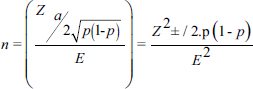| Original Article | ||
Open Vet. J.. 2022; 12(5): 668-675 Open Veterinary Journal, (2022), Vol. 12(5): 668–675 Original Research Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, ColombiaDiana María Bulla-Castañeda1, Henry Alexander Lopez Buitrago1, Deisy Johana Lancheros-Buitrago1, Adriana María Díaz-Anaya1, Diego Jose Garcia-Corredor1, Julio Cesar Tobón-Torreglosa2, Diego Ortiz Ortega3 and Martín Orlando Pulido-Medellín1*1Grupo de Investigación en Medicina Veterinaria y Zootecnia – GIDIMEVETZ, Universidad Pedagógica y Tecnológica de Colombia, Tunja, Colombia 2Empresa Colombiana de Productos Veterinarios – VECOL, Bogotá, Colombia 3Corporación Colombiana de Investigación Agropecuaria - Agrosavia, Bogotá, Colombia *Corresponding Author: Martin Orlando Pulido Medellin. Grupo de Investigación en Medicina Veterinaria y Zootecnia – GIDIMEVETZ. Universidad Pedagógica y Tecnológica de Colombia, Tunja, Colombia. Email: martin.pulido [at] uptc.edu.co Submitted: 09/05/2022 Accepted: 12/08/2022 Published: 14/09/2022 © 2022 Open Veterinary Journal
AbstractBackground: Bovine leptospirosis is a zoonotic, infectious, and cosmopolitan disease of worldwide distribution, caused by the spirochete Leptospira spp., which has been diagnosed in humans; domestic mammals, such as dogs, sheep, goats, swine, horses and cattle; and wild animals. It is considered a significant cause of economic losses in livestock because it causes infertility, abortion and reduced milk production. Aim: To establish the prevalence and the main risk factors associated with Leptospira spp. in cattle in the municipality of Sotaquirá, Colombia. Methods: An observational, descriptive, cross-sectional study with simple random sampling was carried out. 1,000 cattle of Ayrshire, Holstein, Jersey, Normande, Zebu, and crossbreeds were sampled. Blood samples were taken by coccygeal venipuncture and processed by microscopic agglutination technique; animals were considered positive when titers were ≥1:100. The data obtained were processed with the statistical program EpiInfo®. Results: A general apparent prevalence (AP) of 16% (160/1,000) was established, where the crossbreeds (20.5% AP), the 2–4 years age group (17% AP), and the serovars Leptospira interrogans serogroup Pomona (5.1%) and L. interrogans serogroup Sjroe serovar Hardjo (3.4%) presented the highest seropositivity. The variables barnyard, artificial insemination, and use of certified semen were identified as protective factors against the disease, while diarrhea was considered a risk factor. Conclusion: The prevalence in this study is within the range of those reported at the national level; however, it is essential to establish plans to control and prevent the disease. Keywords: Cattle, Leptospira, Leptospirosis, Prevalence. IntroductionLeptospirosis is a zoonotic, infectious, and cosmopolitan disease that occurs worldwide and is caused by obligate pathogenic bacteria of the genus Leptospira (Fávero et al., 2017; Vincent et al., 2019). Almost all mammals can be exposed to Leptospira spp. and can become lifelong carriers. The disease affects dairy and beef cattle, causing infertility, abortion, and reduced milk production, reflecting in economic losses (Ruano et al., 2020). Leptospira can be divided into three lineages that correlate with the level of pathogenicity of the species: saprophytic, intermediate, and pathogenic (Vincent et al., 2019), of which at least 300 serovars are known, divided into 28 serogroups for convenience (Levett, 2015). Cattle can be recognized as maintenance hosts of serovar Hardjo and other members of the Sejroe serogroup. However, serovars Pomona, Icterohaemorrhagiae, and Grippotyphosa may also be associated with bovine infection (Grippi et al., 2020). Bovine leptospirosis can result in abortions, fetal death, premature calving, and the birth of weak, low-weight calves. This infection is more closely associated with more subtle syndromes, such as subfertility and early embryonic death. Thus, this disease can go undetected and undiagnosed, compromising reproductive efficiency and decreasing herd productivity over long periods (Loureiro and Lilenbaum, 2020). Signs and symptoms of leptospirosis are often varied, allowing it to be confused with other causes of acute febrile syndromes; therefore, early diagnosis and identification of a specific agent in clinical specimens are crucial for effective treatment (Ali et al., 2021). Rodents are the main reservoirs for this disease; however, cattle are responsible for maintaining the disease (Fávero et al., 2017). In Latin America, a high prevalence of infection has been established (75.0% at herd level and 44.2% at animal level), with a predominance of Sejroe serogroup strains (80.3%) (da Silva Pinto et al., 2016). In 2019, the Colombian agricultural sector represented 6.74% of the country’s gross domestic product (GDP); in addition to this, the livestock sector contributed 28.9% of the agricultural sector (DANE, 2020). In this sense, livestock is one of the most important agricultural activities in Colombia, which participated with 48.7% of livestock GDP (FEDEGAN, 2018). Taking this into account, it is important to mention that the productivity of a farm begins with an adequate state of animal health, which depends mainly on the efficient management of pathogens (Washburn, 2020), where the control of etiological agents of infectious diseases generates significant negative impacts on the reproductive efficiency of bovines, as well as concomitant problems for human health and the environment (Newcomer and Givens, 2016; Gilbert, 2018). In Colombia, research has been conducted on the epidemiology of leptospirosis in several country regions, where outbreaks have occurred, mainly in the Atlantic Coast, Urabá Antioqueño and the Eje Cafetero (Carreño et al., 2017). However, at the regional level, few studies have established the presence of the disease in the Department of Boyacá (Moreno et al., 2017; Pulido-Medellín et al., 2017). Therefore, the objective was to establish the prevalence and the main risk factors associated with the presentation of Leptospira spp. in cattle in the municipality of Sotaquirá, Colombia. Materials and MethodsGeographical locationSotaquirá is a Colombian municipality in the Department of Boyacá, located in central-eastern Colombia, in the Alto Chicamocha region, 39 km from the city of Tunja. The municipal area is 268.65 km2, of which 258.55 km2 correspond to the extension of the rural area, in which agricultural and livestock activities are developed (Alcaldía Municipal, 2019). Sample sizeThe registered cattle population in Sotaquirá was 19,333 heads of cattle (Instituto Colombiano Agropecuario, 2019). Based on the above, a sample size of 1,000 individuals with a sampling fraction of 5.17% was determined, considering an accepted error of 3.1%, confidence level of 95%, and expected prevalence rate of 50%. The following formula was used:
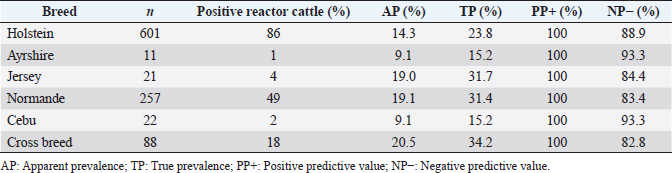
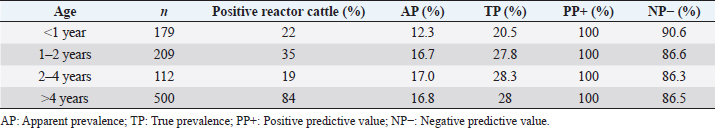
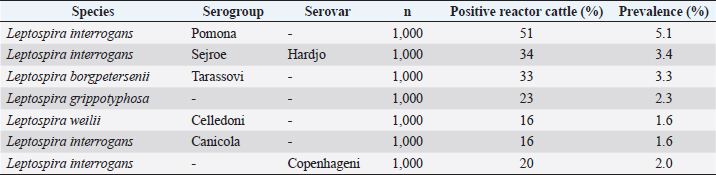
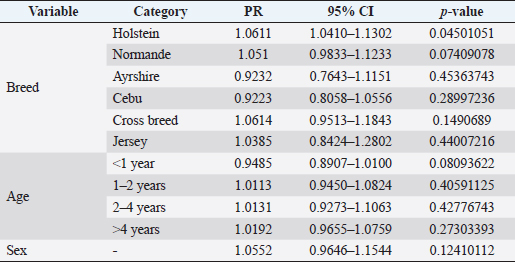
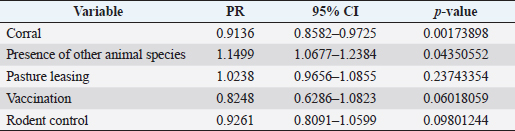
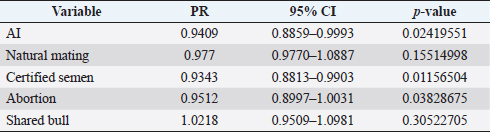
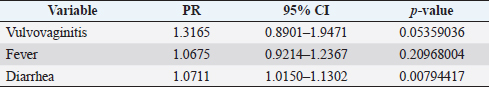
where Z =confidence level; n =sample size; E =accepted error; p=expected value of the proportion; and α=tail probability. Variables evaluatedThe variables were divided into two categories: those related to the animal, considering the age, breed, sex, and reproductive events of the cattle evaluated, and those associated with the farm, prioritizing the management practices implemented on the farms that participated in the study. Sample collection and processingThe samples were obtained from females and males of different ages belonging to the Ayrshire, Holstein, Jersey, Normande, Zebu, and crossbreeds unvaccinated. 7 ml of blood was extracted by coccygeal venipuncture and stored in Vacutainer® tubes for subsequent refrigeration (4°C) and transport to the Veterinary Parasitology laboratory of the Universidad Pedagógica y Tecnológica de Colombia (Uptc). The samples were centrifuged (2,500 rpm/10 minutes) to obtain the serum, which was transferred to an Eppendorf tube for storage at −20°C. The samples were analyzed by microscopic agglutination test (MAT) (OIE, 2021). The selected strains were cultivated in liquid culture medium for leptospires at 30°C ± 2°C for 4–10 days. Live cultures with densities of approximately 2 × 108 leptospires per ml were used as antigens. To standardize culture density, cultures may need to be adjusted to a concentration of 2 × 108 leptospires per ml prior to testing. A selection of antigens was made with a serum dilution of 1/50. A volume of each antigen, equal to the volume of the diluted serum, was added to each well to make a final serum dilution of 1/100 in the screening test. Microtiter plates were incubated at 30°C ± 2°C for 1.5–4 hours. Plates are examined by darkfield microscopy (OIE, 2021). Animals were considered positive when titers were ≥1:100, with a sensitivity of 60% and a specificity of 100%. Statistical analysisThe study was an observational, descriptive, cross-sectional study with simple random sampling. The real prevalence and MAT’s predictive values were found with WinEpi statistical program. With the database consolidated and cleaned, the analyses were performed with the EpiInfo® statistical program. The determining factors were defined by calculating the prevalence ratio (PR). The dependent variable (Y) included the serological results obtained, while the independent variables (X) were all the determining factors established in the structured epidemiological survey implemented during sample collection; the association between the presentation of the disease and the variables evaluated was determined using Fisher’s exact test. Once these factors were established, a final model was constructed using logistic regression analysis. Ethical approvalThe study was conducted under Law 576 of 2,000 and Law 84 of 1,989 of the Republic of Colombia. Informed consent was obtained from the cattle owners before sample collection. ResultsThe apparent prevalence (AP) of bovine leptospirosis was 16% (160/1,000), where 15.4% (134/869) of the females and 19.8% (26/131) of the males were positive for the disease. A true prevalence (TP) of 26.7% was established with a positive predictive value (PP+) of 100% and a negative predictive value (NP−) of 87.3%. Concerning the breeds evaluated, the crossbreeds (20.5% AP; 34.2% TP) and the Normande breed (19.1% AP; 31.4% TP) had the highest prevalence of leptospirosis, while the Cebu cattle had the lowest prevalence (9.1% AP; 15.2% TP) (Table 1). Regarding the age groups evaluated, individuals <1 year old presented the lowest seropositivity (12.3% PA; 20.5% TP), followed by cattle aged 1–2 years (16.7% PA; 27.8% TP), >4 years (16.8% PA; 28% TP), and animals aged 2–4 years (17% PA; 28.3% TP) (Table 2). Likewise, a seroprevalence of 5.1% (51/1,000) was determined for Leptospira interrogans serogroup Pomona; 3.4% for L. interrogans serogroup Sjroe serovar Hardjo (34/1,000); 3.3% for L. borgpetersenii serogroup Tarassovi (33/1,000); 2.5% for Bratislava (25/1000); 2.3% for L. grippotyphosa (23/1,000); 2% for L. interrogans serovar Copenhageni (20/1,000); and 1.6% for L. weilii serogroup Celledoni and L. interrogans serogroup Canicola (16/1,000) (Table 3). No significant statistical association was found between the age and sex of the cattle evaluated with positivity to bovine leptospirosis (p ≥ 0.05). However, an association was established between the Holstein breed and the presence of the disease ( p=0.04501051). Likewise, this variable was identified as a possible risk factor for the presentation of Leptospira spp. (Table 4). A significant statistical association was found between the variables: the presence of barnyard ( p=0.00173898) and other species in the farms evaluated ( p =0.04350552). The barnyard was identified as a protective factor against leptospirosis, while other animal species’ presence was considered a possible risk factor associated with the presentation of the disease (Table 5). Regarding the reproductive variables evaluated, a significant statistical association was found with artificial insemination (AI) ( p=0.02419551), the use of certified semen ( p=0.01156504), and abortion ( p=0.03828675); the first two variables were identified as possible protective factors associated with bovine leptospirosis (Table 6). In relation to clinical manifestations, diarrhea presented a significant statistical association ( p=0.00794417) and a possible risk factor for the presentation of the disease (Table 7). Logistic regression allowed establishing that the only risk factor associated with bovine leptospirosis is the presentation of diarrhea in the cattle evaluated (Table 8). DiscussionThe seroprevalence of bovine leptospirosis for this study was lower than the 54.2% found in the municipality of Toca, Boyacá (Pulido-Medellín et al., 2017). Other studies at the national level reported prevalences of 41% in Montería (Córdoba) (Hurtado et al., 2013), 6.1%–46.5% in Caquetá (Motta et al., 2014), and 5.82% in Pasto (Nariño) (Benavides-Romo and Marcillo, 2016). Table 1. PA and PR of leptospirosis by breed in cattle from Sotaquirá, Boyacá.
Table 2. PA and PR of leptospirosis by age in cattle from Sotaquirá, Boyacá.
Table 3. Leptospirosis prevalence by serogroups and serovars in cattle from Sotaquirá, Boyacá.
Table 4. Analysis of race, age, and sex are possible risk factors associated with Leptospira spp. infections. Results are presented as PR and 95% CI.
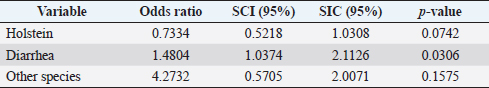
Table 5. Analysis of management practices as possible risk factors associated with Leptospira spp. infections. Results are presented as PR and 95% CI.
Studies conducted in South America show higher prevalence, such as those observed in the Department of Boquerón, Paraguay (45.78%) (Szwako et al., 2015) and in the province of Manabí, Ecuador (56.21%) (Ruano et al., 2020). However, this is above the values established in Santa Catarina (6.44%), Brazil, and the stables of Lima and Lima (6.44%), Peru (Fávero et al., 2017), and in the Lima and Huancayo stables in Peru (14.8% and 12.3%, respectively) (Llanco et al., 2017). This variation in the reported prevalence may be due to the risk of animal infection associated with environmental factors such as rainfall and climate, management factors and farming systems such as co-breeding with other productive species such as pigs or the presence of domestic animals (canines) and wild animals (rodents) (Llanco et al., 2017). It is also important to note that the MAT test achieves maximum agglutination 2–3 weeks after infection so that seropositivity may vary depending on the time of sample collection (Mullan and Panwala, 2016). Concerning the species found L. interrogans serogroup Pomona and L. interrogans serogroup Sjroe serovar Hardjo were the most prevalent in the municipality of Sotaquirá. Khalili et al. (2014), Szwako et al. (2015), Llanco et al. (2017), and Ruano et al. (2020) indicated that the most prevalent serovars belonging to the genomospecies L. interrogans were L. pomona; L. wolffi, L. hardjo, and L. icterohaemorrhagiae in cattle. However, it agrees with the studies found at the national level, where one of the serovars with the highest seroprevalence was L. hardjo (Hurtado et al., 2013; Motta et al., 2014; Moreno et al., 2017). Table 6. Analysis of reproductive variables as possible risk factors associated with Leptospira spp. infections. Results are presented as PR and 95% CI.
Table 7. Analysis of clinical manifestations as possible risk factors associated with Leptospira spp. infections. Results are presented as PR and 95% CI.
Table 8. Analysis of variables as possible risk factors associated with Leptospira spp. infections.
The presence of the different serovars of Leptospira spp. varies according to latitude; natural regions of a country; the interaction of susceptible species, reservoirs, time of the year in which the study is conducted or productive systems (Motta et al., 2014). In addition, in tropical and subtropical countries, climatic conditions, such as heat, summer rains, and low soil in some areas, are considered factors that favor the pathogen's presence and survival in the environment (Carvalho et al., 2015; Szwako et al., 2015). Regarding the age of the cattle evaluated, it is important to highlight that cattle aged 2–4 years presented the highest seroprevalence; however, this does not agree with what was reported by Hurtado et al. (2013), who found the highest seropositivity in individuals older than 7 years (43.2%). Likewise, no significant statistical association was found between the age groups evaluated and bovine leptospirosis, which differs from that reported by Ruano et al. (2020), who indicated that at the animal level, only age was associated with seropositivity to the disease (Ruano et al., 2020). The high prevalence of older cattle is due to the passive protection transmitted by the dams in the first 3–4 months of life, which decreases with advancing age (Rebhum, 1999; Gómez, 2005; Álvarez et al., 2018). The highest prevalence was found in crossbreeds and the Normande breed. Additionally, a significant statistical association was found between the presentation of the disease and Holstein cattle, which differs from that reported by Benavides-Romo and Marcillo-Arévalo (2016). This study shows that there was no relationship between leptospirosis and the Holstein, Jersey, Brown, and crossbreeds. This may occur because the different strains of Leptospira spp. do not have a breed predilection in the bovine species, so the level of seropositivity is the same for all breeds (Awosanya et al., 2013). Within the management practices evaluated in the farms, rodent control did not present a significant statistical association with the presentation of bovine leptospirosis. However, it is important to indicate that rodents are ubiquitous, difficult to eliminate without adequate sanitary measures, have a high reproduction rate; and are always in contact with feed and water offered to cattle, which can be easily contaminated with the leptospires that mice intermittently shed with urine (Llanco et al., 2017). Likewise, the presence of other species within the herds presented an association with seropositivity to Leptospira spp., which indicates that the presentation of the disease is related to the presence of other animals. This is because leptospirosis can occur in more than 160 domestic or wild mammalian animals, in addition to humans, which facilitates the circulation of spirochetes among the different species present in the same environment (Torres et al., 2018). In addition to the above, it has been shown that tropical regions have many unique aspects that affect the occurrence of infection, where husbandry practices and management factors can affect serovars' overall seroprevalence and distribution in the tropics (Martins and Lilenbaum, 2017). Therefore, the presence of penning on farms behaved as a protective factor for the pathology, as it has been shown that in dairy cattle, the subdivision of animals into smaller flocks and avoidance of co-grazing during herd management decreases Leptospira seropositivity (Martins et al., 2012; Mughini-Gras et al., 2014; Martins and Lilenbaum, 2017). A significant statistical association was established between seropositivity to Leptospira spp. and the occurrence of abortion in the cattle evaluated. It has been reported that the occurrence of reproductive disorders is significantly related to leptospirosis (p=0.01) (Fávero et al., 2017). This pathology is an important cause of production drops associated with reproductive problems (Marianelli et al., 2007; de Oliveira et al., 2018). In addition, in infected pregnant females, the bacterium can cross the placenta at any stage of pregnancy, causing embryonic losses, abortions, or stillbirths; in addition to this, the repetition of estrus is a characteristic that can be frequently observed (Menezes et al., 2006; de Oliveira et al., 2018; Loureiro and Lilenbaum, 2020). The statistical association found between the presentation of antibodies against Leptospira spp. and AI differs from that reported by Benavides-Romo and Marcillo-Arévalo (2016), who found no relationship with this variable. It is important to highlight that AI and certified semen behaved as protective factors for Leptospira spp. This may be due to the transmission of the disease occurring mainly through contact with urine from infected animals (Ospina-Pinto and Hernández, 2015). It has also been shown that bulls are often subclinically infected and represent an important infection source for females (Ellis, 2015). Considering the above, it should be highlighted that leptospires could remain in the vaginal environment for an unknown period and form biofilms, which indicates a possible sexual transmission and suggest that it not only occurs from male to female but also from female to male during natural mating (Loureiro et al., 2017). The results also suggest that they are sensitive to antimicrobials commonly used to prepare diluted semen for AI, such as streptomycin (Givens, 2018). This indicates that implementing reproductive biotechnologies and certified semen would decrease the chances of disease presentation in cattle. It is necessary to indicate that the presentation of diarrhea behaved as a risk factor for the presentation of bovine leptospirosis. The presence of diarrhea does not represent a pathognomonic sign of any disease; however, it may be associated with the presence of other pathogens of reproductive importance. For instance, Olmo et al. (2019) showed that Neospora caninum and bovine viral diarrhea virus (BVDv) were more prevalent in females than males of cattle exposed to L. interrogans serovar Hardjo and an association with higher titers of BVDv was also found. García et al. (2020) reported a significant statistical association between BVDV seropositivity and cattle with a clinical history of diarrhea, which was accompanied by the presence of antibodies against Leptospira spp. in 16% of the sampled individuals, so diarrhea possibly occurs due to the presence of other pathogens in the herds that participated in this study. Finally, it has been reported that the presence of antibodies against Leptospira spp. could be associated with various factors such as contact with other animal species, management, biosecurity practices, presence of different serotypes in the same region, as well as climatic and environmental conditions (Higino et al., 2013); however, in the present investigation, no other risk factors associated with its presentation were found. ConclusionA seroprevalence of 16% was found in the municipality of Sotaquirá, Boyacá (Colombia), with serovars L. interrogans serogroup Pomona and L. interrogans serovar Hardjo, the latter associated with the presentation of abortion in cattle. It is considered that prevention and control strategies should be established that include management practices, vaccination, and elimination of seropositive animals since this would favor the reduction of seroprevalence in herds and the consequent economic losses for producers. Authors’ contributionsAll authors contributed to the study. All authors read and approved the final manuscript. Conflict of interestAll authors declare that there is no conflict of interest. ReferencesAlcaldía Municipal, S. 2019. Alcaldía Municipal de Sotaquirá en Boyacá. Available via http://www.sotaquira-boyaca.gov.co/tema/informacion-adicional (Accessed 02 December 2021). Ali, M.R.M., Sum, J.S., Baki, N.N.A., Choong, Y.S., Amdan, N.A.N., Amran, F. and Lim, T.S. 2021. Development of monoclonal antibodies against recombinant LipL21 protein of pathogenic Leptospira through phage display technology. Int. J. Biol. Macromol. 168, 289–300. Álvarez, M.Á.L., Escatell, G.S., Arzate, J.J.M., Ibarra, J.M.O. and Rivera, E.M.L. 2018. Anticuerpos contra Leptospira spp en caprinos lecheros en Guanajuato, México. Rev. Investig. Vet. Perú. 29, 611–618. Awosanya, E.J., Nguku, P., Oyemakinde, A. and Omobowale, O. 2013. Factors associated with probable cluster of leptospirosis among kennel workers in Abuja, Nigeria. Pan. Afr. Med. J. 16, 144–149. Benavides-Romo, K.L.A. and Marcillo-Arévalo, A.R. 2016. Seroprevalecia de Leptospira spp en hembras bovinos de fincas lecheras en el municipio de Pasto, Colombia. REVIP 4, 27–32. Carreño, L.A., Salas, D. and Beltrán, K.B. 2017. Prevalencia de leptospirosis en colombia: revisión sistemática de literatura. Rev. Salud. Publica. 19, 204–209. Carvalho, O.S., Gonzaga, L.N.R, Albuquerque, A.S., Bezerra, D.C. and Chaves, N.P. 2015. Occurrence of Brucella abortus, Leptospira interrogans and bovine herpesvirus type 1 in buffalo (Bubalus bubalis) herd under extensive breeding system. Afr. J. Microbiol. Res. 9, 598–603. DANE. 2020. Cuentas nacionales anuales, base 2015 [Base de datos]. Available via https://www.dane.gov.co/index.php/estadisticas-por-tema/cuentas-nacionales/cuentas-nacionales-anuales. (Accessed 11 July 2022). da Silva Pinto, P., Libonati, H., Penna, B. and Lilenbaum, W. 2016. A systematic review on the microscopic agglutination test seroepidemiology of bovine leptospirosis in Latin America. Trop. Anim. Health. Prod. 48, 239–248. Ellis, W.A. 2015. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 387, 99–137. Fávero, J.F., de Araújo, H.L., Lilenbaum, W., Machado, G., Tonin, A.A., Baldissera, M.D., Stefani, L.M. and Da Silva, A.S. 2017. Bovine leptospirosis: prevalence, associated risk factors for infection and their cause-effect relation. Microb. Pathog. 107, 149–154. FEDEGAN. 2018. Ganadería colombiana: hoja de ruta 2018–2022. Bogotá, Colombia: Federación Colombiana de Ganaderos (FEDEGAN). Available via http://static.fedegan.org.co.s3.amazonaws.com/publicaciones/Hoja_de_ruta_Fedegan.pdf. (Accessed 11 July 2022). García, A.A., Torreglosa, J.T., Marín, D.D., Bernal, M.K.M., Filho, S.T.R. and Pereira, W.L.A. 2021. Leptospirosis, bovine viral diarrhea and infectious bovine rhinotracheitis: prevalence in Colombian cattle and buffaloes. Acta. Sci. 44, 1–8; doi: 10.4025/actascianimsci.v44i1.54875. Givens, M.D. 2018. Review: risks of disease transmission through semen in cattle. Animal 12, 165–171. Gilbert, R.O. 2018. Reproductive diseases. In Rebhun’s diseases of dairy cattle, 3rd ed. Eds., Peek, S.F. and Divers, T.J. st.Louis, MO: ELSEVIER, pp: 466–507. Gómez Reynoso, M. 2005. Determinación de anticuerpos maternos transferidos a becerros nacidos de vacas vacunadas contra leptospirosis en un rancho de Atilaquia, Hidalgo, Bachelor thesis, Universidad Nacional Autonoma de Mexico, Cautitlan, México. Grippi, F., Giudice, E., Di Pietro, S., Sciacca, C., Santangelo, F., Galluzzo, P., Barreca, S. and Guercio, A. 2020. Leptospira interrogans serogroup sejroe serovar hardjo in aborting cows: two herd cases in Sicily (Italy). J. Vet. Res. 64, 73–78. Higino, S.S.S., Santos, F.A., Costa, D.F., Santos, C.S.A.B., Silva, M.L.C.R., Alves, C.J. and Azevedo, S.S. 2013. Flock-level risk factors associated with leptospirosis in dairy goats in a semiarid region of Northeastern Brazil. Prev. Vet. Med. 109, 158–161. Hurtado, C.B., Uribe, A.O. and Tous, M.G. 2013. Seroepidemiología de la leptospirosis en bovinos con trastornos reproductivos en el municipio de Montería, Colombia. Rev. Med. Vet. 1, 47. Khalili, M., Sakhaee, E., Afatoonian, M.R., Abdollahpour, G., Tabrizi, S.S., Damaneh, E.M. and Hossini-nasab, S. 2014. Seroprevalence of bovine leptospiral antibodies by microscopic agglutination test in Southeast of Iran. Asian. Pac. J. Trop. Biomed. 4, 354–357. Levett, P.N. 2015. Systematics of leptospiraceae. Curr. Top. Microbiol. Immunol. 387, 11–20. Llanco, A.L., Suárez, A.F., Huanca, L.W. and Rivera, G.H. 2017. Frecuencia y Riesgo de Infección de Leptospirosis Bovina en Dos Establos Lecheros de la Costa y Sierra Peruana. Rev. Investig. Vet. Perú. 28, 696–702. Loureiro, A.P., Pestana, C., Medeiros, M.A. and Lilenbaum, W. 2017. High frequency of leptospiral vaginal carriers among slaughtered cows. Anim. Reprod. Sci. 178, 50–54. Loureiro, A.P. and Lilenbaum, W. 2020. Genital bovine leptospirosis: a new look for an old disease. Theriogenology 141, 41–47. Marianelli, C., Tarantino, M., Astarita, S., Martucciello, A., Capuano, F. and Galiero, G. 2007. Molecular detection of Leptospira species in aborted fetuses of water buffalo. Vet. Rec. 161, 310–312. Martins, G. and Lilenbaum, W. 2017. Control of bovine leptospirosis: aspects for consideration in a tropical environment. Res. Vet. Sci. 112, 156–160. Martins, G., Penna, B. and Lilenbaum, W. 2012. Differences between seroreactivity to leptospirosis in dairy and beef cattle from the same herd in Rio de Janeiro, Brazil. Trop. Anim. Health. Prod. 44, 377–378. Menezes, A.T., de Contador, T.L., Rodrigues, R.V., Barcelos, F., Bonadia, G.O., Souza, M.I.L. and Patelle, T.H.C. 2006. A Leptospirose e seus efeitos na reprodução. Rev. Científ. Eletrôn. Med. Vet. 3, 1–4. Moreno, F.G., Benavides, O.E., Guerrero, B. and Cruz, C.A. 2017. Asociación entre Seropositividad al Virus de la Diarrea Viral Bovina, Leptospira interrogans y Neospora caninum, y la Ocurrencia de Abortos en Fincas de Pequeños Productores del Cordón Lechero de Boyacá, Colombia. Rev. Investig. Vet. Perú. 28, 1002–1009. Motta, G.J.L., Clavijo, H.J.A., Waltero, G.I. and Abeledo, M.A. 2014. Prevalencia de anticuerpos a Brucella abortus, Leptospira sp. y Neospora caninum en hatos bovinos y bubalinos en el departamento de Caquetá, Colombia. Rev. Salud. Anim. 36, 80–89. Mughini-Gras, L., Bonfanti, L., Natale, A., Comin, A., Ferronato, A., La Greca, E., Patregnani, T., Lucchese, L. and Marangon, S. 2014. Application of an integrated outbreak management plan for the control of leptospirosis in dairy cattle herds. Epidemiol. Infect. 142, 1172–1181. Mullan, S. and Panwala, T.H. 2016. Polymerase chain reaction: an important tool for early diagnosis of leptospirosis cases. J. Clin. Diagn. Res. 10, DC08–DC11. Newcomer, B.W. and Givens, D. 2016. Diagnosis and control of viral diseases of reproductive importance: infectious bovine rhinotracheitis and bovine viral diarrhea. Vet. Clin. North. Am. Food. Anim. Pract. 32(2), 425–441. OIE. 2021. Chapeter 3.1.1.2 leptospirosis. In Terrestrial Manual OIE. 1–13. https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.01.12_LEPTO.pdf de Oliveira, P.R.F., Soares, L.B.F., Borges, J.de.M., Barrosa, N.de.C., Langoni, H., Brandespim, D.F., Junior, J.W.P. and Mota, R.A. 2018. Occurrence of serological reactions for serogroup Sejroe (CTG and Prajtino) in female buffalo in the state of Pernambuco, Brazil. Braz. J. Microbiol. 49, 795–800. Olmo, L., Reichel, M.P., Nampanya, S., Khounsy, S., Wahl, L.C., Clark, B.A., Thomson, P.C., Windsor, P.A. and Bush, R.D. 2019. Risk factors for neospora caninum, bovine viral diarrhoea virus, and Leptospira interrogans serovar hardjo infection in smallholder cattle and buffalo in Lao PDR. PLoS One 14, 1–25. Ospina-Pinto, M.C. and Hernández Rodríguez, P. 2015. Utilidad de las herramientas moleculares para la identificación de Leptospira spp. en muestras humanas, animales y ambientales. Rev. Cubana. Med. Trop. 67(3), ISSN 0375–0760. Pulido-Medellín, M., Díaz-Anaya, A. and Giraldo-Forero, J. 2017. Determinación de Leptospira spp. en humanos y bovinos pertenecientes al municipio de Toca, Boyacá. Vet. Zoo. 11, 55–66. Rebhum, W. 1999. Enfermedades del ganado vacuno lechero, 1st ed. Zaragoza, Spain: Acribia. Ruano, M.P., Macías, D.I.B., Goicochea, C.A.B., Aguayo, M.D.Z., Valencia, H.P.S., Flores, M.A.F., Loor, L.A.V., Ruales, A.P.R. and Fonseca-Rodríguez, O. 2020. Seroprevalence and risk factors of bovine leptospirosis in the province of Manabí, Ecuador. Comp. Immunol. Microbiol. Infect. Dis. 72, 101527. Szwako, A., Acuña, L., Rolón, C., Glatzle, F., Lemkemeyer, C., Unger, N. and Wiebe, J. 2015. Seroprevalencia De Leptospirosis Bovina En El Chaco Central, Departamento De Boqueron, Paraguay. Compend. Cienc. Vet. 5, 26–30. Torres-Castro, M., Hernández-Betancourt, S., Agudelo-Flórez, P., Arroyave-Sierra, E., Zavala-Sierra, J. and Puerto, F.I. 2018. Leptospirosis: enfermedad zoonótica endémica en América. Salud (i) Ciencia 22, 778–780. Vincent, A.T., Schiettekatte, O., Goarant, C., Neela, V.K., Bernet, E., Thibeaux, R., Ismail, N., Khalid, M.K.N.M., Amran, F., Masuzawa, T., Nakao, R., Korba, A.A., Bourhy, P., Veyrier, F.J. and Picardeau, M. 2019. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLOS. Negl. Trop. Dis. 13, 1–25. Washburn, K.E. 2020. Management of pathogens in cattle. In Animal agriculture: sustainability, challenges and innovations. Eds., Bazer, F.W., GuoYao, W. and Lamb, G.C. Amsterdam, Netherlands: Elsevier Inc, pp: 493–499. | ||
| How to Cite this Article |
| Pubmed Style Castañeda DMB, Buitrago HAL, Buitrago DJL, Anaya AMD, Corredor DJG, Torreglosa JCT, Ortega DO, Medellin MOP. Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia. Open Vet. J.. 2022; 12(5): 668-675. doi:10.5455/OVJ.2022.v12.i5.11 Web Style Castañeda DMB, Buitrago HAL, Buitrago DJL, Anaya AMD, Corredor DJG, Torreglosa JCT, Ortega DO, Medellin MOP. Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia. https://www.openveterinaryjournal.com/?mno=34915 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i5.11 AMA (American Medical Association) Style Castañeda DMB, Buitrago HAL, Buitrago DJL, Anaya AMD, Corredor DJG, Torreglosa JCT, Ortega DO, Medellin MOP. Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia. Open Vet. J.. 2022; 12(5): 668-675. doi:10.5455/OVJ.2022.v12.i5.11 Vancouver/ICMJE Style Castañeda DMB, Buitrago HAL, Buitrago DJL, Anaya AMD, Corredor DJG, Torreglosa JCT, Ortega DO, Medellin MOP. Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia. Open Vet. J.. (2022), [cited January 25, 2026]; 12(5): 668-675. doi:10.5455/OVJ.2022.v12.i5.11 Harvard Style Castañeda, D. M. B., Buitrago, . H. A. L., Buitrago, . D. J. L., Anaya, . A. M. D., Corredor, . D. J. G., Torreglosa, . J. C. T., Ortega, . D. O. & Medellin, . M. O. P. (2022) Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia. Open Vet. J., 12 (5), 668-675. doi:10.5455/OVJ.2022.v12.i5.11 Turabian Style Castañeda, Diana María Bulla, Henry Alexander Lopez Buitrago, Deisy Johana Lancheros Buitrago, Adriana Maria Diaz Anaya, Diego Jose Garcia Corredor, Julio Cesar Tobón Torreglosa, Diego Ortiz Ortega, and Martin Orlando Pulido Medellin. 2022. Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia. Open Veterinary Journal, 12 (5), 668-675. doi:10.5455/OVJ.2022.v12.i5.11 Chicago Style Castañeda, Diana María Bulla, Henry Alexander Lopez Buitrago, Deisy Johana Lancheros Buitrago, Adriana Maria Diaz Anaya, Diego Jose Garcia Corredor, Julio Cesar Tobón Torreglosa, Diego Ortiz Ortega, and Martin Orlando Pulido Medellin. "Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia." Open Veterinary Journal 12 (2022), 668-675. doi:10.5455/OVJ.2022.v12.i5.11 MLA (The Modern Language Association) Style Castañeda, Diana María Bulla, Henry Alexander Lopez Buitrago, Deisy Johana Lancheros Buitrago, Adriana Maria Diaz Anaya, Diego Jose Garcia Corredor, Julio Cesar Tobón Torreglosa, Diego Ortiz Ortega, and Martin Orlando Pulido Medellin. "Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia." Open Veterinary Journal 12.5 (2022), 668-675. Print. doi:10.5455/OVJ.2022.v12.i5.11 APA (American Psychological Association) Style Castañeda, D. M. B., Buitrago, . H. A. L., Buitrago, . D. J. L., Anaya, . A. M. D., Corredor, . D. J. G., Torreglosa, . J. C. T., Ortega, . D. O. & Medellin, . M. O. P. (2022) Seroprevalence and risk factors associated with the presence of bovine leptospirosis in the municipality of Sotaquirá, Colombia. Open Veterinary Journal, 12 (5), 668-675. doi:10.5455/OVJ.2022.v12.i5.11 |