| Case Report | ||
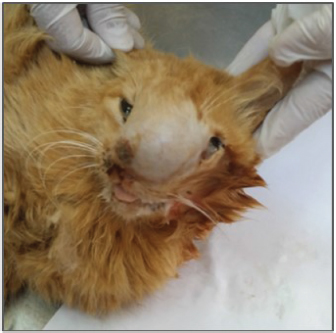
Open Vet. J.. 2019; 9(4): 331-334 doi: 10.4314/ovj.v9i4.10 Open Veterinary Journal, (2019), Vol. 9(4): 331–334 Case Report DOI: http://dx.doi.org/10.4314/ovj.v9i4.10 Clinical, radiological, and pathological findings of primary nasal osteosarcoma in a Libyan catMohamed H. Abushhiwa1*, Seham A. Al-Azreg2, Samer K. Tmumen1, Abdulrhman M. Alrtib3, Abdulkareem K. Elbaz4, Mahir A. Kubba2, Al-Asayed R. Al-Attar2, and Emad M. Bennour51Department of Surgery and Theriogenology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 2Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 3Department of Anatomy, Histology and Embryology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 4Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 5Department of Internal Medicine, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Mohamed H. Abushhiwa. Department of Surgery and Theriogenology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: m.abushhiwa [at] uot.edu.ly Submitted: 21/04/2019 Accepted: 23/11/2019 Published: 20/12/2019 © 2019 Open Veterinary Journal AbstractBackground: Although bone tumors are common pathologies in companion animals, limited reports describe nasal osteosarcoma (OSA) in cats. Case description: A case of nasal OSA in a local Libyan cat was admitted to the Veterinary Teaching Hospital at the Faculty of Veterinary Medicine, University of Tripoli–Libya, with nasal swelling and discharges and facial deformity. The radiological findings revealed nasal osteolysis with the absence of evidence of lung metastasis. In addition, fungal growth was not identified in microbiological culture. Furthermore, the pathological examination has grossly revealed a destructed nasal bone due to the presence of a tumor mass, with a mucohemorrhagic nasal discharge and absence of metastasis. OSA was confirmed histopathologically. Conclusion: This report presents the clinical, radiological, and pathological findings of a primary nasal OSA in a Libyan cat with no tumor metastasis to other body organs. Keywords: Cat, Nasal cavity, Primary osteosarcoma. IntroductionOsteosarcoma (OSA) is a malignant bone neoplasm. Although it is the most commonly reported primary bone neoplasm in cats (Durham et al., 2008; Liu et al., 1974), it is rarely encountered clinically (Heldmann et al., 2000). Malignant neoplasms of the nasal cavity represent around 1%–5% of feline neoplasms (Mellanby et al., 2002). These neoplasms include lymphosarcoma (most common), fibrosarcoma, chondrosarcoma, OSA, hemangiosarcoma, and undifferentiated sarcoma (Mellanby et al., 2002; Moore and Ogilvie, 2001). Clinically, nasal neoplasms are characterized by sneezing, epistaxis, facial and oral deformity, exophthalmos, and neurological signs (Mellanby et al., 2002). OSA could be of medullary, axial, or extraskeletal origin (Heldmann et al., 2000). Feline medullary OSA occurs in the appendicular skeleton, and the axial OSA originates most commonly in the skull, pelvis, ribs, or vertebrae (Bitetto et al., 1987; O’Brien et al., 1980). The extraskeletal OSA has been reported in ocular (Groskopf et al., 2010), mammary (Heldmann et al., 2000), and duodenal (Stimson et al., 2000) tissues and as postvaccination tumor (Esplin et al., 1993). In contrast to the appendicular OSA, the medical management of feline axial OSA mostly carries poor prognosis because of the difficulty of surgical resection of the tumor mass due to its location and local invasion (Heldmann et al., 2000). Limited reports have described OSA as an intranasal tumor (Malinowski, 2006). This report presents the clinical, radiological, and pathological findings of a primary nasal OSA in a Libyan cat with no tumor metastasis to other body organs. Case DetailsA female eight-year-old local Libyan cat was admitted to the Veterinary Teaching Hospital at the Faculty of Veterinary Medicine, University of Tripoli–Libya. The cat presented with a soft swelling in the rostral part of the skull and anorexia. A thorough clinical examination was carried out. The radiographical examination was performed to investigate bone involvement, and the right lateral view of the skull was acquired. In addition, a lateral chest radiograph was taken to investigate the presence of lung metastasis. Nasal swabs were collected from the nasal cavity and submitted for microbiological examination to investigate a fungal or bacterial infection. The decision for euthanasia was made, and the cadaver was submitted for postmortem and histopathological examinations. The clinical examination revealed that there was a severe soft swelling involving the left craniorostral area of the skull causing pressure and deforming the left orbital area (see Fig. 1). In addition, there was malodorous, mucohemorrhagic nasal discharge with severe dyspnea and stridor. The auscultation did not reveal any abnormal lung sounds. No signs of other body systems involvement were noticed.
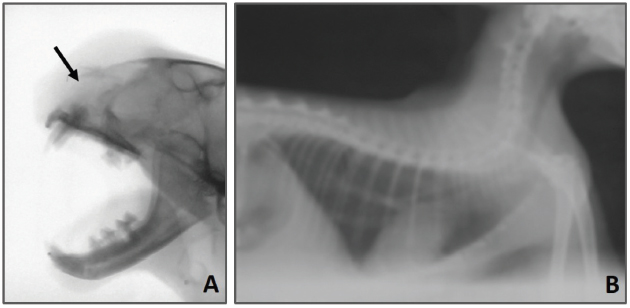
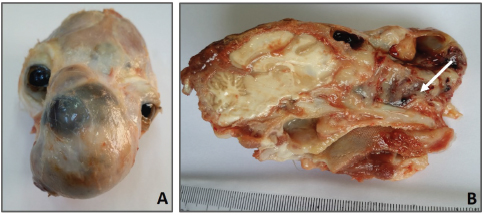
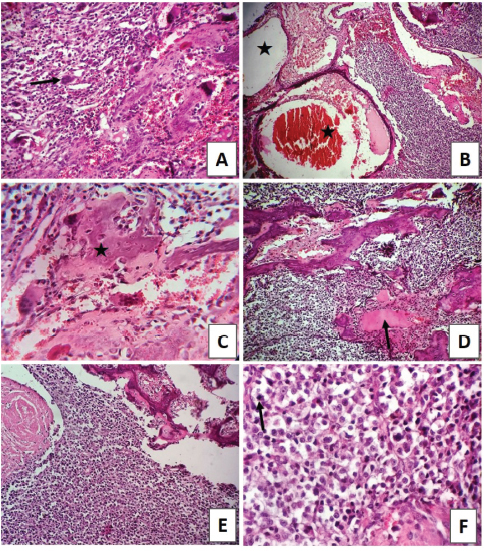
Fig. 1. Facial swelling involving the left craniorostral area of the skull causing pressure and deforming the left eye area. Open mouth for breathing is obvious due to nasal obstruction. The radiographic examination of the skull revealed that there was bone lysis involving the nasal bone with no evidence of bony proliferation (see Fig. 2A). In addition, the thorax radiograph showed no signs of tumor metastasis to the lung (see Fig. 2B). On the microbiological examination, the culture plates showed no fungi or yeast growth on Sabouraud Dextrose Agar. However, there was bacterial growth on blood and MacConkey agar. The isolates were Gram-negative rods, lactose fermenting on MacConkey agar, and oxidase negative. Analytical Profile Index (API) 20E biochemical reactions showed the characteristics of Enterobacter species (data not shown). At necropsy, the rostral part of the head was enlarged (see Fig. 3A). In the skull section, there were nasal deformity and partial bilateral obstruction of nostrils due to the presence of a tumoral mass with a mucohemorrhagic exudate as well as a proliferation of soft tissue, part of the tumor, involving the nasal structures. This caused bone deviation from right to left, affecting the eyeballs (see Fig. 3B). Blood-filled multilocular cavities of different sizes and shapes were present. No signs of metastasis were observed in the thoracic and abdominal cavities, cervical lymph nodes, lung, liver, or kidney (data not shown). The histopathological examination has revealed the characteristics of poorly differentiated OSA. The tissue sections showed highly proliferating anaplastic mesenchymal cells in different shapes and types with pronounced osteogenic properties, where different stages of bony differentiation were seen. Blood cysts and spaces of variable shape and size were seen throughout the section. In addition, osteoclast-like tumor giant cells, angiogenesis, cartilaginous metamorphosis, and multiple necrotic areas were detected (see Fig. 4). No relevant microscopic lesions were noticed in other major body organs.
Fig. 2. (A) Lateral radiographic view of the skull showing an osteolysis involving the nasal bone (arrow). (B) Lateral radiographic view of thorax revealing the absence of evidence of metastasis.
Fig. 3. (A) Presence of a tumoral mass on the rostral part of the skull. (B) Lateral skull section showing nasal bone destruction and nasal obstruction due to the presence of tumor mass and mucohemorrhagic nasal discharges (arrow). Lesions were absent in the brain or buccal tissues.
Fig. 4. (A) Irregular neoplastic osteoid tissue with osteoclast-like tumor giant cells (arrow) (H&E X100). (B) Large cavernous blood-filled spaces surrounded with neoplastic cells (star) (H&E X100). (C) Irregular neoplastic osteoid masses (star) surrounded by poorly differentiated hyperchromatic polygonal or fusiform cells (H&E X400). (D) Pleomorphic neoplastic osteogenic cells with irregular homogeneous eosinophilic masses of osteoid tissue (arrow) (H&E X100). (E) Large tumor mass of atypical highly undifferentiated cells with dark round to oval nuclei having little cytoplasm. There is osteoid formation by the tumor cells (H&E X100). (F) Individual neoplastic cells with variable degrees of pleomorphism, moderate hyperchromacia, and mitotic figures (arrow) (H&E X400). DiscussionReports describing nasal OSA in cats are limited. This makes it interesting to present the current case of feline nasal OSA. Clinically, the major signs seen in the affected cat were a severe soft swelling involving the left craniorostral area of the skull which causing pressure and deforming the left orbital area, malodorous, mucohemorrhagic nasal discharge with severe dyspnea and stridor, and absence of abnormal lung sounds. This is well correlating with the classical clinical presentation of cases of OSA in cats (Mellanby et al., 2002). OSA is radiographically characterized by bony proliferation, lysis, or both (Thrall, 2017). In the current case, OSA was osteolytic with no radiographic evidence of bone proliferation. In addition, no radiographic evidence of tumor metastasis to the lung was identified. Because bone lysis of the nasal cavity may be attributed to the primary bone tumor or mycotic lesions (Thrall, 2017), a further microbiological investigation was carried out to explore the presence of fungal infection. This investigation did not reveal fungal infection but bacterial growth. Infection and bacterial growth were considered as a secondary infection. As the prognosis of nasal OSA is poor (Heldmann et al., 2000), the decision for euthanasia was made and both postmortem and histopathological examinations were performed. Grossly, nasal bone lysis was present with no involvement of nearby cranial or buccal tissues nor other body organs. These findings are similar to those previously reported, which indicate that the tendency of feline OSA to metastasize is very low due to the nonaggressive nature of this type of tumor in cats (Heldmann et al., 2000). However, this low capacity to produce metastases does not rule out a high local aggressiveness in this case and not very prolonged survival time in feline cases of OSA (Heldmann et al., 2000). To conclude, this report describes the clinical, radiological, microbiological, and pathological findings of a case of feline nasal osteolytic OSA with no metastasis. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionMohamed H. Abushhiwa, Samer K. Tmumen, and Abdulrhman M. Alrtib carried out the radiological assessment. Abdulkareem K. Elbaz carried out the microbiological examination. Seham A. Al-Azreg, Mahir A. Kubba, and Al-Asayed R. Al-Attar carried out the postmortem and histopathological examinations. Emad M. Bennour carried out the clinical assessment. Mohamed H. Abushhiwa and Emad M. Bennour contributed largely, among all other authors, to the writing and the revision of this manuscript. ReferencesBitetto, W.V., Patnaik, A.K., Schrader, S.C. and Mooney, S.C. 1987. Osteosarcoma in cats: twenty-two cases (1974–1984). J. Am. Vet. Med. Assoc. 190, 91–93. Durham, A.C., Popvitch, C.A. and Goldschmidt, M.H. 2008. Feline chondrosarcoma: a retrospective study of 67 cats (1987–2005). J. Am. Anim. Hosp. Assoc. 44, 124–130. Esplin, D.G., McGill, L.D., Meininger, A.C. and Wilson, S.R. 1993. Postvaccination sarcomas in cats. J. Am. Vet. Med. Assoc. 8, 1245–1246. Groskopf, B.S., Dubielzig, R.R. and Beaumont, S.L. 2010. Orbital extraskeletal osteosarcoma following enucleation in a cat: a case report. Vet. Ophthalmol. 13, 179–183. Heldmann, E., Anderson, M.A. and Wagner-Mann, C. 2000. Feline osteosarcoma: 145 cases (1990–1995). J. Am. Anim. Hosp. Assoc. 36, 518–521. Liu, S., Dorfman, H.D. and Patnaik, A.K. 1974. Primary and secondary bone tumours in the cat. J. Small Anim. Pract. 15, 141–156. Malinowski, C. 2006. Canine and feline nasal neoplasia. Clin. Tech. Small Anim. Pract. 21, 89–94. Mellanby, R.J., Herrtage, M.E. and Dobson, J.M. 2002. Long-term outcome of eight cats with non-lymphoproliferative nasal tumours treated by megavoltage radiotherapy. J. Feline Med. Surg. 4, 77–81. Moore, A.S. and Ogilvie, G.K. 2001. Tumors of the respiratory tract. In Feline oncology: a comprehensive guide to compassionate care. Eds., Ogilvie, G.K. and Moore, A.S. Trenton, NJ: Veterinary Learning Systems, pp: 368–384. O’Brien, D., Parker, A.J. and Tarvin, G. 1980. Osteosarcoma of the vertebra causing compression of the thoracic spinal cord in a cat. J. Am. Anim. Hosp. Assoc.16, 497–499. Stimson, E.L., Cook, W.T., Smith, M.M., Dru Forrester, S., Moon, M.L. and Saunders, G.K. 2000. Extraskeletal osteosarcoma in the duodenum of a cat. J. Am. Anim. Hos p. Assoc. 36, 332–336. Thrall, D. 2017. Textbook of veterinary diagnostic radiology, 7th ed., Elsevier, St. Louis, Missouri, USA. | ||
| How to Cite this Article |
| Pubmed Style Abushhiwa MH, Azreg SA, Tmumen SK, Alrtib AM, Elbaz AK, Kubba MA, Attar AARA, , Bennour EM. Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat. Open Vet. J.. 2019; 9(4): 331-334. doi:10.4314/ovj.v9i4.10 Web Style Abushhiwa MH, Azreg SA, Tmumen SK, Alrtib AM, Elbaz AK, Kubba MA, Attar AARA, , Bennour EM. Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat. https://www.openveterinaryjournal.com/?mno=42113 [Access: January 26, 2026]. doi:10.4314/ovj.v9i4.10 AMA (American Medical Association) Style Abushhiwa MH, Azreg SA, Tmumen SK, Alrtib AM, Elbaz AK, Kubba MA, Attar AARA, , Bennour EM. Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat. Open Vet. J.. 2019; 9(4): 331-334. doi:10.4314/ovj.v9i4.10 Vancouver/ICMJE Style Abushhiwa MH, Azreg SA, Tmumen SK, Alrtib AM, Elbaz AK, Kubba MA, Attar AARA, , Bennour EM. Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat. Open Vet. J.. (2019), [cited January 26, 2026]; 9(4): 331-334. doi:10.4314/ovj.v9i4.10 Harvard Style Abushhiwa, M. H., Azreg, . S. A., Tmumen, . S. K., Alrtib, . A. M., Elbaz, . A. K., Kubba, . M. A., Attar, . A. A. R. A., , . & Bennour, . E. M. (2019) Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat. Open Vet. J., 9 (4), 331-334. doi:10.4314/ovj.v9i4.10 Turabian Style Abushhiwa, Mohamed H, Seham Al Azreg, Samer K Tmumen, Abdulrhman M Alrtib, Abdulkareem K Elbaz, Mahir A Kubba, Al Asayed R Al Attar, , and Emad M Bennour. 2019. Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat. Open Veterinary Journal, 9 (4), 331-334. doi:10.4314/ovj.v9i4.10 Chicago Style Abushhiwa, Mohamed H, Seham Al Azreg, Samer K Tmumen, Abdulrhman M Alrtib, Abdulkareem K Elbaz, Mahir A Kubba, Al Asayed R Al Attar, , and Emad M Bennour. "Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat." Open Veterinary Journal 9 (2019), 331-334. doi:10.4314/ovj.v9i4.10 MLA (The Modern Language Association) Style Abushhiwa, Mohamed H, Seham Al Azreg, Samer K Tmumen, Abdulrhman M Alrtib, Abdulkareem K Elbaz, Mahir A Kubba, Al Asayed R Al Attar, , and Emad M Bennour. "Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat." Open Veterinary Journal 9.4 (2019), 331-334. Print. doi:10.4314/ovj.v9i4.10 APA (American Psychological Association) Style Abushhiwa, M. H., Azreg, . S. A., Tmumen, . S. K., Alrtib, . A. M., Elbaz, . A. K., Kubba, . M. A., Attar, . A. A. R. A., , . & Bennour, . E. M. (2019) Clinical, radiological and pathological findings of non-metastatic nasal osteosarcoma in a Libyan cat. Open Veterinary Journal, 9 (4), 331-334. doi:10.4314/ovj.v9i4.10 |