| Original Article | ||
Open Vet. J.. 2021; 11(2): 228-237 Open Veterinary Journal, (2021), Vol. 11(2): 228–237 Original Research Role of insulin, insulin sensitivity, and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield ratesEnas Elmeligy1*, Shin Oikawa2, Sabry A. Mousa3, Sara A. Bayoumi4, Ahmed Hafez5, Ragab H. Mohamed6, Al-lethie A. Al-lethie7, Dalia Hassan8 and Arafat Khalphallah91Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 2Departments of Veterinary Herd Health, School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Japan 3Division of Internal Medicine, Department of medicine and infectious disease, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 4Division of Clinical Laboratory Diagnosis, Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 5Department of Pharmacology, Faculty of Veterinary, Medicine, Aswan University, Aswan, Egypt 6Theriogenology Department, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt 7Department of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt 8Department of Animal & Poultry Hygiene and Environmental Sanitation, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 9Division of Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt *Corresponding Author: Enas Elmeligy. Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. Email: enaselmeligy [at] yahoo.com Submitted: 29/01/2021 Accepted: 01/04/2021 Published: 30/04/2021 © 2021 Open Veterinary Journal
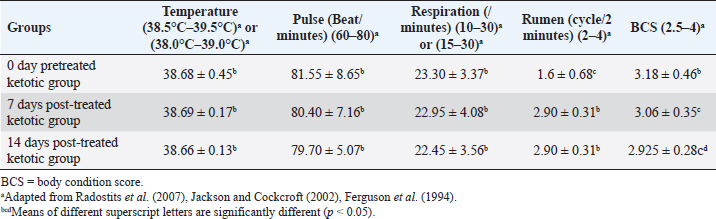
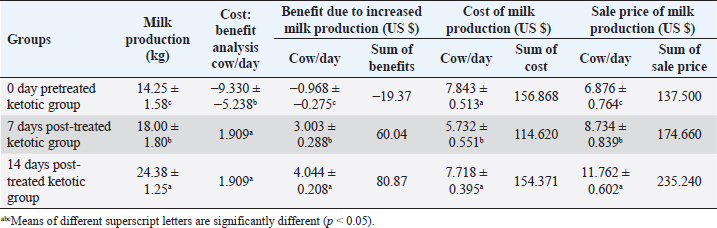
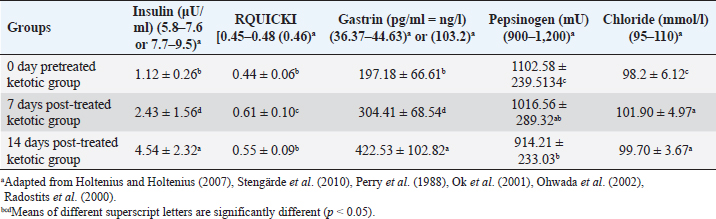
AbstractBackground: Ketosis is one of the most critical metabolic disorders that occur in dairy cows after parturition due to negative energy balance around calving. Aim: The study evaluated a specific therapeutic regimen of ketosis in Holstein dairy cattle by using the combination therapy including hormones, corticosteroids, propylene glycol, and vitamin B12 as well as the use of milk yield rates, insulin, insulin sensitivity, and abomasal functions monitors as diagnostic biomarkers for the recovery of ketotic cows either pre-therapy (0 days) or post-therapy (7 and 14 days). Methods: This study was conducted on ketotic cattle (n=20) belonged to different dairy farms in Cairo and Giza governorates, Egypt. The diseased cows were undergoing clinical and biochemical investigations for the estimation of serum insulin. Quantitative Insulin Sensitivity Check Index (RQUICKI) and abomasal functions monitor mainly serum levels of gastrin, pepsinogen, and chloride. Results: The milk production rates, cost: benefit analysis ratio, and benefit of the dairy farm in ketotic animals were significantly increased post-treatment. An improvement of insulin sensitivity was stated as serum insulin, and RQUICKI were remarkably increased in post-therapeutic ketotic cows. Monitors of the abomasal function revealed abomasal functions improvement through the significant elevation of blood gastrin and a substantial reduction in serum pepsinogen due to treatment. Conclusion: The study revealed high efficacy of the applied therapeutic strategy regime. It led to a high recovery rate and a very low relapse rate for ketosis. An improvement in milk yield rates, insulin sensitivity, and abomasal function monitors was reported. Hypoinsulinaemia was still reported, however, serum insulin was improved. Keywords: Abomasal functions monitors, Cattle, Insulin resistance, Ketosis, RQUICKI. IntroductionThe postpartum period of the dairy cows is critical in the production life of the cow during this time; where dairy cows are at risk of certain diseases such as metritis, abomasal displacement (DA), and ketosis (Curtis et al., 1985; Khalphallah et al., 2015; Khalphallah et al., 2018a). The most common diseases of transition periods reported at the first 50 days in milk (DIM) in dairy cattle included retained placenta, ketosis, DA, milk fever, mastitis, and dystocia. Retained placenta, ketosis, and DA, i.e., energy-related diseases, were sometimes associated with one or more disease states such as milk fever, mastitis, and dystocia (Khalphallah et al., 2018a). Iwersen et al. (2009) and Khalphallah et al. (2018a) added that retained placenta, ketosis, DA, milk fever, mastitis, and dystocia were the most commonly reported diseases of transition periods (50 DIM) in dairy cattle. Ketosis is one of the most critical metabolic disorders that occur in dairy cows after parturition due to negative energy balance (NEB) around calving (Cameron et al., 1998). From the possible ways in the diagnosis of ketosis are evaluating clinical findings including (rectal temperature, pulse rate, respiratory rate, and ruminal movement) also monitoring of body condition score (BCS) are vital to evaluate the nutritional and health status of animals (Upham, 1996). Secondary, or type II, ketosis occurs earlier in lactation than type I ketosis and is usually concurrent with other diseases (Holtenius and Holtenius, 1996). Hyperketonemia and varying serum glucose and insulin concentrations were reported in ketotic cattle (type II). Affected cows were often over-conditioned (Agenäs et al., 2003). Insulin facilitates the cellular uptake of glucose and downregulates adipose tissue mobilization. Insulin concentration is high before calving and declines to low levels after parturition (Kokkonen et al., 2005; Van Knegsel et al., 2007), so the low postpartum concentration of insulin stimulates adipose tissue breakdown to counteract the NEB in early lactation. Insulin resistance is a multi-factorial phenomenon characterised in humans by metabolic acidosis, hyperglycaemia, glucose intolerance, glucosuria, ketonaemia, ketonuria, diuresis, hypovolaemia, dehydration, polydipsia, central nervous system depression, and shock (McCance and Huether, 1994). These physiopathological events are also seen in ruminant animals with induced or spontaneous hepatic lipidosis and ketosis (DeBoer et al., 1985; Veenhuizen et al., 1991; Drackley, 1999). The previous reports concerned with the monitoring of abomasal functions in ketotic cattle associated with DA through the estimation of serum levels of gastrin and pepsinogen (Khalphallah et al., 2018a). Other studies mentioned a strong association between many postpartum diseases such as ketosis and increase risk of DA (Curtis et al., 1985; Duffield and Bagg, 2002) as well as serum pepsinogen and gastrin showed variable changes due to DA only or DA with ketosis problems (Ozturk et al., 2013; Khalphallah et al., 2018b); therefore, the current study needed to focus on this relationship between ketotic cows and blood gastrin, pepsinogen, and chloride. Several articles referred to the efficacy of different therapeutic regimens in the treatment and management of ketosis in dairy cattle that were reported at different lactation stages (Tufani et al., 2011) as some used complex treatment regimens (Tufani et al., 2011; Madreseh-Ghahfarokhi et al., 2018) where others used simple treatments (Radostits et al., 2007; McArt et al., 2011). In cattle, several effective treatments were available for ketosis, but in some affected animals, the response was only transient; in rare cases, the disease might persist and cause death or necessitate the animals' slaughter (Constable et al., 2017). The prognosis and response to therapy were related to the type of ketosis. Ketosis (type I) that occurred at the peak of lactation usually responded quickly to the treatment, but relapses were common if the diet was not corrected. Periparturient ketosis (type II) responds less rapidly to treatment (Anderson and Rings, 2009). Therefore, the present study aimed to evaluate a specific therapeutic regimen of ketosis in Holstein dairy cattle by using combination therapy including hormones, corticosteroids, propylene glycol, and vitamin B12, through the innovative use of insulin, insulin sensitivity, and abomasal functions monitors (Serum gastrin, pepsinogen, and chlorides) as diagnostic biomarkers for the recovery of ketotic cows either pre-therapy (0 days) or post-therapy (7 and 14 days) as well as estimation of milk yield rates. Materials and MethodsAnimalsThe study was conducted on ketotic Holstein dairy cattle (n=20) belonged to different dairy farms in Cairo and Giza governorates, Egypt. Their body weight ranged between 350 and 400 kg, and age ranged between 3 and 5 years. The average 305-days milk yield was approximately 4,600 kg. They were fed on total mixed ration (TMR). The one cow consumed about 18–19 kg TMR. The farm's system was a tie stall. Milking times were two; 6 am and 6 pm. The diseased cattle were subjected to a therapeutic strategy extended to 5 days to relieve them from ketosis. All cattle were examined and sampled at day 0 (pretreatment) then at days 7 and 14 post-treatment. 0-day pretreated ketotic group was considered as a control group. SamplesBlood serum samples were collected from the jugular vein on plain vacutainer tubes and stored at −20 Ċ until analysis. The animals were sampled at day 0 before treatment and days 7 and 14 following treatment. Therapeutic strategyDiagnosis of all ketotic cows was mainly based on clinical findings and estimation of serum β-hydroxybutyric acid (BHBA) at day 0 (lactating cows 5–50 days in milk). The diseased animals were either subclinically ketotic cows (SCK) in which serum BHBA levels were 1.2–2.4 mmol/l or clinically ketotic ones (CK) in which serum BHBA levels were ≥2.5 mmol/l. This classification was according to Sakha et al. (2007) and Tehrani-Sharif et al. (2012). The diseased cattle were treated at day 0 after clinical examination, and serum sample collection was conducted before the first treatment, according to Radostits et al. (2007) and Constable et al. (2017). The treatment continued for successive 5 days as following: The IV injection of 500 ml of a 50% solution of glucose (5%® El Fath for Pharmaceutical and Cosmetics Industries (FIPCO), Cairo, Egypt) q24h alternatively with IV injection of 500 ml of a 50% dextrose solution (Dextrose 50%® VETWIC, Cairo, Egypt) for 5 successive days. Drenching with propylene glycol as glucose precursor, i.e., propane-1, 2-diol, once or twice daily for successive 5 days. The traditional dose is 300 ml twice daily for 2 days, followed by 150 ml once daily for 3 days to cattle. Treatment continued for 5 days until feed intake improved [El Nasr Pharmaceutical Chemical Co., Cairo, Egypt]. Dexamethasone [Dexamethasone® ADWIA Co., Cairo, Egypt] as glucocorticoid administration [0.04 mg/kg (20 mg/450 kg) of Dexamethasone IM once daily every other day], following adequate fluid volume replacement (IV 50% solution of glucose), steroids were given as a single IM injection over a 1–3 minutes’ period. The treatment should continue for successive 5 days/3 doses. Insulin (Mixtard® 30 HM Vial 100 IU/ml, Novo Nordisk A/S, Denmark) was administered in conjunction with glucose and glucocorticoid (Dexamethasone). The dose of Mixtard ®30 HM was 2 ml (200 IU) per animal administered SC every 48 hours for 5 days. 1 vial contained 10 ml (1,000 IU). The animals were treated with IV 50% glucose solution, IM dexamethasone, and SC Insulin together in alternation with IV 50% dextrose. Vitamin B12 (KENAZAL® 10 solution, KENANA) was administered 5 ml/100 kg IM every other day three times (days 1, 3, and 5). The feed additive, i.e., Smartamine (Smartamine® M, Kemin company, Des Moines, IA, imported by United Bio-med Co., Cairo, Egypt) in a dose of 15 g daily, per os, for successive 60 days. Smartamine included amino acids (methionine and lysine). Clinical examinationAll animals underwent a thorough clinical examination as described by Cockcroft (2015). The animals were examined on day 0 before treatment and days 7 and 14 following treatment. BCS of all cows was measured depended on a 5-point scale (Ferguson et al., 1994). Rates (per kg) and cost (US $) of milk production for each cow were estimated either pre-therapy or post-therapy. Furthermore, the price (US $) of milk production (kg) per cow was estimated after adding the cost of therapy per ketotic cow at 0 days. Cost: benefit analysis (per cow)=cost of milk production per cow per day [with (at day 0) or without (at days 7 and 14) the fees of strategic treatment] / benefit of milk production per cow per day was determined for each cow at each sampling time. Benefit (US $) was estimated by subtraction cost of milk production per cow per day (with or without the fees of strategic treatment) from the sale price of milk production per cow per day. The sum (US $) of each of the benefits, cost of milk production, and sale prices of milk yield for all investigated cows were also measured at each sampling time. Laboratory examinationSerum samples were collected, and all precautions for collection and preparation of samples to ensure accurate evaluation of biochemical indices, as described by Coles (1986), were taken into consideration. Serum biochemical assays were estimated at day 0 before treatment and days 7 and 14 following treatment. Serum BHBA was measured for all cows only at day 0 by using the kinetic enzymatic method by the Optizen 32,220 UV/Visible spectrophotometer (Mecasys Co., Ltd. South Korea) with commercial kits (Ranbut, Randox Ltd, UK). Insulin was measured using a commercial ELISA kit supplied by CALBIOTECH, the USA, imported by a beta-diagnostic company, Egypt. Quantitative Insulin Sensitivity Check Index (RQUICKI values) was based on fasting plasma glucose, insulin, and non-esterified fatty acid (NEFA), and a low value indicates decreased insulin sensitivity. RQUICKI as a metabolic index was estimated=1/ [log10 (glucose, mg/dl) + log10 (insulin, μU/ml) + log10 (NEFA, mmol/l)] (Perseghin et al., 2001; Rabasa-Lhoret et al., 2003). Serum levels of glucose (Glucose, Gamma Trade Company, Egypt) and NEFAs (NEFA, Randox Ltd, UK) were determined using the Optizen 32,220 UV/Visible spectrophotometer (Mecasys Co., Ltd. South Korea) with commercial kits. Serum gastrin levels were determined by double-antibody gastrin 125I radioimmunoassay (RIA Kit, DPC, USA) according to Kataria et al. (2008). Serum pepsinogen was determined by sandwich enzyme-linked immunosorbent assay (ELISA- Sandwich Protocol) using commercial kits (Bovine Pepsin A (PGA) ELISA Kit, AssayGenie, Dublin, Ireland). Serum chlorides were determined using Optizen 32,220 UV/Visible spectrophotometer (Mecasys Co., Ltd. South Korea) with reagent kits (Spinreact Company, Spain). Statistical analysisComputer Software (SPSS version 16.0, Chicago, IL) was used to analyse all obtained data. Data were shown as mean ± standard deviation values. The data obtained from clinical examination and biochemical analyses were analysed by general linear model repeated measures analysis of variance, and the significance level of results was set at p < 0.05. The significance of differences was evaluated between the means at selected sampling days (days 0, 7, and 14). Ethical approvalAll experimental protocols were approved by Institutional Animal Care and Use Committee guidelines of Assiut University which were in accordance with the Guide for Laboratory Animals Care and Use of the National Institutes of Health in the USA (NIH publication No. 86–23, revised 1996). ResultsThe ketotic cattle were usually considered risk factors for many postparturient diseases such as retained placenta, DA, and/or mastitis. Out of the 20 (100%) cows, 8 (40%) were suffering from DA, 5 (25%) were suffering from the retained placenta, 2 (10%) were suffering from mastitis, and 5 (25%) of the cows had no complications. The clinical findings in diseased ketotic cows, including temperature, pulse rate, and respiratory rate (Table 1), showed no significant changes between days 0 (pre-treatment), 7, and 14. All clinical findings values were within the physiological reference range. Ruminal movement values in diseased ketotic cows (Table 1) showed significant improvement (p < 0.05) at days 7 and 14 (post-treatment) when their values were compared with those at day 0 (pre-treatment). BCS in ketotic cows showed a significant decrease (p < 0.05) at days 7 and 14 (post-treatment) when compared with their values at day 0 (pre-treatment). This substantial reduction in BCS was also reported at day 14 when their values compared with those at day 7. BCS values still within the physiological reference values (Table 1). The rates of milk production (kg) in diseased animals (Table 2) were significantly increased (p < 0.05) at days 7 and 14 post-treatment when they were compared with their values at day 0 (pre-treatment). This significant elevation (p < 0.05) in mean values of milk production was also reported between days 7 and 14 when values at day 14 compared with those at days 7. These findings confirm the impact of treatment in improving the health and productivity states of ketotic cows. Cost: benefit analysis reported a significant (p < 0.05) increase at day 0 (−9.330 ± −5.238) compared with those at days 7 (1.909) and 14 (1.909). The benefit (US $) of the dairy farm due to increased milk production per cow as a result of the success in therapeutic strategy regimen of ketosis showed a remarkable (p < 0.05) elevation post-therapy at days 7 (3.003 ± 0.288) and 14 (4.044 ± 0.208) compared with values of pre-therapy benefit (−0.968 ± −0.275) at day 0. The sum of sale prices (US $) of milk production of all investigated dairy cows was improved due to therapy at days 7 (174.660) and 14 (235.240) compared with those at day 0 (137.500) (Table 2). Blood insulin levels showed a remarkable increase (p < 0.05) in post-treatment cows at days 7 and 14 when their values compared with that at day 0 (pre-treatment). This significant elevation in insulin levels was also shown on day 14 compared with that on day 7 (Table 3). Insulin concentrations were gradually increased after treatment on day 0, however, their values did not reach the reference values on days 14. The RQUICKI (Table 3) were remarkably increased (p < 0.05) in ketotic cows at days 7 and 14 (post-treatment) compared with the values at day 0 (pre-treatment). The elevation in RQUICKI values with treatment (at days 7 and 14) in ketotic cows indicated an improvement in insulin sensitivity and the success of ketosis's therapeutic program. Post-treated RQUICKI values were higher than the reference values. The reported results mentioned that several changes in abomasal functions monitors, including gastrin, pepsinogen and chlorides. Generally, an improvement of the abomasal functions was reported. Serum gastrin levels (Table 3) in diseased ketotic cows throughout the present study, either before or after treatment, were higher than the reference values of blood gastrin in cows. Serum gastrin value was significantly increased (p < 0.05) in post-treated ketotic cows at days 7 and 14 compared to day 0. Serum pepsinogen concentration was remarkably (p < 0.05) reduced after therapy, particularly on day 14 (Table 3). However, the pepsinogen levels were still within the physiological reference range. There were no significant changes in serum levels of chloride (Table 3) within the physiological reference range during the entire study period. DiscussionKetosis was one of the common metabolic diseases of the periparturient period as it was associated with several concurrent diseases and economic losses. CK and SCK were described (Dohoo and Martin, 1984; Dohoo et al., 1984). Iwersen et al. (2009) mentioned that SCK often was still hidden in the absence of clinical findings, and the true prevalence was underestimated in dairy herds. Ketosis had damaging effects on healthy cow performance, productivity and reproductivity. Table 1. Mean values of body weight, temperature, pulse, respiration and rumen movement in pretreated and post-treated ketotic cows (n=20).
Table 2. Mean values of cost: benefit analysis, benefit, cost and sale price of milk production in pretreated and post-treated ketotic cows (n=20).
Table 3. Mean values of serum insulin, RQUICKI, gastrin, pepsinogen and chloride in pretreated and post-treated ketotic cows (n=20).
The current results found that ketotic cattle were usually considered risk factors for many postparturient diseases such as retained placenta, DA and/or mastitis. Khalphallah et al. (2018a) added that retained placenta, ketosis, DA, milk fever, mastitis and dystocia were the most commonly reported diseases of transition periods (50 DIM) in dairy cattle. Ketosis, DA and retained placenta as energy-related diseases were sometimes related to one or more of each milk fever, mastitis, and/or dystocia. Curtis et al. (1985) and Duffield and Bagg (2002) said that herds with ketosis (SCK) in early lactation cows were at an increased frequency of DA. The previous articles revealed that ketosis predisposed cows to other diseases and reproductive disorders and was associated with depressed milk production. Despite energy-rich feed rations, NEB frequently developed in dairy cattle's periparturient period (Hejel et al., 2018). Reduction of dry matter intake during early lactation and the energy and fat and protein demands were increased; therefore, cows mobilised body fat to meet the lactation needs (Cao et al., 2017). The clinical findings in diseased ketotic cows showed no significant changes in temperature, pulse, and respiration indices at day 0 (pre-treatment) and at days 7 and 14 (post-treatment). All clinical findings values were still within the physiological reference range reported by Radostits et al. (2000) and Jackson and Cockcroft (2002). The ruminal cycle in diseased ketotic cows (pretreated) revealed a remarkable decrease compared to the physiological reference range reported by Radostits et al. (2000). This might be due to the effect of ketogenic acids on rumen movement; after treatment at days 7 and 14, the rumen cycle values significant increase but still within the physiological reference range reported by Radostits et al. (2007). These findings agreed with Tufani et al. (2011), who added that a characteristic sweetish odour of the breath, milk and urine was reported in most cases. The mean values of milk production showed a significant increase at days 7 and 14 (post-treatment) compared with those at day 0, reflecting the role of treatment regimen in improving both productivity and health condition of cows. The milk production was sharply decreased at day 0 then improved after treatment. Edwards and Tozer (2004) reported that ketosis was usually associated with moderate to marked decline in milk yield over 5-6 days before the onset of obvious clinical signs that can persist (up to 5 l per day) 2 weeks after diagnosis. Regarding the success of the ketosis therapeutic program in the current article, which was reflected through the significant increase in milk production per cow as well as the exclusion of fees of therapeutic regimen (at days 7 and 14), the cost: benefit analysis reported a significant reduction post-therapy (days 7 and 14). This indicated the low cost and high benefit. The high benefit was due to a significant elevation in milk yield per cow per day and the zero cost of therapy. Therefore, the dairy farm's benefit showed also a remarkable elevation post-therapy compared with those on day 0 (pre-therapy). Van Winden and Kuiper (2003) and Khalphallah et al. (2015) reported economic losses due to milk loss, cost of treatment and increased culling rate either in DA SCK or DA CK. On the other side, the present study added that the sum of sale prices of all investigated dairy cows' milk production was improved due to therapy and due to the significant improvement in milk yield. These findings supported by McArt et al. (2012), who said that cows that had SCK within the first-week post-calving (3–7 DIM) produced less milk (Produced 2.2 kg less milk per day) in the first 30 DIM than cows developing SCK after the first week of lactation (at 8 DIM or later). BCS values in ketotic cows either before treatment at day 0 or after treatment at days 7 and 14 were within the physiological reference range reported by Ferguson et al. (1994). These results relatively were supported by Khalphallah et al. (2018a), who stated that BCS was not significantly changed either in close-up or post-fresh periods when its values in control cattle were compared with those of diseased one. Edwards and Tozer (2004) mentioned that ketosis was usually associated with a gradual drop of body condition over several days or weeks. Previous studies reported that most dairy cows face NEB in early lactation; postpartum feed intake was lower in cows with higher BCS prepartum, leaving them in NEB for a more extended period than cattle with normal or low BCS (Hayirli et al., 2002). The insulin concentrations were gradually increased with therapy after treatment at day 0, however, their values did not reach the reference values which were reported by Holtenius and Holtenius (2007) at days 14 that indicated hypoinsulinaemia, as well as further observation of the animals, was requested until restoring physiological reference values of insulin. The other studies mentioned that the metabolic pattern in induced or spontaneous hepatic lipidosis and ketosis included low serum insulin concentration and plasma glucose (Veenhuizen et al., 1991; Drackley, 1999; Khalphallah et al., 2015). Cows with primary ketosis have a low concentration of plasma glucose and insulin (Hejel et al., 2018). A reduced insulin concentration further facilitated the net mobilisation of adipose tissue and thus increased the hepatic uptake of fatty acids, as reviewed by Hayirli (2006). Agenäs et al. (2003) added that reduced feed intake leads to a rapid decrease in glucose and insulin concentrations. The current work revealed the high efficacy of therapy in ketotic cows with insulin (200 IU SC) parallel with IV glucose 50% (500 ml) and dexamethasone (20 mg/450 kg) once daily every other day. This regime should be used alternatively with dextrose 50% and for successive 5 days. Sakai et al. (1993) stated that ketotic cows treated with daily intravenous glucose recovered later than those treated with intravenous glucose and subcutaneous insulin injection for 4 days. Furthermore, Radostits et al. (2007) and Constable et al. (2017) supported the current results. They reported that cows' response with primary ketosis to treatment with corticosteroids and IV glucose was superior to therapy with corticosteroids or IV glucose alone, with fewer relapses. Other reports added that insulin enhanced cellular uptake of glucose, inhibit lipolysis and stimulated hepatic gluconeogenesis. It was administered together with either glucose, or a glucocorticoid and might be efficient in early-onset ketosis, which was not responded to glucose or corticosteroid (Djokovic et al., 2007; Radostits et al., 2007); consequently, IV dextrose administration should always be considered as a dual treatment of glucose and insulin (Djokovic et al., 2007; Constable et al., 2017). Tufani et al. (2011) concluded that hormonal therapy with dexamethasone or nandrolone associated with parenteral glucose and B-complex (B1, B2 and B3) provided an excellent recovery rate and significantly less evidence of relapse. McArt et al. (2011) added that Oral propylene glycol administration was effective sharply preventive for ketosis prevention and treatment in dairy cows and produced a prominent elevation in milk yield. The importance of administration of exogenous insulin in bovine ketosis therapy that was concluded in the present article was confirmed by Hayirli et al. (2002) and Hayirli (2006), who said that the occurrence of lowered responsiveness and sensitivity of extrahepatic tissues to insulin as a result of NEB should be relieved by improving nutritional status, which increases the efficiency of exogenous insulin for treating hepatic lipidosis and ketosis to maintain health and improve performance. Grünberg et al. (2006), Radostits et al. (2007) and Grünberg et al. (2011) stated that 500 ml of glucose solution 50% when IV administrated, it led to an increase insulin secretion and a decrease in glucagon release as well as transient hyperglycemia with lowered plasma concentration of NEFAs. Most diseased cattle showed marked improvement after IV Glucose, but relapses were reported commonly if repeated treatments were not applied. The obtained results revealed that RQUICKI values were remarkably increased in treated cows at days 7 and 14 when their values compare with value at day 0 (pre-treatment). RQUICKI was also over the physiological reference range reported by Stengärde et al. (2010). The previous reports added that decreased RQUICKI values indicated reduced insulin sensitivity (Perseghin et al., 2001; Rabasa-Lhoret et al., 2003; Bossaert et al., 2009). A negative linear relationship was proved between RQUICKI and body condition in lactating dairy cows (Holtenius and Holtenius 2007). Over-conditioned cows had been shown to have a decreased insulin response or reduced insulin sensitivity (Rukkwamsuk et al., 1998; Holtenius et al., 2003). The current study stated that the elevation in RQUICKI values with treatment (at days 7 and 14) in ketotic cows indicated an improvement in insulin sensitivity and the success of ketosis's therapeutic program. In contrast, the previous articles reported that RQUICKI values were not correlated with insulin sensitivity in ketotic cows and cattle with signs of puerperal metritis (Kerestes et al., 2009). Here, the reported results said that the insulin sensitivity was improved on therapy in ketotic cows that indicated the high efficacy of the applied therapeutic regimen. The other previous report stated that cows with ketosis had low tissue responsiveness to insulin (Grünberg et al., 2006), and ketoacidosis was one reason for insulin resistance (Van Putten et al., 1985; Steen et al., 1997). The importance of estimation of serum gastrin, pepsinogen and chloride here were due to the complications which occurred as a consequence to ketosis in the present study as out of the 20 (100%) cow, 8 (40%) was suffering from DA and 5 (25%) was suffering from the retained placenta, and 2 (10%) was suffering from mastitis. 5 (25%) of the cows had no complications. On the other hand, the reported results mentioned several changes in abomasal functions monitors, including gastrin, pepsinogen and chlorides. Generally, an improvement of the abomasal functions was reported as out of the ketotic cows (n=20), many cows (n=13) were associated with DA and other fatty liver-related diseases (retained placenta), and therefore, the changes in abomasal function monitors was related to the recovery of ketotic cattle and the high efficacy of the therapeutic strategy. The current study stated that serum gastrin value was significantly increased in post-treated ketotic cows at days 7 and 14. Throughout the study, gastrin levels in ketotic cow had higher serum gastrin concentration than the physiological reference range mentioned by Perry et al. (1988) and Ok et al. (2001). Khalphallah et al. (2018b) stated that serum gastrin levels in DA cow with SCK and DA with CK either before or after operation were higher than the physiological reference values of blood gastrin in cows. Ozturk et al. (2013) stated an elevation in serum gastrointestinal motility hormone concentrations, including ghrelin, motilin and gastrin in DA cattle. Increases in the serum ghrelin, motilin and gastrin concentrations were attributable to activation of gastrointestinal motility hormones to facilitate gastric emptying in impaired gastric motility and/or outlet occlusion in DA. Serum pepsinogen concentrations were remarkably reduced after therapy, particularly on day 14. Throughout the study (pre-and post-therapy), serum pepsinogen levels in the ketotic cows were within the physiological reference values mentioned by Ohwada et al. (2002). Khalphallah et al. (2018b) mentioned that serum pepsinogen concentrations were not remarkably changed in DA cattle with either SCK or CK when their values at days 7 or 30 compared with those at day 0. They were within the physiological reference values. In contrast, Ohwada et al. (2002) proved that serum pepsinogen concentrations in DA cows were remarkably decreased compared with a healthy one. Some authors thought that the estimation of pepsinogen levels in the blood could be diagnostic for cattle's abomasal changes (Mesarič et al., 2002). Mesarič (2005) found that serum pepsinogen concentration was a fair reflection of the damage to the abomasal mucosa. Low or normal levels of serum pepsinogen activity (<5.0 U/l) might be useful as a predictor for low susceptibility for significant changes to the mucous membrane of the abomasum. Mesarič et al. (2002) concluded that serum pepsinogen levels above 5.0 IU/l in cattle denote a severe injury of the abomasal mucosa. From the obtained results in the current study, there were no significant changes in serum chlorides levels throughout the study period, either pre-treatment or post-treatment. Chloride concentrations were within the physiological reference range as recorded by Radostits et al. (2007). In contrast, it was reported that cows with ketosis were associated with hypochloremia, as reported by Ok et al. (2001). ConclusionThe reported results revealed the high efficacy of the applied therapeutic strategy regime. The exposure of diseased ketotic dairy cattle for this therapeutic strategy greatly impacted their recovery from ketosis and improved their health and productivity status. The combination between hormonal therapy as insulin (2 ml per animal SC/48 hours for 5 days=3 doses), and glucocorticoids as dexamethasone (0.04 mg/kg IM /48 hours for 5 days=3 doses), as a single IM injection over a 1–3 minutes’ period following adequate fluid volume replacement (IV of 500 ml glucose solution 50%/48 hours for 5 days=3 doses alternative with IV of 500 ml dextrose solution 50%/48 hours for 5 days=2 doses) with drenching of propylene glycol (300 ml/12 hours for 2 days followed by 150 ml/24 hours for 3 days) for successive 5 days and IM injection of Vitamin B12 (5 ml/100 kg/48 hours for 5 days=3 doses) led to high recovery rate and very low relapse rate for ketosis. This was reflected through clear improvement in clinical findings, milk yield rates, insulin sensitivity and abomasal function monitors. Hypoinsulinaemia was still reported until day 14, however, serum insulin was improved. Therefore, further observation (>14 days) of the animals was required until insulin levels restored their physiological reference values. Conflict of interestThe authors declare that they have no conflicts of interest. Authors' contributionAK, EE, and SAM conceived, designed, and supervised the project. AK, EE, DH, SAM, RHM, and AAA conducted the practical part of the study. AK, AH, and EE conducted the laboratory assays. SO, AH, DH, and SAB conducted the statistical analyses. All the authors interpreted the data, wrote, and critically revised the manuscript for intellectual content and approved the final version. SO, AK and EE had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ReferencesAgenäs, S., Dahlborn, K. and Holtenius, K. 2003. Changes in metabolism and milk production during and after feed deprivation in primiparous cows selected for different milk fat content. Livest. Prod. Sci. 83, 153–164. Anderson, D.E. and Rings, D.M. 2009. Current veterinary therapy. In: Food animal practice. Eds. 5th ed. Philadelphia, PA: W.B. Saunders Company, pp: 756. Bossaert, P., Leroy, J.L.M.R., De Campeneere, S., De Vliegher, S. and Opsomer, G. 2009. Differences in the glucose-induced insulin response and the peripheral insulin responsiveness between neonatal calves of the Belgian Blue, Holstein-Friesianand East Flemish breeds. J. Dairy Sci. 92(9), 4404–4411. Cameron, R.E.B., Dyk, P.B., Herdt, T.H., Kaneene, J.B., Miller, R., Bucholtz, H.F., Liesman, J.S., Vandehaar, M.J. and Emery, R.S. 1998. Dry cow diet, management, and energy balance as risk factors for displaced abomasum in high producing dairy herds. J. Dairy Sci. 81, 132–139. Cao, Y., Zhang, J., Yang, W., Xia, C., Zhang, H.Y., Wang, Y.H. and Xu, C. 2017. Predictive value of plasma parameters in the risk of postpartum ketosis in dairy cows. J. Vet. Res. 61, 91–95. Cockcroft, P.D. 2015. Diagnosis and clinical reasoning in cattle practice. In: Bovine medicine. Eds., Cockcroft, P. D. 3rd ed. Hoboken, NY: John Wiley and Sons, pp: 124–132. Coles, E.H. 1986. Veterinary clinical pathology, 4th ed. Philadelphia, PA, W.B. Saunders, pp: 132–139. Constable, P.D., Hinchcliff, K.W., Done, S.H. and Grünberg, W. 2017. Veterinary medicine. A textbook of the diseases of cattle, horses, sheep, pigs and goats, 11th ed. Philadelphia, PA: Saunders Elsevier, pp: 1708–16. Curtis, C.R., Erb, H.N., Sniffen, C.J., Smith, R.D. and Kronfeld, D.S. 1985. Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows. J. Dairy Sci. 68(9), 2347–2360. DeBoer, G., Trenkle, A. and Young, J.W. 1985. Glucagon, insulin, growth hormone, and some blood metabolites during energy restriction ketonemia of lactating cows. J. Dairy Sci. 68, 326–337. Djokovic, R., Šamanc, H., Nikolić, Z. and Bošković Bogosavljević, S. 2007. Changes in blood values of glucose, insulin and inorganic phosphorus in healthy and ketotic dairy cows after intravenous infusion of propionate solution. Acta. Vet. Brno. 76(4), 533–539. Dohoo, I.R. and Martin, S.W. 1984. Subclinical ketosis: prevalence and associations with production and disease. Can. J. Comp. Med. 48, 1–5. Dohoo, I.R., Martin, S.W., Meek, A.H. and Sandals, W.C.D. 1984. Disease, production and culling in Holstein Friesian cows. I. The data. Prevent. Vet. Med. 1, 321–334. Drackley, J.R. 1999. Biology of dairy cows during the transition period: the final frontier? J. Dairy Sci. 82, 2259–2273. Duffield, T. and Bagg, R. 2002. Herd level indicators for the prediction of high-risk dairy herds for subclinical ketosis. Proc. Am. Assoc. Bov. Pract. 35, 175–176. Edwards, J.L. and Tozer, P.R. 2004. Using activity and milk yield as predictors of fresh cow disorders. J. Dairy Sci. 87, 524–531. Ferguson, J.D., Galligan, D.T. and Thomsen, N. 1994. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 77, 2695–2703. Grünberg, W., Hartmann, H., Burfeind, O., Heuwieser, W. and Staufenbiel, R. 2011. Plasma potassium-lowering effect of oral glucose, sodium bicarbonate and the combination thereof in healthy neonatal dairy calves. J. Dairy Sci. 94, 5646–5655. Grünberg, W., Morin, D.E., Drackley, J.K. and Constable, P.D. 2006. Effect of rapid intravenous administration of 50% dextrose solution on phosphorus homeostasis in postparturient dairy cows. J. Vet. Intern. Med. 20, 1471–1478. Hayirli, A. 2006. The role of exogenous insulin in the complex of hepatic lipidosis and ketosis associated with insulin resistance phenomenon in postpartum dairy cattle. Vet. Res. Commun. 30, 749–774. Hayirli, A., Grummer, R.R., Nordheim, E.V. and Crump, P.M. 2002. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J. Dairy Sci. 85, 3430–3443. Hejel, P., Zechner, G., Csorba, C. and KÖnyves, L. 2018. Survey of ketolactia, determining the main predisposing management factors and consequences in Hungarian dairy herds by using a cow-side milk test. Vet. Rec. Open 5, e000253. Holtenius, K., Agenas, S., Delavaud, C. and Chilliard, Y. 2003. Effects of feeding intensity during the dry period. 2. Metabolic and hormonal responses. J. Dairy Sci. 86, 883–891. Holtenius, P. and Holtenius, K. 1996. New aspects of ketone bodies in energy metabolism of dairy cows: a review. J. Vet. Med. A. 43(10), 579–587. Holtenius, P. and Holtenius, K. 2007. A model to estimate insulin sensitivity in dairy cows. Acta. Vet. Scand. 49, 29. Iwersen, M., Falkenberg, U., Voigtsberger, R., Forderung, D. and Heuwieser, W. 2009. Evaluation of an electronic cowside test to detect subclinical ketosis in dairy cows. J. Dairy Sci. 92, 2618–2624. Jackson, P.G.G. and Cockcroft, P.D. 2002. Clinical examination of farm animals, 1st ed. Ames, IA: Wiley-Blackwell Science Ltd, pp: 301–305. Kataria, N., Kataria, A.K. and Gahlot, A.K. 2008. Use of plasma gastrin and pepsinogen levels as diagnostic markers of abomasal dysfunction in marwari sheep of arid tract. Slov. Vet. Res. 45(4), 121–126. Kerestes, M., Faigl V., Kulcsár, M., Balogh, O., Földi, J., Fébel, H., Chilliard, Y. and Huszenicza, G. 2009. Periparturient insulin secretion and whole-body insulin responsiveness in dairy cows showing various forms of ketone pattern with or without puerperal metritis. Domest. Anim. Endocrin. 37(4), 250–261. Khalphallah, A., Aamer, A.A., AbdelAll, TH., Elmeligy, E., Oikawa, S. and Nakada, K. 2018a. Changes in clinical and blood lipid metabolism parameters in holstein dairy cattle during the transition period. Bulg. J. Vet. Med. 21(4), 420–428. Khalphallah, A., Aamer, A.A., AbdelAll, TH., Katoh, H., Oikawa, S., Nakada, K. and Elmeligy, E. 2015. Assessment of insulin and insulin resistance in dairy cattle with displaced abomasum before and after surgery. Sch. Adv. Anim. Vet. Res. 2(3), 162–176. Khalphallah, A., Elmeligy, E., Aamer, A.A., AbdelAll, TH., Oikawa, S. and Nakada, K. 2018b. Diagnostic and prognostic significance of serum gastrin and pepsinogen in displaced abomasum dairy cows. Bulg. J. Vet. Med. 21(1), 67–75. Kokkonen, T., Taponen, J., Anttila, T., Syrjälä-Qvist, L., Delavaud, C., Chilliard, Y., Tuori, M. and Tesfa, AT. 2005. Effect of body fatness and glucogenic supplement on lipid and protein mobilization and plasma leptin in dairy cows. J. Dairy Sci. 88(3), 1127–1141. Madreseh-Ghahfarokhi, S., Dehghani-Samani, AZ. and Dehghani-Samani, AM. 2018. Ketosis (acetonaemia) in dairy cattle farms: practical guide based on importance, diagnosis, prevention and treatments. J. Dairy. Vet. Anim. Res. 7(6), 299‒302. McArt, J.A.A., Nydam, D.V. and Oetzel, G.R. 2012. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 95, 5056–5066. McArt, J.A.A., Nydam, D.V., Ospina, P.A. and Oetzel, G.R. 2011. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J. Dairy Sci. 94(12), 6011‒6020. McCance, K.L. and Huether, S.E. 1994. Alterations of hormonal regulations. pathophysiology, 2nd ed. St Louis, MO: Mosby, pp: 674–692. Mesarič, M. 2005. Role of serum pepsinogen in detecting cows with abomasal ulcer. Vet. Arh. 75, 111–118. Mesarič, M., Zadnik T. and Klinkon, M. 2002. Comparison of serum pepsinogen activity between enzootic bovine leukosis (EBL) positive beef cattle and cows with abomasal ulcers. Slov. Vet. Res. 39, 227–232. Ohwada, S., Oikawa, S., Mori, F., Koiwa, M., Nitanai, A., Kurosawa, T. and Sato, H. 2002. Serum pepsinogen concentrations in healthy cows and their diagnostic significance with abomasal diseases. J. Rakuno Gakuen Univ. 26, 289–293. Ok, M., Sen, I., Turgut, K. and Irmak, K. 2001. Plasma gastrin activity and the diagnosis of bleeding abomasal ulcers in cattle. J. Vet. Med. A. 48, 563–568. Ozturk, A.S., Guzel, M., Askar, T.K. and Aytekin, I. 2013. Evaluation of the hormones responsible for the gastrointestinal motility in cattle with displacement of the abomasum; ghrelin, motilin and gastrin. Vet. Rec. 172(24), 1. Perry, K.W., Weekes, T.E., Rooke, J.A. and Parker, D.S., Armstrong, D.G. 1988. Effect of protein intake on gastrin secretion in ruminants. J. Exp. Physiol. 73, 985–993. Perseghin, G., Caumo, A., Caloni, M., Testolin, G. and Luzi, L. 2001. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in non-obese individuals. J. Clin. Endocrinol. Metab. 86, 4776–4781. Rabasa-Lhoret, R., Bastard, J.P., Jan, V., Ducluzeau, P.H., Andreelli, F., Guebre, F., Bruzeau, J., Louche-Pellissier, C., Maîtrepierre, C., Peyrat, J., Chagne, J., Vidal, H. and Laville, M. 2003. Modified quantitative insulin sensitivity check index is better correlated to hyperinsulinemic glucose clamp than other fasting-based index of insulin sensitivity in different insulin- resistant states. J. Clin. Endocrinol. Metab. 88, 4917–4923. Radostits, O.M., Gay, C.C., Blood, D.C. and Hinchcliff, K.W. 2000. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats and horses, 9th ed. London, UK: W. B. Saunders, pp: 1819–1822. Radostits, O.M., Gay, C.C., Hinchcliff, K.W. and Constable, P.D. 2007. Veterinary Medicine. A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th ed. Philadelphia, PA, Saunders Elsevier, pp: 3–37, 1661–1668. Rukkwamsuk, T., Wensing, T. and Geelen, M.J.H. 1998. Effect of overfeeding during the dry period on regulation of adipose tissue metabolism in dairy cows during the periparturient period. J. Dairy Sci. 81(11), 2904–2911. Sakai, T., Hayakawa, T., Hamakawa, M., Ogura, K. and Kubo, S. 1993. Therapeutic effects of simultaneous use of glucose and insulin in ketotic dairy cows. J. Dairy Sci. 76, 109–114. Sakha, M., Ameri, M., Sharifi, H. and Taheri, I. 2007. Bovine subclinical ketosis in dairy herds in Iran. Vet. Res. Commun. 31, 673–679. Steen, A., Grantor, H. and Tureen, P.A. 1997. Glucose and insulin responses to glucagon injection in dairy cows with ketosis and fatty liver. Zentralbl Veterinarmed A. 44, 521–530. Stengärde, L.U., Holtenius, K., Traven, M., Hultgren, J., Niskanen, R. and Emanuelson, U. 2010. Blood profiles in dairy cows with displaced abomasum. J. Dairy Sci. 93, 4691–4699. Tehrani-Sharif, M., Tehrani-Sharif, M., Hadadi, M., Noughabi, H.H., Mohammadi, A., Rostami, F. and Sharifi, H. 2012. Bovine subclinical ketosis in dairy herds in Nishaboor, Iran. Comp. Clin. Pathol. 21, 1637–1641. Tufani, N.A., Hafiz, A., Muhee, A. and Makhdoomi, D.M. 2011. Therapeutic management of ketosis in bovine. Indian J. Vet. Med. 31(1), 38–39. Upham, G.L. 1996. A practitioner approach to management of metritis/endometritis early detection and supportive treatment. Tulare, CA, Veterinarian's Outlet, vol 29, pp: 19–21. Van Knegsel, A.T.M., van den Brand, H., Graat, E.A.M., Dijkstra, J., Jorritsma, R., Decuypere, E., Tamminga, S. and Kemp, B. 2007. Dietary energy source in dairy cows in early lactation: metabolites and metabolic hormones. J. Dairy Sci. 90(3), 1477–1485. Van Putten, J., Wieringa, P.T. and Krans, H.M. 1985. Low pH and ketoacidosis induce insulin receptor binding and postbinding alterations in cultured 3T3 adipocytes. Diabetes 34, 744–750. Van Winden, S.C.L. and Kuiper, R. 2003. Left displacement of the abomasum in dairy cattle: recent developments in epidemiological and etiological aspects. Vet. Resch. 34, 47–56. Veenhuizen, J.J., Drackley, J.K., Richard, M.J., Sanderson, T.P., Miller, L.D. and Young, J.W. 1991. Metabolic changes in blood and liver during development and early treatment of experimental fatty liver and ketosis in cows. J. Dairy Sci. 74, 4238–4253. | ||
| How to Cite this Article |
| Pubmed Style Elmeligy E, Oikawa S, Mousa SA, Bayoumi SA, Hafez A, Mohamed RH, Al-lethie AA, Khalphallah A. Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. Open Vet. J.. 2021; 11(2): 228-237. doi:10.5455/OVJ.2021.v11.i2.7 Web Style Elmeligy E, Oikawa S, Mousa SA, Bayoumi SA, Hafez A, Mohamed RH, Al-lethie AA, Khalphallah A. Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. https://www.openveterinaryjournal.com/?mno=50157 [Access: January 17, 2026]. doi:10.5455/OVJ.2021.v11.i2.7 AMA (American Medical Association) Style Elmeligy E, Oikawa S, Mousa SA, Bayoumi SA, Hafez A, Mohamed RH, Al-lethie AA, Khalphallah A. Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. Open Vet. J.. 2021; 11(2): 228-237. doi:10.5455/OVJ.2021.v11.i2.7 Vancouver/ICMJE Style Elmeligy E, Oikawa S, Mousa SA, Bayoumi SA, Hafez A, Mohamed RH, Al-lethie AA, Khalphallah A. Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. Open Vet. J.. (2021), [cited January 17, 2026]; 11(2): 228-237. doi:10.5455/OVJ.2021.v11.i2.7 Harvard Style Elmeligy, E., Oikawa, . S., Mousa, . S. A., Bayoumi, . S. A., Hafez, . A., Mohamed, . R. H., Al-lethie, . A. A. & Khalphallah, . A. (2021) Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. Open Vet. J., 11 (2), 228-237. doi:10.5455/OVJ.2021.v11.i2.7 Turabian Style Elmeligy, Enas, Shin Oikawa, Sabry A. Mousa, Sara A. Bayoumi, Ahmed Hafez, Ragab H. Mohamed, Al-lethie A. Al-lethie, and Arafat Khalphallah. 2021. Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. Open Veterinary Journal, 11 (2), 228-237. doi:10.5455/OVJ.2021.v11.i2.7 Chicago Style Elmeligy, Enas, Shin Oikawa, Sabry A. Mousa, Sara A. Bayoumi, Ahmed Hafez, Ragab H. Mohamed, Al-lethie A. Al-lethie, and Arafat Khalphallah. "Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates." Open Veterinary Journal 11 (2021), 228-237. doi:10.5455/OVJ.2021.v11.i2.7 MLA (The Modern Language Association) Style Elmeligy, Enas, Shin Oikawa, Sabry A. Mousa, Sara A. Bayoumi, Ahmed Hafez, Ragab H. Mohamed, Al-lethie A. Al-lethie, and Arafat Khalphallah. "Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates." Open Veterinary Journal 11.2 (2021), 228-237. Print. doi:10.5455/OVJ.2021.v11.i2.7 APA (American Psychological Association) Style Elmeligy, E., Oikawa, . S., Mousa, . S. A., Bayoumi, . S. A., Hafez, . A., Mohamed, . R. H., Al-lethie, . A. A. & Khalphallah, . A. (2021) Role of insulin, insulin sensitivity and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. Open Veterinary Journal, 11 (2), 228-237. doi:10.5455/OVJ.2021.v11.i2.7 |