was at a lower level, suggesting its un-conspicuous role in quails’ E. tsunodai infection. The immune improvement in supplemented birds was supported by the enhanced relative weight of immune organs, especially in the Coxan group compared to infected birds. Many studies indicated increasing the relative weights of lymphoid organs in chickens by adding the extract or EOs of herbs (Hanieh et al., 2010; Rahimi et al., 2011; Elbaz et al., 2022). ConclusionCoxan (oregano and garlic oil), an herbal alternative product, proved a comparable anticoccidial with the chemical Clazo-Fort product, together with additional benefits including body weight gain and immune enhancement of quails. The relative safety of green medications should encourage their use on a large scale with a more comprehensive evaluation. Conflict of interestAll authors declare that they have no conflict of interest regarding the publication of this article. FundingThere was no funding source for this study; it was all as a contribution from the authors. Author’s contributionAll authors contributed to the aim and design of the work; Amal A.M. Eid and Reham M. ElBakrey visualized and planned the design and conception of this study. Preparation of the material, data collection and entry were performed by Ahmed A. ElKholy, Mohamed A. El-Morsy, Mohamed R. Mousa, and Reham M. ElBakrey and Walaa S. Abdelaziz. Data analysis and investigation were performed by Reham M. ElBakrey, Amal A.M. Eid, and Mohamed R. Mousa. The original draft of the manuscript was written by Reham M. ElBakrey, Mohamed R. Mousa and Walaa S. Abdelaziz. All authors reviewed and edited the prior drafts of the manuscript. The final form of the manuscript was revised and editing by Amal A.M. Eid and Reham M. ElBakrey Data availabilityThe manuscript contains all the data supporting the findings of this study. Any additional information needed is obtainable from the corresponding author upon justifiable request. ReferencesAbbas, R.Z., Colwell, D.D. and Gilleard, J. 2012. Botanicals: an alternative approach for the control of avian coccidiosis. Worlds Poult. Sci. J. 68(2), 203–215. Abou-Elkhair, R., Gaafar, K.M., Elbahy, N.M., Helal, M.A., Mahboub, H.D. and Sameh, G. 2014. Bioactive effect of dietary supplementation with essential oils blend of oregano, thyme and garlic oils on performance of broilers infected with Eimeria species. Glob. Vet. 13(6), 977–985. Adedokun, S.A. and Olojede, O.C. 2019. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front. Vet. sci. 5, 348. Al-Hijazeen, M., Lee, E.J., Mendonca, A. and Ahn, D.U. 2016. Effect of oregano essential oil (Origanum vulgare subsp. hirtum) on the storage stability and quality parameters of ground chicken breast meat. Antioxidants 5(2), 18. Ali, A.M., El Agrab, H.M., Hamoud, M.M., Gamal, A.M., Mousa, M.R., Nasr, S.A.E., El Shater, M.A.H., Laban, S.E., Zahran, O.K. and Ali, M.M. 2020. Effect of acidified drinking water by organic acids on broiler performance and gut health. Adv. Anim. Vet. Sci. 8(12), 1301–1309. Ali, M., Chand, N., Khan, R. U., Naz, S. and Gul, S. 2019. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 47(1), 79–84. Alvarez, K.L.F., Poma-Acevedo, A., Fernández-Sánchez, M. and Fernández-Díaz, M. 2020. An EdU-based flow cytometry assay to evaluate chicken T lymphocyte proliferation. BMC Vet. Res. 16, 1–12. Amoudi, M.A. 1987. Eimeria tahamensis N. Sp. (Apicomplexa: Eimeriidae) from the Arabian Quail (Coturnix delegorguei arabica) 1. J. Protozool. 34(4), 455–456. Anbarasi, P., Ponnudurai, G., Senthilvel, K., Puvarajan, B. and Arulmozhi, A. 2016. A note on incidence of coccidiosis in Japanese quail (Coturnix coturnix japonica). Indian Vet. J. 93(2), 29–31. Arafat, N. and Abbas, I. 2018. Coccidia of Japanese quail: from identification, prevalence, infection, and immunization. J. Parasitol. 104(1), 23–30. Awaad, M.H.H., Abdel-Alim, G.A., Sayed, K.S.S., Ahmed, A., Nada, A.A., Metwalli, A.S.Z. and Alkhalaf, A.N. 2010. Immunostimulant effects of essential oils of peppermint and eucalyptus in chickens. Pak. Vet. J. 30(2), 61–66. Awad, W., Ghareeb, K. and Böhm, J. 2008. Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing Enterococcus faecium and oligosaccharides. Int. J. Mol. Sci. 9(11), 2205–2216. Bahar, S., Shahrokh, R. and Mohsen, M. 2014. Study on parasitic infections of Quails in Garmsar, Iran. Int. J. Adv. Biol. Biomed. Res. 2, 262–266. Balestrin, P.W., Balestrin, E., Santiani, F., Cristo, T.G.D., Pereira, D.G., Bonatto, G.R., Bilick, J.V. and Casagrande, R.A. 2022. Comparison of macroscopy, histopathology and PCR for diagnosing Eimeria spp. in broiler chickens. Pesq. Vet. Bras. 42, e06968. Bancroft, J.D. and Gamble, M. (ed). 2008. Theory and practice of histological techniques. England, UK: Elsevier Health Sciences. Bashtar, A.R, Abdel-Ghaffar, F., Al-Rasheid, K.A.S., Mehlhorn, H. and Al Nasr, I. 2010. Light microscopic study on Eimeria species infecting Japanese quails reared in Saudi Arabian farms. Parasitol. Res. 107, 409–416. Blake, D.P. and Tomley, F.M. 2014. Securing poultry production from the ever-present Eimeria challenge. Trends parasitol. 30(1), 12–19. Bozkurt, M., Ege, G., Aysul, N., Akşit, H., Tüzün, A. E., Küçükyılmaz, K., Borum, A.E., Uygun, M., Aksit, D., Aypak, S., Simsek, E., Seyrek, K., Kocer, B., Bintas, E. and Orojpour, A. 2016. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 95(8), 1858–1868. Cetin, E., Yibar, A.R.T.U.N., Yesilbag, D., Cetin, I. and Cengiz, S.S. 2016. The effect of volatile oil mixtures on the performance and ilio-caecal microflora of broiler chickens. Br. Poult. Sci. 57(6), 780–787. Chang, L.Y., Di, K.Q., Xu, J., Chen, Y.F., Xi, J.Z., Wang, D.H., Hao, E., Xu, L., Chen, H. and Zhou, R.Y. 2021. Effect of natural garlic essential oil on chickens with artificially infected Eimeria tenella. Vet. Parasitol. 300, 109614. Choi, K.D., Lillehoj, H.S., Song, K.D. and Han, J.Y. 1999. Molecular and functional characterization of chicken IL-15. Dev. Comp. Immunol. 23(2), 165–177. Clarke, L., Fodey, T.L., Crooks, S.R., Moloney, M., O’Mahony, J., Delahaut, P., O’Kennedy, R. and Danaher, M. 2014. A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci. 97(3), 358–374. Cobaxin-Cárdenas, M.E. 2018. Natural compounds as an alternative to control farm diseases: avian coccidiosis. In Farm Animals Disease. Recent Omic Trends and New Strategies of Treatment. Eds., Quiroz-Castañeda R.E. IntechOpen: London, UK, pp: 135–149. da Silva, M.A., de Sousa Pessotti, B.M., Zanini, S.F., Colnago, G.L., Rodrigues, M.R.A., de Carvalho Nunes, L., Zanini, M.S. and Martins, I.V.F. 2009. Intestinal mucosa structure of broiler chickens infected experimentally with Eimeria tenella and treated with essential oil of oregano. Ciência Rural. 39(5), 1471–1477. De Pablos, L.M., dos Santos, M.F., Montero, E., Garcia-Granados, A., Parra, A. and Osuna, A. 2010. Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens. Parasitol. Res. 107, 601–604. Dhama, K., Latheef, S.K., Mani, S., Samad, H.A., Karthik, K., Tiwari, R., Farag, M.R. and Tufarelli, V. 2015. Multiple beneficial applications and modes of action of herbs in poultry health and production-a review. Int. J. Pharmacol. 11(3), 152–176. Dkhil, M.A., Abdel-Baki, A.S., Wunderlich, F., Sies, H. and Al-Quraishy, S. 2011. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet. Parasitol. 175(1–2), 66–72. Duszynski, D.W. 2001. Eimeria. eLS. Chichester, UK: John Wiley & Sons, Ltd. Duszynski, D.W. and Gutiérrez, R.J. 1981. The coccidia of quail in the United States. J. Wildl. Dis. 17(3), 371–379. Duszynski, D.W. and Wilber, P.G. 1997. A guideline for the preparation of species descriptions in the Eimeriidae. J. Parasitol. 83(2), 333–336. Economou, K.D., Oreopoulou, V. and Thomopoulos, C.D. 1991. Antioxidant properties of some plant extracts of the Labiatae family. J. Am. Oil Chem. Soc. 68, 109–113. El garhy, O.H.M., Salem, A.A. and Abdel-rahman, H.M. 2015. Influence of the integration among oxytetracycline, oregano essential oil or garlic powder on intestine microbial population and productive performance of Japanese quail. Annals of Agric. Sci., Moshtohor, 53, 585–596. El-Banna, H.A., El-Bahy, M.M., El-Zorba, H.Y. and El-Hady, M. 2005. Anticoccidial efficacy of drinking water soluble diclazuril on experimental and field coccidiosis in broiler chickens. J. Vet. Med. Series A. 52(6), 287–291. Elbaz, A.M., Ashmawy, E.S., Salama, A.A., Abdel-Moneim, A.M.E., Badri, F.B. and Thabet, H.A. 2022. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet. Res. 18(1), 1–12. El-Gaos, M.I.A. 2014. Studies on coccidial sensitivity to certain drugs. Ph. D. thesis, Poultry and Rabbit Diseases Department, Veterinary Medicine Faculty, Mansoura Univ., Egypt. El-Madawy, R.S. 2001. Studies on some protozon parasites in birds M. S. thesis, Veterinary Medicine Faculty, Zagazig, Egypt: Zagazig University. El-Morsy, M.A., Abou El-Azm, K.I. and Awad, S.S. 2016. Efficacy of some anticoccidial drugs on experimentally induced cecal coccidiosis (E. tsunodai) in Japanese quails. Egypt J. Vet. Sci. 47(2), 165–177. Elmorsy, M.A., Das, M., Senapati, S.K., Jena, G.R., Mishra, S., Panda, S.K., Kundu, A.K. and Kumar, D. 2021b. Efficacy of Immunization of Japanese Quail (Coturnix coturnix japonica) Against the Challenge with Different Eimeria Species. Indian J. Anim. Res. 1, 10. Elmorsy, M.A., Das, M., Senapati, S.K., Jena, G.R., Panda, S.K., Kundu, A.K., Mishra, S. and Kumar, D. 2021a. Efficacy of immunization compared to an anticoccidial drug combination in the management of challenged coccidiosis in Japanese quail. Vet. Parasitol. 295, 109451. Elmorsy, M.A., Jena, G.R., Panda, S.K., Kundo, A.K., Kumar, D., Mishra, S.K., Senapati, S.K., Majhi, C., Panda, S.K. and Das, M.R. 2020. Isolation, identification, and clinical impact of coccidiosis in Japanese quail farms in and around Bhubaneswar, Odisha, India. J. Entomol. Zool. stud. 8(6), 302–307. El-Sayed, Z.S., El-Garhy, O.H.M., Abdella, M.M. and El-Sayaad, G.A. 2017. Effect of adding antibiotic, oregano essential oil and garlic powder on growth performance, carcass traits and intestinal pathogenic microrganisms of Quails. Egypt. J. Anim. Prod. 54(1), 55–66. Fernando, M.A. and McCraw, B.M. 1973. Mucosal morphology and cellular renewal in the intestine of chickens following a single infection of Eimeria acervulina. J. Parasitol. 59(3), 493–501. Franciosini, M.P., Casagrande-Proietti, P., Forte, C., Beghelli, D., Acuti, G., Zanichelli, D., dal Bosco, A., Castellini, C. and Trabalza-Marinucci, M. 2016. Effects of oregano (Origanum vulgare L.) and rosemary (Rosmarinus officinalis L.) aqueous extracts on broiler performance, immune function and intestinal microbial population. J. Appl. Anim. Res. 44(1), 474–479. Franz, C., Baser, K.H.C. and Windisch, W. 2010. Essential oils and aromatic plants in animal feeding–a European perspective. A review. Flavour Fragr. J. 25(5), 327–340. Gadde, U., Kim, W.H., Oh, S.T. and Lillehoj, H.S. 2017. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 18(1), 26–45. Gebreyohannes, G. and Gebreyohannes, M. 2013. Medicinal values of garlic: a review. Int. J. Med. Med. Sci. 5(9), 401–408. Gesek, M., Welenc, J., Tylicka, Z., Otrocka-Domagała, I., Paździor, K. and Rotkiewicz, A. 2014. Pathomorphological changes in the alimentary system of Japanese quails naturally infected with Eimeria tsunodai. Bull. Vet. Inst. Pulawy. 58(1), 41–45. Ghazi, S., Amjadian, T. and Norouzi, S. 2015. Single and combined effects of vitamin C and oregano essential oil in diet, on growth performance, and blood parameters of broiler chicks reared under heat stress condition. Int. J. Biometeorol. 59, 1019–1024. Gholami-Ahangaran, M., Ahmadi-Dastgerdi, A., Azizi, S., Basiratpour, A., Zokaei, M. and Derakhshan, M. 2022. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 8(1), 267–288. Giannenas, I., Bonos, E., Christaki, E. and Florou-Paneri, P. 2018. Oregano: a feed additive with functional properties. In Therapeutic Foods, Handbook of Food Engineering, (Vol 8). Eds., Holban, A.M. and Grumezescu, A.M. Elsevier, Academic Press: London, UK, pp: 179–208. Giannenas, I., Florou-Paneri, P., Papazahariadou, M., Christaki, E., Botsoglou, N.A. and Spais, A.B. 2003. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 57(2), 99–106. Gopi, M., Karthik, K., Manjunathachar, H. V., Tamilmahan, P., Kesavan, M., Dashprakash, M., Balaraju, B.L. and Purushothaman, M.R. 2014. Essential oils as a feed additive in poultry nutrition. Adv. Anim. Vet. Sci. 2(1), 1–7. Greathead, H. and Kamel, C. 2006. Encapsulated plant extracts to fight coccidiosis. Feed Mix. 14, 18–21. Habibi, H., Firouzi, S., Nili, H., Razavi, M., Asadi, S.L. and Daneshi, S. 2016. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: in vitro and in vivo study. J. Parasitic Dis. 40, 401–407. Hanieh, H., Narabara, K., Piao, M., Gerile, C., Abe, A. and Kondo, Y. 2010. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in White Leghorn chickens. Anim. Sci. J. 81(6), 673–680. Hein, H. 1971. Pathogenic effects of Eimeria necatrix in young chickens. Exp. Parasitol. 30, 321–330. Herigstad, B., Hamilton, M. and Heersink, J. 2001. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Meth. 44(2), 121–129. Heydarian, M., Ebrahimnezhad, Y., Meimandipour, A., Hosseini, S.A. and Banabazi, M.H. 2020. Effects of dietary inclusion of the encapsulated thyme and oregano essential oils mixture and probiotic on growth performance, immune response and intestinal morphology of broiler chickens. Poult. Sci. J. 8(1), 17–25. Hodgson, J.N. 1970. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 28(1), 99–102. Hughes, H.P.A., Gonzalez, A., Guhl, F. and Hudson, L. 1985. Antigen-specific lymphocyte transformation in congenital toxoplasmosis. Immunol. Lett. 10(2), 95–98. Kaingu, F., Liu, D., Wang, L., Tao, J., Waihenya, R. and Kutima, H. 2017. Anticoccidial effects of Aloe secundiflora leaf extract against Eimeria tenella in broiler chicken. Trop. Anim. Health Prod. 49, 823–828. Kemp, L.E., Yamamoto, M. and Soldati-Favre, D. 2013. Subversion of Host Cellular Functions by the Apicomplexan Parasites. FEMS Microbiol. Rev. 37(4), 607–631. Khan, R.U., Naz, S., Nikousefat, Z., Tufarelli, V., Javdani, M., Qureshi, M.S. and Laudadio, V. 2012. Potential applications of ginger (Zingiber officinale) in poultry diets. World’s Poult. Sci. J. 68(2), 245–252. Khater, H.F., Ziam, H., Abbas, A., Abbas, R.Z., Raza, M.A., Hussain, K., Younis, E.Z., Radwan, I.T. and Selim, A. 2020. Avian coccidiosis: Recent advances in alternative control strategies and vaccine development. Agrobiol. Rec. 1, 11–25. Landers, T.F., Cohen, B., Wittum, T.E. and Larson, E.L. 2012. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 127(1), 4–22. Lee, Y., Lu, M. and Lillehoj, H.S. 2022. Coccidiosis: recent progress in host immunity and alternatives to antibiotic strategies. Vaccines. 10(2), 215. Lestari, D., Murtini, S., Ulupi, N., Gunawan, A. and Sumantri, C. 2023. Flow cytometric evaluation of CD4+ and CD8+ T-cell in IPB-D2 chickens with different Newcastle disease antibody titers level. Vet. World. 16(5), 1161–1164. Lillehoj, H., Liu, Y., Calsamiglia, S., Fernandez-Miyakawa, M.E., Chi, F., Cravens, R.L., Oh, S. and Gay, C.G. 2018. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 49, 1–18. Lillehoj, H.S. and Choi, K.D. 1998. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 42, 307–314. Lillehoj, H.S., Ding, X., Quiroz, M.A., Bevensee, E. and Lillehoj, E.P. 2005. Resistance to intestinal coccidiosis following DNA immunization with the cloned 3-1E Eimeria gene plus IL-2, IL-15, and IFN-γ. Avian Dis. 49(1), 112–117. Lu, C., Yan, Y., Jian, F. and Ning, C. 2021. Coccidia-microbiota interactions and their effects on the host. Front. cell. infect. microbiol. 11, 751481. Lupp, C., Robertson, M.L., Wickham, M.E., Sekirov, I., Champion, O.L., Gaynor, E.C. and Finlay, B.B. 2007. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2(2), 119–129. Marien, M. and Gussem, D.M. 2007. Coccidiosis rotation programmes are a must!. Poult. World. 23, 34–35. Masood, A., Qureshi, A.S., Shahid, R.U. and Jamil, H. 2020. Effects of oral administration of essential oil (Mix Oil®) on growth performance and intestinal morphometry of Japanese Quails (Coturnix coturnix japonica). Pak. Vet. J. 40(3), 385–389. Masood, S., Abbas, R.Z., Iqbal, Z., Mansoor, M.K., Sindhu, Z.U.D., Zia, M.A. and Khan, J.A. 2013. Role of natural antioxidants for the control of coccidiosis in poultry. Pak. Vet. J. 33(4), 401–407. McDougald, L.R., Da Silva, J.M., Solis, J. and Braga, M. 2015. A survey of sensitivity to anticoccidial drugs in 60 isolates of coccidia from broiler chickens in Brazil and Argentina. Avian Dis. 31, 287–92. McSorley, S.J., Cookson, B.T. and Jenkins, M.K. 2000. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 164(2), 986–993. Mohiti-Asli, M. and Ghanaatparast-Rashti, M. 2017. Comparison of the effect of two phytogenic compounds on growth performance and immune response of broilers. J. Appl. Anim. Res. 45(1), 603–608. Nweze, N.E. and Obiwulu, I.S. 2009. Anticoccidial effects of Ageratum conyzoides. J. Ethnopharmacol. 122(1), 6–9. Paraskeuas, V., Fegeros, K., Palamidi, I., Hunger, C. and Mountzouris, K.C. 2017. Growth performance nutrient digestibility, antioxidant capacity, blood biochemical biomarkers and cytokines expression in broiler chickens fed different phytogenic levels. Anim. Nutr. 3(2), 114–120. Peek, H.W. and Landmanab, W.J.M. 2011. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 31(3), 143–161. Pourali, M., Kermanshahi, H., Golian, A., Razmi, G.R. and Soukhtanloo, M. 2014. Antioxi-dant and anticoccidial efects of garlic powder and sulfur amino acids on Eimeria-infected and uninfected broiler chickens. Iran. J. Vet. Res. 15(3), 227–32. Qaid, M.M., Al-Mufarrej, S.I., Azzam, M.M. and Al-Garadi, M.A. 2021. Anticoccidial effectivity of a traditional medicinal plant, Cinnamomum verum, in broiler chickens infected with Eimeria tenella. Poult. Sci. 100(3), 100902. Quiroz-Castaneda, R.E. and Dantan-Gonzalez, E. 2015. Control of avian coccidiosis: Future and present natural alternatives. Biomed. Res. Int. 2015(1), 430–610. Rahimi, S., Teymori Zadeh, Z., Torshizi, K., Omidbaigi, R. and Rokni, H. 2011. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agric. Sci. Technol. 13(4), 527–539. Ramadan, M., Elmadawy, R. and Tolba, I. 2021. Coccidiosis in Japanese quails (Coturnix coturnix japonica) in Kalioubia governorate: prevalence and treatment trials. Benha Vet. Med. J. 40(2), 131–136. Rose, M.E. and Hesketh, P. 1979. Immunity to coccidiosis: T-lymphocyte- deficient animals B-lymphocyte-. Immunology. 26(2), 630–637. Rose, M.E., Hesketh, P. and Wakelin, D. 1992. Immune control of murine coccidiosis: CD4+ and CD8+ T lymphocytes contribute differentially in resistance to primary and secondary infections. Parasitology. 105(3), 349–354. Rothwell, L., Gramzinski, R.A., Rose, M.E. and Kaiser, P. 1995. Avian coccidiosis: changes in intestinal lymphocyte populations associated with the development of immunity to Eimeria maxima. Parasite immunol. 17(10), 525–533. Ruff, M.D. 1985. Life cycle and biology of Eimeria lettyae sp. n. (Protozoa: Eimeriidae) from the northern bobwhite, Colinus virginianus (L.). J. Wildl. Dis. 21(4), 361–370. Ruff, M.D. and Edgar, S.A. 1982. Reduced intestinal absorption in broilers during Eimeria mitis infection. Am. J. Vet. Res. 43(3), 507–509. Ruff, M.D., Fagan, J.M. and Dick, J.W. 1984. Pathogenicity of coccidia in Japanese quail (Coturnix coturnix japonica). Poult. Sci. 63(1), 55–60. Schlee, M., Harder, J., Köten, B., Stange, E.F., Wehkamp, J. and Fellermann, K. 2008. Probiotic lactobacilli and VSL# 3 induce enterocyte β-defensin 2. Clin. Exp. Immunol. 151(3), 528–535. Seok, S., Park, J., Cho, S., Baek, M., Lee, H. and Kim, D. 2003. Coccidian (Eimeria spp) in small intestine of Japanese quail (coturnix coturnix japonica). Kor. J. Lab. Ani. Sci. 19(2), 90–91. Shetshak, M., Suleimn, M., Jatau, I., Ameh, M. and Akefe, I. 2021.Anticoccidial efficacy of Garcinia kola (Heckel H.) against experimental Eimeria tenella infection in chicks. J. Parasit. Dis. 45(4), 1034–1048. Sidiropoulou, E., Skoufos, I., Marugan-Hernandez, V., Giannenas, I., Bonos, E., Aguiar-Martins, K., Lazari, D., Blake, D.P. and Tzora, A. 2020. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Vet. Sc. 7, 420. Singh, G.J.P. and Gill, S.S. 1976. Activity of amprolium against different of Eimeria Necatrix infection and effect of medication on development of immunity. Rev. Parasitol. 37, 63–70. Song, X., Li, Y., Chen, S., Jia, R., Huang, Y., Zou, Y., Li, Y., Zhao, X. and Yin, Z. 2020. Anticoccidial effect of herbal powder “Shi Ying Zi” in chickens infected with Eimeria tenella. Animals. 10(9), 1484. Sreeranjini, A.R., Lyyangar, M.P. and Pramod kumar, D. 2010. Histological study on the fibrous architecture of kidney and ureter of Japanese quail (coturnix coturnix japonica). Tam. J. Vet. Ani. Sci. 6,107–110. Su, G., Wang, L., Zhou, X., Wu, X., Chen, D., Yu, B., Huang, Z., Luo, Y., Mao, X., Zheng, P., Yu, J., Luo, J. and He, J. 2021. Effects of essential oil on growth performance, digestibility, immunity, and intestinal health in broilers. Poult. Sci. 100(8), 101242. Tanweer, A.J., Chand, N., Saddique, U., Bailey, C.A. and Khan, R.U. 2014. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol. Res. 113, 2951–2960. Teixeira, M. and Lopes, C.W.G. 2000. Eimeria minima n. sp. (Apicomplexa: Eimeriidae) from the Japanese quail (Cuturnix cuturnix japonica) in Brazil. Revista Brasileira de Ciência Veterinária. 7(3), 157–158. Teixeira, M., Teixeira-Filho, W.L. and Lopes, C.W.G. 2004. Coccidiosis in Japanese quails (Coturnix japonica): characterization of a naturally occurring infection in a commercial rearing farm. Braz. J. Poult. Sci. 6(2), 129–134. Tsinas, A., Giannenas, I., Voidarou, C., Tzora, A. and Skoufos, J. 2011. Effects of an oregano based dietary supplement on performance of broiler chickens experimentally infected with Eimeria acervulina and Eimeria maxima. Poult. Sci. J. 48(3), 194–200. Tsutsumi, Y. 1972. Eimeria tsunodai sp. nov. (protozoa: Eimeriidae). A caecal coccidium of Japanese quails (Coturnix coturnix japonica). Nihon Juigaku Zasshi. 34(1), 1–9. Tzora, A., Giannenas, I., Karamoutsios, A., Papaioannou, N., Papanastasiou, D., Bonos, E., Skoufos, S., Bartzanas, T. and Skoufos, I. 2017. Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and Intestinal morphology. J. Poultry Sci. 54(3), 218–227. Umar, H.A., Lawal, I.A., Okubanjo, O.O. and Wakawa, A.M. 2014. Morphometric identification, gross and histopathological lesions of Eimeria species in Japanese quails (Coturnix coturnix japonica) in Zaria, Nigeria. J. Vet. Med. 2014(1), 1–6. Verma, J., johri, T.S., Swain, B.K. and Ameena, S. 2004. Effect of graded level of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. Br. Poult. Sci. 45(4), 512–518. Witcombe, D.M. and Smith, N.C. 2014. Strategies for anti-coccidial prophylaxis. Parasitology. 141(11), 1379–1389. WOAH Terrestrial Manual. 2021. Newcastle Disease (Infection with Newcastle Disease Virus). In Manual of diagnostic tests and vaccines for terrestrial animals (Chapter 3.3.14). Paris, France: WOAH. Available via https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.14_NEWCASTLE_DIS.pdf/ (Accessed 15 March 2022). Youn, H.J. and Noh, J.W. 2011. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet. Parasitol. 96(4), 257–263. Zhang, D.F., Sun, B.B., Yue, Y.Y., Zhou, Q.J. and Du, A.F. 2012. Anticoccidial activity of traditional Chinese herbal Dichroa febrifuga Lour. extract against Eimeria tenella infection in chickens. Parasitol. Res. 111, 2229–2233. | ||
| How to Cite this Article |
| Pubmed Style Elbakrey RM, Eid AA, Elkholy AA, Mousa MR, El-morsy MA, Abdelaziz WS. Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact. Open Vet. J.. 2025; 15(3): 1446-1467. doi:10.5455/OVJ.2025.v15.i3.35 Web Style Elbakrey RM, Eid AA, Elkholy AA, Mousa MR, El-morsy MA, Abdelaziz WS. Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact. https://www.openveterinaryjournal.com/?mno=240737 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.35 AMA (American Medical Association) Style Elbakrey RM, Eid AA, Elkholy AA, Mousa MR, El-morsy MA, Abdelaziz WS. Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact. Open Vet. J.. 2025; 15(3): 1446-1467. doi:10.5455/OVJ.2025.v15.i3.35 Vancouver/ICMJE Style Elbakrey RM, Eid AA, Elkholy AA, Mousa MR, El-morsy MA, Abdelaziz WS. Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1446-1467. doi:10.5455/OVJ.2025.v15.i3.35 Harvard Style Elbakrey, R. M., Eid, . A. A., Elkholy, . A. A., Mousa, . M. R., El-morsy, . M. A. & Abdelaziz, . W. S. (2025) Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact. Open Vet. J., 15 (3), 1446-1467. doi:10.5455/OVJ.2025.v15.i3.35 Turabian Style Elbakrey, Reham M., Amal A.m. Eid, Ahmed A. Elkholy, Mohamed R. Mousa, Mohamed A. El-morsy, and Walaa S. Abdelaziz. 2025. Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact. Open Veterinary Journal, 15 (3), 1446-1467. doi:10.5455/OVJ.2025.v15.i3.35 Chicago Style Elbakrey, Reham M., Amal A.m. Eid, Ahmed A. Elkholy, Mohamed R. Mousa, Mohamed A. El-morsy, and Walaa S. Abdelaziz. "Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact." Open Veterinary Journal 15 (2025), 1446-1467. doi:10.5455/OVJ.2025.v15.i3.35 MLA (The Modern Language Association) Style Elbakrey, Reham M., Amal A.m. Eid, Ahmed A. Elkholy, Mohamed R. Mousa, Mohamed A. El-morsy, and Walaa S. Abdelaziz. "Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact." Open Veterinary Journal 15.3 (2025), 1446-1467. Print. doi:10.5455/OVJ.2025.v15.i3.35 APA (American Psychological Association) Style Elbakrey, R. M., Eid, . A. A., Elkholy, . A. A., Mousa, . M. R., El-morsy, . M. A. & Abdelaziz, . W. S. (2025) Cecal coccidiosis in Japanese quails (Coturnix Japonica): Eimeria tsunodai identification and comparative prophylactic phytochemotherapy with immune stimulant impact. Open Veterinary Journal, 15 (3), 1446-1467. doi:10.5455/OVJ.2025.v15.i3.35 |