| Research Article | ||
Open Vet. J.. 2025; 15(6): 2518-2531 Open Veterinary Journal, (2025), Vol. 15(6): 2518-2531 Research Article A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattleAsmaa I. Zin Eldin1, Heba F. Hozyen2, Eman Shafik1 and Nourhan Eissa3*1Department of Microbiology and Immunology, National Research Center (NRC), Giza, Egypt 2Department of Animal Reproduction and AI, National Research Center (NRC), Giza, Egypt 3Department of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt *Correspondence to: Nourhan Eissa. Department of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt. Email: vet_noura [at] yahoo.com Submitted: 07/02/2025 Revised: 11/05/2025 Accepted: 28/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
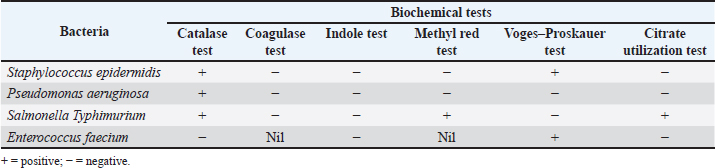
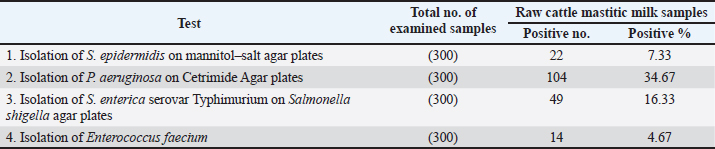
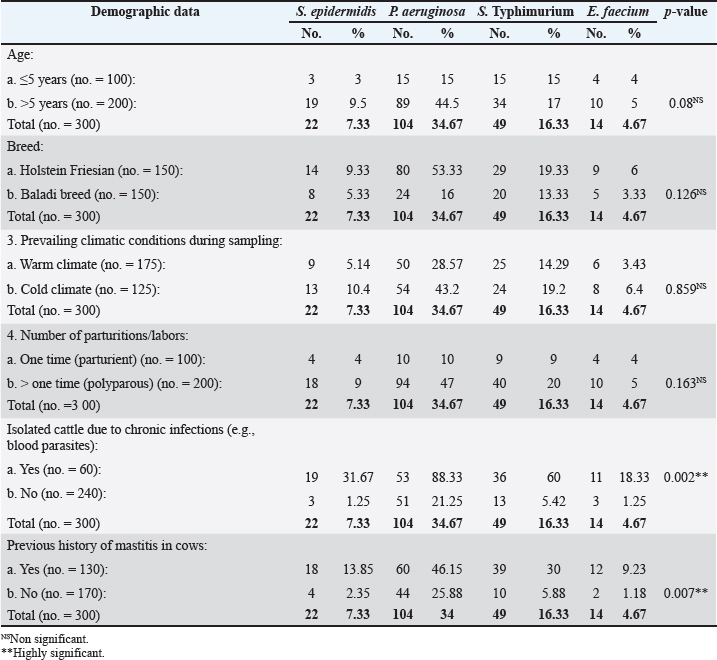
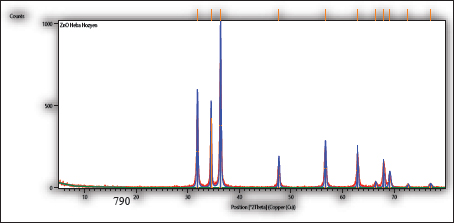
AbstractBackground: Cattle mastitis is a widespread and affluent illness that threatens the dairy industry. Numerous common infectious diseases, mostly zoonotic, impact Egypt’s dairy cow production. According to their remarkable affordability, high safety, and biocompatibility, and their exceptional effectiveness against microbes, zinc oxide nanoparticles (ZnO-NPs) have shown great promise in biomedicine, particularly in the areas of antibacterial and anticancer treatment, and so have become the most broadly used nanoparticles of metal oxides in various biological treatments throughout the last 20 years. Aim: The present study emphasized the potential for dispersed, non-agglomerated ZnO-NPs produced by sonochemical irradiation with starch serving as a capping agent to be considered more economical and effective germicidal agents in the eradication of bacteria of bovine mastitis, such as Staphylococcus epidermidis, Pseudomonas aeruginosa, Salmonella enterica serovar Typhimurium (S. Typhimurium), and Enterococcus faecium. Methods: To improve their antibacterial effectiveness against specific zoonotic pathogens that can cause bovine mastitis, three suspensions of ZnO-NPs were created as follows: auto-combustion reaction-synthesized ZnO-NPs, auto-combustion reaction-prepared uncapped ZnO-NPs, and sonochemically prepared starch-capped ZnO-NPs. The three suspensions were exposed to the zoonotic pathogens S. epidermidis, P. aeruginosa, S. Typhimurium, and E. faecium at concentrations ranging from 1 to 50 mg/ml. Results: The prepared three nano-ZnO suspensions showed minimum bactericidal concentration (MBC) total suppression of S. epidermidis at doses of 1–10 mg/ml of nano-ZnO and minimum inhibitory concentration (MIC) against S. epidermidis at concentrations <1–5 mg/ml. According to the suspension (No. 1), MBC was detected at a concentration of 30 mg/ml, and the MIC was 20 mg/ml for P. aeruginosa. Concerning S. Typhimurium, the MIC was found at a concentration of 5 mg/ml, while MBC was measured at 10 mg/ml. Lastly, at a concentration of 30 mg/ml, the nano-ZnO suspension (No. 1) demonstrated MBC activity against E. faecium, with MIC activity at a concentration of 2 mg/ml. Conclusion: This point will be useful for future research on ZnO-NPs because it focuses on their biological and antibacterial applications. Keywords: Zoonoses, Bovine mastitis, Zinc oxide nanoparticles, Egypt, Antibiotic residues.. IntroductionDiseases that naturally spread from vertebrates to humans and vice versa are known as zoonotic diseases (Eissa and Harb, 2023; Zin Eldin et al., 2023; Bedair et al., 2024; Eissa, 2024a,b,c; Elgendy et al., 2024; Elsobky et al., 2024; Salman et al., 2024a,b; Zain Eldeen et al., 2024; Ramzy et al., 2025). Mastitis, specific to the bovine mammary glands where inflammation or infection occurs, is a popular, prevalent disease threatening dairy farms globally. It reduces milk production and causes significant financial losses because of the high expense of treatment and the requirement to discard possibly contaminated milk (Eissa and Harb, 2023; Salman et al., 2024a). The two main types of inflammatory response in the parenchymal tissue of the mammary gland are symptomatic clinical mastitis (CM) and asymptomatic subclinical mastitis (SCM). These criteria are based on pathological signs (Prajapati et al., 2024). Furthermore, by consuming milk, mastitis-affected animals can spread harmful infections to humans, a public health risk that necessitates indirect diagnosis (Ahmed et al., 2020). In order to prevent and treat mastitis, adult dairy calves are also given a lot of antibiotics, which has a detrimental effect on the health of consumers (Ruegg, 2017; Byomi et al., 2018, 2019b; Eissa and Harb, 2023; Salman et al., 2024a,b; Zain Eldeen et al., 2024). In Egypt, Azooz et al. (2020a) calculated that the yearly economic losses as a result of CM were 1,196,871.4 LE because of direct and indirect failure and preventive expenses. While they calculated expenditures associated with medications, rejected milk, decreased milk yield, and veterinary services to prevent conversion to CM, labor, product quality, diagnostics, culling, materials, and investments were all included in the 21,933,258.60 LE for SCM that they recorded as annual cost loss. Based on how the pathogens are passed on, there are two types of zoonotic bovine mastitis: zooanthroponotic [from humans to animals, such as Pseudomonas aeruginosa and Salmonella enterica serovar Typhimurium (S. Typhimurium)] and anthroponotic (from animals to humans, such as Staphylococcus epidermidis and Enterococcus faecium) (Prajapati et al., 2024). To stop and manage the onset and spread of dairy foodborne zoonoses, Egypt and others must place a high priority on knowledge assessment and awareness (Fagnani et al., 2021). In some instances, S. Typhimurium usually spreads in a herd through an animal’s feces, but it can also be passed on through the milk of mastitic and asymptomatic shedders, making it more likely that other animals will get sick (Holschbach and Peek, 2018). In addition, P. aeruginosa bacteria have been found in dairy mastitic cows in both clinical and subclinical studies (Meng et al., 2025). Bovine pseudomonas mastitis can cause fever, severe toxemia symptoms, watery, clotted, or bloody milk, and swollen and red mammary glands. It can also manifest as long-lasting illnesses with high somatic cell counts (SCC). The most common progression starts with moderate mastitis and ends with persistent infection (Kawai et al., 2017). Conversely, the most prevalent zooanthroponotic pathogen that causes bovine CM and SCM with high SCC is S. epidermidis, one of the coagulase-negative staphylococci members (Kløve et al., 2025). The function and impact of these pathogens on the health of the mammary glands are little understood, despite their obvious significance for the quality of dairy products and animal welfare (De Buck et al., 2021). Additionally, E. faecium, formerly known as fecal streptococci, accounts for 90% of enterococci that cause inframammary infections (Nam et al., 2010). Enterococci are the primary environmental pathogens of mastitis, not from udder tissue, and the bacteria that cause these diseases have a high potential for zoonotic transmission and can infect cows and quarters during the milking process (Kwit et al., 2023). Furthermore, the seriousness of the current research presented by the WHO BPPL (2024) that covered 24 pathogens of antibiotic-resistant bacterial pathogens showing their difficulty in treatment, in which Salmonella spp. and P. aeruginosa were included in high-burden resistant pathogens where they occupied levels 7 and 10 in the 2024 priority list, underscoring their global impact in terms of burden. Numerous antibiotics have been used to treat cattle mastitis up to this point (Eissa and Harb, 2023; Salman et al., 2024a,b; Zain Eldeen et al., 2024). This technique has numerous drawbacks, including a poor efficiency rate, increasing antibiotic resistance, and the emergence of various antibiotic remnants in the milk that may be hazardous to human health (Eissa and Harb, 2023; Salman et al., 2024a; Zain Eldeen et al., 2024). The rise of antibiotic-resistant pathogens is an enormous global health hazard, second only to terrorism and global warming, according to the World Health Organization (WHO) assessment (WHO, 2014). Thus, there is an urgent need to develop novel, safe, and affordable antibiotic formulation techniques. Numerous metal oxide nanoparticles have become attractive antibacterial materials as the clinical and veterinary fields have become increasingly interested in less dangerous drugs (Low et al., 2013; Kadiyala et al., 2018; Salman et al., 2024a; Zain Eldeen et al., 2024). Zinc oxide nanoparticles (ZnO-NPs), which are the most common oxides, have sparked a lot of interest around the world for biological uses, and they are generally thought to be safe for both humans and animals (Sirelkhatim et al., 2015). Because of their remarkable antibacterial, anti-inflammatory, and regenerative qualities, ZnO-NPs have attracted attention (Mandal et al., 2022). However, ZnO-NPs’ propensity to aggregate limits their use and decreases their accessible surface area (Anders et al., 2015) because it might inhibit their ability to react with bacterial cell walls, which would inhibit their antibacterial action (Slavin et al., 2017). Accordingly, a previous study performed by Hozyen et al. (2019) suggested that ZnO-NPs made by auto-combustion reaction, uncapped nanoparticles made by auto-combustion reaction, and starch-capped nanoparticles made by the sonochemical method may have improved the germicidal property against pathogens of mastitis. The current study used the aforementioned hypotheses to apply the germicidal qualities and dose impact of ZnO-NPs on zoonotic microbes originating from the mastitic milk of cows with different modes of transmission (S. Typhimurium, P. aeruginosa, S. epidermidis, and E. faecium). Material and MethodsAnimal samplingA total number of 300 clinical mastitic lactating cows were examined at the farm of El-Nubaria, one of the most prolific dairy farms in the Beheira Governorate of Egypt, between September 2023 and August 2024. Mastitis was identified by udder examination, udder palpation, milk clots, and inflammation of the mammary glands and was routinely recorded by the farm veterinarians using the California Mastitis Test and SCC techniques, where an automated somatic NucleoCounter SCC-100 was used. Positive SCC values (>200 × 103) were taken into account during sampling as described by Abd El-Razik et al. (2021). The animals were not treated with antibiotics for a minimum of 30 days prior to sample assembly. Samples of milk were collected from each cow using CM and detailed demographic data. Teats were wiped with 70% ethanol before sampling. Milk amount of 10 ml from each cow was aseptically collected into sterile tubes, tagged, and kept chilled in an icebox until it was delivered to the laboratory, as described by Salman et al. (2024a) and Amran et al. (2025). Moreover, within two hours of the sample being collected, a bacteriological study was carried out. Isolation and identification of zoonotic bacteriaTo isolate S. epidermidis, P. aeruginosa, Salmonella spp., and E. faecium, each milk sample was cultivated in 10-µl increments on mannitol salt agar (CM0085, Oxoid), Cetrimide agar (CIE) (CM0579, Oxoid), Salmonella Shigella agar (SSA) (CM0099, Oxoid), and blood agar media (CM0271, Oxoid). Each culture was kept at 37°C for 24 hours, gram stained, and subjected to a variety of biochemical tests (coagulase, DNase, catalase, and IMViC tests, which stand for Indole, Methyl Red, Voges–Proskauer, and Citrate), as stated by Hozyen et al. (2019). Additionally, S. Typhimurium was identified by serotyping putative colonies and sending them to the bacteriology laboratory of the National Research Center, Egypt. For the identification of E. faecium, smears from suspected colonies were examined under a microscope before being placed onto semisolid slope agar (Quinn et al., 2011), and the manufacturer’s instructions for the Vitek2 compact system method of staphylococci identification were followed (Ibrahim et al., 2022). ZnO-NPs synthesisAs usual, sodium hydroxide pellets (Merck, Germany), deionized water, glycine (Merck, Germany), and zinc nitrate (Sigma-Aldrich, India) were used. For the first two ZnO-NP preparations, the uncapped preparation was created using an auto-combustion process in which glycine was used as a fuel, while the capped ZnO-NPs were created using a sonochemical method in line with Luo et al.’s method (Luo et al., 2018) and Ahmed et al.’s approach (Ahmed et al., 2014), respectively. Furthermore, a third preparation of ZnO-NPs was also generated using auto-combustion practice with glycine used as a fuel. Characterization of ZnO-NPsUsing high-resolution transmission electron microscopy (HR-TEM) at an accelerating voltage of 200 kV, the form and dispersion of ZnO-NPs were examined. On a PANalytical (Empyrean) X-ray diffractometer, X-ray diffraction (XRD) patterns were captured using Cu Kα1 radiation (wavelength 1.5406 Å) with a scan angle range of 5°–80°, 40 kV acceleration voltage, a scan step of 0.02°, and a current of 30 mA. According to Hozyen et al. (2019), the distribution of particle sizes was determined using Zetasizer nano-Zs90 (Malvern, UK). Assessment of the antibacterial activity of ZnO-NPs using the agar well diffusion testThe antibacterial activity of ZnO-NPs was evaluated using a culture medium of Mueller–Hinton agar (MH) (CM0337, Oxoid), as depicted for the diffusion disc technique, which is always employed to measure antibiotic susceptibility (Hozyen et al., 2019; Salman et al., 2024a). When freshly cultured bacteria were added to nutrient-rich broth (CM0001, Oxoid), turbidity was measured and compared with the McFarland standard at 0.5. Wells with a diameter of 5 mm were placed on the top of the infected test organisms after the bacterial suspensions had been dispersed across the Petri plates with MH agar media. ZnO-NPs in varying concentration ranges of (1, 3, 5, 10, 20, 30, 40, and 50 mg/ml) were then added to the wells. The inhibition zones surrounding the wells in the Petri dishes were evaluated following a 24-hour incubation period at 37°C in order to assess the antibacterial activity (Salman et al., 2024a). Determination of ZnO-NPs’ minimum bactericidal concentration and minimum inhibitory concentrationUsing 96-well microplates and a modified version of the microdilution technique, the minimum inhibitory concentration (MIC) was determined (Hozyen et al., 2019). Briefly, each well of 96-well plates received 5 μl of bacterial inoculant and 95 μl of MH broth medium. In order to find the MIC of ZnO-NPs, 100 μl of each solution (1–50 mg/ml) was added to each well and left to stand at 37°C for 24 hours. To determine the growth rate, a 600-nm wavelength was used for absorbance through a microplate reader. An Eppendorf tube was filled with 200 ml of the contents of each well as 2 ml of Brain Heart Infusion (BHI) medium (CM1135, Oxoid) in order to test the germicidal effect. The tube was then incubated at 37°C for 12 hours. The cultures were inoculated onto BHI agar and incubated for 24 hours at 37°C after 20 ml of the media when no turbidity was observed. The lack of colonies on the agar plate served as the basis for selecting the bactericidal ZnO-NP concentrations. In comparison to the positive control (no ZnO-NP treatment), the minimum bactericidal concentration (MBC) assessment was represented by the concentration of ZnO-NPs at which no bacterial growth appeared (100% inhibited). Statistical analysisThe Statistical Package for the Social Sciences version 22.0 (IBM, USA, released 2013) was used to perform all statistical analyses for Windows 2, 3, 5, 13, and 43. One-way analysis of variance was performed to statistically investigate the inhibitory zones and effects of all types of ZnO-NPs on the examined microbes at a significance threshold of 0.05. The GraphPad Prism software (GraphPad, San Diego, CA) was used to display the inhibition zone graph (Salman et al., 2024a,b; Ramzy et al., 2025). Ethics approval and consent to participateIn accordance with the Institutional Animal Care and Use Committee’s guidelines, research ethics were conducted while handling animals under the supervision of the University of Sadat City’s Faculty of Veterinary Medicine (permission No: VUSC-011-1-24). ResultsIdentification of zoonotic microbes with different transmission patternsStaphylococcus epidermidis was initially identified using gram staining, catalase, biochemical tests, coagulase activity, and DNase assays. It was found to be present in 7.33% (22/300) of the milk samples analyzed in this investigation (Table 2 and Fig. 1). Additionally, P. aeruginosa was detected in the positive results by urease, citrate, and catalase assays, and biochemical identification using IMViC tests confirmed that 34.67% (104/300) of the samples were greenish blue on CIE, a characteristic of the bacteria. Furthermore, 16.33% (49/300) of the samples on SSA plates had lactose nonfermenting colonies with black cores, a characteristic of S. Typhimurium that was biochemically positive for catalase. Furthermore, 4.67% (14/300) of the samples had an acquired prevalence of E. faecium in the examined bovine milk. Occurrence of the isolated bacteria in relation to demographic risk factors in the examined cattleGenerally, Table 3 illustrated that the prevalence percentage of the four examined zoonotic pathogens (S. epidermidis, P. aeruginosa, S. Typhimurium, and E. faecium) is higher among cattle aged more than 5 years, Holestein Friesian breeds, samples collected during cold climate, polyparous cows, cattle with history of chronic infections (such as blood parasites), and those with the previous history of mastitis than among those of younger ages, Baladi breeds, samples collected during warm climate, parturient, and those without chronic infections and without previous signs of mastitis. Characterization of ZnONPsThe ZnO-NPs produced by the auto-combustion reaction are displayed in Figure 2 of the current investigation. Furthermore, for the ZnO-NPs generated by the auto-combustion reaction, the XRD pattern revealed diffraction peaks at 2θ = 36.3092°, 31.844°, and 34.4971°, which were consistent with the JCPDS file (Joint Committee on Powder Diffraction Standards) No. 01-083-6338 (101), (100), and (002) crystal planes of nano zinc oxide. The production of nano-ZnO structures was confirmed by the observed XRD signals. In the HR-TEM microscopic study shown in Figure 3, ZnO-NPs produced by the auto-combustion process were visible. The size of these hexagon-shaped particles was approximately 41 nm. Table 1. Results of biochemical testing of examined bacteria.
Table 2. Occurrence of zoonotic bacteria with different transmission patterns isolated from raw cattle mastitic milk samples.
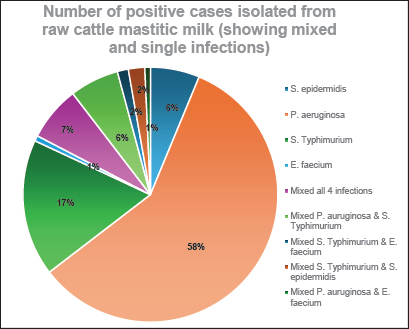
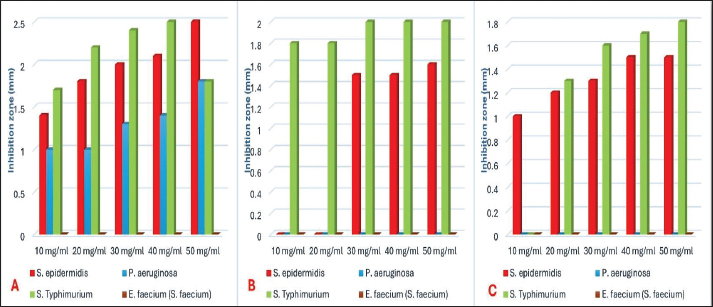
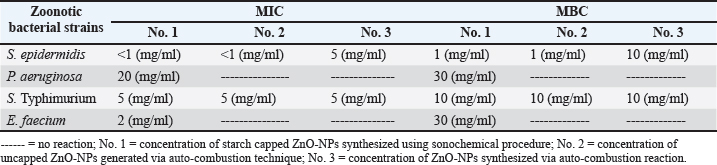
Fig. 1. Number of single and mixed infections of S. epidermidis, P. aeruginosa, S. enterica serovar Typhimurium, and E. faecium cases. Antibacterial activity of ZnONPs against S. epidermidis, P. aeruginosa, S. Typhimurium, and E. faeciumIn the current investigation, Figure 4 illustrated that ZnO-NPs (A) showed higher antibacterial activities against S. Typhimurium and S. epidermidis with Max zone of inhibition 3 and 2.5 mm, respectively, followed by P. aeruginosa with maximum zone of inhibition 1.4 mm. On the other hand, it had no effect against Streptococcus faecium. Concerning ZnO-NPs (B), they have the highest antibacterial effect against S. Typhimurium with a maximum inhibition zone of 2 mm (bacteriostatic effect) followed by S. epidermidis with a maximum inhibition zone of 1.6 mm. On the other hand, P. aeruginosa and S. faecium showed no effect on their growth. Regarding the concentration of ZnO-NPs (C), a higher antibacterial effect against S. Typhimurium with Max zone of inhibition of 1.8 mm was presented, followed by S. epidermidis with Max zone of 1.5 mm. However, it showed no effect against P. aeruginosa and S. faecium (E. faecium). MBC and MIC of capped, non-capped, and synthesized ZnO-NPs nanoparticles via combustion reaction against S. epidermidis, P. aeruginosa, S. Typhimurium, and E. faeciumTable 4 illustrates how the bactericidal and bacteriostatic effects of nano-ZnO suspensions were assessed by measuring the MBC and MIC values against the pathogens under investigation, respectively. Three nano-ZnO solutions ranging in concentration from 1 to 50 mg/ml were examined in this instance. The results indicated that MBC (S. epidermidis) was completely suppressed at doses of 1 to 10 mg/ml of nano-ZnO. The nano-ZnO solution exhibited inhibitory kinetics (MIC) against S. epidermidis at concentrations <1–5 mg/ml. According to the suspension (No. 1), MBC was detected at a concentration of 30 mg/ml and the MIC was 20 mg/ml for P. aeruginosa. When it came to S. Typhimurium, the MIC was found at a concentration of 5 mg/ml, whereas MBC was measured at 10 mg/ ml. Finally, the nano-ZnO suspension (No. 1) showed MBC activity against E. faecium at a concentration of 30 mg/ml, and MIC activity at a concentration of 2 mg/ ml. DiscussionMilk is one of the most plentiful dietary sources of vital minerals and nutritional components that are physiologically active and necessary for the function of the cardiovascular and immune systems (Byomi et al., 2018, 2019b; Yang et al., 2020; Eissa and Harb, 2023; Eissa, 2024c; Salman et al., 2024a). Perhaps because cattle mastitis has not been a particularly vocal killer, it has been a chronic infection that has been irksomely persistent since ancient times. Due to complicated infections and the development of bacteria that spread to other locations, it has been demonstrated to have a high rate of death and morbidity (Mazzariol et al., 2018). By overcoming multiple hierarchical barriers, the germs that cause mastitis may go undetected and infiltrate our lives. As the line between people and animals gets less clear, it is easier for zoonotic diseases to spread, even though it should not be possible in this case (McDaniel et al., 2014; Byomi et al., 2018, 2019a, b; Zin Eldin et al., 2023; Bedair et al., 2024; Eissa, 2024a,b,c; Elgendy et al., 2024; Elsobky et al., 2024; Salman et al., 2024a,b; Zain Eldeen et al., 2024; Ramzy et al., 2025). Along with pathogenic intervention being the main cause of infection, breast dysbiosis is caused by a change in the ecology of the mammary glands, with fewer commensals and more native pathobionts (Catozzi et al., 2019). Table 3. Occurrence of S. epidermidis, P. aeruginosa, S. enterica, serovar Typhimurium, and E. faecium in relation to demographic data of the examined cattle.
Fig. 2. Auto-combustion reaction-prepared ZnO-NPs’ X-ray diffraction patterns isolated from the raw cattle mastitic milk samples.
Fig. 3. Transmission electron microscopy micrograph of ZnO-NPs synthesized by performance of auto-combustion reaction. Anthropogenic bacterial infections such as S. Typhimurium and P. aeruginosa were found to be 16.33% (49/300) and 34.67% (104/300) prevalent in raw cattle milk in the Beheira governorate, Egypt, according to the current study. The S. Typhimurium occurrence result was similar to that reported by Azooz et al. (2020b), who found that 13.33% (20/150) of cattle milk samples had S. Typhimurium. They obtained this information by studying 13 governorates in detail: Alexandria, Beheira, Qaluiobia, Sharkia, Dakahlia, Cairo, Giza, Fayoum, Monofia, Asuit, Sohag, Bani Suif, and Aswan. Additionally, based on previous studies conducted in different regions of Egypt, El-Baz et al. (2017) showed that the Dakahlia governorate had a higher incidence rate of S. Typhimurium [25.93% (7/27)]. However, El-Tawab et al. (2017) reported that the prevalence of S. Typhimurium was lower in five governorates (Bani Suif, Ismailia, Giza, Fayoum, and Monofia governorates) at 2.35% (5/213). Bedassa et al. (2023) and Parolini et al. (2024) observed greater prevalence rates of S. Typhimurium in other regions of the world. They found prevalence rates of 21.88% in Ethiopia (42/192) and 23.33% in Italy (28/120).
Fig. 4. How big are the zinc oxide nanoparticlesʼ (ZnO-NPs) zones of inhibition against S. epidermidis, P. aeruginosa, S. enterica serovar Typhimurium, and E. faecium (S. faecium). Concentrations of ZnO-NPs produced by auto-combustion reaction, uncapped nanoparticles created by auto-combustion procedure, and starch-capped nanoparticles created using sonochemical technique are shown in A, B, and C, respectively. Table 4. Evaluation of MIC and MBC of the three preparations of ZnO-NPs against S. epidermidis, P. aeruginosa, S. enterica serovar Typhimurium, and E. faecium.
Pseudomonas aeruginosa prevalence results were linked with the findings of Ibrahim et al. (2017) and Gamal et al. (2022) from Egypt, which reported prevalence rates of 34% (34/100) and 38.55% (32/83) of P. aeruginosa in cattle milk in the Dakahlia governorate, respectively. Conversely, the prevalence of P. aeruginosa in cattle milk in the Dakahlia governorate was lower, at 10% (5/50) and 15% (30/200), respectively, according to Badawy et al. (2023) and Salem et al. (2023). Furthermore, among the dairy cattle in the Asuit governorate, Hassanein et al. (2023) reported a lower incidence of P. aeruginosa at 14% (7/50). Mahmood (2023) and Kováčová et al. (2024) reported significant prevalence rates of P. aeruginosa in cattle milk of 80% (8/10) and 47.19% (151/320), respectively. In contrast, the current investigation found that the occurrence of zooanthroponotic infections, specifically S. epidermidis, was 7.33% (22/300) of the raw cattle milk analyzed, whereas the prevalence of E. faecium (S. faecium) was 4.67% (14/300). The species-level connections between non-aureus staphylococci and mammaliicocci and mammary gland health are unclear because of the paucity of published data in Egypt and other nations. On the other hand, they are associated with the development of bovine mastitis and a rise in the SCC in milk (Addis et al., 2024). Ahmed et al. (2014) reported a higher prevalence rate of 11.76% (2/17) of S. epidermidis in cattle milk in the governorates of Qaluiobia and Monofia based on published Egyptian research that is currently available. Furthermore, a higher prevalence rate of 25% (6/24) in the Monofia governorate was shown by Nayel et al. (2020). Conversely, Walaa and Al-Shimaa (2023) found that the Alexandria governorate had a lower prevalence of S. epidermidis, at 4%. Previous research reported that S. epidermidis was more common in Belgium (11.4%, or 57/500) and Finland (11.52%) than in other parts of the world based on data from other studies (Reydams et al., 2023; Taponen et al., 2023). In the case of E. faecium (S. faecium), the prevalence rate of S. faecium was 2.08% (33/1,584) in Korea (Yoon and Lee, 2021), whereas Hammad et al. (2022) presented a higher prevalence rate of 29.17% (7/24) in Monofia governorate, Egypt. In Iraq, a previous study recorded a lower prevalence rate of 3.33% (10/300) (Musawi and Abdullaziz, 2023). By further studying the demographic data of the examined cattle to provide a risk assessment of pathogen acquisition for future researchers, the present investigation demonstrated that the prevalence of the four examined zoonotic pathogens increased with cattle age, among Holestein Friesian breeds, during cold climate, polyparous cows, those with infestation with blood parasites, and those with a previous history of mastitis. The present findings are in agreement with those of Veling et al. (2002) and Fossler et al. (2005), who reported that the incidence of S. Typhimurium increased in cattle with age in the Netherlands and USA, respectively. Moreover, Asamenew et al. (2013) revealed that the prevalence rates of Streptococcus spp. and P. aeruginosa increased among adult, Friesian, and polyparous cattle in Ethiopia. In addition, Nyakiti (2017) concluded that the prevalence of Streptococcus spp. and P. aeruginosa was higher in adult and polyparous cattle. However, he failed to find any influence of the cattle breed on the incidence of these pathogens in Kenya. Furthermore, Ngotho et al. (2022) reported higher prevalence rates of Staphylococcus spp. and Pseudomonas spp. infections among Friesian cattle, polyparous cattle, and cattle with the previous history of mastitis in Kenya. Concerning prevailing climate and infestation with blood parasites, Kamal et al. (2024) revealed similar findings to the present research, where they declared that the incidence of P. aeruginosa was higher among Egyptian cattle during cold season times and among cattle infested with blood parasites such as Babesia spp. and Theileria spp. than during warm season times and cattle without blood parasites. Conversely, Fossler et al. (2005) and Pangloli et al. (2008) found that the prevalence of S. Typhimurium in cattle in the USA and Tennessee is higher in warm climates than in cold climates. The results of the current study could be attributed to hormonal changes over the course of several gestational periods, with cattle becoming older with more contact with pathogen sources throughout their lives. This resulted in a higher prevalence of pathogens in adults, polyparous cattle, and cattle with a history of mastitis (Byomi et al., 2018, 2019b; Eissa and Harb, 2023; Eissa, 2024c; Elgendy et al., 2024; Salman et al., 2024a). Furthermore, the role of fomites and climate change in Egypt, which has been suggested to alter the habitats of fomites and vectors during colder seasons compared to warmer ones, particularly in regions where they were not previously observed, could be responsible for the increased prevalence of the examined pathogens during cold climates. In addition, factors such as increased crowding and decreased immunity can occur during cold seasons (Martínez-García et al., 2021; Kamal et al., 2024). Regarding the elevated prevalence of pathogens in cattle with blood parasites, Lokken et al. (2018) demonstrated that ferritin, a necessary nutrient that promotes bacterial growth and rescue, would rise in response to the rupture of red blood cells caused by blood parasites. Because of their exceptional photocatalytic activity, large surface area, adjustable bandgap, and environmentally friendly characteristics, ZnO-NPs have become a popular and effective photocatalyst for environmental remediation (Gaur et al., 2024). According to previous reports, they may also be helpful in treating infections caused by bovine mastitis (Bozkurt et al., 2024). The issue of bovine mastitis and the resulting antibiotic resistance in dairy cows is well-established (Byomi et al., 2019b; Eissa and Harb, 2023; Elgendy et al., 2024; Salman et al., 2024a). In the current research, ZnO-NPs were created using three distinct methods: auto-combustion reaction synthesis of uncapped ZnO-NPs using glycine as fuel according to a formula, auto-combustion reaction synthesis of ZnO-NPs using glycine as fuel according to another formula, and sonochemical synthesis of capped ZnO-NPs. The three synthesized formulations demonstrated antibacterial efficacy against zoonotic pathogens of various transmission patterns that were isolated from cows with CM, including S. epidermidis, P. aeruginosa, S. Typhimurium, and E. faecium, at dosages ranging from 1 to 50 mg/ml, according to Table 3. ZnO-NPs created via the auto-combustion approach may have mild bactericidal and bacteriostatic activity against S. epidermidis because ZnO is almost water insoluble and quickly clumps with it during dissolving because water is highly polar (Padmavathy and Vijayaraghavan, 2008). On the other hand, sonochemical irradiation and starch capping were combined to create stable and uniformly dispersed ZnO-NPs. The quantity of nanoparticles has a significant impact on their germicidal activity (Patil et al., 2018). Moreover, Wahab et al. (2010) discovered that the growth inhibition rose as the ZnO-NP concentrations in the discs increased. High nanoparticle concentrations can result in considerable ion release, and their concentration directly affects toxicity (Soltani Nezhad et al., 2014). The fundamental mechanisms of action and the capacity of ZnO-NPs to regulate microbes are still debated. Since ZnO-NPs are 250 times smaller than bacteria, their antibacterial qualities can be linked to their small size. This facilitates the adhesion of nanoparticles to the bacterial cell wall, thereby killing the bacterium (Chwalibog et al., 2010). Additionally, it has been proposed that zinc ions emitted by tiny nanoparticles with high surface reactivity and simple cellular absorption may harm the macromolecules of bacterial cells (Mandal et al., 2022). The metal ions in solution lose their distinct localization in the surrounding environment and become evenly distributed when they encounter bacterial cells. However, the bacterial cells become more dangerous when nanoparticles engage with the cell wall to create a concentrated source for continuous ion release (Karaky et al., 2024). Cellular penetration is further promoted by the high ion concentration produced. Thus, the cell membrane of bacteria is the site of nanoparticle dissolution, and the size and shape of the nanoparticle affect the rate of dissolution (Sánchez-López et al., 2024). According to a different concept, ZnO-NPs can result in reactive oxygen species production and membrane dysfunction (Chakroborty et al., 2024). The fact that nanoparticles appear to activate many systems simultaneously makes explanation challenging, but it also explains the benefits of nanoparticle exposure. No single component is likely to be the cause of the bacterial fatality; this mixture appears to be hazardous in and of itself. The multi-target action of nanoparticles makes them perfect for eliminating microorganisms and treating diseases (Slavin et al., 2017). ConclusionThe current study’s findings indicate that (1) ZnO-NPs, a novel, affordable, economically and environmentally friendly material, have potent germicidal qualities against a variety of zoonotic bacteria, including S. epidermidis, P. aeruginosa, S. Typhimurium, and E. faecium, which were identified from samples of mastitic bovine milk. (2) Various synthesis techniques and capping agents may have an impact on the aggregation, size, and shape of synthesized ZnONPs, which may in turn have an impact on the particles’ antibacterial capabilities. (3) The sonochemical method and starch capping used to create ZnO-NPs prevented them from clumping together and improved their antibacterial activity against the mastitic bovine infections. LimitationsThere is less available research concerning the examined bacteria and their role in mastitis, particularly in Egypt. Furthermore, the lack of obvious data recording mastitis status in Egypt and the associated risk factors of different pathogens, all these factors were limitations facing the current research. AcknowledgmentsNot applicable. Conflict of interestNo conflict of interest. FundingNot applicable. Authors’ contributionsAIZE, HFH, ES, and NE designed the experiments and collected samples. HFH and ES synthesized and characterized the nanoparticles and evaluated their antibacterial activity. AIZE and NE wrote the scientific manuscript and performed data analysis. HFH and ES helped draft the manuscript and efficiently reviewed the manuscript. All authors have read and approved the final manuscript. Data availabilityAll data were provided in the manuscript. ReferencesAbd El-Razik, K.A., Arafa, A.A., Fouad, E.A., Younes, A.M., Almuzaini, A.M. and Abdou, A.M. 2021. Isolation, identification and virulence determinants of Streptococcus agalactiae from bovine subclinical mastitis in Egypt. J. Infect. Dev. Ctries. 15(8), 1133–1138; doi:10.3855/jidc.12668. Addis, M.F., Locatelli, C., Penati, M., Poli, S.F., Monistero, V., Zingale, L., Rota, N., Gusmara, C., Piccinini, R., Moroni, P. and Bronzo, V. 2024. Non-aureus staphylococci and mammaliicocci isolated from bovine milk in Italian dairy farms: a retrospective investigation. Vet. Res. Commun. 48(1), 547–554; doi:10.1007/s11259-023-10187-x. Ahmed, M.A., Mansour, S.F., El-Dek, S.I. and Abu-Abdeen, M. 2014. Conduction and magnetization improvement of BiFeO3 multiferroic nanoparticles by Ag+ doping. Mater. Res. Bull. 49, 352–359; doi:10.1016/j.materresbull.2013.09.011. Ahmed, W., Neubauer, H., Tomaso, H., El Hofy, F.I., Monecke, S., Abdeltawab, A.A. and Hotzel, H. 2020. Characterization of Staphylococci and Streptococci isolated from milk of bovides with mastitis in Egypt. Pathogens 9(5), 381; doi:10.3390/ pathogens9050381. Amran, R., Alhimaidi, A. and Ammari, A. 2025. Evaluation of the California mastitis test, pH and milk colour as indicators for subclinical mastitis detection in local dairy cows. Trop. J. Pharmaceut. Res. 24(1), 15–20; doi:10.4314/tjpr.v24i1.3. Anders, C.B., Chess, J.J., Wingett, D.G. and Punnoose, A. 2015. Serum proteins enhance dispersion stability and influence the cytotoxicity and dosimetry of ZnO nanoparticles in suspension and adherent cancer cell models. Nanoscale. Res. Lett. 10, 1–22; doi:10.1186/s11671-015-1158-y. Asamenew Tesfaye, A.T., Aster Yohannes, A.Y., Addisalem Hunde, A.H., Tegegnework Tezera, T.T. and Tsadik, Z.G. 2013. Mastits: prevalence, risk factors and antimicrobial sensitivity patterns of bacterial isolates in dairy cattle at Holeta farm in Ethiopia. Afr. J. Agric. Res. 8(23), 2837–2842. Available via http://www.academicjournals.org/ ajar/PDF/pdf2013/20Jun/Tesfaye%20et%20al.pdf Azooz, M.F., El-Wakeel, S.A. and Yousef, H.M. 2020a. Financial and economic analyses of the impact of cattle mastitis on the profitability of Egyptian dairy farms. Vet. World. 13(9), 1750–1759; doi:10.14202/ vetworld.2020.1750-1759. Azooz, M.F., El-Wakeel, S.A. and Yousef, H.M. 2020b. Risk factor analysis of Salmonella Typhimurium, Staphylococcus aureus, standard plate count and somatic cell count in bulk tank milk in cattle dairies. World Vet. J. 10(3), 338–361; doi:10.36380/ scil.2020.wvj44. Badawy, B., Moustafa, S., Shata, R., Sayed-Ahmed, M.Z., Alqahtani, S.S., Ali, M.S., Alam, N., Ahmad, S., Kasem, N., Elbaz, E. and El-Bahkiry, H.S. 2023. Prevalence of multidrug-resistant Pseudomonas aeruginosa isolated from dairy cattle, milk, environment, and workers’ hands. Microorganisms 11(11), 2775; doi:10.3390/ microorganisms11112775. Bedair, N.M., Sakr, M.A., Mourad, A., Eissa, N., Mostafa, A. and Khamiss, O. 2024. Molecular characterization of the whole genome of H9N2 avian influenza virus isolated from Egyptian poultry farms. Arch. Virol. 169(5), 1; doi:10.1007/ s00705-024-06018-2. Bedassa, A., Nahusenay, H., Asefa, Z., Sisay, T., Girmay, G., Kovac, J., Vipham, J.L. and Zewdu, A. 2023. Prevalence and associated risk factors for Salmonella enterica contamination of cow milk and cottage cheese in Ethiopia. Food Saf. Risk. 10(1), 2; doi:10.1186/s40550-023-00101-3. Bozkurt, M.A., Keyvan, E., Donmez, S. and Sen, E. 2024. Eco-friendly preparation of zinc oxide nanoparticles and effects on Staphylococcus aureus isolated from subclinical mastitis cow milk. J. Hellenic. Vet. Med. Soc. 75(1), 7089–7098; doi:10.12681/jhvms.34155. Byomi, A., Zidan, S., Salama, A., Elsify, A., Hadad, G. and Eissa, N. 2018. Public health implications of toxoplasmosis in animals and women in selected localities of Menoufia governorate, Egypt. Assiut. Vet. Med. J. 64, 120–130; doi:10.21608/ avmj.2018.168920. Byomi, A., Zidan, S., Elkamshishi, M., Sakr, M., Elsify, A., Eissa, N. and Hadad, G. 2019a. Some associated risk factors with Coxiella burnetii in sheep, humans and ticks in Menoufiya governorate, Egypt. Biosci. Res. 16(S1–2), 121–138. Available via https://www. researchgate.net/publication/338336617_Some_ associated_risk_factors_with_Coxiella_burnetii_ in_sheep_humans_and_ticks_in_Menoufiya_ governorate_Egypt Byomi, A., Zidan, S., Sakr, M.A., Elsify, A., Hussien, H., Eissa, N. and Hadad, G. 2019b. Coxiella burnetii infection in milk of cattle and the risk of human infection in Menoufia Governorate. J. Curr. Vet. Res. 1, 140–148; doi:10.21608/jcvr.2019.65030. Catozzi, C., Cuscó, A., Lecchi, C., De Carlo, E., Vecchio, D., Martucciello, A., D’Angelo, L., Francino, O., Sanchez Bonastre, A. and Ceciliani, F. 2019. Impact of intramammary inoculation of inactivated Lactobacillus rhamnosus and antibiotics on the milk microbiota of water buffalo with subclinical mastitis. PLoS One 14(1), e0210204; doi:10.1371/ journal.pone.0210204. Chakroborty, S., Nath, N., Soren, S., Barik, A. and Kaur, K. 2024. Plasmonic-based TiO2 and TiO2 nanoparticles for photocatalytic CO2 to methanol conversion in energy applications: current status and future prospects. Top. Catal. 67(1), 232–245; doi:10.1007/s11244-023-01816-5. Chwalibog, A., Sawosz, E., Hotowy, A., Szeliga, J., Mitura, S., Mitura, K., Grodzik, M., Orlowski, P. and Sokolowska, A. 2010. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomed. 6, 1085–1094; doi:10.2147/IJN.S13532. De Buck, J., Ha, V., Naushad, S., Nobrega, D.B., Luby, C., Middleton, J.R., De Vliegher, S. and Barkema, H.W. 2021. Non-aureus staphylococci and bovine udder health: current understanding and knowledge gaps. Front. Vet. Sci. 8, 1–16; doi:10.3389/ fvets.2021.658031. Eissa, N. 2024a. Fungal diseases of dogs and cats. In Introduction to diseases, diagnosis, and management of dogs and cats. Ed., Rana T. Cambridge, MA: Academic Press, pp: 523–532. Eissa, N. 2024b. Mycoplasma, rickettsia, and chlamydia diseases of dogs and cats. In Introduction to diseases, diagnosis, and management of dogs and cats. Ed., Rana T. Cambridge, MA: Academic Press, pp: 489–499. Eissa, N. 2024c. Q fever (Queensland fever). In The handbook of zoonotic diseases of goats. Ed., Rana T. Wallingford, UK: CABI, pp: 260–267; doi:10.1079/9781800622852.0022. Eissa, N. and Harb, O.H. 2023. Diseases of the ear. In Key questions in clinical farm animal medicine, volume 2: types, causes and treatments of infectious disease. Ed., Rana T. Wallingford, UK: CABI, pp: 232–276; doi:10.1079/9781800624818.0007. El-Baz, A.H., El-Sherbini, M., Abdelkhalek, A. and Al-Ashmawy, M.A. 2017. Prevalence and molecular characterization of Salmonella serovars in milk and cheese in Mansoura city, Egypt. J. Adv. Vet. Anim. Res. 4(1), 45–51; doi:10.5455/javar.2017.d189. Elgendy, S.M., Zaghawa, A.A., Nayel, M.A., El Sify, A.M., Salama, A.A., Dawood, M. and Eissa, N. 2024. Seroprevalence of Neospora caninum, Toxoplasma gondii and Brucella species in Sheep. J. Curr. Vet. Res. 6(1), 54–69; doi:10.21608/ jcvr.2024.352702. Elsobky, Y., Eltholth, M., Abdalla, E., Eissa, N., Hadad, G., Nayel, M., Salama, A., Mousa, W., Kamal, A., Abu-Seida, A.M. and Gaidan, O.K. 2024. Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: advancing outbreak management through customized regional strategies in Egypt. Open Vet. J. 14(11), 2911; doi:10.5455/OVJ.2024.v14.ill.20. El-Tawab, A., Ashraf, A., Nabih, A.M., Agag, M.A., Ali, A. and Marwah, H. 2017. Molecular studies of virulence genes of Salmonella Typhimurium causing clinical mastitis in dairy cattle. Benha Vet. Med. J. 33(2), 27–37. Availablre via https://bvmj.journals.ekb.eg/ article_29985_14d3aa2d0220ef8517c071 6ed51ba331.pdf Fagnani, R., Nero, L.A. and Rosolem, C.P. 2021. Why knowledge is the best way to reduce the risks associated with raw milk and raw milk products. J. Dairy Res. 88(2), 238–243; doi:10.1017/ S002202992100039X. Fossler, C.P., Wells, S.J., Kaneene, J.B., Ruegg, P.L., Warnick, L.D., Eberly, L.E., Godden, S.M., Halbert, L.W., Campbell, A.M., Bolin, C.A. and Zwald, A.G. 2005. Cattle and environmental sample-level factors associated with the presence of Salmonella in a multi-state study of conventional and organic dairy farms. Prev. Vet. Med. 67(1), 39–53; doi:10.1016/j.prevetmed.2004.10.005. Gamal, H., El-Diasty, M., Dapgh, A., El-Sherbini, M., El-Baz, A. and Abdelkhalek, A. 2022. Virulence genes of multi-drug resistance Pseudomonas species isolated from milk and some dairy products. J. Adv. Vet. Res. 12(4), 415–421. Available via https://advetresearch.com/index.php/AVR/article/ view/1037/696 Gaur, J., Kumar, S., Pal, M., Kaur, H., Batoo, K.M. and Momoh, J.O. 2024. Current trends: zinc oxide nanoparticles preparation via chemical and green method for the photocatalytic degradation of various organic dyes. Hybrid Adv. 5, 100128; doi:10.1016/j.hybadv.2023.100128. Hammad, A.M., Aly, S.S., Hassan, H.A., Abbas, N.H., Eltahan, A., Khalifa, E. and Shimamoto, T. 2022. Occurrence, phenotypic and molecular characteristics of vancomycin-resistant enterococci isolated from retail raw milk in Egypt. Foodborne Pathog. Dis. 19(3), 192–198; doi:10.1089/ fpd.2021.0054. Hassanein, S.A., Elsherif, W.M., Elshater, M.A. and Sayed, M. 2023. Pseudomonas aeruginosa and Staphylococcus aureus profile in some dairy farms. Assiut. Vet. Med. J. 69(178), 119–123; doi:10.21608/avmj.2023.200439.1133. Holschbach, C.L. and Peek, S.F. 2018. Salmonella in dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 34(1), 133–154; doi:10.1016/j.cvfa.2017.10.005. Hozyen, H.F., Ibrahim, E.S., Khairy, E.A. and El-Dek, S.I. 2019. Enhanced antibacterial activity of capped zinc oxide nanoparticles: a step towards the control of clinical bovine mastitis. Vet. World. 12(8), 1225; doi:10.14202/vetworld.2019.1225-1232. Ibrahim, E.S., Dorgham, S.M., Mansour, A.S., Abdalhamed, A.M. and Khalaf, D.D. 2022. Genotypic characterization of mecA gene and antibiogram profile of coagulase-negative staphylococci in subclinical mastitic cows. Vet. World. 15(9), 2186; doi:10.14202/vetworld.2022.2186-2191. Ibrahim, N.A., Farag, V.M., Abd-El-Moaty, A.M. and Atwa, S.M. 2017. Resistant gene of Pseudomonas aeruginosa in mastitic cattle with reference to some biochemical and immunological parameters. World Vet. J. 7(1), 5–13; doi:10.5455/wvj.20170488. Kadiyala, U., Kotov, N.A. and VanEpps, J.S. 2018. Antibacterial metal oxide nanoparticles: challenges in interpreting the literature. Curr. Pharm. Des. 24(8), 896–903; doi:10.2174/13816128246661802.19130659. Kamal, E., Zaghawa, A., Salma, A., Nayel, M. and Dawoud, M. 2024. Risk factors associated with common infectious diseases in beef cattle in Menofia Governorate. J. Curr. Vet. Res. 6(1), 39–53; doi:10.21608/jcvr.2024.352701. Karaky, N., Tang, S., Ramalingam, P., Kirby, A., McBain, A.J., Banks, C.E. and Whitehead, K.A. 2024. Multidrug-resistant Escherichia coli eemains susceptible to metal ions and graphene-based compounds. Antibiotics 13(5), 381; doi:10.3390/ antibiotics13050381. Kawai, K., Shinozuka, Y., Uchida, I., Hirose, K., Mitamura, T., Watanabe, A., Kuruhara, K., Yuasa, R., Sato, R., Onda, K. and Nagahata, H. 2017. Control of Pseudomonas mastitis on a large dairy farm by using slightly acidic electrolyzed water. Anim. Sci. J. 88(10), 1601–1605; doi:10.1111/asj.12815. Kløve, D.C., Strube, M.L., Heegaard, P.M. and Astrup, L.B. 2025. Mapping antimicrobial resistance in Staphylococcus epidermidis isolates from subclinical mastitis in Danish dairy cows. Antibiotics 14(1), 67. Kováčová, M., Výrostková, J., Regecová, I., Dudriková, E., Zahumenská, J. and Marcinčák, S. 2024. Effect of selected bacteria of the genus Pseudomonas on the quality of raw cow’s milk. Polish J. Vet. Sci. 27(2), 229–239; doi:10.24425/pjvs.2024.149353. Kwit, R., Zając, M., Śmiałowska-Węglińska, A., Skarżyńska, M., Bomba, A., Lalak, A., Skrzypiec, E., Wojdat, D., Koza, W., Mikos-Wojewoda, E. and Pasim, P. 2023. Prevalence of Enterococcus spp. and the whole-genome characteristics of Enterococcus faecium and Enterococcus faecalis strains isolated from free-living birds in Poland. Pathogens 12(6), 836; doi:10.3390/pathogens12060836. Lokken, K.L., Stull-Lane, A.R., Poels, K. and Tsolis, R.M. 2018. Malaria parasite-mediated alteration of macrophage function and increased iron availability predispose to disseminated nontyphoidal Salmonella infection. Infect. Immun. 86(9), 10–128; doi:10.1128/iai.00301-18. Low, W.L., Martin, C., Hill, D.J. and Kenward, M.A. 2013. Antimicrobial efficacy of liposome-encapsulated silver ions and tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Lett. Appl. Microbiol. 57(1), 33–39; doi:10.1111/lam.12082. Luo, Z., Dong, K., Guo, M., Lian, Z., Zhang, B. and Wei, W. 2018. Preparation of zinc oxide nanoparticles-based starch paste and its antifungal performance as a paper adhesive. Starch-Stärke 70(7–8), 1700211; doi:10.1002/star.201700211. Mahmood, M.W. 2023. Detection of pseudomonas contamination in milk and some dairy products in Diyala Province. Diyala J. Vet. Sci. 1(2), 110–117. Available via https://www.iasj.net/iasj/ download/030613ff3d456eec Mandal, A.K., Katuwal, S., Tettey, F., Gupta, A., Bhattarai, S., Jaisi, S., Bhandari, D.P., Shah, A.K., Bhattarai, N. and Parajuli, N. 2022. Current research on zinc oxide nanoparticles: synthesis, characterization, and biomedical applications. Nanomaterials 12(17), 3066; doi:10.3390/ nano12173066. Martínez-García, G., Santamaría-Espinosa, R.M., Lira-Amaya, J.J. and Figueroa, J.V. 2021. Challenges in tick-borne pathogen detection: the case for Babesia spp. identification in the tick vector. Pathogens 10(2), 92; doi:10.3390/pathogens10020092. Mazzariol, S., Corrò, M., Tonon, E., Biancani, B., Centelleghe, C. and Gili, C. 2018. Death associated to methicillin resistant Staphylococcus aureus ST8 infection in two dolphins maintained under human care, Italy. Front. Immunol. 9, 2726; doi:10.3389/ fimmu.2018.02726. McDaniel, C.J., Cardwell, D.M., Moeller, R.B. and Gray, G.C. 2014. Humans and cattle: a review of bovine zoonoses. Vector-Borne Zoonotic Dis. 14(1), 1–9; doi:10.1089/vbz.2012.1164. Meng, Y., Zhu, W., Han, S., Jiang, H., Chen, J., Zhou, Z., Hao, X., Xu, T., Qin, A., Yang, Z. and Shang, S. 2025. Presence of aminoglycoside and β-lactam-resistant Pseudomonas aeruginosa in raw milk of cows. Dairy 6(2), 13. Musawi, M.H. and Abdullaziz, A.A. 2023. Isolation and characterization of antibiotics resistance Enterococcus faecium from mastitic cow’s milk. Iraqi J. Vet. Sci. 37(Supplement I-IV), 21–27; doi:10.33899/ijvs.2023.1391120.2884. Nam, H.M., Lim, S.K., Moon, J.S., Kang, H.M., Kim, J.M., Jang, K.C., Kim, J.M., Kang, M.I., Joo, Y.S. and Jung, S.C. 2010. Antimicrobial resistance of enterococci isolated from mastitic bovine milk samples in Korea. Zoonoses Pub. Health. 57(7–8), e59–64; doi:10.1111/j.1863-2378.2009.01307.x. Nayel, M., Abdeen, E., Basiony, E., Younis, G. and Elnahriry, S. 2020. Phenotypic and genotypic characterization of methicillin-resistant coagulase-negative staphylococci as an etiological agent of bovine mastitis in Egypt. J. Curr. Vet. Res. 2(2), 31–40; doi:10.21608/jcvr.2020.121500. Ngotho, M., Kagira, J., Nkoiboni, D., Njoroge, J. and Maina, N. 2022. Risk factors associated with sub-clinical mastitis and antibacterial resistance in small-holder dairy farms of Kajiado North Sub-County, Kenya. J. Vet. Physiol. Pathol. 1(3), 49–55; doi:10.58803/jvpp.v1i3.8. Nyakiti, A.A. 2017. Prevalence of mastitis and antimicrobial resistance among dairy cattle in Uasin Gishu County–Kenya. Doctoral dissertation. University of Eldoret, Eldoret, Kenya. Ohnishi, M., Sawada, T., Hirose, K., Sato, R., Hayashimoto, M., Hata, E., Yonezawa, C. and Kato, H. 2011. Antimicrobial susceptibilities and bacteriological characteristics of bovine Pseudomonas aeruginosa and Serratia marcescens isolates from mastitis. Vet. Microbiol. 154(1–2), 202–207; doi:10.1016/j.vetmic.2011.06.023. Padmavathy, N. and Vijayaraghavan, R. 2008. Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Sci. Technol. Adv. Mater. 9(3), 35004; doi:10.1088/1468-6996/9/3/035004. Pangloli, P., Dje, Y., Ahmed, O., Doane, C.A., Oliver, S.P. and Draughon, F.A. 2008. Seasonal incidence and molecular characterization of Salmonella from dairy cows, calves, and farm environment. Foodborne Pathog. Dis. 5(1), 87–96; doi:10.1089/ fpd.2007.0048. Parolini, F., Ventura, G., Rosignoli, C., Rota Nodari, S., D’incau, M., Marocchi, L., Santucci, G., Boldini, M. and Gradassi, M. 2024. Detection and phenotypic antimicrobial susceptibility of Salmonella enterica serotypes in dairy cattle farms in the Po Valley, Northern Italy. Animals 14(14), 2043; doi:10.3390/ ani14142043. Patil, B.N., Taranath T.C. and Limonia acidissima, L. 2018. leaf mediated synthesis of silver and zinc oxide nanoparticles and their antibacterial activities. Microb. Pathog. 115, 227–232; doi:10.1016/j. micpath.2017.12.035. Prajapati, P., Gangil, D., Yadav, J.S., Yadav, D.S., Gupta, V.K., Ahirwar, S., Prajapati, D., Parihar, S.P. and Sharma, S. 2024. Assessment of knowledge of dairy farmers regarding zoonotic diseases in indore district. Ann. Res. 67(2), 1. Available via https://afr-journal.com/index.php/ afr/article/view/195/195 Quinn, P.J., Markey, B.K., Leonard, F.C., Hartigan, P., Fanning, S. and Fitzpatrick, E. 2011. Veterinary microbiology and microbial disease. Hoboken, NJ: John Wiley & Sons; doi:10.1016/S1090-0233(02)00137-5. Ramzy, Gehad., Mousa, W., Gaidan, O.K., Zaghawa, A., Nayel, M., Elsify, A., Eissa, N., Abu-Seida, A.M. and Salama, A. 2025. Molecular characterization and associated risk factors of zoonotic cryptosporidiosis in bovine calves and humans in Menoufia governorate, Egypt. Open Vet. J. 15(1), 1–12. Reydams, H., Toledo-Silva, B., Mertens, K., Piepers, S., de Souza, F.N., Haesebrouck, F. and De Vliegher, S. 2023. Comparison of non-aureus staphylococcal and mammaliicoccal species found in both composite milk and bulk-tank milk samples of dairy cows collected in tandem. J, Dairy Sci. 106(11), 7974–7990; doi:10.3168/jds.2022-23092. Ruegg, P.L. 2017. A 100-year review: mastitis detection, management, and prevention. J. Dairy Sci. 100(12), 10381–10397; doi:10.3168/jds.2017-13023. Salem, M., Awad, A. and Younis, G. 2023. Antibiotic susceptibility and molecular detection of virulent Pseudomonas aeruginosa isolated from bovine mastitis milk in Egypt. J. Adv. Vet. Res. 13(4), 664–671. Available via https://advetresearch.com/ index.php/AVR/article/view/1314/757 Salman, M.B., Eldin, A.I., Eissa, N., Maher, A., Aish, A.E. and Abd El-Moez, S.I. 2024a. Evaluation of the effectiveness of some essential oils against zoonotic methicillin-resistant Staphylococcus aureus isolated from dairy products and humans. JAVAR 11(2), 306; doi:10.5455/javar.2024.k778. Salman, M.B., Zin Eldin, A.I., Ata, N.S., AbdElfatah, E.B. and Eissa, N. 2024b. Prevalence, antimicrobial resistance and risk factors of zoonotic foodborne pathogens isolated from camel meat. J. Curr. Vet. Res. 6(1), 272–284; doi:10.21608/ jcvr.2024.352718. Sánchez-López, P., Hernández-Hernández, K.A., Fuentes Moyado, S., Cadena Nava, R.D. and Smolentseva, E. 2024. Antimicrobial and virus adsorption properties of Y-zeolite exchanged with silver and zinc cations. ACS Omega 9(7), 7554–7563; doi:10.1021/acsomega.3c06462. Sirelkhatim, A., Mahmud, S., Seeni, A., Kaus, N.H., Ann, L.C., Bakhori, S.K., Hasan, H. and Mohamad, D. 2015. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro. Lett. 7, 219–242; doi:10.1007/s40820-015-0040-x. Slavin, Y.N., Asnis, J., Hńfeli, U.O. and Bach, H. 2017. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15, 1–20; doi:10.1186/s12951-017-0308-z. Soltani Nezhad, S., Rabbani Khorasgani, M., Emtiazi, G., Yaghoobi, M.M. and Shakeri, S. 2014. Isolation of copper oxide (CuO) nanoparticles resistant Pseudomonas strains from soil and investigation on possible mechanism for resistance. World J. Microbiol. Biotechnol. 30, 809–817; doi:10.1007/ s11274-013-1481-3. Taponen, S., Tölli, H.T. and Rajala-Schultz, P.J. 2023. Antimicrobial susceptibility of staphylococci from bovine milk samples in routine microbiological mastitis analysis in Finland. Front. Vet. Sci. 10, 1235417; doi:10.3389/fvets.2023.1235417. Veling, J., Wilpshaar, H., Frankena, K., Bartels, C. and Barkema, H.W. 2002. Risk factors for clinical Salmonella enterica subsp. enterica serovar Typhimurium infection on Dutch dairy farms. Prev. Vet. Med. 54(2), 157–168; doi:10.1016/S0167-5877(02)00023-5. Wahab, R., Kim, Y.S., Mishra, A., Yun, S.I. and Shin, H.S. 2010. Formation of ZnO micro-flowers prepared via solution process and their antibacterial activity. Nanoscale Res. Lett. 5, 1675–1681; doi:10.1007/s11671-010-9694-y. Walaa, I.A. and Al-Shimaa, A.S. 2023. Correlation between biofilm formation and antibacterial resistance pattern among coagulase negative Staphylococcus. Egypt J. Anim. Health. 3(4), 105–118; doi:10.21608/ejah.2023.315383. WHO (World Health Organization). 2014. News release WHO 1st global report on antibiotic resistance reveals serious, worldwide threat to public health-30 WHO report Available via http:// www.who.int/media centre/news/releases/2014/ amr-report/en (Accessed 28 February 2019). WHO BPPL (WHO bacterial priority pathogens list). 2024.Bacterialpathogensofpublichealthimportance to guide research, development and strategies to prevent and control antimicrobial resistance. Available via https://iris.who.int/bitstream/ handle/10665/376776/9789240093461eng. pdf?sequence=1 Yang, H.G., Li, H.Y., Li, P., Bao, X.Y., Huang, G.X., Xing, L., Zheng, N. and Wang, J.Q. 2020. Modulation activity of heat-treated and untreated lactoferrin on the TLR-4 pathway in anoxia cell model and cerebral ischemia reperfusion mouse model. J. Dairy Sci. 103(2), 1151–163; doi:10.3168/ jds.2019-17002. Yoon, S. and Lee, Y.J. 2021. Molecular characteristics of Enterococcus faecalis and Enterococcus faecium from bulk tank milk in Korea. Animals 11(3), 661; doi:10.3390/ani11030661. Zin Eldin, A.I., Salman, M.B., Salah Eldin, D.A. and Eissa, N. 2023. An overview on: bacterial fish zoonoses. J. Curr. Vet. Res. 5(2), 196–215; doi:10.21608/jcvr.2023.320449. Zain Eldeen, A., Younes, A.M., Bazid, A., Eissa, N.M., Salman, M. and AboElkhair, M. 2024. Influence of some herbals on immunostimulation of cellular immunity in experimentally Ndv-vaccinated chicks. Egypt J. Vet. Sci. 55(5), 1165–1174; doi:10.21608/ ejvs.2024.256822.1734. | ||
| How to Cite this Article |
| Pubmed Style Eldin AIZ, Hozyen HF, Shafik E, Eissa N. A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle. Open Vet. J.. 2025; 15(6): 2518-2531. doi:10.5455/OVJ.2025.v15.i6.24 Web Style Eldin AIZ, Hozyen HF, Shafik E, Eissa N. A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle. https://www.openveterinaryjournal.com/?mno=241650 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.24 AMA (American Medical Association) Style Eldin AIZ, Hozyen HF, Shafik E, Eissa N. A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle. Open Vet. J.. 2025; 15(6): 2518-2531. doi:10.5455/OVJ.2025.v15.i6.24 Vancouver/ICMJE Style Eldin AIZ, Hozyen HF, Shafik E, Eissa N. A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2518-2531. doi:10.5455/OVJ.2025.v15.i6.24 Harvard Style Eldin, A. I. Z., Hozyen, . H. F., Shafik, . E. & Eissa, . N. (2025) A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle. Open Vet. J., 15 (6), 2518-2531. doi:10.5455/OVJ.2025.v15.i6.24 Turabian Style Eldin, Asmaa I. Zin, Heba F. Hozyen, Eman Shafik, and Nourhan Eissa. 2025. A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle. Open Veterinary Journal, 15 (6), 2518-2531. doi:10.5455/OVJ.2025.v15.i6.24 Chicago Style Eldin, Asmaa I. Zin, Heba F. Hozyen, Eman Shafik, and Nourhan Eissa. "A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle." Open Veterinary Journal 15 (2025), 2518-2531. doi:10.5455/OVJ.2025.v15.i6.24 MLA (The Modern Language Association) Style Eldin, Asmaa I. Zin, Heba F. Hozyen, Eman Shafik, and Nourhan Eissa. "A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle." Open Veterinary Journal 15.6 (2025), 2518-2531. Print. doi:10.5455/OVJ.2025.v15.i6.24 APA (American Psychological Association) Style Eldin, A. I. Z., Hozyen, . H. F., Shafik, . E. & Eissa, . N. (2025) A new Egyptian approach to the antibacterial effect of zinc oxide nanoparticles on zoonotic bacteria with different transmission patterns isolated from cattle. Open Veterinary Journal, 15 (6), 2518-2531. doi:10.5455/OVJ.2025.v15.i6.24 |