| Original Article | ||
Open Vet. J.. 2021; 11(3): 370-378 Open Veterinary Journal, (2021), Vol. 11(3): 370–378 Original Research Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, BrazilHenrique M. Freitas1, André T. Somma1, Bret A. Moore2 and Fabiano Montiani-Ferreira1*1Veterinary Medicine Department, Comparative Ophthalmology Laboratory (LABOCO), Federal University of Paraná, Curitiba, Brazil 2Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL 32608, USA *Corresponding Author: Fabiano Montiani-Ferreira. Comparative Ophthalmology Laboratory (LABOCO), Veterinary Medicine Department, Federal University of Paraná, Rua dos Trabalhadores, Curitiba, Brazil. Email: montiani [at] ufpr.br Submitted: 13/01/2021 Accepted: 02/07/2021 Published: 22/07/2021 © 2021 Open Veterinary Journal
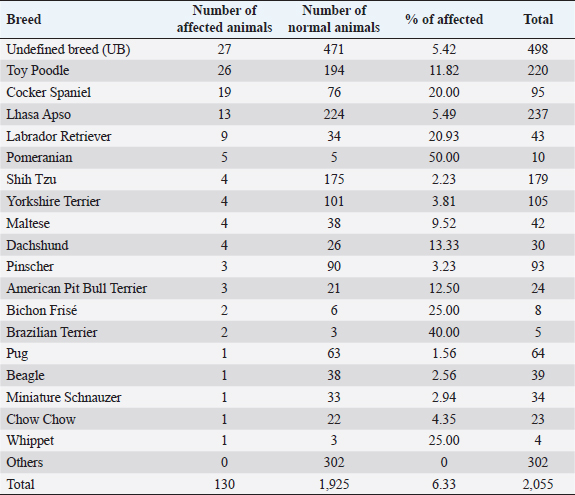
AbstractBackground: Progressive retinal atrophy (PRA) is one of the main causes of blindness in dogs. Despite its clinical importance, there is limited epidemiological information available, particularly in South America. Aim: The main objective of this study was to perform a retrospective, and prospective analysis of PRA in dogs admitted at the Veterinary Hospital of the Federal University of Paraná, Brazil. Methods: Medical records of dogs admitted between 2014 and 2018 were selected through the archives of the Comparative Ophthalmology Laboratory. A total of 130 dogs with medical records indicating clinical signs suggestive of PRA, independent of the electroretinography confirmation, were selected. In order to investigate common characteristics, each patient’s clinical history, ophthalmic examination, and visual status were reviewed (obstacle course, pupillary light reflex, dazzle reflex, visual tracking to a cotton ball, and menace responses). Additionally, a prospective study was performed, where flash electroretinography was performed on 30 animals with clinical signs suggestive of PRA, and 14 animals were selected for fundus photography. Data were assessed through descriptive and inferential statistics. Results: A total of 2,055 dogs were evaluated between January 2014 and December 2018. Of those, 130 animals were presumptively diagnosed with PRA (6.33%), consisting of 18 different breeds and 27 dogs with a mixed pedigree. Poodles were the most prevalent breed (n=26; 20.00%), followed by Cocker Spaniels (n=19; 14.62%). In the reported caseload, Pomeranians showed a considerably higher odds ratio for PRA development (15.36%). Conclusion: Pomeranians presented a high odds ratio, suggesting that further studies may be performed with breeds with a high potential for developing this disease. Keywords: PRA, ERG, Retinal dystrophy. IntroductionRetinal dystrophies are a group of inherited diseases that can cause blindness in humans and animals. Progressive retinal atrophy (PRA) is a group of inherited diseases characterized by progressive loss of photoreceptors and atrophy of the retina secondary to gene mutations (Magnusson, 1911; Petersen-Jones, 1998a), and it is one of the main types of retinal dystrophies affecting domestic animals. PRAs were first described in dogs in 1911 by Magnusson in Gordon Setters in Sweden but has also been reported in pigs (Li et al., 1998), sheep (Banin et al., 2015), horses (Winkler et al., 2020), non-human primates (Winkler et al., 2020), laboratory animals (Huang et al., 1993; Naash et al., 1993), and several breeds of cats (Rubin and Lipton, 1973, Giuliano and van DerWoerdt, 1999, Narfström et al., 2009). Since first reported in Gordon Setters, more than 100 breeds of dogs have been described with various PRAs (Petersen-Jones, 1998b; Petersen-Jones, 2005; Beltran, 2009). Histological and genetic analyzes have enabled PRAs to be further classified into different categories, most broadly based on whether the rods or cones are primarily affected and by the age of onset. Early-onset forms of PRAs are usually caused by a developmental abnormality of the photoreceptors (i.e., dysplasias) and are characterized by the onset of clinical signs prior to 1 year of age and rapid progression. Late-onset forms occur after normal maturation of the photoreceptors (i.e., degenerations) and affect middle-aged or older dogs, such as progressive rod-cone degeneration (PRCD) (Petersen-Jones, 2005). Clinically, PRAs are characterized by the bilateral degeneration of the retina, causing progressive vision loss culminating in blindness, similar to retinitis pigmentosa, a homologous group of retinal degenerative disorders that are important causes of blindness in humans (Petersen-Jones, 1998a; Dias et al., 2018). Across breeds, nyctalopia (or night-blindness) is the most common initial presenting clinical sign, although initial presentation with more advanced signs is not uncommon, such as mydriasis, bright eye-shine, secondary cataract formation, and loss of daytime vision and/or total blindness(Whitley et al., 1995; Petersen-Jones, 1998a; Petersen-Jones, 2005). Diagnosis is based primarily on history and clinical signs, with electroretinography (ERG) being the definitive test. ERG usually changes precedes funduscopic changes. Progression of clinical signs varies with breeds and form of PRA, but PRAs culminate in blindness (Petersen-Jones,1998a; Petersen-Jones, 2005). Up until now, no therapeutic approach to limit progression or reverse vision loss associated with PRAs has been developed that is widely accepted, accessible, or feasible. However, gene therapy is showing promise in both veterinary and human ophthalmology (Petersen-Jones, 2012; Al-Saikhan, 2013; Apte, 2018; Takahashi et al., 2018; Arbabi et al., 2019). Studying domestic veterinary species with natural forms in PRAs has been pivotal in developing our understanding of the genetic basis of PRAsand new therapeutic strategies that may minimize the occurrence in both human and animal populations (Beltran, 2009; Petersen-Jones, 2012; Zeiss, 2013). Previous studies have focused primarily on the clinical features and prevalence of PRAs in a specific breed or small group of breeds or have evaluated in detail the genetic aspects and molecular biology of a particular form of PRA. However, aspects including breed distribution have not been described. Considering the paucity of data information on PRAs in Brazil, the main objective of the present study was to evaluate the presence of this group of diseases and describe the nature of PRAs across the population of dogs presenting to the Laboratory of Comparative Ophthalmology (LABOCO) of the Veterinary Hospital of the Federal of Paraná (UFPR), as a means to identify new patterns of the disease and that may contribute to more objective studies in the future. Materials and MethodsData from 2,055 dogs presenting to the LABOCO with or without signs of retinal degeneration consistent with PRAs were collected by a clinic management system software (Vetus®, São Paulo, SP, Brazil) and through medical records of animals admitted between January 2014 and December 2018, regardless of diagnosis. Information about the breed, age, sex, presence of concomitant systemic and ocular diseases, presence of visual impairment or nyctalopia, ophthalmoscopy signs of retinal degeneration, presence of cataracts suspected to be secondary to retinal degeneration (due to clinical appearance, clinical history evidence, and ERG results), and personal data of the owners of the animals were collected and transcribed into an Excel spreadsheet (Microsoft®, Redmond, WA) for retrospective analysis. At each visit, a thorough ophthalmic examination was performed. Anterior segment exam was performed with a portable slit lamp (Hawk-Eye, Dioptrix, L`Union, France). Vision testing was assessed by the ability to track falling cotton balls and the ability to negotiate an obstacle course in both photopic and scotopic conditions. Neurophthalmicparameters tested included the direct and consensual pupillary light reflex (PLR) and dazzle reflex using a 3.5 V Finoff halogen fiber optic transilluminator (Welch Allyn®, Skaneateles Falls, NY), as well as the menace response. Indirect ophthalmoscopy was performed after inducing mydriasis using 1% tropicamide (Mydriacyl®, Novartis®, São Paulo, SP, Brazil) with a 3.5 V Finoff halogen fiber optic transilluminator (Welch Allyn®, Skaneateles Falls, NY) and a 20D lens (OPTOMED OY LTD., Finland). In 14 dogs without advanced cataracts or nuclear sclerosis, fundus photography was performed using a ClearViewfundus camera (ClearView Optical Imaging System Optibrand, Ft Collins, CO). By nature of a retrospective analysis, not all animals had complete data recorded, including vision testing, in the medical record. Therefore, the resulting percentages reflect the number of eyes examined by each technique, and that was entered in the patient’s record. ERG was performed on a total of 26 dogs within the study period and 4 dogs within the LABOCO archives, all of which had visual impairment with signs of retinal degeneration and/or cataract. Dogs with other causes of abnormal ERGs, such as posterior uveitis, retinal detachment, sudden acquired retinal degeneration syndrome (SARDS), and glaucoma, were excluded. The dogs were prepared for ERG with the instillation of a drop of Tropicamide 1% (Mydriacyl; AlconTM, São Paulo, SP, Brazil) combined with Phenylephrine 10% (Allergan, Guarulhos, SP, Brazil) in each eye every 5 minutes until complete mydriasis was achieved. Following mydriasis, dogs were dark-adapted for 20 minutes. A mini portable Ganzsfield (HMsERG model 1000, Ocuscience®, NV) was used to perform the flash electroretinogram. ERGs were performed using manual restraint without the use of anesthesia or sedation. Animals were placed in sternal recumbency with the active electrode (ERG-Jet, Fabrinal SA, La Chaux-de-Fonds, Switzerland) positioned on the cornea, and hypodermic platinum needles (Model E2, Grass Technologies, Warvick, NY) positioned as reference and ground electrodes. The reference electrode was positioned about 2 cm from the lateral canthus, and the ground electrode was positioned at the base of the neck. The protocol used was a short protocol, consisting of light flash intensities of 10, 3,000, and 10,000 mcd.s/m2, based on the protocol described in Somma et al. (2016). A-wave and b-wave amplitudes, and implicit times (ITs), were measured by ERGVIEW 4.380V software (Ocuscience®, NV), and the data generated were analyzed using descriptive and inferential statistics. A Shapiro–Wilk test was used to assess data normality. For the sex-related data, the Chi-square test was used. The mean age of onset of the disease was compared in each breed by an unpaired Student’s t-test. Both analyses used a significance level of p < 0.05 and were performed using the GraphpadQuickCalcs statistical software (Graphpad Software Inc., La Jolla, CA). The analysis of non-normal data, such as ERG implicit time, was performed through the Mann–Whitney test (p < 0.05). The odds ratio or the odds of a breed having PRA compared to the total population presenting to the LABOCO for PRA development in each breed was calculated by the MEDCALC® software (MedCalc Software bvba, Ostend, Belgium). The odds ratio values were considered significant when they did not present the value “1” in their confidence interval. Descriptive statistical data were calculated using the Excel software (Microsoft®, Redmond, WA). Ethical approvalThe project was registered in the Research Ethics Committee on Animal Use (Comissão de Ética no Uso de Animais—CEUA, in Portuguese) of UFPR, protocol number 045/2017. ResultsRetrospective evaluationA total of 2,055 privately owned animals were admitted at LABOCO between 2014 and 2018. Females (1,107, 53.87%) outnumbered males (948, 46.13%). PRA was diagnosed in 130 dogs (6.33%), with no statistical difference (Chi Squared test) between sex (males 67, 51.53%; females 63, 48.47%; p < 0.05). A total of 18 breeds were diagnosed with PRA, the most prevalent being the Miniature Poodles (n=26, 20.00%), followed by English Cocker Spaniels (n=19, 14.62%), and Lhasa Apsos (n=13, 10.00%). When considering the prevalence of PRAs in relation to the entire population presenting to the LABOCO, the Pomeranian had a significantly higher odds ratio (Tables 1 and 2). Presenting clinical signs reported by owners were nyctalopia (93, 71.54%) and general vision impairment (6, 4.62%). The mean age of onset of presenting clinical signs reported by the owners was 8.55 ± 3.92 years, and the mean age at the time of diagnosis of PRA was 9.06 ± 3.91 years (range 3 months to 16 years). No significant difference in the mean age of presentation or prevalence of PRAs was found across breeds when compared to the total population (p <0.05) (Table 3). Clinical evaluationAmong the 130 PRAs affected dogs, 208 eyes were evaluated through direct and consensual pupillary reflex, while 206 eyes were evaluated through dazzle reflex, menace response, and tracking (cotton wool ball) response tests. In addition, 72 were submitted to the obstacle course test in photopic and scotopic conditions. Menace and tracking responses were the most commonly abnormal tests: 65.05% of the animals showed a negative response/absence of response. Moreover, 32.31% of the evaluated dogs presented at least scotopic impairment in the obstacle course, and 19.23% showed complete both night and day blindness (Tables 4 and 5). Table 1. Distribution of the affected and normal animals admitted to LABOCO relative to the total population of dogs of the same breed (2014–2018).
Table 2. Distribution of breeds diagnosed with PRAs relative to the total number of affected animals and their respective of odds ratio (2014–2018).
Seventy-two animals (55.38%) were diagnosed with secondary cataracts. Of these, the highest rate was detected in poodles (21; 29.16%), followed by Cocker Spaniel (15; 20.83%) and mixed breed dogs (13; 18.05%). Equatorial and posterior cortical vacuoles were the most common initial presentation. All stages of cataracts maturity were found, with the incipient stage most common (48 eyes; 36.09%) (Table 6). Fundus abnormalities consistent with PRAs, including early signs such as a granular tapetum, or more advanced sigs as tapetal hyperreflectivity, vascular attenuation, and optic disc atrophy, were found in 141 eyes (54.23%). The most prevalent fundus abnormality was retinal vascular attenuation, noted in 120 of the affected eyes (85.10%). The main funduscopic changes can be seen in Table 7. Eye fundus photographyFundus photographs were obtained from 14 dogs with confirmed PRA. In total, 11 animals showed signs of retinal thinning manifested as tapetal hyperreflectivity, 10 had granularity of the tapetal fundus, and 8 showed signs of optic disc atrophy. One image was difficult to evaluate due to cataracts, and therefore the only tapetal hyperreflectivity was identified in this animal. A decrease in retinal vascular caliber was evident in most fundus images (n=12, 85.71%), regardless of breed or age. Representative pictures of both a normal fundus and one showing typical features of those affected by PRAs are shown in Figure 1. ElectroretinographyOf the 30 dogs evaluated by ERG, 23 (76.66%) had PRA confirmed by a subnormal or absent electroretinographic response, and 7 (23.33%) dogs had a normal ERG response, thus excluding the initial presumptive PRA diagnosis in these individuals. The ITs of the a-and b-waves were always higher in affected individuals, and the a-wave and b-wave amplitudes were subnormal to absent (Fig. 1). DiscussionRetrospective studies may provide a baseline for understanding the prevalence of disease in the specific population and thus are essential steps in targeting areas in need of focused research. Considering limitations, the present investigation may have suffered an influence from a selection bias for the analysis, introduced by the selection of specific dogs that have been diagnosed with PRA in a Brazilian veterinary ophthalmology service (influenced by the most common local breeds). Thus, proper randomization was not achieved because the dogs were not randomly selected from the general population, and the general population's size was not determined. Other sources of bias should be considered as well; for example, Miniature Poodle owners might be more likely to have brought affected dogs to the facility for evaluation than owners of Miniature Schnauzers. Thus, extrapolation of results should be done with caution. Determining the number of cases among dogs presenting to LABOCO does not measure incidence within a breed or among all dogs in Brazil, but only among dogs that presented to the institution. Presumably, dogs presented to the LABOCO because they have health issues, and healthy dogs would likely be under-represented in this index population. Nevertheless, the present study provides the first report of the prevalence of PRAs in any canine population in South America. The prevalence of PRAs in the LABOCO was highest in Miniature Poodles (20.00%) and English Cocker Spaniels (14.62%), similar to what was previously reported in North America by Petersen-Jones (1998a). Lhasa Apso dogs were the third most prevalent; however, the odds ratio was not significant (p =0.05). Considering all PRAs affected dogs, Pomeranians and the Brazilian Terrier had significantly higher odds ratio when compared to the total population, followed by Cocker Spaniel, Labrador retriever, and Toy Poodle. There is no published description of PRAs in Brazilian Terriers, an uncommonly presented breed at the Veterinary Hospital of the UFPR. The popularity of Pomeranians as a pet was increasing and were the second most common breed recorded in the Brazilian Cynophilia Confederation (CBKC) in 2018, with a total of 18,828 registered dogs (CBKC, personal communication). Therefore, it is important to consider future studies on PRAs in Pomeranians to establish the features of these diseases and, in the future, make pedigree evaluations and molecular analysis studies to identify mutant genes and minimize the incidence and number of unaffected genetic carriers within the population. Table 3. Mean age of onset of clinical signs as described by owners and mean age at the time of diagnosis of PRA for different breeds of dogs.
Table 4. The number of eyes with PRA resulting in abnormal clinical test results.
Table 5. Obstacle course test results for dogs diagnosed with PRAs.
Table 6. Number of eyes diagnosed with cataracts secondary to PRAs and classified by stage of maturity in dogs with PRAs in LABOCO’s case series (2014–2018).
Table 7. Main funduscopic changes in dogs diagnosed with PRAs in LABOCO’s case series (2014–2018).
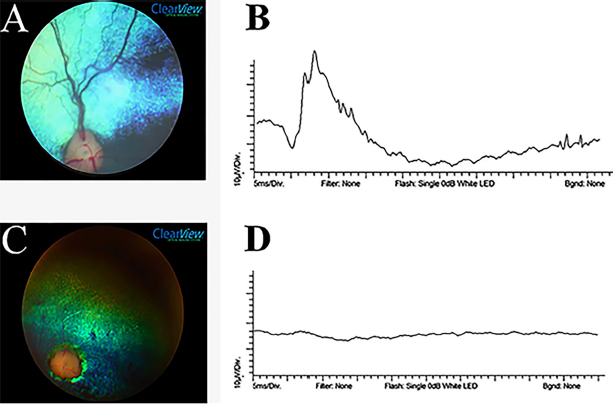
Fig. 1. Ocular fundus photographed by a ClearView camera and representative ERG tracings of dogs referred to LABOCO. A—Normal ocular fundus of a 5-month-old Pomeranian, showing a blue-green tapetal zone, adequate retinal vasculature emanating from the optic disc, dark spots representing the non-tapetal areas, and a well-myelinated optical disc. B—Normal 10,000 mcd.s/m2 ERG tracing of the same dog as in (A), showing a well-defined a-wave (negative) and b-wave (positive). C—A 2-year-old Pomeranian with evidence of advanced PRA funduscopically, showing pronounced vascular attenuation, tapetal hyperreflectivity, optic disc atrophy. D—ERG tracing of the same dog as in (C) at 10,000 mcd.s/m2 showing an absent ERG waveform. The mean age of onset of clinical signs first noted by owners of the dogs affected with PRAs was 8.55 ± 3.92 years. However, the true age of onset of clinical signs could be earlier due to the owner’s difficulty recognizing the signs if subtle or if their pet was successfully adapting to visual impairment. American Pit Bull Terriers, Pomeranians, and Yorkshire Terriers have a lower mean age of onset. These findings are supported by Kijas et al. (2004) and Safatle et al. (2005), who identified a form of cone-rod dystrophy in young Pit Bulldogs with early-onset and rapid progression. However, a previous report described Yorkshire Terriers and Pomeranians with late-onset forms of PRAs (Downs et al., 2014). Nyctalopiais typically the first clinical sign noted due to initial rod photoreceptor degeneration, often described by owners as their pets having insecurity to navigate their environment in low light situations. Other behavioral changes, such as increased aggression or lethargy, have also been described (Barnett,1965; Levin, 1998). Despite the ability to detect subtle changes of early PRAs on clinical examination, this opportunity does not frequently arise in practice. Due to the high level of adaptability that some dogs achieve as PRAs progress or due to the inability of an owner to detect subtle behavioral changes related to visual impairment, unfortunately, PRAs are often presented to a veterinarian for evaluation when in more advanced stages. Early stages of PRAs are most often detected incidentally on examination, often upon referral from a primary care veterinarian for secondary cataracts. In order to detect early PRA, younger close relatives of affected dogs should be evaluated (e.g., offspring of affected dogs). Funduscopic signs of PRAs are usually the first recognized on clinical examination. Early signs include light tapetal granularity and peripheral vascular taper, followed by tapetal hyperreflectivity and vascular attenuation coincident with progressive loss of photoreceptors and retinal thinning (Petersen-Jones, 1998a). The most prevalent lesions in the present study were vascular attenuation (85.10%) and tapetal hyperreflectivity (55.31%). Additionally, different types of PRAs have distinctive patterns of retinal degeneration. For example, PRCD in the Toy Poodle tends to present with early signs 5 years of age. Histologically, the photoreceptors at the posterior pole and central retina are affected before peripheral photoreceptors in this breed (Aguirre and Acland, 1988). PRCD in the Cocker Spaniel is typically slower, and the retina's nasal and temporal corner aspects are spared until late, leaving healthy photoreceptor islands (Aguirre and Acland, 1988). In this study, no specific differences between the PRAs of different breeds were detected clinically. Other common abnormalities detected on ophthalmic exam, especially in dogs with advanced disease, include reduced or absent dazzle reflex and menace response. Direct and indirect PLR usually somewhat preserved, although diminished with longer to return to a resting state following light stimulation, due to the need for only a few functional photoreceptors to initiate the reflex pathway. Secondary effects of PRAs are common, most notably being the development of cataracts (Petersen-Jones, 1998a; Park et al., 2009). In this study, 72 dogs (55.38%) were diagnosed with cataracts, of which 61 (84.72%) were bilateral. Cases of anterior cortical incipient cataracts were excluded because this is not the typical presentation for cataracts secondary to PRAs. This value is higher than those shown in a previous study, which reported that 44% of dogs with PRAs had cataracts (Kraijer-Huver et al., 2008). Poodles were the breed most commonly affected by cataracts, mostly of the incipient stage, consistent with previous reports (Park et al., 2009, Donzel et al., 2017). Between August 2016 and July 2018, 205 animals were diagnosed by the LABOCO with cataracts secondary to PRAs, and of these, 40 (19.51%) were Toy Poodles. Several owners reported the presence of a cloudy aspect in their dog’s eyes, and soon after, they lost their sight. The owner’s ability to detect cataracts is often limited to the late immature to mature stage, contributing significantly to visual impairment. ERG can be useful to differentiate causes of visual impairment, such as SARDS, optic neuritis, and PRAs. It is also a helpful diagnostic tool when examining the posterior segment is limited due to alteration in the ocular media transparency, such as cataracts (Narfstrom and Petersen-Jones, 2013; Ekesten et al., 2013; Drazek et al., 2014). In this study, a flash ERG protocol was used in conscious animals under manual restraint, similar to that used preoperatively for cataract surgery staging (Ekesten et al., 2013; Somma et al., 2016). This technique does not allow the differentiation of cone and rod responses and is rarely used in studies of retinal dystrophies that require more complete protocols to evaluate with more detail the retinal electrical activity. Freeman et al. (2013) found that noise levels in non-sedated and non-anesthetized animals were not different than those with sedation or anesthesia, but that anesthesia and sedation both significantly reduced a- and b-wave amplitudes as well as ITs. Most generically, when using a short ERG protocol, PRAs cause a decrease in a-wave and b-wave amplitudes and an increase in their implicit time (Safatle et al., 2005; Balicki et al., 2013; Freeman et al., 2013; Good et al., 2015). Our study corroborates these results; the a-wave and the b-wave of animals with PRAs were decreased at all light intensities or even absent in lower light intensities. In addition, both a-wave and b-wave ITs were significantly higher in the PRAs affected dogs, at light intensities of 3,000 and 10,000 mcd.s/m2 (p < 0.05). Dogs with a clinical history of SARDs were excluded. However, some of the animals were selected for ERG even in the absence of a retinal abnormality on clinical examination, mainly because owners reported suspicious abnormalities in vision at home (e.g., especially in dim light). As a result, some normal ERG results were found. Although the results only apply to the population of dogs presented at LABOCO and cannot be extrapolated to the entire breed, this study showed that Miniature Poodles and Cocker Spaniels were the breeds most commonly affected by PRAs presented at LABOCO. Further research on the prevalence of PRAs in populations of dogs throughout Brazil is recommended, which together may provide new avenues for research of inherited retinal diseases in domestic dogs. In addition, it is important to note that most dogs are highly adaptable to progressing visual impairment until a critical point is reached. Therefore, it is important to educate owners and raise awareness among the general population of signs to monitor for, particularly in high-risk breeds. Additional education is recommended for those owners with dogs diagnosed with PRAs, to help them successfully navigate the coming changes in their pet’s lifestyle as visual impairment progresses. Pomeranians showed a high odds ratio within that population, and considering the increase in the popularity of this breed in recent years, monitoring the incidence of PRAs is recommended. Several commercially available tests for PRA-causing genetic variants for forms of PRA that occur in many breeds (e.g., see https://www.pawprintgenetics.com or https://breeder.wisdompanel.com). Blood samples were collected from all affected dogs in this investigation and will be screened for known PRA gene sequence variants in the near future. AcknowledgmentsThe author would like to thank the Academic Publishing Advisory Center (Centro de Assessoria de Publicação Acadêmica—CAPA) of the UFPR for language support for this version of the article. Conflict of interestThe authors declare that there are no conflicts of interest. FundingThis work was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). Authors contributionHenrique de Moura Freitas: Main executor of this project. Participated in the organization and preparation of all stages of this project including the schedule preparation, in the review of medical records and literature data, in the tabulation of data in tables, statistical analyses, in the registration of ophthalmoscopy and ERG images, writing, formatting and editing this work. Fabiano Montiani-Ferreira: Project advisor. Helped with the main idea and in the preparation and organization of all the stages of this project. Actively participated in the ophthalmoscopy and ERG and in the review of this paper. Also, participated in the edition of the images. André Tavares Somma: Secondary author of this paper. Participated in the writing, review of the data, formatting and editing the figures and tables. Bret A. Moore: Secondary author of this paper. Participated in the writing, review of the data, formatting and editing the figures and tables. ReferencesAguirre, G.D. and Acland, G.M. 1988. Variation in retinal degeneration phenotype inherited at the prcd locus. Exp. Eye Res. 46(5), 663–687. Al-Saikhan, F.I. 2013. The gene therapy revolution in ophthalmology. Saudi J. Ophthalmol. 27, 107–111. Apte, R.S. 2018. Gene therapy for retinal degeneration. Cell 173(1), 5. Arbabi, A., Liu, A. and Ameri, H. 2019. Gene therapy for inherited retinal degeneration. J. Ocul. Pharmacol. Ther. 35(2), 79–97. Balicki, I., Nestorowicz, N. and Ofri, R. 2013. Funduscopic abnormalities and electroretinography in cases of retinopathy in German Shepherd dogs. Vet. Ophthalmol. 16(6), 397–408. Banin, E., Gootwine, E., Obolensky, A., Ezra-Elia, R., Ejzenberg, A., Zelinger, L., Honig, H., Rosov, A., Yamin, E., Sharon, D. and Averbukh, E. 2015. Gene augmentation therapy restores retinal function and visual behavior in a sheep model of CNGA3 achromatopsia. Mol. Ther. 23(9), 1423–1433. Barnett, K.G. 1965. Canine retinopathies—II the Miniature and Toy Poodle. J. Small Anim. Pract. 6(2), 93–109. Beltran, W.A. 2009. The use of canine models of inherited retinal degeneration to test novel therapeutic approaches. Vet. Ophthalmol. 12(3), 192–204. Dias, M.F., Joo, K., Kemp, J.A., Fialho, S.L., da Silva Cunha Jr, A., Woo, S.J. and Kwon, Y.J. 2018. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog. Retin. Eye Res. 63, 107–131. Donzel, E., Arti, L. and Chahory, S. 2017. Epidemiology and clinical presentation of canine cataracts in France: a retrospective study of 404 cases. Vet. Ophthalmol. 20(2), 131–139. Downs, L.M., Hitti, R., Pregnolato, S. and Mellersh, C.S. 2014. Genetic screening for PRA-associated mutations in multiple dog breeds shows that PRA is heterogeneous within and between breeds. Vet. Ophthalmol. 17(2), 126–130. Drazek, M., Lew, M., Lew, S. and Pomianowski, A. 2014. Electroretinography in dogs: a review. Vet. Med. 59(11), 515–526. Ekesten, B., Komáromy, A.M., Ofri, R., Petersen-Jones, S.M. and Narfström, K. 2013. Guidelines for clinical electroretinography in the dog: 2012 update. Doc. Ophthalmol. 127(2), 79–87. Freeman, K.S., Good, K.L., Kass, P.H., Park, S.A., Nestorowicz, N. and Ofri, R. 2013. Effects of chemical restraint on electroretinograms recorded sequentially in awake, sedated, and anesthetized dogs. Am. J. Vet. Res. 74(7), 1036–1042. Giuliano, E.A. and van Der Woerdt, A. 1999. Feline retinal degeneration: clinical experience and new findings (1994-1997). J. Am. Anim. Hosp. Assoc. 35(6), 511–514. Good, K.L., Komáromy, A.M., Kass, P.H. and Ofri, R. 2015. Novel retinopathy in related Gordon setters: a clinical, behavioral, electrophysiological, and genetic investigation. Vet. Ophthalmol. 19(5), 398–408. Heitmann, M., Hamann, H., Brahm, R., Grussendorf, H., Rosenhagen, C.U. and Distl, O. 2005. Analysis of prevalence of presumed inherited eye diseases in Entlebucher Mountain Dogs. Vet. Ophthalmol. 8(3), 145–151. Huang, P.C., Gaitan, A.E., Hao, Y., Petters, R.M. and Wong, F. 1993. Cellular interactions implicated in the mechanism of photoreceptor degeneration in transgenic mice expressing a mutant rhodopsin gene. Proc. Natl. Acad. Sci. USA. 90(18), 8484–8488. Kijas, J.K., Zangerl, B., Miller, B., Nelson, J., Kirkness, E.F., Aguirre, G.D. and Acland, G.M. 2004. Cloning of the canine ABCA4 gene and evaluation in canine cone-rod dystrophies and progressive retinal atrophies. Mol. Vis. 10, 223–233. Kraijer-Huver, I.M., Gubbels, E.J., Scholten, J., Djajadiningrat-Laanen, SC., Boevé, M.H. and Stades, F.C. 2008. Characterization and prevalence of cataracts in Labrador Retrievers in The Netherlands. Am. J. Vet. Res. 69(10), 1336–1340. Levin, C.D. 1998. Living with blind dogs: a resource book and training guide for the owners of blind and low-vision dogs. New York, NY: Lantern Publications. Li, Z.Y., Wong, F., Chang, J.H., Possin, D.E., Hao, Y., Petters, R.M. and Milam, A.H. 1998. Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 39(5), 808–819. Magnusson, H. 1911. Uber retinitis pigmentosa und Konsinguinitat beim Hunde. Arch. Vergl. Ophthalmol. 2, 147–149. Naash, M.I., Hollyfield, J.G., Al-Ubaidi, M.R. and Baehr, W. 1993. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc. Natl. Acad. Sci. U S A. 90(12), 5499–5503. Narfström, K., David, V., Jarret, O., Beatty, J., Barrs, V., Wilkie, D. and Menotti-Raymond, M. 2009. Retinal degeneration in the Abyssinian and Somali cat (rdAc): correlation between genotype and phenotype and rdAc allele frequency in two continents. Vet. Ophthalmol. 12(5), 285–291. Narfstrom, K. and Petersen-Jones, S. 2013. Diseases of the canine ocular fundus. In Veterinary ophthalmology. Eds., Gelatt, K.N., Gilger, B.C. and Kern, T.J. 5th ed. Weinheim, Germany: Wiley-Blackwell, pp: 1303–1392. Park, S.A., Yi, N.Y., Jeong, M.B., Kim, W.T., Kim, S.E., Chae, J.M. and Seo, K.M. 2009. Clinical manifestations of cataracts in small breed dogs. Vet. Ophthalmol. 12(4), 205–210. Pawprintgenetics. 2021. Available via https://www.pawprintgenetics.com (Accessed ??/??/2021). Petersen-Jones, S.M. 2005. Advances in the molecular understanding of canine retinal diseases. J. Small Anim. Pract. 46(8), 371–80. Petersen-Jones, S.M. 1998a. A review of research to elucidate the causes of the generalized progressive retinal atrophies. Vet. J. 155(1), 5–18. Petersen-Jones, S.M. 1998b. Animal models of human retinal dystrophies. Eye. 12(3b), 566. Petersen-Jones, S.M. 2012. Viral vectors for targeting the canine retina: a review. Vet. Ophthalmol. (15), 29–34. Rubin, L.F. and Lipton, D.E. 1973. Retinal degeneration in kittens. J. Am. Vet. Med. Assoc. 162, 467–469. Safatle, A., Salomão, S., Berezovsky, A., Sacai, P., Fantoni, D., Yasbek, K. and Barros, P.S. 2005. Retinal degeneration in a Pit Bull dog: electroretinographic findings. Arch. Vet. Sci. 10(2), 119–124. Somma, A.T., Moreno, J.C.D., Sato, M.T., Rodrigues, B.D., Bacellar-Galdino, M., Occelli, L.M. and Montiani-Ferreira, F. 2016. Characterization of a novel form of progressive retinal atrophy in Whippet dogs: a clinical, electroretinographic, and breeding study. Vet. Ophthalmol. 20(5), 450–459. Takahashi, V.K., Takiuti, J.T., Jauregui, R. and Tsang, S.H. 2018. Gene therapy in inherited retinal degenerative diseases, a review. Ophthalmic genet. 39(5), 560–568. Whitley, R.D., Mclaughlin, S.A. and Gilger B.C. 1995. Update on eye disorders among purebred dogs. Vet. Med. 90, 574–592. Winkler, P.A., Occelli, L.M. and Petersen-Jones, S.M. 2020. Large animal models of inherited retinal degenerations: a review. Cells 9(4), 882. Zeiss, C.J. 2013. Translational models of ocular disease. Vet. Ophthalmol. 16, 15–33. | ||
| How to Cite this Article |
| Pubmed Style Freitas HM, Somma AT, Moore BA, Montiani-ferreira F. Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil. Open Vet. J.. 2021; 11(3): 370-378. doi:10.5455/OVJ.2021.v11.i3.6 Web Style Freitas HM, Somma AT, Moore BA, Montiani-ferreira F. Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil. https://www.openveterinaryjournal.com/?mno=44279 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i3.6 AMA (American Medical Association) Style Freitas HM, Somma AT, Moore BA, Montiani-ferreira F. Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil. Open Vet. J.. 2021; 11(3): 370-378. doi:10.5455/OVJ.2021.v11.i3.6 Vancouver/ICMJE Style Freitas HM, Somma AT, Moore BA, Montiani-ferreira F. Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil. Open Vet. J.. (2021), [cited January 25, 2026]; 11(3): 370-378. doi:10.5455/OVJ.2021.v11.i3.6 Harvard Style Freitas, H. M., Somma, . A. T., Moore, . B. A. & Montiani-ferreira, . F. (2021) Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil. Open Vet. J., 11 (3), 370-378. doi:10.5455/OVJ.2021.v11.i3.6 Turabian Style Freitas, Henrique Moura, Andr T Somma, Bret A. Moore, and Fabiano Montiani-ferreira. 2021. Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil. Open Veterinary Journal, 11 (3), 370-378. doi:10.5455/OVJ.2021.v11.i3.6 Chicago Style Freitas, Henrique Moura, Andr T Somma, Bret A. Moore, and Fabiano Montiani-ferreira. "Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil." Open Veterinary Journal 11 (2021), 370-378. doi:10.5455/OVJ.2021.v11.i3.6 MLA (The Modern Language Association) Style Freitas, Henrique Moura, Andr T Somma, Bret A. Moore, and Fabiano Montiani-ferreira. "Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil." Open Veterinary Journal 11.3 (2021), 370-378. Print. doi:10.5455/OVJ.2021.v11.i3.6 APA (American Psychological Association) Style Freitas, H. M., Somma, . A. T., Moore, . B. A. & Montiani-ferreira, . F. (2021) Retrospective and prospective study of progressive retinal atrophy in dogs presented to the veterinary hospital of the Federal University of Parana, Brazil. Open Veterinary Journal, 11 (3), 370-378. doi:10.5455/OVJ.2021.v11.i3.6 |