| Review Article | ||
Open Vet. J.. 2025; 15(6): 2312-2328 Open Veterinary Journal, (2025), Vol. 15(6): 2312-2328 Review Article Rift Valley fever: A zoonotic disease with global potentialEka Pramyrtha Hestianah1*, Aswin Rafif Khairullah2, Mustofa Helmi Effendi3, Budiastuti Budiastuti4, Wiwiek Tyasningsih5, Budiarto Budiarto3, Dian Ayu Permatasari3, Ikechukwu Benjamin Moses6, Riza Zainuddin Ahmad2, Bantari Wisynu Kusuma Wardhani7, Muhammad Khaliim Jati Kusala2, Dea Anita Ariani Kurniasih8, Ima Fauziah2, Syahputra Wibowo9, Emmanuel Nnabuike Ugbo6 and Kartika Afrida Fauzia10,111Division of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Study Program of Pharmacy Science, Faculty of Health Science, Universitas Muhammadiyah Surabaya, Surabaya, Indonesia 5Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 6Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 7Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 8Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 9Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 11Department of Environmental and Preventive Medicine, Faculty of Medicine, Oita University, Yufu, Japan *Corresponding Author: Eka Pramyrtha Hestianah. Division of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: eka-p-h [at] fkh.unair.ac.id Submitted: 05/01/2025 Revised: 05/05/2025 Accepted: 17/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
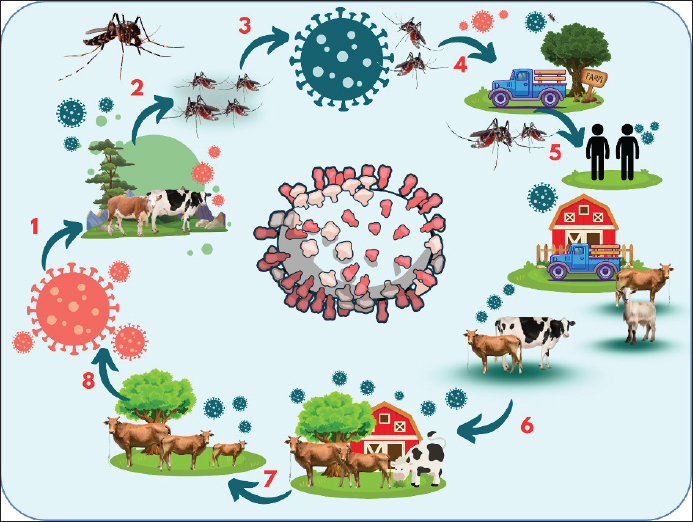
AbstractAn arthropod-borne zoonotic disease called “Rift Valley fever (RVF)” spreads widely among ruminant animals and humans. RVF is caused by the RVF Virus (RVFV), a round-enveloped RNA virus belonging to the genus Phlebovirus and family Bunyaviridae. RVF is found exclusively in African nations, and it is primarily associated with high rainfall and dense vector mosquito populations. The virus moves from its initial replication site to vital organs, such as the brain, liver, and spleen, after infection. These organs either recover due to both general and particular host responses, or they are affected by the pathogenic effects of the virus or immunological pathological processes. The main lesion observed in the RVF is hepatic necrosis. RVF can be diagnosed in clinical laboratories using a variety of techniques. RVF is defined by high abortion rates and high newborn deaths, which typically follow periods of intense precipitation. Commonly observed pathologies include gastrointestinal hemorrhage, splenomegaly, and liver necrosis. Transmission of the virus between Aedes and Culex mosquitoes in flood water has been demonstrated to occur transovarially. A number of ecological, anthropogenic, environmental, and viral evolutionary risk factors combine to make it more likely for RVFV to spread and establish in new locations. Although there is no specific treatment for human or animal RVF, supportive care can be beneficial. RVF can be prevented in a number of ways, such as by detecting climatic change, controlling mosquito populations, immunizing animals in endemic areas, and managing travel. Keywords: RVF, RVFV, Mosquito, Public health, Virus. IntroductionAn arthropod-borne zoonotic disease called “Rift Valley fever (RVF)” spreads widely among ruminant animals and humans (Kwaśnik et al., 2021). RVF, caused by the Rift Valley Fever Virus (RVFV), is regarded as one of Africa’s most significant diseases (Wright et al., 2019). RVFV is one of nine species of the genus Phlebovirus and family Bunyaviridae, which also includes the sand fly fever virus, Punta Toro virus, and severe fever syndrome with thrombocytopenia virus (Hornak et al., 2016). Although the disease is now only seen in Africa and some parts of the Middle East, it has the potential to spread worldwide (Lapa et al., 2024). The disease has spread to previously unreported places due to international travel and livestock trade. In 1931, an outbreak of unexpected sheep fatalities and abortions along the shores of Lake Naivasha in Kenya’s Rift Valley led to the first identification of RVF (Pepin et al., 2010). Since then, the majority of Southern and East African nations have recorded cases of the disease. Large-scale RVF outbreaks followed by intense rains and widespread floods in East Africa in 1997–1998 and 2006–2007 resulted in huge financial losses for livestock owners (Munyua et al., 2010). Although it is believed that domesticated ruminants such as sheep, buffalo, goats, cattle, and camels, are the most vulnerable, certain other wild animals, including impala, giraffes, pigs, and warthogs, have also been shown to have serological signs of infection (Hartman, 2017). The disease is transmitted through the bite of an infected mosquito, typically belonging to the Aedes or Culex genera (Tinto et al., 2023). The hatching of RVFV eggs during years of intense rainfall and local floods has been connected to epidemics, indicating that the virus may survive in dry Aedes mosquito eggs (Manore and Beechler, 2015). RVFV can also be spread by other vectors, including a variety of mosquito species and possibly other biting insects like gnats and ticks (Socha et al., 2022). RVF is transmitted to hosts based on their ability to harbor the virus, their vulnerability to infection, and the environmental and climatic factors that promote mosquito survival and reproduction. For both humans and animals, exposure to skilled mosquito vectors poses a serious risk, especially for those who handle livestock (Kwaśnik et al., 2021). Although infected mosquito bites can also infect humans, contact with diseased livestock is the primary way through which humans contract RVF (Mansfield et al., 2015). In ruminants, signs of infection typically start with fever and might include liver necrosis, respiratory illness, abortion, stillbirth, and death (Ikegami and Makino, 2011). Most commonly, this virus causes no symptoms or a self-limiting feverish illness in people. However, in extreme circumstances, neurological abnormalities, encephalitis, hemorrhagic illness, and liver damage (Connors and Hartman, 2022). Less than 1% of instances of this disease are associated with severe and occasionally deadly symptoms; however, it can produce a widespread febrile sickness in people (Hartman, 2017). Although RVFV outbreaks in endemic places have resulted in miscarriages among pregnant women, there is currently a dearth of data to corroborate these clinical consequences (Baudin et al., 2016). RVF is categorized as an overlapping select agent by the U.S. Department of Agriculture (USDA) and the Centers for Disease Control and Prevention (CDC). This is a notifiable illness referred to by the World Organization for Animal Health. The economic impact of this disease extends beyond its effects on human and animal health (Peyre et al., 2015). It can also include the costs of surveillance and control measures, quarantine restrictions, livestock movement bans, and a decline in product demand as a result of public perceptions of risk (Himeidan et al., 2016). Understanding the risks associated with RVF is crucial for implementing measures to curtail the spread of the illness. Therefore, the aim of this review was to review the issues related to RVF disease. EtiologyRVF is caused by the RVF Virus (RVFV), a round-enveloped RNA virus belonging to the genus Phlebovirus and family Bunyaviridae (Sall et al., 1999). The viral genome, which is a tripartite form of RNA, is composed of three segments: short (S), medium (M), and large (L). The virus has a diameter of 80–120 nm (Fawzy and Helmy, 2019). The viral RNA polymerase is encoded by the L segment, whereas the envelope glycoproteins Gc and Gn, as well as the nonstructural proteins NSm1 and NSm2, are encoded by the M segment (Gerrard et al., 2007). The nonstructural protein NSs and nucleoprotein (N protein) are encoded by the S segment, which employs an ambisense approach. The genomic segment is linked to RNA-dependent RNA polymerase and numerous copies of the N protein and forms a viral ribonucleoprotein complex (Wang et al., 2022). The envelope of RVFV consists of a lipid bilayer and glycoproteins Gn and Gc, which combine to form heterodimers (Allen et al., 2018). These heterodimers are then further formed into icosahedral pentamer and hexamers. The viral glycoproteins Gn and Gc interact with the cellular C-type lectins DC-SIGN and I-SIGN to allow RVFV to adhere to cells. The Gn and Gc glycoproteins are exposed on the virus’s outer surface, which allows the host immune system to detect them and produce neutralizing antibodies (Phoenix et al., 2016). Following cell infection by receptor-mediated viral endocytosis and pH-mediated fusing of the viral and endosomal membranes, the nucleocapsid is released into the cytoplasm, where translation, transcription, and genome replication occur (Harmon et al., 2016). One important virulence component that enables the virus to evade the host’s innate immune response is the nonstructural protein NSs, which suppresses the type I interferon response (Ly and Ikegami, 2016). Sherman and Smith (2025) recently described the visualization of the three-dimensional (3D) organization of RVFV by cryogenic electron microscopy (Cryo-EM) of single particles. In their study, they described spherical enveloped RVFV virions with a diameter of 100 nm with icosahedral symmetry (T = 12) and 720 prominent protrusions (composed of two viral glycoproteins, Gn and Gc) on their surface (Sherman and Smith, 2025). Although it can withstand alkaline conditions, this virus becomes inactive at pH 6.8, with a pH range of 7–8 being optimal (Liu et al., 2008). The virus can survive at neutral or alkaline pH for up to 4 months at 4°C and for 8 years if stored below 0°C (32°F), particularly if protein material like serum is present (Fawzy and Helmy, 2019). If the conditions are favorable, RVFV can persist in aerosols for more than an hour at 23 °C and 50%–80% relative humidity (Xu et al., 2023). The virus is vulnerable to lipid solvents (sodium or calcium hypochlorite and acetic acid) because the virion envelope contains a double lipid layer (Boshra et al., 2011). HistoryThe name RVF was given by Daubney et al. (1931) in 1930–1931 after an outbreak in the Rift Valley, Kenya, which affected at least 50 cattle, 1200 ewes, 3,500 sheep, and 200 humans. Nonetheless, comparable illnesses were noted in the same region in 1912 and 1926. RVFV was initially isolated in 1944 by Smithburn et al. (1949) from a population of wild-caught mosquitoes in the barren Semliki Forest in western Uganda. Host rangeHumans, cattle, goats, antelopes, buffalo, camels, monkeys, rats, and sheep are all known to naturally contract RVFV (Kroeker et al., 2020). There have been reports of significant RVF-related mortality and morbidity in people (Anywaine et al., 2024), cattle (Johnson et al., 2023), and sheep (Moreno et al., 2024). RVFV can infect a variety of domestic, agricultural, and lab animal species (Hartman, 2017). Mice, hamsters, lambs, puppies, kittens, and children are particularly vulnerable to RVFV (Kasye et al., 2016). No reptile or amphibian hosts have been reported for RVFV (Rissmann et al., 2020). EpidemiologyRVF is found exclusively in African nations, and it is primarily associated with high rainfall and dense vector mosquito populations. Since RVFV was initially identified in 1931, significant outbreaks have affected both humans and livestock in a number of African nations, including Kenya (Sang et al., 2017), Egypt (Fawzy and Helmy, 2019), Madagascar (Tantely et al., 2024), South Africa (Nanyingi et al., 2015), Mauritania (Jäckel et al., 2013), and Senegal (Sow et al., 2014). Severe RVF outbreaks are believed to be caused by a combination of factors, including greater vegetation greenness index, recurring flooding, and climatic circumstances. As a result, mosquito vectors that infect vulnerable ruminant hosts emerge (Bashir and Hassan, 2019). Cyclical epidemics occur in arid regions every 5 to 20 years. It is believed that the virus remains dormant in mosquito eggs throughout the time between epidemics (Evans et al., 2008). Numerous nations have been known to have multiple cases, sporadic viral isolation, or serological evidence of RVF, including Cameroon (Sado et al., 2022), Guinea (Grobbelaar et al., 2011), Angola (Liu et al., 2016), Botswana (Sanderson et al., 2020), Congo (Shariff et al., 2023), Burkina Faso (Boussini et al., 2014), Tanzania (de Glanville et al., 2022a), Gabon (Becquart et al., 2024), Ethiopia (Jaleta et al., 2022), Malawi (Kainga et al., 2022), Mali (Tong et al., 2019), Niger (Lagare et al., 2019), Nigeria (Oragwa et al., 2023), Somalia (Hassan-Kadle et al., 2021), Chad (Durand et al., 2003), and Uganda (Tumusiime et al., 2023). Humans and other animal species have been connected to this illness. Between 1950 and 1951, the disease was later identified in South Africa, where it led to severe losses, human cases, and epizootics in sheep and cattle (McMillen and Hartman, 2018). Outside of sub-Saharan Africa, only RVF epizootic outbreaks were documented in humans and animals in Egypt in 1977–1978; Mauritania in 1987; and Egypt in 1993 (Kenawy et al., 2018). Both the 1997–1998 and 2006–2007 large-scale RVF epidemics in East Africa were followed by intense rains, extensive flooding, and severe financial losses for cattle owners (Baba et al., 2016). In recent years, epidemics have struck most sub-Saharan nations, including those in West Africa. Since 2010, epidemics have spread to the Middle East, killing people and causing massive financial losses in livestock (Mahmoud et al., 2021). For the first time, significant RVF outbreaks outside of Africa were documented in Saudi Arabia and Yemen in 2000 and 2001, respectively (Balkhy and Memish, 2003). The importation of disease-carrying insects or animals (cattle and camels) from endemic regions are contributing reasons to the outbreak. In Mayotte, a French island, RVF cases were discovered in 2007. These cases were probably brought in by importing infected ruminants from an area where the virus was endemic (Cêtre-Sossah et al., 2012). Since 2004, retrospective serological investigations have demonstrated the existence of RVFV in cattle (Métras et al., 2016). In 2018, 142 cases in humans and many ruminant groups were documented, marking the resurgence of RVF (Youssouf et al., 2020). There is some seropositivity in both humans and animals, according to previous research done in the Western Sahara and the Mediterranean region (Iraq, Turkey, Algeria, Tunisia, and Iran), where no human or animal illness cases have ever been documented (Gür et al., 2017; Kalthoum et al., 2021; Fakour et al., 2021; Bron et al., 2021; Trabelsi et al., 2023). Conclusions from the data may be challenging due to the small sample size and restricted geographic range of the animals studied in the majority of these investigations. Two RVF outbreaks were documented in the Al Kufrah district of southeast Libya in January 2020 (Mahmoud et al., 2021). Only positive serological data were taken into consideration, and one case was reported in each epidemic (one involving sheep and one involving sheep and goats). There were no fatalities (Fafetine et al., 2013). The first RVF epidemic in Burundi occurred in 2022, when Mauritania also reported an outbreak (Tabassum et al., 2023; Ndishimye et al., 2024). The illness impacts a nation’s livestock, which is an important source of revenue and a crucial component of maintaining proper nutrition and food security. RVFV is present in a wide range of ecological climates, such as the humid highlands of Madagascar, sub-humid regions of eastern Africa, moist forests of central Africa, arid regions of western Africa and the Arabian Peninsula, and the dam-irrigated agricultural lands of Egypt, Mauritania, and Sudan (Tantely et al., 2015). According to reports from different groups, recurrent epidemic intervals usually last between 5 and 15 years. (Chambaro et al., 2022). PathogenesisOnce RVFV enters target tissues through mosquito bites, percutaneous wounds, or oropharyngeal aerosols, it replicates quickly at extremely high titers (Reed et al., 2013). The virus moves from its initial replication site to vital organs, such as the brain, liver, and spleen, after infection. These organs either recover due to both general and particular host responses, or they are harmed by the pathogenic effects of the virus or immunological pathological processes (Nair et al., 2023). From the site of inoculation, the virus is transported via lymphatic drainage to the lymph nodes, where it multiplies and cycles out into the bloodstream, resulting in viremia and systemic infection (Salimi et al., 2016). In extreme situations, there is noticeable liver necrosis, and individuals may have necrotic foci in their brains, which could be an indication of a less common type of encephalitis (Boyles et al., 2021). Because RVFV replication in cells is extremely cytotoxic, the majority of acute disease-related cellular damage is probably caused by the virus directly destroying host cells (Gwon et al., 2021). Depending on the animal’s susceptibility or resistance, three different infection patterns are typically observed (in both naturally infected and experimentally infected animals), although infection exhibits a different pathophysiology in each animal model (Xu et al., 2023). High blood viral loads are strongly linked to fatal outcomes, and infected animals die quickly from this infection, which can be a severe acute infection with uncontrolled viremia. The second pattern is characterized by a sharp reduction in viremia and mild-to-asymptomatic illness (Kitandwe et al., 2022). The third pattern involves fever and viremia in the first phase, followed by the possibility of more febrile episodes in the second phase and delayed onset of infectious sequelae (Hassanain et al., 2010). This pattern of virus dissemination to other organs, particularly the retina and central nervous system organs, after the virus has passed through the blood-brain barrier is frequently linked to major long-term repercussions (Quellec et al., 2024). In animals and humans, RVF-induced lesions largely occur in the liver (Ikegami and Makino, 2011). Histopathological analysis of sheep tissue that had been experimentally infected has proven that these results are constant throughout severe instances (Balaraman et al., 2024). Virus tropism is limited to hepatocytes and monocytes. Hepatocellular alterations caused by infection may develop into necrosis, which is characterized by elevated liver enzyme levels, leukopenia, or thrombocytopenia (Smith et al., 2012). Immune responseThe production of neutralizing antibodies against Gn and Gc has provided a good correlation with protection against RVFV, despite the fact that the precise mechanistic correlate of immune protection against this virus in humans or animals is unknown (Bian et al., 2023). As a result, Gn and Gc are now the primary antigen targets in RVF vaccine research (Wright et al., 2020). When RVFV and other Bunyavirus infections occur, the N nucleoprotein causes elevated levels of IgG and IgM antibodies, but these antibodies do not neutralize the virus (Lapa et al., 2024). However, as this protein is a target for complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC), as well as a strong human CD8+ T cell antigen, anti-N immune responses appear to contribute to protection against RVFV (Clémenceau et al., 2006). Early CD4+ and CD8+ T-cell proliferation and Th1 cytokine production were linked to nonlethal outcomes in African green monkeys infected with RVFV (McElroy and Nichol, 2012). Higher levels of the cytokine IL-10, which inhibits Th1 responses, are linked to fatal occurrences in humans, as opposed to nonfatal instances (van Vuren et al., 2015). PathologyThe main lesion observed in RVF is hepatic necrosis (Kamal, 2009). The liver is mushy, swollen, friable, and yellowish to dark brown in newborns and aborted fetuses (Lubisi et al., 2023). The gallbladder wall also showed signs of edema and hemorrhage, hemorrhagic enteritis, enlarged peripheral and visceral lymph nodes, large-scale skin hemorrhages, buildup of blood-stained fluid in bodily cavities, and large-scale subcutaneous and serous hemorrhages (Odendaal et al., 2021). Severe liver injury may cause the carcass to decompose quickly. Hepatocytes in the afflicted livers of sheep exhibit focal to widespread coagulative necrosis (Ikegami and Makino, 2011). DiagnosisRVFV can be diagnosed in clinical laboratories using a variety of techniques. These clinical laboratory techniques include the following: Isolation and identification of the causative agent: A diagnosis can be made by identifying and isolating the infectious virus from suitable specimens. The most accurate way to diagnose viral illnesses is to isolate the infected viruses in cell culture (Rangel et al., 2024). In order to isolate viruses, clinical specimens such as lymph nodes, liver, brain, spleen, heart, kidney, and blood should be aseptically collected in sterile containers (Kasye et al., 2016). When a fetus is performing autolysis, the brain is an appropriate sample for ice export to the laboratory for diagnosis (Quellec et al., 2024). Numerous cell cultures, such as primary kidney and testis cell cultures of sheep and laboratory animals, including mice and hamsters, baby hamster kidney (BHK), chicken embryo reticulum (CER), and African green monkey kidney (Vero), can be used to extract viruses (Lapa et al., 2024). Serological diagnosis: A key diagnostic technique for many viral infections is the identification of certain virus antibodies and the measurement of those antibodies at various phases of the illness. RVFV has a wide geographic range and an explosive potential to spread to new places where cattle husbandry is common, making laboratory confirmation of the virus a diagnostic urgency (Hartman, 2017). To show antibodies to RVF using the enzyme linked immune sorbent assay (ELISA), Hemagglutination (HI), agar gel immune diffusion (AGID), and virus neutralization techniques, two serum samples should be collected at 30-day intervals (Paweska et al., 2005). Reverse transcriptase polymerase chain reaction (RT-PCR) is a method for detecting viral antigens (Sall et al., 2002). For the early diagnosis of RVF, this highly sensitive and specific molecular technique. RVFV identification in mosquito populations is accomplished through the use of PCR, which provides fast diagnosis for antigen detection (Mwaengo et al., 2012). Phylogenetic analysis employs RT-PCR and nucleocapsid protein-coding area sequencing (Ikegami, 2012). Virus neutralization (test specified for international trade): Although the detection of RVFV antibodies in the sera of many species is highly specific and will document the first reaction; this technique cannot distinguish between the presence of antibodies in animals that are naturally sick and those that have received the RVF vaccine (Mansfield et al., 2015). It is not recommended to utilize this test outside of endemic areas or in laboratories lacking the necessary biosecurity equipment and vaccinated staff because it can only be conducted using live virus (Mahmoud et al., 2021). ELISA: This technique can be applied in nations free of RVF because it can be performed using inactivated antigens (McElroy et al., 2009). Interactions could occur between the RVF virus and other phlebovirus. Recently, recombinant nucleocapsid protein has taken the place of a complete inactivated virus or mouse liver antigen. Recent infection can be diagnosed using IgM capture ELISA (Fukushi et al., 2012). RVF-free nations can apply hemagglutination inhibition because it can be performed using inactivated antigens (Soliman et al., 1988). In nonendemic regions, this inhibition works incredibly well. Differential diagnosisThe differential diagnosis of RVF in animals includes bluetongue, water heart disease, Wessel’s disease, foot and mouth disease, transient fever, brucellosis, pest des petites rumenitis, and Nairobi sheep disease. Newborn lambs are not affected by Nairobi sheep illness, which does not have hepatitis. Lesions in the mouth and feet (coronitis) are common in cases of blue tongue, but there is no hepatitis present. Heart water illness frequently manifests as neurologic symptoms and serous fluid in bodily cavities. In contrast to brucellosis, which does not occur in conjunction with heavy rainfall, transitory fever causes muscle weakness, recovers quickly, and is rare in sheep and goats. RVF is more severe than Wessel’s disease, which is an uncommon viral illness (Gerdes, 2004). Clinical signs and symptomsRVF causes fetal malformations, neonatal mortality, and abortion storms in livestock ruminants (Pavulraj et al., 2025). In some cases, RVF is defined by high abortion rates and high newborn death, which typically follow periods of intense precipitation (Wright et al., 2019). Commonly observed pathologies include gastrointestinal hemorrhage, splenomegaly, and liver necrosis (Ikegami and Makino, 2011). In cattle: Calves suffer from fever (104°F–106°F/40°C–41°C), weakness, diarrhea, jaundice, depression, and loss of appetite (Hartman, 2017). Adult cattle often have inapparent infections; clinical disease is characterized by fever lasting 24–96 hours, dry or dull coat, excessive salivation, anorexia, nasal discharge, bloody diarrhea, weakness, low milk production, lacrimation, and high abortion rates in pregnant cows (Melkamu, 2018). In sheep and goats: Biphasic fever (40°C–41°C), anorexia, lethargy, stomach pain, rapid breathing, and mortality occur in newborn lambs (less than 2 weeks old) within 24–36 hours (McMillen and Hartman, 2018). Fever lasting 24 to 96 hours, anorexia, lethargy, depression, elevated respiratory rate, vomiting, mucopurulent nasal discharge, bloody diarrhea, jaundice, and abortion rates close to 100% are among the symptoms experienced by lambs (older than 2 weeks), adult sheep, and goats experience (Hartman, 2017). In humans: In humans, RVF manifests as an influenza-like illness that includes fever (37.8°C–40°C), headache, myalgia, weakness, nausea, and light sensitivity (Anywaine et al., 2022). Retinopathy, petechiae, hemorrhagic syndrome with jaundice, meningoencephalitis, blindness, and death are possible outcomes of complications (Rahman et al., 2023; Kasongamulilo et al., 2025; Pavulraj et al., 2025). A recent study revealed evidence of a silent human carriage of the RVFV in central and western Zambia, especially among slaughter-house workers (16.67%) and livestock farmers (14.41%) who are in close contact with animals and animal products (Kasongamulilo et al., 2025). In this study by Kasongamulilo et al. 2025), an overall occupational seropositivity of 9.90% was reported. The identified risk factor for RVF-seropositivity was found to be associated with the movement of animals in search of greener pastures (Kasongamulilo et al., 2025). TransmissionTransmission of the virus between Aedes and Culex mosquitoes in flood water has been demonstrated to occur transovarially (Pepin et al., 2010). The virus flourishes for long periods of time in mosquito eggs laid on the edges of dry depressions called ambos, which are common in grassy highland areas (Schreur et al., 2021). Infected mosquitoes emerge after the eggs hatch; the surrounding pets and wildlife are affected when it rains or when dams get flooded (Baba et al., 2016). RVFV can spread without the use of an insect vector, which raises concerns about the possibility that the virus could infect humans or other livestock species, pollute items, or reach non-enzootic areas by these means (Socha et al., 2022). Humans may be at risk of health problems if they ingest raw or unpasteurized milk with low RVFV levels (Grossi-Soyster et al., 2019). Animals in uninfected locations may contract the RVF virus from humans through mosquito bites. The transmission cycle of the RVFV is shown in Figure 1.
Fig. 1. Understanding the transmission cycle of RVF Virus (RVFV). RVF spreads through humans, animals, and mosquitoes. Infected livestock develop viremia, allowing Culex and Aedes mosquitoes to become carriers while feeding (1). The virus replicates in the mosquito’s salivary glands and spreads through bites (2). Mosquitoes transmit RVFV to animals, and infected livestock spread the virus through movement (3). Humans can contract it via mosquito bites or contact with infected animal fluids (4). In animals, transmission occurs through direct contact and in contaminated environments (5). Pregnant animals can transmit RVPV to their offspring, often leading to abortion storms (6). Infected animals act as reservoirs while maintaining the cycle going (7). Effective vector control, biosecurity, and surveillance are crucial for preventing RVF outbreaks and reducing health and economic impacts. Direct transmission can occur from one animal to another or from one animal to a human. Human infection is primarily contracted through contact with the blood, bodily fluids, or organs and tissues of infected animals and fetuses (McMillen et al., 2022). Additionally, mosquito bites represent a risk factor for human RVFV infection. The occasional vertical transmission of RVFV from mothers to their infants has been described; however, no direct horizontal transmission from humans to humans has been reported (Kwaśnik et al., 2021). Animals in uninfected areas may contract RVFV from humans through mosquito bites (Tinto et al., 2023). The potential for African buffalo (Syncerus caffer) or other local or endemic wild ruminants, wild rodents, or bats to contribute to the virus’s propagation has not been confirmed, but it is also not ruled out when considering wild animals as an RVFV reservoir (Olive et al., 2012). Risk factorsA number of ecological, anthropogenic, environmental, and viral evolutionary risk factors combine to make it more likely for RVFV to spread and establish in new locations. The range and seasonality of insect vectors and vertebrate hosts are expanding due to global variations in temperature and precipitation zones (Gibson et al., 2023). The number and magnitude of outbreaks each year can be made worse by the intensity and regularity of extreme weather events, and the volume and frequency of international travel by people, animals, and goods keeps growing, opening up new avenues for virus transmission by infected individuals, animals, or vectors (Odendaal et al., 2021). In endemic and developing regions, suburban and peri-urban development merges with cattle pastures and irrigated croplands, creating novel habitats that support vertebrate host groups and mosquito vectors, facilitating interepizootic transmission and low-level maintenance of RVFV (de Glanville et al., 2022b). If the virus spreads to a new location, unexpected transmission patterns could result from opportunities for virus evolution (Behl et al., 2022). The virus spreads through a variety of routes, such as mosquito bites, aerosol droplets inhaling from an infected vertebrate host, potential human-to-human sexual and placental transmission, and direct contact from handling infected tissue, such as by slaughterhouse workers or veterinarians. These routes may be more severe or tolerant to broader environmental restrictions or additional hosts (Tantely et al., 2024). Although RVF vaccines for animal use in endemic areas have been approved and are in use, they have not yet been approved for human use. To be successful and cost-effective, they must be targeted in both time and space (Kitandwe et al., 2022). This approach may not be practical in developing nations, especially if the virus infiltrates communities of wild ungulates or is not identified until it quickly spreads to livestock (Domenech et al., 2006). In addition to being an important livestock and human pathogen, RVFV is also a potential bioweapon, especially due to its ability to be spread by aerosols (Sherman and Smith, 2025). Public health importanceRVF is a high-consequence pathogen that can cause serious disease in both humans and animals during an outbreak, making it a major zoonotic danger that has the ability to transmit a list “A” disease internationally (Tomori and Oluwayelu, 2023). There is a very high risk of infection among occupational groups like herders, farmers, and agricultural workers; slaughterhouse workers; veterinarians; and animal health workers (Tinto et al., 2023). The virus can be spread via direct or indirect contact with an infected animal’s blood or tissue (Fawzy and Helmy, 2019). This can involve performing veterinary surgical procedures, handling animal tissue during slaughter, cutting or skinning animals, helping with animal birth, or getting rid of carcasses or fetuses (McMillen and Hartman, 2018). Less frequent ways of transmission include inoculation, such as through contact with broken skin or wounds from infected knives or needlesticks; inhaling aerosols released during the autopsy or slaughter of infected animals; getting bitten by an infected mosquito (usually Aedes); and consuming raw (unpasteurized or uncooked) milk from infected animals (Al-Hamdan et al., 2015). No transmission from one person to another has ever been reported. Economic impactThere are serious economic repercussions from RVF disease, such as widespread animal abortions, risks to food security, and stringent trade restrictions (Tinto et al., 2023). In addition, this disease results in significant losses in animal production (meat and milk), expensive management expenses, and the closure of livestock markets (Fawzy and Helmy, 2019). Pastoralists are especially affected by RVF because they reside in communities that are at risk and have poor levels of adversity resistance. Livestock farmers are the first group to directly suffer the socioeconomic effects of RVF because of the high rates of animal mortality and abortion (Muga et al., 2015). This constitutes a large stock loss, particularly for young ruminants. The long-term effects of disruptions in herd dynamics can also include production losses that last for years or even generations of animals (Peyre et al., 2015). These effects become apparent over time and are contingent on the combined impact of multiple economic factors and strict herd dynamics. The effects of RVF on producers will have an effect on the livestock value chain and the services linked to it. Compared with individual farms, RVF might have a greater overall effect on the economy and other service providers in the livestock supply chain (Gerken et al., 2023). The effects may be short-term (less than a year) or long-term (more than a year), qualitative (reconfiguration of value chains), quantitative (performance and socioeconomic value), or both (Xiao et al., 2015). Differences in the cost and accessibility of animals on the market are partly to blame for this effect. Other agricultural value chains, such as transportation and tourism, may be impacted in addition to the livestock value chain; for instance, the importation of other agricultural products from impacted nations may be prohibited (Rich and Wanyoike, 2010). An RVF outbreak could hinder the export of live animals and animal products due to international health standards (Chengula et al., 2013). Exports are a significant component of the national trade balance, and their prohibition can negatively impact the national economy. A series of trade sanctions related to RVF could have a detrimental impact on national treasury, national currency value, and import expenses (Pendell et al., 2016). Exporting livestock is a major source of foreign cash, income, and jobs. Livestock exports provide the majority of jobs, revenue, and foreign exchange. Export limitations have caused cattle prices to decline and trading conditions to deteriorate, further undermining herders’ purchasing power and standard of living (Himeidan et al., 2016). Marketing cattle is negatively impacted, particularly during religious holidays. The danger of RVF infection increases during these periods because of high animal population densities and religious commitment (de Glanville et al., 2022a). As a result, the zoonotic nature of RVF has caused major economic and social effects on all sectors (livestock and other industries), induced long-term trade restrictions on livestock and animal products during outbreaks, and caused a decline in confidence in importing countries. Outbreaks of RVF have a detrimental effect on human populations and the livestock industry, particularly in emerging and transitional nations (Himeidan et al., 2016). In low-resource countries, the risk is significant because public health infrastructure is insufficient to conduct routine disease surveillance, preventive, and control operations, particularly when outbreaks occur every 5 to 15 years (Gerken et al., 2022). TreatmentAlthough there is no specific treatment for human or animal RVF, supportive care can be beneficial. Nonetheless, epidemics can be prevented with the use of certain vaccines. Most RVF instances are moderate, and most people get better in 2 to 7 days on their own (Anywaine et al., 2024). Ibuprofen and acetaminophen are examples of over-the-counter drugs that might help treat moderate symptoms like fever and body aches (Yin et al., 2022). Serious patients may need to be admitted to a hospital where they will receive supportive care, including painkillers and water. Curcumin treatment decreases RVFV infection to almost undetectable levels (Narayanan et al., 2012). One of the rivals with the most nontoxic capacity to reduce viral levels is sorafenib. Researchers confirmed sorafenib’s efficacy against RVFV infection was confirmed by observing its effects in other in vitro and in vivo models (Brahms et al., 2017). The best option for creating a successful defense against a variety of viral illnesses is to use broad-spectrum antivirals. Respiratory syncytial virus infections may benefit from nebulized ribavirin, which is also used off-label to treat viral disease pathogens like Lassa (Hoover et al., 2018). It has since been discovered that a number of viruses, most notably RVFV, are resistant to ribavirin in both in vitro and in vivo settings. The treatment course started 1 hour after infection (HPI) and was continued twice a day for 10 days. The study included 70 mcg of ribavirin (positive control) and a 0.4% CMC placebo. Some RNA viruses have shown strong antiviral activity against favipiravir (T-705; 6-flouro-3-hydroxy-2-pyrazinecarboxamine), a possible pyrazine derivative (Scharton et al., 2014). In rhesus monkeys, prophylactic treatment with polyriboinosinic-polyribocytidylic acid stabilized with poly-L-lysine and carboxymethyl cellulose, or poly (IRCLC), has shown effectiveness in preventing viral illnesses, including rabies, yellow fever, and simian hemorrhagic fever (Arishi et al., 2006). According to more studies, convalescent phase plasma, immune modulators, and interferon may also be useful in treating people with RVF (Bouloy and Flick, 2009). VaccinationAt the moment, there are no licensed RVF vaccines for humans; however, several potential vaccine candidates with high promise are in early-stage development. The development of safe and effective antivirals is necessary and very urgent, in order to curtail the spread of RVFV. Livestock vaccination against RVF is a crucial disease control measure in both endemic and nonendemic regions. RVF vaccines have been developed using a variety of approaches. Early vaccines, including the live-attenuated Smithburn preparation and the inactivated vaccine, were created using virulent RVFV strains (Faburay et al., 2017). Formalin was used to inactivate the first RVF vaccine, which was based on the Entebbe strain NDBR103 obtained from a Ugandan mosquito (Randall et al., 1962). The US Army Medical Research Institute of Infectious Diseases (USAMRIID) developed TSI-GSD200, a new generation of formalin-inactivated RVFV vaccine, by further developing this vaccine. The BHK-21 hamster kidney cell method has been used to generate RVFV vaccines for animal usage that have been inactivated with formalin or ethylenediamine. The primary disadvantages of inactivated vaccines are their high cost and the need for repeated immunization schedules (Faburay et al., 2017). One of the earliest and most popular vaccines for preventing RVF is the live-attenuated Smithburn vaccine. This strain of Eretmapodites spp. was identified in Uganda in 1944 (Ikegami and Makino, 2009). The Smithburn vaccine is effective and affordable, but it has a number of disadvantages, including residual pathogenicity, which can result in prenatal deformities and abortion, and the potential for reversion to full virulence. Live-attenuated vaccines can only be used in nations free of RVFV and cannot be used in the prophylactic treatment of pregnant animals. Additionally, when vaccines are administered during an outbreak, reassortment may take place; thus, increasing the diversity of viruses (Botros et al., 2006). Using the virulent ZH548 and ZH501 strains that were obtained from Egyptian patients and serially passaged 12 times in MRC-5 cells with 5-fluorouracil as a mutagen, USAMRIID created the next live-attenuated vaccine, MP-12, for use in humans and animals. The MP-12 strain contains repetitive mutations in all three genome segments and is temperature sensitive. In general, MP-12 can generate enough antibody titers to shield vaccinated animals from RVFV, and immunizing pregnant animals may have positive side effects, such as protecting the unborn (Caplen et al., 1985). Attenuated vaccination of Clone 13 was created by naturally deleting 549 nucleotides (70%) from the NS gene. The 74HB59 clone, identified from plaques in the Central African Republic, served as the basis for this vaccination. The Diagnostic Interview for ADHD in Adults (DIVA) test makes it simple to differentiate Clone 13-vaccinated animals from naturally infected animals. A significant drawback of the vaccine is that the mutant virus does not generate a sustained immunological response and cannot replicate well in inoculated animals (Muller et al., 1995). Subunit protein vaccines, DNA vaccines (Lagerqvist et al., 2009; Spik et al., 2006), virus-like particles (VLPs) (Mandell et al., 2010), viral replicon particle vaccines (Oreshkova et al., 2013), viral vector vaccines (Warimwe et al., 2016), and live genetically modified vaccines (made from recombinant viruses engineered using reverse genetics) are a new class of vaccines made using recombinant nucleic acid technology (Bird et al., 2008). The most promising treatments appear to be recombinant protein-based vaccinations, VLPs vaccinations, and DIVA-compatible DNA vaccines, as they are very safe and entail minimal environmental concerns (Faburay et al., 2017). Currently, no vaccination is permitted for use in the European Union (EU), and no vaccine is available for human use. The Gc protein of RVFV was identified as the primary target of the neutralizing antibody response, as a new therapeutic idea for treating RVFV infection (Zhao et al., 2025). A recent study reported a Gc-specific neutralizing antibody (NA137), which was isolated from an alpaca, and a bispecific antibody (E2-NA137), and also evaluated their protective efficacy in A129 mice. In this prophylactic study, the survival rates of the NA137 and E2-NA137 groups were both 80%, whereas in the treatment study, the survival rates were 20% and 60%, respectively. This study further showed that NA137 and E2-NA137 provide a potential approach for the treatment of RVFV-associated illnesses either prophylactically or therapeutically (Zhao et al., 2025). In a previous study, researchers engineered a BoHV-1qmv-vectored subunit RVF virus (RVFV) vaccine (BoHV-1qmv Sub-RVFV) for cattle, in which a chimeric polyprotein coding for RVFV Gc, Gn, and bovine granulocyte–macrophage colony-stimulating factor (GMCSF) proteins was fused but cleaved proteolytically in infected cells into individual membrane-anchored Gc and secreted Gn-GMCSF proteins. Calves vaccinated with the BoHV-1qmv Sub-RVFV vaccine were noted to generate moderate levels of RVFV-specific serum-neutralizing (SN) antibodies and cellular immune responses (Pavulraj et al., 2025). Authors from the same study also recently revealed that sheep immunized with a newly developed live BoHV-1qmv Sub-RVFV vaccine using a combined intranasal–subcutaneous and prime–boost regimen is safe and stimulated significantly high SN antibody titers against the subunit vaccine vector (BoHV-1) and RVFV-MP-12 (Pavulraj et al., 2025). The researchers in this study concluded that the BoHV-1qmv-vectored subunit RVFV vaccine is safe and highly immunogenic in sheep and can potentially be an efficient subunit vaccine for sheep against RVFV. In June 2024, a Coalition for Epidemic Preparedness Innovations (CEPIs), which included representatives from international health organizations, multiple RVF-endemic countries, and key global collaborators, convened a 2-day workshop in Nairobi, Kenya, to discuss RVF epidemiology, modeling priorities, and specific gaps relevant to human RVF vaccine development (Gharpure et al., 2025). In the high-powered meeting, delegates identified some key priorities: looking beyond outbreaks, better data for better models, New, improved, and accessible diagnostics and serological assays, defining use cases, regulatory pathways, and implementation strategies for human vaccines, and people-centered approaches, as key to the success of human vaccine research and development and implementation, particularly as the virus impacts livestock and livelihoods (Gharpure et al., 2025). ControlRVF can be prevented in a number of ways, such as by detecting climatic change, controlling mosquito populations, immunizing animals in endemic areas, and managing travel. RVFV progression is highly dependent on the susceptibility of the physical, microbial, cellular, and immune response barriers of the carriage vectors. Importantly, these barriers, which are predominantly shaped by the genetic makeup of the mosquito species and the surrounding environmental temperature, exert strong selective pressure on the virus; thus, affecting its replication, evolution, and spread (Azerigyik et al., 2025). The changing climate coupled with the listed bottlenecks/barriers are potential significant drivers of RVF epidemics and their expansion into areas that were previously considered not to be endemic (Azerigyik et al., 2025). Vector control methods can be implemented at different phases of mosquito development (Bergren et al., 2020). Adult mosquitoes can be controlled by concentrating on pesticide application. It can directly target flying or resting adult mosquitoes or employ thermal fogging or extremely low-volume spraying to target resting adult mosquitoes (Dunbar et al., 2019). Mosquito larvae must reside in water to survive. They can live in any kind of standing water, such as a birdbath, tarp, pond, or old tire. Since mosquito larvae continue to reside in the same bodies of water where they hatch from their eggs, control at this stage is centered on the continuous management of the egg-laying locations (Romoser et al., 2011). To prevent the virus from entering, establishing, and spreading in high-risk locations, a thorough quarantine program is necessary (Fawzy and Helmy, 2019). This preventive measure prevents the spread of tainted animals, goods, and fomites from contaminated or suspicious places. The frequency of RVF can be decreased by management techniques such as relocating cattle to pastures with improved drainage and wind protection or containing animals in pens equipped with mosquito-repellent equipment (Kitandwe et al., 2022). Effective disease control and prevention in nonendemic nations, particularly those that share borders with nearby endemic nations, depend on routine surveillance and prediction (Himeidan et al., 2016). Finding probable mosquito species that spread RVF aids in gathering biological data, including biting patterns and egg-laying regions (Islam et al., 2024). ConclusionIn conclusion, RVF is a significant zoonotic disease that is primarily transmitted by mosquitoes and is known to affect ruminants and humans. The outbreak of COVID-19 can cause severe public health implications and economic losses; thereby, highlighting the importance of understanding and managing its risks in endemic regions. This review further highlights the need and importance of educating the public, especially livestock framers and veterinarians, slaughterhouse workers, and even communities, on the establishment and implementation of cost-effective preventive and control measures, including diagnosis and surveillance for early detection and response to RVF, especially due to its substantial health and socioeconomic consequences. The factors influencing the transmission of RVF require a multisectoral, transdisciplinary, and integrated One Health strategy that prioritizes strengthening preparedness and prevention strategies, while at the same time, improving case management. Early preparedness and prevention strategies by improving diagnosis and surveillance strategies for monitoring the dynamics of RVFV vectors will also be very helpful in reducing the RFV burden. Additionally, the use of Artificial Intelligence (AI)-powered drones for vector surveillance and control, as well as the delivery of developed vaccines and samples to regional laboratories for confirmation tests, should be encouraged. This will further strengthen and reinforce pandemic preparedness, prevention, and response frameworks against the spread or outbreak of RFV. AcknowledgmentsThe authors thank the Faculty of Veterinary Medicine, Universitas Airlangga. Author’s contributionsARK, BB, ENU, and MHE drafted the manuscript. IBM, WT, DAAK, and EPH revised and edited the manuscripts. BB, MKJK, SW, and DAP participated in the preparation and critical checking of the manuscript. BWKW, RZA, KAF, and IF edited the references. All authors read and approved the final manuscript draft. Conflict of interestThe authors declare no conflict of interest. FundingThe authors would like to acknowledge Ebonyi State University, Abakaliki, Nigeria, and Lembaga Penelitian dan Pengabdian Masyarakat, Universitas Airlangga, Indonesia, for their support. This study was partly supported by the International Research Consortium, Lembaga Penelitian dan Pengabdian Masyarakat, Universitas Airlangga, Surabaya, Indonesia, in the year 2024 (grant number: 171/UN3.LPPM/PT.01.03/2024. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAl-Hamdan, N.A., Panackal, A.A., Al Bassam, T.H., Alrabea, A., Al Hazmi, M., Al Mazroa, Y., Al Jefri, M., Khan, A.S. and Ksiazek, T.G. 2015. The risk of nosocomial transmission of rift valley fever. PLoS Negl. Trop. Dis. 9(12), e0004314. Allen, E.R., Krumm, S.A., Raghwani, J., Halldorsson, S., Elliott, A., Graham, V.A., Koudriakova, E., Harlos, K., Wright, D., Warimwe, G.M., Brennan, B., Huiskonen, J.T., Dowall, S.D., Elliott, R.M., Pybus, O.G., Burton, D.R., Hewson, R., Doores, K.J. and Bowden, T.A. 2018. A protective monoclonal antibody targets a site of vulnerability on the surface of Rift Valley fever virus. Cell Rep. 25(13), 3750–3758.e4. Anywaine, Z., Hansen, C., Warimwe, G.M., Mustapher, G.A., Nyakarahuka, L., Balinandi, S., Ario, A.R., Lutwama, J.J., Elliott, A. and Kaleebu, P. 2024. Severe morbidity and hospital-based mortality from Rift Valley fever disease between November 2017 and March 2020 among humans in Uganda. Virol. J. 21(1), 104. Anywaine, Z., Lule, S.A., Hansen, C., Warimwe, G. and Elliott, A. 2022. Clinical manifestations of Rift Valley fever in humans: systematic review and meta-analysis. PLoS Negl. Trop. Dis. 16(3), e0010233. Arishi, H.M., Aqeel, A.Y. and Al Hazmi, M.M, 2006. Vertical transmission of fatal Rift Valley fever in a newborn. Ann. Trop. Paediatr. 26(3), 251–253. Azerigyik, F.A., Cagle, S.M., Wilson, W.C., Mitzel, D.N., Kading, R.C, 2025. The temperature-associated effects of Rift Valley fever virus infections in mosquitoes and climate-driven epidemics: a review. Viruses 17(2), 217. Baba, M., Masiga, D.K., Sang, R. and Villinger, J. 2016. Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg. Microbes Infect. 5(6), e58. Balaraman, V., Indran, S.V., Kim, I.J., Trujillo, J.D., Meekins, D.A., Shivanna, V., Zajac, M.D., Urbaniak, K., Morozov, I., Sunwoo, S.Y., Faburay, B., Osterrieder, K., Gaudreault, N.N., Wilson, W.C. and Richt, J.A. 2024. Rift Valley fever phlebovirus reassortment study in sheep. Viruses 16(6), 880. Balkhy, H.H. and Memish, Z.A. 2003. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents 21(2), 153–157. Bashir, R.S.E. and Hassan, O.A. 2019. A One Health perspective to identify environmental factors that affect Rift Valley fever transmission in Gezira state, Central Sudan. Trop. Med. Health 47(1), 54. Baudin, M., Jumaa, A.M., Jomma, H.J.E., Karsany, M.S., Bucht, G., Näslund, J., Ahlm, C., Evander, M. and Mohamed, N. 2016. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health 4(11), e864–e871. Becquart, P., Kombila, L.B., Mebaley, T.N., Paupy, C., Garcia, D., Nesi, N., Olive, M.M., Vanhomwegen, J., Boundenga, L., Mombo, I.M., Piro-Mégy, C., Fritz, M., Lenguiya, L.H., Ar Gouilh, M., Leroy, E.M., N’Dilimabaka, N., Cêtre-Sossah, C. and Maganga, G.D. 2024. Evidence for circulation of Rift Valley fever virus in wildlife and domestic animals in a forest environment in Gabon, Central Africa. PLoS Negl. Trop. Dis. 18(3), e0011756. Behl, A., Nair, A., Mohagaonkar, S., Yadav, P., Gambhir, K., Tyagi, N., Sharma, R.K., Butola, B.S. and Sharma, N. 2022. Threat, challenges, and preparedness for future pandemics: a descriptive review of phylogenetic analysis based predictions. Infect. Genet. Evol. 98(1), 105217. Bergren, N.A., Patterson, E.I., Blair, H., Ellis, R.P. and Kading, R.C. 2020. Methods for successful inactivation of Rift Valley fever virus in infected mosquitoes. J. Virol. Methods 276(1), 113794. Bian, T., Hao, M., Zhao, X., Zhao, C., Luo, G., Zhang, Z., Fu, G., Yang, L., Chen, Y., Wang, Y., Yu, C., Yang, Y., Li, J. and Chen, W. 2023. A Rift Valley fever mRNA vaccine elicits strong immune responses in mice and rhesus macaques. NPJ Vaccines 8(1), 164. Bird, B.H., Albariño, C.G., Hartman, A.L., Erickson, B.R., Ksiazek, T.G. and Nichol, S.T. 2008. Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol. 82(6), 2681–2691. Boshra, H., Lorenzo, G., Busquets, N. and Brun, A. 2011. Rift valley fever: recent insights into pathogenesis and prevention. J. Virol. 85(13), 6098–6105. Botros, B., Omar, A., Elian, K., Mohamed, G., Soliman, A., Salib, A., Salman, D., Saad, M. and Earhart, K. 2006. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J. Med. Virol. 78(6), 787–791. Bouloy, M. and Flick, R. 2009. Reverse genetics technology for Rift Valley fever virus: current and future applications for the development of therapeutics and vaccines. Antiviral Res. 84(2), 101–118. Boussini, H., Lamien, C.E., Nacoulma, O.G., Kaboré, A., Poda, G. and Viljoen, G. 2014. Prevalence of Rift Valley fever in domestic ruminants in the central and northern regions of Burkina Faso. Rev. Sci. Tech. 33(3), 893–901. Boyles, D.A., Schwarz, M.M., Albe, J.R., McMillen, C.M., O’Malley, K.J., Reed, D.S. and Hartman, A.L. 2021. Development of Rift valley fever encephalitis in rats is mediated by early infection of olfactory epithelium and neuroinvasion across the cribriform plate. J. Gen. Virol. 102(2), 001522. Brahms, A., Mudhasani, R., Pinkham, C., Kota, K., Nasar, F., Zamani, R., Bavari, S. and Kehn-Hall, K. 2017. Sorafenib impedes Rift Valley fever virus egress by inhibiting valosin-containing protein function in the cellular secretory pathway. J. Virol. 91(21), e00968-17. Bron, G.M., Strimbu, K., Cecilia, H., Lerch, A., Moore, S.M., Tran, Q., Perkins, T.A. and Bosch, Q.A.T. 2021. Over 100 years of Rift Valley fever: a patchwork of data on pathogen spread and spillover. Pathogens 10(6), 708. Caplen, H., Peters, C.J. and Bishop, D.H. 1985. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J. Gen. Virol. 66(Pt 10), 2271–2277. Cêtre-Sossah, C., Pédarrieu, A., Guis, H., Defernez, C., Bouloy, M., Favre, J., Girard, S., Cardinale, E. and Albina, E. 2012. Prevalence of Rift Valley fever among ruminants, Mayotte. Emerg. Infect. Dis. 18(6), 972–975. Chambaro, H.M., Hirose, K., Sasaki, M., Libanda, B., Sinkala, Y., Fandamu, P., Muleya, W., Banda, F., Chizimu, J., Squarre, D., Shawa, M., Qiu, Y., Harima, H., Eshita, Y., Simulundu, E., Sawa, H. and Orba, Y. 2022. An unusually long Rift valley fever inter-epizootic period in Zambia: evidence for enzootic virus circulation and risk for disease outbreak. PLoS Negl. Trop. Dis. 16(6), e0010420. Chengula, A.A., Mdegela, R.H. and Kasanga, C.J. 2013. Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania. Springerplus 2(1), 549. Clémenceau, B., Congy-Jolivet, N., Gallot, G., Vivien, R., Gaschet, J., Thibault, G. and Vié, H. 2006. Antibody-dependent cellular cytotoxicity (ADCC) is mediated by genetically modified antigen-specific human T lymphocytes. Blood 107(12), 4669–4677. Connors, K.A. and Hartman, A.L. 2022. Advances in understanding neuropathogenesis of Rift Valley fever virus. Annu. Rev. Virol. 9(1), 437–450. Daubney, R., Hudson, J.R. and Garnham, P.C. 1931. Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from east Africa. J. Pathol. Bacteriol. 34(4), 545– 579. de Glanville, W.A., Allan, K.J., Nyarobi, J.M., Thomas, K.M., Lankester, F., Kibona, T.J., Claxton, J.R., Brennan, B., Carter, R.W., Crump, J.A., Halliday, J.E.B., Ladbury, G., Mmbaga, B.T., Mramba, F., Nyasebwa, O.M., Rubach, M.P., Rostal, M.K., Sanka, P., Swai, E.S., Szemiel, A.M., Willett, B.J. and Cleaveland, S. 2022b. An outbreak of Rift Valley fever among peri-urban dairy cattle in northern Tanzania. Trans. R. Soc. Trop. Med. Hyg. 116(11), 1082–1090. de Glanville, W.A., Nyarobi, J.M., Kibona, T., Halliday, J.E.B., Thomas, K.M., Allan, K.J., Johnson, P.C.D., Davis, A., Lankester, F., Claxton, J.R., Rostal, M.K., Carter, R.W., de Jong, R.M.F., Rubach, M.P., Crump, J.A., Mmbaga, B.T., Nyasebwa, O.M., Swai, E.S., Willett, B. and Cleaveland, S. 2022a. Inter-epidemic Rift Valley fever virus infection incidence and risks for zoonotic spillover in northern Tanzania. PLoS Negl. Trop. Dis. 16(10), e0010871. Domenech, J., Lubroth, J., Eddi, C., Martin, V. and Roger, F. 2006. Regional and international approaches on prevention and control of animal transboundary and emerging diseases. Ann. N. Y. Acad. Sci. 1081(1), 90–107. Dunbar, M.W., Correa-Morales, F., Dzul-Manzanilla, F., Medina-Barreiro, A., Bibiano-Marín, W., Morales-Ríos, E., Vadillo-Sánchez, J., López-Monroy, B., Ritchie, S.A., Lenhart, A., Manrique-Saide, P. and Vazquez-Prokopec, G.M. 2019. Efficacy of novel indoor residual spraying methods targeting pyrethroid-resistant Aedes aegypti within experimental houses. PLoS Negl. Trop. Dis. 13(2), e0007203. Durand, J.P., Bouloy, M., Richecoeur, L., Peyrefitte, C.N. and Tolou, H. 2003. Rift Valley fever virus infection among French troops in Chad. Emerg. Infect. Dis. 9(6), 751–752. Evans, A., Gakuya, F., Paweska, J.T., Rostal, M., Akoolo, L., Van Vuren, P.J., Manyibe, T., Macharia, J.M., Ksiazek, T.G., Feikin, D.R., Breiman, R.F. and Njenga, M.K. 2008. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol. Infect. 136(9), 1261–1269. Faburay, B., LaBeaud, A.D., McVey, D.S., Wilson, W.C. and Richt, J.A. 2017. Current status of Rift Valley fever vaccine development. Vaccines 5(3), 29. Fafetine, J., Neves, L., Thompson, P.N., Paweska, J.T., Rutten, V.P. and Coetzer, J.A. 2013. Serological evidence of Rift Valley fever virus circulation in sheep and goats in Zambézia Province, Mozambique. PLoS Negl. Trop. Dis. 7(2), e2065. Fakour, S., Naserabadi, S. and Ahmadi, E. 2021. A serological and hematological study on Rift valley fever and associated risk factors in aborted sheep at Kurdistan province in west of Iran. Comp. Immunol. Microbiol. Infect. Dis. 75(1), 101620. Fawzy, M. and Helmy, Y.A. 2019. The one health approach is necessary for the control of Rift Valley fever infections in Egypt: a comprehensive review. Viruses 11(2), 139. Fukushi, S., Nakauchi, M., Mizutani, T., Saijo, M., Kurane, I. and Morikawa, S. 2012. Antigen-capture ELISA for the detection of Rift Valley fever virus nucleoprotein using new monoclonal antibodies. J. Virol. Methods 180(1–2), 68–74. Gerdes, G.H. 2004. Rift Valley fever. Rev. Sci. Tech. 23(2), 613–623. Gerken, K.N., LaBeaud, A.D., Mandi, H., Jackson, M.L., Breugelmans, J.G. and King, C.H. 2022. Paving the way for human vaccination against Rift Valley fever virus: a systematic literature review of RVFV epidemiology from 1999 to 2021. PLoS Negl. Trop. Dis. 16(1), e0009852. Gerken, K.N., Maluni, J., Mutuku, F.M., Ndenga, B.A., Mwashee, L., Ichura, C., Shaita, K., Mwaniki, M., Orwa, S., Seetah, K. and LaBeaud, A.D. 2023. Exploring potential risk pathways with high-risk groups for urban Rift Valley fever virus introduction, transmission, and persistence in two urban centers of Kenya. PLoS Negl. Trop. Dis. 17(1), e0010460. Gerrard, S.R., Bird, B.H., Albariño, C.G. and Nichol, S.T. 2007. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359(2), 459–465. Gharpure, R., Vegvari, C., Abdissa, A., Alimi, Y., Anyamba, A., Auerbach, J. et al. 2025. Meeting report: CEPI workshop on Rift Valley fever epidemiology and modeling to inform human vaccine development, Nairobi, 4–5 June 2024. Vaccine 54, 126860. Gibson, S., Noronha, L.E., Tubbs, H., Cohnstaedt, L.W., Wilson, W.C., Mire, C., Mitzel, D., Anyamba, A., Rostal, M. and Linthicum, K.J. 2023. The increasing threat of Rift Valley fever virus globalization: strategic guidance for protection and preparation. J. Med. Entomol. 60(6), 1197–1213. Grobbelaar, A.A., Weyer, J., Leman, P.A., Kemp, A., Paweska, J.T. and Swanepoel, R. 2011. Molecular epidemiology of Rift Valley fever virus. Emerg. Infect. Dis. 17(12), 2270–2276. Grossi-Soyster, E.N., Lee, J., King, C.H. and LaBeaud, A.D. 2019. The influence of raw milk exposures on Rift Valley fever virus transmission. PLoS Negl. Trop. Dis. 13(3), e0007258. Gür, S., Kale, M., Erol, N., Yapici, O., Mamak, N. and Yavru, S. 2017. The first serological evidence for Rift Valley fever infection in the camel, goitered gazelle and Anatolian water buffaloes in Turkey. Trop. Anim. Health Prod. 49(7), 1531–1535. Gwon, Y.D., Mahani, S.A.N., Nagaev, I., Mincheva-Nilsson, L. and Evander, M. 2021. Rift Valley fever virus propagates in human villous trophoblast cell lines and induces cytokine mRNA responses known to provoke miscarriage. Viruses 13(11), 2265. Harmon, B., Bird, S.W., Schudel, B.R., Hatch, A.V., Rasley, A. and Negrete, O.A. 2016. A genome-wide RNA interference screen identifies a role for Wnt/ β-catenin signaling during rift valley fever virus infection. J. Virol. 90(16), 7084–7097. Hartman, A. 2017. Rift Valley fever. Clin. Lab. Med. 37(2), 285–301. Hassanain, A.M., Noureldien, W., Karsany, M.S., Saeed, el N.S., Aradaib, I.E. and Adam, I. 2010. Rift Valley Fever among febrile patients at New Halfa hospital, eastern Sudan. Virol. J. 7(1), 97. Hassan-Kadle, A.A., Osman, A.M., Shair, M.A., Abdi, O.M., Yusuf, A.A., Ibrahim, A.M. and Vieira, R.F.C. 2021. Rift Valley fever and Brucella spp. in ruminants, Somalia. BMC Vet. Res. 17(1), 280. Himeidan, Y.E. 2016. Rift Valley fever: current challenges and future prospects. Res. Rep. Trop. Med. 7(1), 1–9. Hoover, J., Eades, S. and Lam, W.M. 2018. Pediatric antiviral stewardship: defining the potential role of ribavirin in respiratory syncytial virus-associated lower respiratory illness. J. Pediatr. Pharmacol. Ther. 23(5), 372–378. Hornak, K.E., Lanchy, J.M. and Lodmell, J.S. 2016. RNA encapsidation and packaging in the phleboviruses. Viruses 8(7), 194. Ikegami, T. 2012. Molecular biology and genetic diversity of Rift Valley fever virus. Antiviral Res. 95(3), 293–310. Ikegami, T. and Makino, S. 2009. Rift valley fever vaccines. Vaccine 27(Suppl 4), D69–D72. Ikegami, T. and Makino, S. 2011. The pathogenesis of Rift Valley fever. Viruses 3(5), 493–519. Islam, M.R., Ahmed, I. and Urmi, T.J. 2024. The pathogenicity and risk evaluation of Rift Valley virus to cause mysterious “Disease X”: an update on recent evidences. Ann. Med. Surg. (Lond). 86(3), 1243–1246. Jäckel, S., Eiden, M., El Mamy, B.O., Isselmou, K., Vina-Rodriguez, A., Doumbia, B. and Groschup, M.H. 2013. Molecular and serological studies on the Rift Valley fever outbreak in Mauritania in 2010. Transbound. Emerg. Dis. 60(Suppl 2), 31–39. Jaleta, M.B., Tefera, M., Negussie, H., Mulatu, T., Berhe, T., Belete, F., Yalew, B., Gizaw, O., Dabasa, G., Abunna, F., Regassa, F., Amenu, K. and Leta, S. 2022. Entomological survey of the potential vectors of Rift Valley fever virus and absence of detection of the virus genome from the vectors in various niches in the southern half of the Great Rift Valley of Ethiopia. Vet. Med. Sci. 8(6), 2716–2725. Johnson, S.A.M., Asmah, R., Awuni, J.A., Tasiame, W., Mensah, G.I., Paweska, J.T., Weyer, J., Hellferscee, O. and Thompson, P.N. 2023. Evidence of Rift Valley fever virus circulation in livestock and herders in Southern Ghana. Viruses 15(6), 1346. Kainga, H., Phonera, M.C., Chatanga, E., Kallu, S.A., Mpundu, P., Samutela, M., Chambaro, H.M., Kajihara, M., Shempela, D.M., Sikalima, J., Muleya, W., Shawa, M., Chulu, J., Njunga, G., Simuunza, M., Takada, A., Sawa, H., Simulundu, E. and Saasa, N. 2022. Seroprevalence and associated risk factors of Rift Valley fever in livestock from three ecological zones of Malawi. Pathogens 11(11), 1349. Kalthoum, S., Arsevska, E., Guesmi, K., Mamlouk, A., Cherni, J., Lachtar, M., Gharbi, R., Mohamed, B.B.H., Khalfaoui, W., Dhaouadi, A., Baccar, M.N., Hajlaoui, H., Mzoughi, S., Seghaier, C., Messadi, L., Zrelli, M., Sghaier, S., Cêtre-Sossah, C., Hendrikx, P. and Squarzoni-Diaw, C. 2021. Risk based serological survey of Rift Valley fever in Tunisia (2017–2018). Heliyon 7(9), e07932. Kamal, S.A. 2009. Pathological studies on postvaccinal reactions of Rift Valley fever in goats. Virol. J. 6(1), 94. Kasongamulilo, C.C., Syakalima, M., Saasa, N., Kainga, H., Pandey, G.S., Mukubesa, A.N., Mwape, I., Kajihara, M., Takada, A., Simuunza, M. 2025. Evidence of human exposure and associated risk factors to Rift Valley fever in selected districts of Central and Western Zambia. PLoS One 20(1), e0309288. Kasye, M., Teshome, D., Abiye, A. and Eshetu, A. 2016. A review on rift valley fever on animal, human health and its impact on live stock marketing. Austin Virol. Retro Virol. 3(1), 1020. Kenawy, M.A., Abdel-Hamid, Y.M. and Beier, J.C. 2018. Rift Valley Fever in Egypt and other African countries: historical review, recent outbreaks and possibility of disease occurrence in Egypt. Acta Trop. 181(1), 40–49. Kitandwe, P.K., McKay, P.F., Kaleebu, P. and Shattock, R.J. 2022. An overview of rift valley fever vaccine development strategies. Vaccines (Basel) 10(11), 1794. Kroeker, A.L., Babiuk, S., Pickering, B.S., Richt, J.A. and Wilson, W.C. 2020. Livestock challenge models of rift valley fever for agricultural vaccine testing. Front. Vet. Sci. 7(1), 238. Kwaśnik, M., Rożek, W. and Rola, J. 2021. Rift Valley fever - a growing threat to humans and animals. J. Vet. Res. 65(1), 7–14. Lagare, A., Fall, G., Ibrahim, A., Ousmane, S., Sadio, B., Abdoulaye, M., Alhassane, A., Mahaman, A.E., Issaka, B., Sidikou, F., Zaneidou, M., Bienvenue, B., Mamoudou, H.D., Diallo, A.B., Kadadé, G., Testa, J., Mainassara, H.B. and Faye, O. 2019. First occurrence of Rift Valley fever outbreak in Niger, 2016. Vet. Med. Sci. 5(1), 70–78. Lagerqvist, N., Näslund, J., Lundkvist, A., Bouloy, M., Ahlm, C. and Bucht, G. 2009. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley Fever virus cDNA constructs. Virol. J. 6(1), 6. Lapa, D., Pauciullo, S., Ricci, I., Garbuglia, A.R., Maggi, F., Scicluna, M.T. and Tofani, S. 2024. Rift Valley fever virus: an overview of the current status of diagnostics. Biomedicines 12(3), 540. Liu, L., Celma, C.C. and Roy, P. 2008. Rift Valley fever virus structural proteins: expression, characterization and assembly of recombinant proteins. Virol. J. 5(1), 82. Liu, W., Sun, F.J., Tong, Y.G., Zhang, S.Q. and Cao, W.C. 2016. Rift Valley fever virus imported into China from Angola. Lancet Infect. Dis. 16(11), 1226. Lubisi, B.A., Mutowembwa, P.B., Ndouvhada, P.N., Odendaal, L., Bastos, A.D.S. and Penrith, M.L. 2023. Experimental infection of domestic pigs (Sus scrofa) with Rift Valley Fever Virus. Viruses 15(2), 545. Ly, H.J. and Ikegami, T. 2016. Rift Valley fever virus NSs protein functions and the similarity to other bunyavirus NSs proteins. Virol. J. 13(1), 118. Mahmoud, A.S., Sawesi, O.K., El-Waer, O.R. and Bennour, E.M. 2021. Rift valley fever in Africa with the emerging interest in Libya. Int. J. One Health 7(2), 237–245. Mandell, R.B., Koukuntla, R., Mogler, L.J., Carzoli, A.K., Freiberg, A.N., Holbrook, M.R., Martin, B.K., Staplin, W.R., Vahanian, N.N., Link, C.J. and Flick, R. 2010. A replication-incompetent Rift Valley fever vaccine: chimeric virus-like particles protect mice and rats against lethal challenge. Virology 397(1), 187–198. Manore, C.A. and Beechler, B.R. 2015. Inter-epidemic and between-season persistence of rift valley fever: vertical transmission or cryptic cycling? Transbound. Emerg. Dis. 62(1), 13–23. Mansfield, K.L., Banyard, A.C., McElhinney, L., Johnson, N., Horton, D.L., Hernández-Triana, L.M. and Fooks, A.R. 2015. Rift Valley fever virus: a review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine 33(42), 5520–5531. McElroy, A.K. and Nichol, S.T. 2012. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology 422(1), 6–12. McElroy, A.K., Albariño, C.G. and Nichol, S.T. 2009. Development of a RVFV ELISA that can distinguish infected from vaccinated animals. Virol. J. 6(1), 125. McMillen, C.M. and Hartman, A.L. 2018. Rift Valley fever in animals and humans: current perspectives. Antiviral Res. 156(1), 29–37. McMillen, C.M., Boyles, D.A., Kostadinov, S.G., Hoehl, R.M., Schwarz, M.M., Albe, J.R., Demers, M.J. and Hartman, A.L. 2022. Congenital Rift Valley fever in Sprague Dawley rats is associated with diffuse infection and pathology of the placenta. PLoS Negl. Trop. Dis. 16(10), e0010898. Melkamu, S. 2018. Review on economic significance and current diagnostic techniques on rift valley fever. Int. J. Adv. Res. Biol. Sci. 5(7), 115–122. Métras, R., Cavalerie, L., Dommergues, L., Mérot, P., Edmunds, W.J., Keeling, M.J., Cêtre-Sossah, C. and Cardinale, E. 2016. The epidemiology of Rift Valley fever in Mayotte: insights and perspectives from 11 years of data. PLoS Negl. Trop. Dis. 10(6), e0004783. Moreno, S., Lorenzo, G., López-Valiñas, Á., de la Losa, N., Alonso, C., Charro, E., Núñez, J.I., Sánchez-Cordón, P.J., Borrego, B. and Brun, A. 2024. Safety and efficacy upon infection in sheep with Rift Valley fever virus ZH548-rA2, a triple mutant rescued virus. Viruses 16(1), 87. Muga, G.O., Onyango-Ouma, W., Sang, R. and Affognon, H. 2015. Sociocultural and economic dimensions of Rift Valley fever. Am. J. Trop. Med. Hyg. 92(4), 730–738. Muller, R., Saluzzo, J.F., Lopez, N., Dreier, T., Turell, M., Smith, J. and Bouloy, M. 1995. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am. J. Trop. Med. Hyg. 53(4), 405– 411. Munyua, P., Murithi, R.M., Wainwright, S., Githinji, J., Hightower, A., Mutonga, D., Macharia, J., Ithondeka, P.M., Musaa, J., Breiman, R.F., Bloland, P. and Njenga, M.K. 2010. Rift Valley fever outbreak in livestock in Kenya, 2006–2007. Am. J. Trop. Med. Hyg. 83(2 Suppl), 58–64. Mwaengo, D., Lorenzo, G., Iglesias, J., Warigia, M., Sang, R., Bishop, R.P. and Brun, A. 2012. Detection and identification of Rift Valley fever virus in mosquito vectors by quantitative real-time PCR. Virus Res. 169(1), 137–143. Nair, N., Osterhaus, A.D.M.E., Rimmelzwaan, G.F. and Prajeeth, C.K. 2023. Rift Valley fever virus-infection, pathogenesis and host immune responses. Pathogens 12(9), 1174. Nanyingi, M.O., Munyua, P., Kiama, S.G., Muchemi, G.M., Thumbi, S.M., Bitek, A.O., Bett, B., Muriithi, R.M. and Njenga, M.K. 2015. A systematic review of Rift Valley fever epidemiology 1931–2014. Infect. Ecol. Epidemiol. 5(1), 28024. Narayanan, A., Kehn-Hall, K., Senina, S., Lundberg, L., Van Duyne, R., Guendel, I., Das, R., Baer, A., Bethel, L., Turell, M., Hartman, A.L., Das, B., Bailey, C. and Kashanchi, F. 2012. Curcumin inhibits Rift Valley fever virus replication in human cells. J. Biol. Chem. 287(40), 33198–33214. Ndishimye, P., Umuhoza, T., Umutoni, B., Zakham, F., Ndayambaje, M., Hewins, B., Gasana, M.N., Ostadgavahi, A.T., Sganzerla, G., Ndayisenga, F., Kelvin, D. and Udahemuka, J.C. 2024. Rift Valley fever outbreaks in the east African community: insights from ProMed data (2010-2024). Front. Public Health 12(1), 1298594. Odendaal, L., Davis, A.S. and Venter, E.H. 2021. Insights into the pathogenesis of viral haemorrhagic fever based on virus tropism and tissue lesions of natural Rift Valley Fever. Viruses 13(4), 709. Olive, M.M., Goodman, S.M. and Reynes, J.M. 2012. The role of wild mammals in the maintenance of Rift Valley fever virus. J. Wildl. Dis. 48(2), 241– 266. Oragwa, A.O., Obishakin, E.T. and Oluwayelu, D.O. 2023. Molecular detection and characterization of Rift Valley fever virus in humans and domestic ruminants in Nigeria. Microbe 1(1), 100016. Oreshkova, N., van Keulen, L., Kant, J., Moormann, R.J. and Kortekaas, J. 2013. A single vaccination with an improved nonspreading Rift Valley fever virus vaccine provides sterile immunity in lambs. PLoS One 8(10), e77461. Pavulraj, S., Stout, R.W., Chowdhury, S.I. 2025. A Novel BoHV-1-vectored subunit RVFV vaccine induces a Robust Humoral and cell-mediated immune response against Rift Valley fever in sheep. Viruses 17(3), 304. Paweska, J.T., Mortimer, E., Leman, P.A. and Swanepoel, R. 2005. An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in humans, domestic and wild ruminants. J. Virol. Methods. 127(1), 10–18. Pendell, D.L., Lusk, J.L., Marsh, T.L., Coble, K.H. and Szmania, S.C. 2016. Economic assessment of zoonotic diseases: an illustrative study of Rift Valley fever in the United States. Transbound. Emerg. Dis. 63(2), 203–214. Pepin, M., Bouloy, M., Bird, B.H., Kemp, A. and Paweska, J. 2010. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 41(6), 61. Peyre, M., Chevalier, V., Abdo-Salem, S., Velthuis, A., Antoine-Moussiaux, N., Thiry, E. and Roger, F. 2015. A systematic scoping study of the socio-economic impact of Rift Valley fever: research gaps and needs. Zoonoses Public Health 62(5), 309–325. Phoenix, I., Nishiyama, S., Lokugamage, N., Hill, T.E., Huante, M.B., Slack, O.A., Carpio, V.H., Freiberg, A.N. and Ikegami, T. 2016. N-Glycans on the Rift Valley fever virus envelope glycoproteins Gn and Gc redundantly support viral infection via DC-SIGN. Viruses 8(5), 149. Quellec, J., Piro-Megy, C., Cannac, M., Nisole, S., Marty, F.H., Gosselet, F., Shimizu, F., Kanda, T., Cêtre-Sossah, C. and Salinas, S. 2024. Rift Valley fever virus is able to cross the human blood-brain barrier in vitro by direct infection with no deleterious effects. J. Virol. 98(10), e0126724. Rahman, M.M., Islam, M.R. and Dhar, P.S. 2023. Recent re-emergence of Rift Valley fever: epidemiology, clinical characteristics, transmission, symptoms, diagnosis, prevention, and treatment. Int. J. Surg. 109(2), 117–119. Randall, R., Gibbs, C.J. Jr., Aulisio, C.G., Binn, L.N. and Harrison, V.R. 1962. The development of a formalin-killed Rift Valley fever virus vaccine for use in man. J. Immunol. 89(1), 660–671. Rangel, M.V., Bourguet, F.A., Hall, C.I. and Weilhammer, D.R. 2024. Evaluation of inactivation methods for Rift Valley fever virus in mouse microglia. Pathogens 13(2), 159. Reed, C., Lin, K., Wilhelmsen, C., Friedrich, B., Nalca, A., Keeney, A., Donnelly, G., Shamblin, J., Hensley, L.E., Olinger, G. and Smith, D.R. 2013. Aerosol exposure to Rift Valley fever virus causes earlier and more severe neuropathology in the murine model, which has important implications for therapeutic development. PLoS Negl. Trop. Dis. 7(4), e2156. Rich, K.M. and Wanyoike, F. 2010. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. Am. J. Trop. Med. Hyg. 83(2 Suppl), 52–57. Rissmann, M., Kley, N., Ulrich, R., Stoek, F., Balkema-Buschmann, A., Eiden, M. and Groschup, M.H. 2020. Competency of amphibians and reptiles and their potential role as reservoir hosts for Rift Valley Fever Virus. Viruses 12(11), 1206. Romoser, W.S., Oviedo, M.N., Lerdthusnee, K., Patrican, L.A., Turell, M.J., Dohm, D.J., Linthicum, K.J. and Bailey, C.L. 2011. Rift Valley fever virus-infected mosquito ova and associated pathology: possible implications for endemic maintenance. Res. Rep. Trop. Med. 2(1), 121–127. Sado, F.Y., Tchetgna, H.S., Kamgang, B., Djonabaye, D., Nakouné, E., McCall, P.J., Ndip, R.N. and Wondji, C.S. 2022. Seroprevalence of Rift Valley fever virus in domestic ruminants of various origins in two markets of Yaoundé, Cameroon. PLoS Negl. Trop. Dis. 16(8), e0010683. Salimi, H., Cain, M.D. and Klein, R.S. 2016. Encephalitic arboviruses: emergence, clinical presentation, and neuropathogenesis. Neurotherapeutics 13(3), 514–534. Sall, A.A., Macondo, E.A., Sène, O.K., Diagne, M., Sylla, R., Mondo, M., Girault, L., Marrama, L., Spiegel, A., Diallo, M., Bouloy, M. and Mathiot, C. 2002. Use of reverse transcriptase PCR in early diagnosis of Rift Valley fever. Clin. Diagn. Lab. Immunol. 9(3), 713–715. Sall, A.A., Zanotto, P.M., Sene, O.K., Zeller, H.G., Digoutte, J.P., Thiongane, Y. and Bouloy, M. 1999. Genetic reassortment of Rift Valley fever virus in nature. J. Virol. 73(10), 8196–8200. Sanderson, C.E., Jori, F., Moolla, N., Paweska, J.T., Oumer, N. and Alexander, K.A. 2020. Silent circulation of rift valley fever in humans, Botswana, 2013-2014. Emerg. Infect. Dis. 26(10), 2453–2456. Sang, R., Arum, S., Chepkorir, E., Mosomtai, G., Tigoi, C., Sigei, F., Lwande, O.W., Landmann, T., Affognon, H., Ahlm, C. and Evander, M. 2017. Distribution and abundance of key vectors of Rift Valley fever and other arboviruses in two ecologically distinct counties in Kenya. PLoS Negl. Trop. Dis. 11(2), e0005341. Scharton, D., Bailey, K.W., Vest, Z., Westover, J.B., Kumaki, Y., Van Wettere, A., Furuta, Y. and Gowen, B.B. 2014. Favipiravir (T-705) protects against peracute Rift Valley fever virus infection and reduces delayed-onset neurologic disease observed with ribavirin treatment. Antiviral Res. 104(1), 84–92. Schreur, P.J.W., Vloet, R.P.M., Kant, J., van Keulen, L., Gonzales, J.L., Visser, T.M., Koenraadt, C.J.M., Vogels, C.B.F. and Kortekaas, J. 2021. Reproducing the Rift Valley fever virus mosquito-lamb-mosquito transmission cycle. Sci. Rep. 11(1), 1477. Shariff, S., Sengar, A.S., Fawaz, L., Rai, A., Nazir, A. and Uwishema, O. 2023. Rift Valley fever and Crimean-Congo hemorrhagic fever in Mauritania: future recommendations and mitigation strategies—a correspondence. Int. J. Surg. 109(3), 641–643. Sherman, M.B. and Smith, T.J. 2025. Cryogenic electron microscopy of rift valley fever virus. Methods Mol Biol. 2893, 57–72. Smith, D.R., Bird, B.H., Lewis, B., Johnston, S.C., McCarthy, S., Keeney, A., Botto, M., Donnelly, G., Shamblin, J., Albariño, C.G., Nichol, S.T. and Hensley, L.E. 2012. Development of a novel nonhuman primate model for Rift Valley fever. J. Virol. 86(4), 2109–2120. Smithburn, K.C., Mahaffy, A.F., Haddow, A.J., Kitchen, S.F. and Smith, J.F. 1949. Rift valley fever: accidental infections among laboratory workers. J. Immunol. 62(2), 213–227. Socha, W., Kwasnik, M., Larska, M., Rola, J. and Rozek, W. 2022. Vector-borne viral diseases as a current threat for human and animal health-one health perspective. J. Clin. Med. 11(11), 3026. Soliman, A.K., Botros, B.A. and Morrill, J.C. 1988. Solid-phase immunosorbent technique for rapid detection of Rift Valley fever virus immunoglobulin M by hemagglutination inhibition. J. Clin. Microbiol. 26(9), 1913–1915. Sow, A., Faye, O., Faye, O., Diallo, D., Sadio, B.D., Weaver, S.C., Diallo, M. and Sall, A.A. 2014. Rift Valley fever in Kedougou, southeastern Senegal, 2012. Emerg. Infect. Dis. 20(3), 504–506. Spik, K., Shurtleff, A., McElroy, A.K., Guttieri, M.C., Hooper, J.W. and SchmalJohn, C. 2006. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine 24(21), 4657–4666. Tabassum, S., Naeem, F., Azhar, M., Naeem, A., Oduoye, M.O. and Dave, T. 2023. Rift Valley fever virus outbreak in Mauritania yet again in 2022: no room for complacency. Health Sci. Rep. 6(5), e1278. Tantely, L.M., Andriamandimby, S.F., Ambinintsoa, M.F., Raharinirina, M.R., Rafisandratantsoa, J.T., Ravalohery, J.-P., Harimanana, A., Ranoelison, N.N., Irinantenaina, J., Ankasitrahana, M.F., Ranoaritiana, D.B., Randrianasolo, L., Randremanana, R.V., Lacoste, V., Dussart, P. and Girod, R. 2024. An entomological investigation during a recent rift valley fever epizootic/epidemic reveals new aspects of the vectorial transmission of the virus in madagascar. Pathogens 13(3), 258. Tantely, L.M., Boyer, S. and Fontenille, D. 2015. A review of mosquitoes associated with Rift Valley fever virus in Madagascar. Am. J. Trop. Med. Hyg. 92(4), 722–729. Tinto, B., Quellec, J., Cêtre-Sossah, C., Dicko, A., Salinas, S. and Simonin, Y. 2023. Rift Valley fever in West Africa: a zoonotic disease with multiple socio-economic consequences. One Health 17(1), 100583. Tomori, O. and Oluwayelu, D.O. 2023. Domestic animals as potential reservoirs of zoonotic viral diseases. Annu. Rev. Anim. Biosci. 11(1), 33–55. Tong, C., Javelle, E., Grard, G., Dia, A., Lacrosse, C., Fourié, T., Gravier, P., Watier-Grillot, S., Lancelot, R., Letourneur, F., Comby, F., Grau, M., Cassou, L., Meynard, J.B., Briolant, S., Leparc-Goffart, I. and de Santi, V.P. 2019. Tracking Rift Valley fever: from Mali to Europe and other countries, 2016. Euro Surveill. 24(8), 1800213. Trabelsi, M.K., Hachid, A., Derrar, F., Messahel, N.E., Bia, T., Mockbel, Y., Khardine, A.F., Degui, D., Bellout, L., Benaissa, M.H., Leulmi, H., Khelef, D., Kaidi, R., Hakem, A., Bouguedour, R., Bitam, I. and Lafri, I. 2023. Serological evidence of Rift Valley fever viral infection among camels imported into Southern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 100(1), 102035. Tumusiime, D., Isingoma, E., Tashoroora, O.B., Ndumu, D.B., Bahati, M., Nantima, N., Mugizi, D.R., Jost, C. and Bett, B. 2023. Mapping the risk of Rift Valley fever in Uganda using national seroprevalence data from cattle, sheep and goats. PLoS Negl. Trop. Dis. 17(5), e0010482. van Vuren, P.J., Shalekoff, S., Grobbelaar, A.A., Archer, B.N., Thomas, J., Tiemessen, C.T. and Paweska, J.T. 2015. Serum levels of inflammatory cytokines in Rift Valley fever patients are indicative of severe disease. Virol. J. 12(1), 159. Wang, X., Hu, C., Ye, W., Wang, J., Dong, X., Xu, J., Li, X., Zhang, M., Lu, H., Zhang, F., Wu, W., Dai, S., Wang, H.W. and Chen, Z. 2022. Structure of rift valley fever virus RNA-dependent RNA polymerase. J. Virol. 96(3), e0171321. Warimwe, G.M., Gesharisha, J., Carr, B.V., Otieno, S., Otingah, K., Wright, D., Charleston, B., Okoth, E., Elena, L.G., Lorenzo, G., Ayman, el-B., Alharbi, N.K., Al-dubaib, M.A., Brun, A., Gilbert, S.C., Nene, V. and Hill, A.V. 2016. Chimpanzee adenovirus vaccine provides multispecies protection against rift valley fever. Sci. Rep. 6(1), 20617. Wright, D., Allen, E.R., Clark, M.H.A., Gitonga, J.N., Karanja, H.K., Hulswit, R.J.G., Taylor, I., Biswas, S., Marshall, J., Mwololo, D., Muriuki, J., Bett, B., Bowden, T.A. and Warimwe, G.M. 2020. Naturally acquired rift valley fever virus neutralizing antibodies predominantly target the Gn glycoprotein. iScience 23(11), 101669. Wright, D., Kortekaas, J., Bowden, T.A. and Warimwe, G.M. 2019. Rift valley fever: biology and epidemiology. J. Gen. Virol. 100(8), 1187–1199. Xiao, Y., Beier, J.C., Cantrell, R.S., Cosner, C., DeAngelis, D.L. and Ruan, S. 2015. Modelling the effects of seasonality and socioeconomic impact on the transmission of rift valley Fever virus. PLoS Negl. Trop. Dis. 9(1), e3388. Xu, Y., Wang, X., Jiang, L., Zhou, Y., Liu, Y., Wang, F. and Zhang, L. 2023. Natural hosts and animal models for Rift Valley fever phlebovirus. Front. Vet. Sci. 10(1), 1258172. Yin, F., Liu, Y. and Guo, H. 2022. Comparison between ibuprofen and acetaminophen in the treatment of infectious fever in children: a meta-analysis. J. Healthc. Eng. 2022(1), 1794258. Youssouf, H., Subiros, M., Dennetiere, G., Collet, L., Dommergues, L., Pauvert, A., Rabarison, P., Vauloup-Fellous, C., Le Godais, G., Jaffar-Bandjee, M.C., Jean, M., Paty, M.C., Noel, H., Oliver, S., Filleul, L. and Larsen, C. 2020. Rift valley fever outbreak, Mayotte, France, 2018–2019. Emerg. Infect. Dis. 26(4), 769–772. Zhao, C., Hao, M., Bian, T., Zhao, X., Chi, X., Chen, Z., Fu, G., Zhu, Z., Fang, T., Yu, C., Li, J. and Chen, W. 2025. Screening of neutralizing antibodies targeting GC protein of RVFV. Viruses 17(4), 559. | ||
| How to Cite this Article |
| Pubmed Style Hestianah EP, Khairullah AR, Effendi MH, Budiastuti B, Tyasningsih W, Budiarto B, Permatasari DA, Moses IB, Ahmad RZ, Wardhani BWK, Kusala MKJ, Kurniasih DAA, Fauziah I, Wibowo S, Ugbo EN, Fauzia KA. Rift Valley fever: A zoonotic disease with global potential. Open Vet. J.. 2025; 15(6): 2312-2328. doi:10.5455/OVJ.2025.v15.i6.5 Web Style Hestianah EP, Khairullah AR, Effendi MH, Budiastuti B, Tyasningsih W, Budiarto B, Permatasari DA, Moses IB, Ahmad RZ, Wardhani BWK, Kusala MKJ, Kurniasih DAA, Fauziah I, Wibowo S, Ugbo EN, Fauzia KA. Rift Valley fever: A zoonotic disease with global potential. https://www.openveterinaryjournal.com/?mno=241293 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i6.5 AMA (American Medical Association) Style Hestianah EP, Khairullah AR, Effendi MH, Budiastuti B, Tyasningsih W, Budiarto B, Permatasari DA, Moses IB, Ahmad RZ, Wardhani BWK, Kusala MKJ, Kurniasih DAA, Fauziah I, Wibowo S, Ugbo EN, Fauzia KA. Rift Valley fever: A zoonotic disease with global potential. Open Vet. J.. 2025; 15(6): 2312-2328. doi:10.5455/OVJ.2025.v15.i6.5 Vancouver/ICMJE Style Hestianah EP, Khairullah AR, Effendi MH, Budiastuti B, Tyasningsih W, Budiarto B, Permatasari DA, Moses IB, Ahmad RZ, Wardhani BWK, Kusala MKJ, Kurniasih DAA, Fauziah I, Wibowo S, Ugbo EN, Fauzia KA. Rift Valley fever: A zoonotic disease with global potential. Open Vet. J.. (2025), [cited January 24, 2026]; 15(6): 2312-2328. doi:10.5455/OVJ.2025.v15.i6.5 Harvard Style Hestianah, E. P., Khairullah, . A. R., Effendi, . M. H., Budiastuti, . B., Tyasningsih, . W., Budiarto, . B., Permatasari, . D. A., Moses, . I. B., Ahmad, . R. Z., Wardhani, . B. W. K., Kusala, . M. K. J., Kurniasih, . D. A. A., Fauziah, . I., Wibowo, . S., Ugbo, . E. N. & Fauzia, . K. A. (2025) Rift Valley fever: A zoonotic disease with global potential. Open Vet. J., 15 (6), 2312-2328. doi:10.5455/OVJ.2025.v15.i6.5 Turabian Style Hestianah, Eka Pramyrtha, Aswin Rafif Khairullah, Mustofa Helmi Effendi, Budiastuti Budiastuti, Wiwiek Tyasningsih, Budiarto Budiarto, Dian Ayu Permatasari, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Bantari Wisynu Kusuma Wardhani, Muhammad Khaliim Jati Kusala, Dea Anita Ariani Kurniasih, Ima Fauziah, Syahputra Wibowo, Emmanuel Nnabuike Ugbo, and Kartika Afrida Fauzia. 2025. Rift Valley fever: A zoonotic disease with global potential. Open Veterinary Journal, 15 (6), 2312-2328. doi:10.5455/OVJ.2025.v15.i6.5 Chicago Style Hestianah, Eka Pramyrtha, Aswin Rafif Khairullah, Mustofa Helmi Effendi, Budiastuti Budiastuti, Wiwiek Tyasningsih, Budiarto Budiarto, Dian Ayu Permatasari, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Bantari Wisynu Kusuma Wardhani, Muhammad Khaliim Jati Kusala, Dea Anita Ariani Kurniasih, Ima Fauziah, Syahputra Wibowo, Emmanuel Nnabuike Ugbo, and Kartika Afrida Fauzia. "Rift Valley fever: A zoonotic disease with global potential." Open Veterinary Journal 15 (2025), 2312-2328. doi:10.5455/OVJ.2025.v15.i6.5 MLA (The Modern Language Association) Style Hestianah, Eka Pramyrtha, Aswin Rafif Khairullah, Mustofa Helmi Effendi, Budiastuti Budiastuti, Wiwiek Tyasningsih, Budiarto Budiarto, Dian Ayu Permatasari, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Bantari Wisynu Kusuma Wardhani, Muhammad Khaliim Jati Kusala, Dea Anita Ariani Kurniasih, Ima Fauziah, Syahputra Wibowo, Emmanuel Nnabuike Ugbo, and Kartika Afrida Fauzia. "Rift Valley fever: A zoonotic disease with global potential." Open Veterinary Journal 15.6 (2025), 2312-2328. Print. doi:10.5455/OVJ.2025.v15.i6.5 APA (American Psychological Association) Style Hestianah, E. P., Khairullah, . A. R., Effendi, . M. H., Budiastuti, . B., Tyasningsih, . W., Budiarto, . B., Permatasari, . D. A., Moses, . I. B., Ahmad, . R. Z., Wardhani, . B. W. K., Kusala, . M. K. J., Kurniasih, . D. A. A., Fauziah, . I., Wibowo, . S., Ugbo, . E. N. & Fauzia, . K. A. (2025) Rift Valley fever: A zoonotic disease with global potential. Open Veterinary Journal, 15 (6), 2312-2328. doi:10.5455/OVJ.2025.v15.i6.5 |