| Review Article | ||
Open Vet. J.. 2021; 11(2): 251-269 Open Veterinary Journal, (2021), Vol. 11(2): 251–269 Review Article The MTT assay application to measure the viability of spermatozoa: A variety of the assay protocolsKakanang Buranaamnuay*Molecular Agricultural Biosciences Cluster, Institute of Molecular Biosciences (MB), Mahidol University, Nakhon Pathom, Thailand *Corresponding Author: Kakanang Buranaamnuay. Molecular Agricultural Biosciences Cluster, Institute of Molecular Biosciences (MB), Mahidol University, Nakhon Pathom, Thailand. Email: ningkakanang [at] yahoo.com Submitted: 29/01/2021 Accepted: 08/04/2021 Published: 08/05/2021 © 2021 Open Veterinary Journal
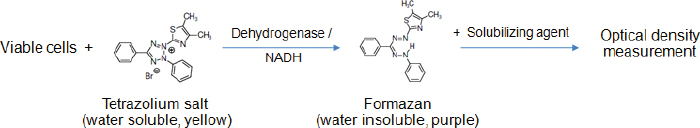
AbstractThe 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay is one of the methods used to evaluate the viability of sperm. In the assay, a tetrazolium component (MTT) is converted into MTT formazan by some specific enzymes in the viable cells. The amount of formazan product in theory is directly correlated with the percentage of viable sperms. It is quantified by measuring the absorbance using a spectrophotometer. The present article compiles the MTT assays that have been used to determine sperm viability in most animal species and humans. In each assay, other factors apart from the number of viable cells that potentially influence the accuracy and precision of results are stated, such as preparations of sperm and MTT solutions, length and conditions of incubation, and a solubilizing agent as well as the formazan detection method. Also, the strengths and shortcomings of the MTT test comparison with the others are summarized at the end of this article. This information may be useful for prospective researchers deciding to implement this colorimetric method in their experiments. Keywords: Colorimetric assay, Metabolic activity, Spectrophotometer, Sperm. IntroductionIn addition to good-quality oocytes, healthy sperms are also important for successful fertilization. One of the sperm characteristics generally evaluated, especially in sperm with low or no motility, is viability. The sperm viability is determined by identifying the cells with an intact plasma membrane, either by carrying out dye exclusion tests using supravital stain or hypo-osmotic swelling test (Kumar et al., 2017). Alternatively, sperm viability can be assessed indirectly through measuring cellular metabolic activity using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay (Aziz et al., 2005). However, the MTT assay protocols reported thus far have been widely divergent, depending on the species of sperm donor and authors who performed the assay, and there are no standardized methods. The present article provides underlying principles, advantages, and disadvantages of the MTT assay. It describes in detail the MTT assay protocols used for measuring the viability of sperm in many species, with the aim of aiding prospective researchers to (1) understand more about how this assay works and (2) be able to identify pivotal points that influence the reliability of the assay which must be considered while carrying out measurements. The principle of the MTT assayThe MTT assay is a colorimetric assay originally described by Mosmann (1983). It is performed to measure metabolic activity in living cells. The underlying principle of this test is based on the reduction of water soluble, yellow colored tetrazolium salt MTT to a non-water-soluble purple formazan crystals by introducing metabolically active cells. The succinate dehydrogenase system of the active mitochondria is responsible for this conversion (Kalina and Palmer, 1968; Altman, 1976). However, it has recently been proposed that the co-enzyme nicotinamide adenine dinucleotide (reduced form) (NADH), which is the product of glycolysis in the cytoplasm and oxidative phosphorylation in the mitochondria, is the major reducing agent accountable for MTT reduction (Diaz et al., 2007; van den Berg, 2015). The needle-shaped formazan crystals are further dissolved in organic solvents or oils. The resulting colored solution is measured for optical density (OD) at a specific wavelength between 500 and 600 nm, using a spectrophotometer or a plate reader (Morgan, 1998; Lin et al., 2019). The intensity of formazan color is proportional to the number of viable, metabolically active cells, which can thus be used to estimate the viability of various cell types, including sperm (Aziz, 2006; Byun et al., 2008). A schematic describing a metabolic reaction of MTT to formazan salts by viable cells is shown in Figure 1. The MTT assay for sperm viability assessmentIrrespective of species, two formats of the MTT assay have been developed to determine sperm viability, i.e., wet (or in tubes/plates) and dry (or on papers) formats. The former is traditional, simpler, and commonly used with most species. At the same time, the latter has been invented recently, which seems complicated in the development process and hence is exploited mainly on humans, by some researchers. The following contents include numerous MTT assay protocols performed on the sperm of animals and humans. In each protocol, sperm type (fresh, refrigerated, or frozen-thawed), preparation of MTT solution, sperm-MTT incubation condition, solubilizing agent, the method for detection of formazan, and interpretation of results are described.
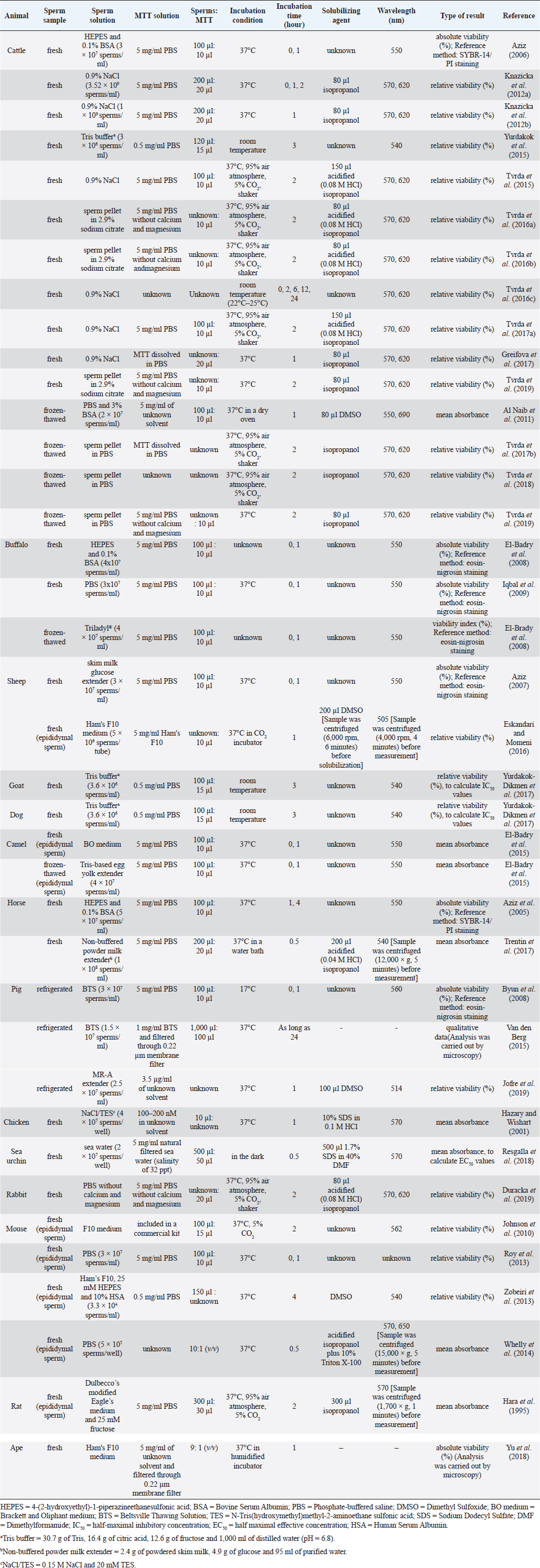
Fig. 1. Reduction of the MTT tetrazolium salt to the formazan in viable cells; Modified from Wang et al. (2014). Conventional MTT assayMTT assay and bull spermAccording to previous reports, the MTT assay was used to determine the viability of bovine sperm both in fresh and frozen-thawed samples. For fresh semen, ejaculates were collected from breeding bulls with Holstein Friesian (HF) (Tvrda et al., 2015), Simmental (Yurdakok et al., 2015), and unidentified breeds (Tvrda et al., 2016c; Greifova et al., 2017). Each semen sample was immediately diluted in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) with 0.1% bovine serum albumin (BSA) (Aziz, 2006), 0.9% NaCl (Knazicka et al., 2012a, 2012b; Tvrda et al., 2016c), and a Tris-based extender (Yurdakok et al., 2015) or was centrifuged and resuspended in 2.9% sodium citrate (pH 7.4) (Tvrda et al., 2016a, 2016b), using a dilution ratio of 1:40 (v/v) (Knazicka et al., 2012a, 2012b) or to acquire sperm concentrations of 3.6 × 106–3 × 107 sperms/ml (Yurdakok et al., 2015; Aziz, 2006). In the 96-well microplate, 100–200 μl of diluted semen sample (3 × 106–3.52 × 109 sperms/ml) and MTT stock solution were placed in each well, at an approximate ratio of 10:1, v/v (Tvrda et al., 2015, 2017a). A stock solution of MTT could be prepared beforehand by dissolving MTT tetrazolium salt (Sigma-Aldrich, St. Louis, MO) in a physiologically balanced solution, e.g., phosphate-buffered saline (PBS) (Aziz, 2006; Knazicka et al., 2012b) and PBS without calcium and magnesium (Tvrda et al., 2016a, 2016b, 2019) to obtain concentrations of 0.5 mg/ml (Yurdakok et al., 2015) or 5 mg/ml (Aziz, 2006; Tvrda et al., 2017a). This stock solution can last up to 18 months if stored in proper conditions, i.e., in a dark and cool (4°C or lower) place without contamination (Barnabe, 2017). Thereafter, the plate was placed at room temperature (Yurdakok et al., 2015) or 37°C (Greifova et al., 2017; Tvrda et al., 2019) in 95% air atmosphere, 5% CO2, and shaker (Tvrda et al., 2015) for 1–3 hours (Aziz, 2006; Yurdakok et al., 2015), where 2 hours was generally preferred (Knazicka et al., 2012a; Tvrda et al., 2015, 2016a, 2016b, 2017a). After incubation, the formazan crystals that had formed were dissolved in 80–150 μl of solubilizing agents, i.e., isopropanol (Greifova et al., 2017) and acidified (0.08 M HCl) isopropanol (Tvrda et al., 2016a, 2016b). The OD of samples was determined using a microplate ELISA reader at a wavelength of 540 nm (Yurdakok et al., 2015), 550 nm (Aziz, 2006), or 570 and, as a reference, 620 nm (Knazicka et al., 2012a, 2012b). The OD for each sample was calculated by concurring the differences between OD570 and OD620 or between the first (0 hour incubation) and second (1 hour incubation) reading (Aziz, 2006). In general, the analysis results were collected during 2–6 repeated measurements of each sperm sample (Aziz, 2006; Tvrda et al., 2016c). The viability of sperm acquired by the MTT assay was reported either as absolute viability percentage or relative viability, compared to 100% of the untreated control. The relative viability was more often found and has traditionally been used in toxicity studies (Yurdakok et al., 2015; Tvrda et al., 2015, 2017a, 2019). To obtain an absolute percentage of sperm viability by the MTT assay, it is necessary to validate this test by comparing its efficiency with a reference method such as SYBR-14/propidium iodide (PI) fluorescence staining with flow cytometric examination (Aziz, 2006). Different proportions of living and dead sperms are made in this study, such as 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10 (v/v), respectively. The prepared samples are analyzed for viability both by MTT assay and the reference method. The relationship between the MTT reduction rate (OD) and sperm viability (%) is created by plotting a standard curve. A regression equation (Y=a + bX) for their relationship is also calculated. This curve and equation are applied later to calculate the percentage of viable sperms in unknown samples based on MTT reduction rates. For instance, in the study reported by Aziz (2006) where X was the OD550 and Y was sperm viability (%), a regression equation of Y=104.583X − 8.877 was obtained. The OD550 of semen in bull number 1 was 0.602 ± 0.05; therefore, the sperm viability of this bull would be Y=104.583 (0.602 ± 0.05) – 8.877=54.06 ± 4.90%, compared with 51.65% ± 5.23% evaluated by SYBR-14/PI. This study found a significant correlation between the sperm viability determined by MTT assay and SYBR-14/PI (r =0.950) (Aziz, 2006). In contrast, to acquire the relative viability of sperm in toxicity studies, it needs to prepare the (negative) control sample composed only of sperms and the medium having its OD. The ODcontrol is set to 100% viable sperms. The OD of samples that have been exposed to substances of interest with different doses and times is measured and then compared with ODcontrol. The ODsamples (relative viability) can therefore be higher, in case of substances having a protective effect, or lower, in the event of substances that are toxic to sperm, than 100%. The examples of the substances that were added to fresh bovine sperms and had effects on their viability as determined by the MTT assay are copper (Cu) (Knazicka et al., 2012a, 2012b), iron (Fe2+, Fe3+) (Tvrda et al., 2015), quercetin (Tvrda et al., 2016b), epicatechin (Greifova et al., 2017; Tvrda et al., 2019), polychlorinated biphenyls (Yurdakok et al., 2015), curcumin (Tvrda et al., 2016a), lycopene (Tvrda et al., 2016c), and Salvia officinalis (Tvrda et al., 2017a). Apart from the fresh semen sample, frozen-thawed bovine sperms were also determined, but to a lesser extent, the mitochondrial activity and viability by the MTT assay. According to the existing data, the semen was collected from HF, Limousin, and Simmental-Fleckvieh breeding bulls and was frozen in a Tris-citric acid-egg yolk-based extender (Al Naib et al., 2011; Tvrda et al., 2017b, 2018). The thawed sperms were either washed by centrifugation (300 × g, 25°C, 5 minutes) and resuspended in PBS (Tvrda et al., 2019) or immediately diluted in PBS containing 3% BSA (Al Naib et al., 2011). In a microplate, the MTT stock solution (5 mg/ml PBS, 10 μl) was administered to the sperm suspension (2 × 107 sperms/ml, 100 μl) and incubated at 37°C for 1 hour in a dry oven (Al Naib et al., 2011) or for 2 hours in 95% air atmosphere, 5% CO2, and shaker (Tvrda et al., 2017b). Following incubation, 80 μl of dimethyl sulfoxide (DMSO) (Al Naib et al., 2011) or isopropanol, as solubilizing agents, was added to each well (Tvrda et al., 2018). The plate was left in the dark for about 1 minute to dissolve formazan crystals. The OD was assessed using a microplate spectrophotometer at both 550 nm (absorbance of formazan) and 690 nm (background absorbance) (Al Naib et al., 2011) or both 570 and 620 nm (Tvrda et al., 2019); then the results were subtracted from each other. The viability of each frozen-thawed sample was acquired from 2 to 5 independent experiments. Like fresh sperm, the viability of frozen-thawed bovine sperm examined by the MTT assay in toxicity studies was reported as relative values that percentage of the untreated control represented 100% (Tvrda et al., 2017b, 2018). Additionally, the results of the MTT assay were recorded as mean absorbance without transforming to percentage; for example, in the study of Al Naib et al. (2011), the mitochondrial metabolic activity in frozen-thawed sperm from high and low fertility HF bulls was not different (p=0.6); the mean OD was 0.34 ± 0.051 and 0.30 ± 0.044 for the high and low fertility bulls, respectively. The MTT assay protocols for bovine sperm viability assessment, both fresh and frozen-thawed samples, are demonstrated in Table 1. MTT assay and buffalo spermEvaluation of buffalo sperm viability using the MTT reduction assay was reported in only a couple of studies. In the first study, the relationship between the MTT reduction rate and sperm viability evaluated by eosin-nigrosin staining was determined in both fresh and frozen-thawed semen of the same buffalo bulls (El-Badry et al., 2008). Fresh semen was diluted with HEPES 0.1% BSA, while frozen semen was diluted with a commercial medium (Triladyl®). The final concentration of both sperm preparations was 4 × 107 sperms/ml. In each preparation, 11 different proportions of viable and freeze-killed sperms were made. The MTT reduction rate and viability of sperm were then evaluated. For the MTT assay, 100 μl of semen and 10 μl of MTT solution (5 mg MTT/ml of PBS) were placed in each of six wells of a 96-well microplate. After incubation, the MTT reduction rates were measured through an ELISA reader at a wavelength of 550 nm. The OD of each sample was read twice at 0 and 1 hour after incubation. The results were calculated from the difference between the first and second readings. The relationship between the MTT reduction rate (OD; X-axis) and sperm viability (%; Y-axis) in fresh and frozen-thawed semen containing different proportions of viable and killed sperms was created as the corresponding correlation curve and regression equation (Y=a + bX). These regression equations were applied as standards to calculate the sperm viability indices based on MTT reduction rates. According to the results, highly positive correlations between the MTT reduction rate and percentage of sperm viability were found in both fresh (r=0.906) and frozen-thawed semen (r=0.958) (El-Badry et al., 2008). In the following year, Iqbal et al. (2009) validated the MTT test to determine the sperm viability of 20 Nili-Ravi buffalo bulls by comparing the test’s efficiency with supravital (eosin-nigrosin) staining and hypo-osmotic swelling test. The fresh semen diluted with PBS (3 × 107 sperms/ml, 100 μl) was placed in each well of the microplate (6 wells/sample). MTT stock solution prepared in PBS (5 mg/ml, 10 μl) was then added. The OD was measured immediately and after incubation at 37°C for 1 hour using a spectrophotometer at a wavelength of 550 nm. MTT reduction rate for each sample was the difference between the first and second reading of the spectrophotometer. A regression equation for the relationship between OD550 (X-axis) and the sperm viability evaluated by eosin-nigrosin and hypo-osmotic swelling test (Y-axis) in 20 samples was calculated, as Y=a + bX, and later used to determine the viability of sperm in other samples. This study found that the OD was significantly correlated with the sperm viability appraised by a combination of eosin-nigrosin and hypo-osmotic swelling test (r=0.995), suggesting that MTT can be used to evaluate the sperm quality in Nili-Ravi buffalo bulls (Iqbal et al., 2009). Table 1. The MTT assay protocols for measuring viability of sperm in animals.
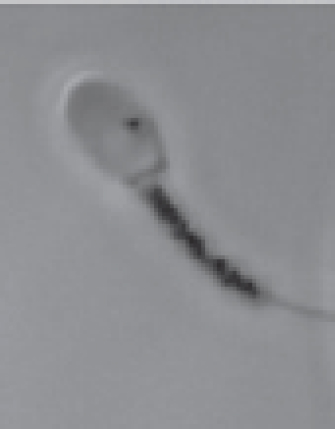
MTT assay and ram spermAccording to existing data, two articles are addressing the MTT assay in ram sperm. The first study aimed at investigating the diagnostic value of the MTT reduction assay compared with eosin-nigrosin staining and the microscopic examination to evaluate the percentage of live sperms. The relationship between MTT and eosin-nigrosin results depicted as the standard curve and regression equation was made. This curve and equation were applied later to calculate the percentage of sperm viability in unknown samples based on the MTT assay. To obtain the standard curve, fresh ejaculates with about 85% viable sperms were diluted with skim milk glucose extender to obtain a concentration of 3 × 107 sperms/ml. One fraction of semen was maintained at 37°C, while the other fraction was plunged into hot water at 60°C to induce sperm death. After combining aliquots of living and dead sperms in ratios, the percentage sperm viability in the samples was evaluated by eosin-nigrosin staining under the microscope and by the MTT reduction test. For the MTT assay, semen samples and MTT stock solution (10:1, v/v) were placed in each well of the 96-well microplate. The stock solution of the MTT was prepared earlier by resuspending tetrazolium salts in PBS to acquire 5 mg/ml in the concentration. The MTT reduction rates were taken immediately and after 1 hour of incubation at 37°C using a Microplate Reader at 550 nm. The mean absorbance of each sample was calculated from six values of six wells. A strong correlation (r2=0.979 to 0.983) between the MTT reduction rate and the result of the microscope was investigated, indicating that the MTT reduction test was applicable to evaluate the viability of ram sperm (Aziz, 2007). On the contrary, another study used the MTT reduction test to determine sperm survival after treating with sodium arsenite and/or silymarin (Eskandari and Momeni, 2016). Sperms were harvested from the caudal epididymis, washed, and resuspended in Ham’s F10 medium. In Eppendorf tubes, 10 μl of MTT stock solution (5 mg/ml of Ham's F10) was added to sperm suspension containing 5 × 106 sperms. The tubes were incubated at 37°C in CO2 incubator for 1 hour and then centrifuged. The precipitate was dissolved in 200 μl DMSO. After centrifugation at 4,000 rpm for 4 minutes, the purple solution was transferred to a 96-well plate. The absorbance was measured at 505 nm using an ELISA reader. The OD of the untreated sample symbolized that 100% of the solution was used for calculating sperm viability percentage. MTT assay and buck and dog spermBuck and dog sperms exposed in vitro to six selected pesticides—cypermethrin, flumethrin, propoxur, carbaryl, chlorpyrifos, and methamidophos—were used to determine the viability by the MTT reduction assay in the same study (Yurdakok-Dikmen et al., 2017). Semen samples collected from Angora buck and German shepherd male dog were used. Collected semen was put in Tris buffer (Tris, citric acid, and fructose in distilled water, pH 6.8) at 3.6 × 106 sperms/ml and was later transferred to 96-well plates (100 μl/well). Different doses of pesticides were added to the wells. The viability of sperm was measured through MTT assay performed according to the previous study on bull sperm (Yurdakok et al., 2015). In brief, 15 μl of MTT stock solution [PBS with 0.5% (w/v) MTT] was mixed with sperm suspension in the wells. The plates were placed at room temperature for 3 hours. They were quantified using a microplate reader at a wavelength of 540 nm. The viability of treated sperm, expressed as a percentage, was calculated with regard to 100% of the untreated cell control (sperms and buffer) and 0% of the dead cell control (sperms, buffer, and Triton-X). A plot of % viability vs. pesticide concentrations was used to calculate the half-maximal inhibitory concentration (IC50). A summary of the assay method is shown in Table 1. MTT assay and camel spermThe quality of the freezable sperm obtained from the caput, corpus, and cauda epididymides of dromedary camels was assessed (El-Badry et al., 2015). Sperms isolated from the epididymides were washed in Brackett and Oliphant (BO) medium. Some aliquots of fluid rich in sperms were further diluted with a Tris-based egg yolk extender to obtain 4 × 107 sperms/ml and were frozen in liquid nitrogen. Freshly harvested and frozen-thawed sperms were evaluated for functional integrity, including mitochondrial activity. For the MTT reduction assay, 100 μl of the semen sample and 10 μl of MTT stock solution (5 mg MTT/ml of PBS) were placed in each of six wells of the 96-well microplate. The OD of fresh and frozen-thawed samples was determined immediately (0 hour) and after 1 hour at 37°C incubation using an ELISA reader at 550 nm. MTT reduction rates for each sample were calculated by subtracting OD at 0 hour from OD at 1 hour. The results showed that fresh and frozen-thawed sperms from the cauda epididymides had significantly higher MTT reduction rates and fertilization rates than the corresponding parameters of caput and corpus sperms (p ≤ 0.05). The MTT assay protocol described in camel sperm is recapitulated in Table 1. MTT assay and stallion spermThe assay of MTT reduction in determining equine sperm viability was validated by Aziz et al. (2005). In this study, the MTT assay efficiencies and the standard flow cytometer method after SYBR-14/PI staining were compared. Fresh semen samples of good quality (n=3) were diluted with HEPES 0.1% BSA to obtain 5 × 107 sperms/ml. An aliquot of the diluted semen was kept at 37°C; the other was killed by freezing-thawing without cryoprotectants. Viable and freeze-killed sperms were mixed at six different ratios. The prepared samples were analyzed for viability by MTT and SYBR-14/PI. Concerning the MTT assay, 100 μl of semen samples and 10 μl of MTT stock solution, at a 5 mg/ml concentration of PBS, were placed in each well. Six wells of the 96-well microplate were used for each sample. MTT reduction rates were determined immediately, 1 and 4 hours after incubation at 37°C using a spectrophotometer at a wavelength of 550 nm. A simple linear regression between the MTT reduction rate (X-axis) and percentage of viable sperms determined by SYBR-14/PI (Y-axis) after 1 and 4 hours of incubation was drawn; an equation Y=a + bX for both incubation times was also created. These standard curves were applied later to obtain the sperm viability (%) in each sample according to the MTT reduction rate. Results of the experiment indicated visible color changes of a yellow tetrazolium salt to purple formazan at increasing incubation times and a high correlation between the MTT reduction rate and the result of sperm viability (r=0.954 and 0.977 for 1 and 4 hours of incubation times, respectively) (Aziz et al., 2005). Additionally, Trentin et al. (2017) used the MTT reduction assay to assess sperm mitochondrial activity of Brazilian ponies. In this study, the gel-free portion of the ejaculate was extended with non-buffered powder milk diluents composing of powdered skim milk and glucose to obtain 1 × 108 sperms/ml. Two aliquots of 200 μl of semen were deposited in 2 ml microcentrifuge tubes. Twenty microliters of tetrazolium solution (5 mg/ml of PBS) were added. After 30 minutes at 37°C incubation, formazan crystals were solubilized by 0.04 M HCl-isopropanol (200 μl). After centrifugation at 12,000 × g for 5 minutes, the OD of the supernatant was measured by spectrophotometry with a wavelength of 540 nm. The mixture of the extender, tetrazolium, and acidified isopropanol was also measured for absorbance and used as the reference sample. Results were reported without transforming to percentages. MTT assay and boar spermEvaluation of boar sperm viability by MTT reduction assay was reported in several studies with different objectives since 2008. Irrespective of the studies that have been conducted, refrigerated, not frozen-thawed semen diluted in commercial semen extenders such as Beltsville Thawing Solution (BTS) (Byun et al., 2008; van den Berg, 2015) and MR-A (Jofre et al., 2019) was used. To determine sperm viability by MTT assay, the diluted semen (1.5–3 × 107 sperms/ml) was added with 0.1 volume of MTT stock solution. The MTT solution was prepared earlier by dissolving MTT in PBS or BTS to obtain a final concentration of 3.5 μg/ml (Jofre et al., 2019), 1 mg/ml (van den Berg, 2015), or 5 mg/ml (Byun et al., 2008). In diluting with BTS, the prepared solution was filtered through a 0.22 μm filter before use. The reaction microplates or vials were placed at 17°C (Byun et al., 2008) or 37°C (Jang et al., 2010; van den Berg, 2015) for as long as 1 hour (Byun et al., 2008; Jofre et al., 2019), 5 hours (Jang et al., 2010), and 24 hours (van den Berg, 2015). At the end of 1 hour of incubation, 100 μl of DMSO was added to solubilize the formazan crystals and stop the reaction (Jofre et al., 2019). The colorimetric reaction was measured in a microplate reader using an absorbance of 514 nm (Jofre et al., 2019), 550 nm (Jang et al., 2010), or 560 nm (Byun et al., 2008). The data obtained were compared with other sperm parameters such as motility, membrane integrity, acrosome integrity, and survival rates (Jang et al., 2010), with the help of eosin-nigrosin staining used as a reference method (Byun et al., 2008) or, in toxicity study, with the non-treated control group set to 100% viability (Jofre et al., 2019). The results of MTT reduction rate were significantly correlated with the percentage viability that was simultaneously determined by eosin-nigrosin staining (r=0.95) (Byun et al., 2008), the sperm motility (r=0.88), membrane and acrosome integrity (r=0.77–0.92), and survival rates (r=0.90) (Jang et al., 2010). Besides using a spectrophotometer, the formation of formazan granules in boar sperm was observed through microscopic analysis (Fig. 2) (van den Berg, 2015). After incubation for varying periods, 5 μl of the MTT stained samples without a solubilizing agent was placed onto microscope slides and covered with cover glasses at room temperature. Microscopic observations performed using a phase-contrast microscope (400×) revealed that the MTT staining process of boar sperm could be divided into a series of morphological events beginning with diffused staining of the midpiece after a few minutes of staining process, more intense staining, and the appearance of formazan granules in the entire midpiece, appearance of a small granule on the sperm head near the edge of the acrosomal cap which increased in size during further incubation, and finally, after about 3 hours of incubation, the disappearance of the formazan granules from the cells due to a process called exocytosis and formation of formazan crystals in the incubation medium (Liu et al., 1997; Molinari et al., 2005). Van den Berg (2015) proposed that the mechanism of the granule formation in the sperm head is different from that in the midpiece since production of reducing NADH by glycolysis has never been reported for the sperm head. The specific position of the head granule may suggest a structure in the sperm head with a reducing capacity that may be related to the acrosomal reaction.
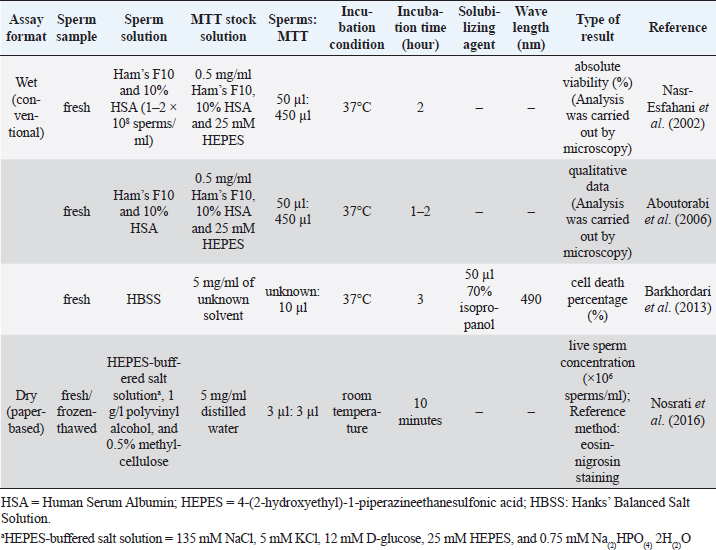
Fig. 2. The appearance of formazan granules in the midpiece and the head of boar sperm after incubation with MTT, observed by a phase-contrast microscope (400×); Modified from Van den Berg (2015). MTT assay and chicken spermThe MTT reduction assay which measures the capacity of chicken sperm to reduce MTT to formazan, has been developed and characterized jointly by Hazary and Wishart (2001) and Hazary et al. (2001). In both studies, MTT reduction was carried out with the semen of ISA brown chickens which was previously diluted 4-fold with N-Tris(hydroxymethyl)methyl-2-aminoethane sulfonic acid (NaCl/TES), comprising 0.15 M NaCl and 20 mM TES. Ten to forty microliters of diluted semen containing approximately 4 × 107–1.6 × 108 sperms was added with MTT solution (100–200 nM) and incubated at 37°C for 1 hour. The formazan product was then solubilized in 10% sodium dodecyl sulfate (SDS) in 0.1 M HCl; the color produced by MTT formazan was assayed by colorimetry (OD570). It was found that the rates of color development throughout the incubation period were positively correlated (r=0.96) with the concentrations of sperm and MTT added to the assay (Hazary and Wishart, 2001). In addition, for samples of semen from individual males, MTT reduction was highly correlated with sperm adenosine triphosphate (ATP) content (r2=0.85), sperm motility (r2=0.62), and fertilizing ability (r2=0.83) (Hazary et al., 2001). MTT assay and sea urchin spermSea urchin sperms had their viability determined by the colorimetric MTT method via in vitro toxicity testing (Resgalla et al., 2018). In the study, 0.5 ml of collected sperms was diluted in 10 ml of seawater and was exposed to different doses of five toxicants (SDS, copper, zinc, cadmium, and ammonium); the MTT stock solution with a concentration of 5 mg/ml was prepared in naturally filtered seawater with a salinity of 32 ppt and kept in a cool dark place to avoid oxidation until use. The MTT reduction assay was carried out in 48-well plates with four replicas per treatment. A volume of 500 μl of sperm solution containing 2 × 107 sperms with/without toxicants was placed in each well. Fifty microliters of MTT solution was added. Incubation period between sperms and MTT solution was 30 minutes in the dark. The formazan granules were dissolved in 500 μl of a solubilizing agent consisting of 1.7% SDS, diluted at room temperature in 40% dimethylformamide (DMF). The absorbance was read in a microplate reader at a wavelength of 570 nm. The results of the MTT test (mean absorbance values) were used to calculate half maximal effective concentration (EC50). MTT assay and rabbit spermThus far, there has been only one study describing the MTT assay in rabbit sperm. In that study, the in vitro effect of Origanum vulgare, a plant extract, on the characteristics of New Zealand rabbit ejaculates was investigated (Duracka et al., 2019). The MTT stock solution was prepared by dissolving tetrazolium salt in PBS without calcium and magnesium, at 5 mg/ml. In the assay, 20 μl of the tetrazolium solution was added to each semen sample, which was previously diluted in PBS without calcium and magnesium using a dilution ratio of 1:40. The sperm-MTT mixture was incubated for 2 hours at 37°C in 95% air atmosphere, 5% CO2, and shaker. The formazan crystals formed were dissolved in 80 μl of acidified (0.08 M HCl) isopropanol. OD was determined at a wavelength of 570 nm against 620 nm as a reference using a microplate photometer. Data were expressed as a percentage of the control containing 0 μg/ml of the plant extract set to 100%. MTT assay and rodent spermMice are laboratory animals commonly used for scientific research to study many aspects of humans. For this reason, the MTT reduction assay conducted on the sperm of this animal is also for the resulting data to be applied to humans. Based on existing reports, male mice must be humanely sacrificed before the collection of sperms from the cauda epididymides. Epididymal sperms in different types of solutions such as M16 and F10 media (Johnson et al., 2010), PBS (Whelly et al., 2014), and Ham’s F10 with 25 mmol/l HEPES and 10% Human Serum Albumin (HSA) (Zobeiri et al., 2013) were submitted to the MTT reduction assay to check for the viability. One hundred to 150 μl of sperm suspension containing 5 × 103–3 × 106 sperms was placed in each of 3–6 wells of a 96-well plate. Then, approximately one-tenth volume of MTT stock solution was added to each well and mixed properly. The MTT solution was included in a commercially available kit (Johnson et al., 2010) or prepared in-house by dissolving tetrazolium salts in PBS to acquire 0.5–5 mg/ml (Roy et al., 2013; Zobeiri et al., 2013). Samples were incubated at 37°C for 30 minutes (Whelly et al., 2014), 1 hour (Roy et al., 2013), 2 hours (Johnson et al., 2010), or 4 hours (Zobeiri et al., 2013). The MTT formazan was dissolved in DMSO (Zobeiri et al., 2013) or acidified isopropanol plus 10% Triton X-100 at 37°C for 1 hour (Whelly et al., 2014). The absorbance of the samples or the supernatant, after being centrifuged for 5 minutes at 15,000 × g, was examined at 540 nm (Zobeiri et al., 2013), 562 nm (Johnson et al., 2010), or 570 and 650 nm (Whelly et al., 2014) using a microplate reader. The results were shown as the OD values (mean±S.E.) (Whelly et al., 2014) or the relative percentage of viable cells compared to the untreated control (Roy et al., 2013). The rat sperm viability was assessed utilizing MTT reduction assay in the in vivo toxicity study conducted by Hara et al. (1995). Male Sprague-Dawley rats were given nitrobenzene dissolved in sesame oil orally for 16 days. The testes and epididymides were harvested from the sacrificed animals. Epididymal fluid rich in sperms was diluted with Dulbecco’s modified Eagle’s medium containing 25 mM fructose. To evaluate the sperm viability, 300 μl of the sperm suspension and 30 μl of MTT stock solution (5 mg/ml of PBS) were mixed in a disposable cuvette and incubated at 37°C for 2 hours in the presence of 5% CO2 and 95% air. Three hundred microliters of isopropanol was then added and mixed thoroughly to dissolve MTT formazan crystals. After centrifugation at 1,700 × g for 1 minute, the supernatant's absorbance was measured at a wavelength of 570 nm with a spectrophotometer. The results of sperm viability in the control and treatment groups were demonstrated, without converting to percentage, as mean absorbance (± SD). MTT assay and ape spermLike many other animals, the MTT reduction assay has been used to determine the viability of ape sperm. Yu et al. (2018) investigated the effects of the collagenase enzyme on the degelification of semen and the quality of sperm. Fresh ejaculates collected from adult chimpanzees (Pan troglodytes) with the aid of artificial vagina were incubated with Ham’s F10 medium at 25°C for 30 minutes for semen liquefaction. Before performing the viability assay, MTT stock solution at a 5 mg/ml concentration was prepared and filtered with a 0.22 μm membrane filter to remove undissolved residues. The solution was stored at 4°C in the dark for not more than 1 week. When used, one volume of MTT stock solution was added into nine volumes of semen suspensions and incubated at 37°C for 1 hour in a humidified incubator. An aliquot of the mixture was dropped on the glass slide to observe the formation of MTT formazan crystals in sperm through a microscope. Sperms with clear granule-like particles aggregated in the mid-piece region were considered viable; on the other hand, sperms without any signs of residue MTT were considered dead cells. The percentage of sperm viability was calculated from a total of 200 sperms examined. MTT assay and human spermSince MTT formazan formation is an enzyme-dependent reaction, changes in incubation conditions such as pH and temperature can directly affect the results. Nasr-Esfahani et al. (2002) investigated the appropriate condition necessary for carrying out sperm MTT viability assay and compared its efficiency with eosin-nigrosin and hypo-osmotic swelling test to discriminate between viable and non-viable human sperms. Among three different buffering bases (i.e., sodium bicarbonate, HEPES, and their combinations), Nasr-Esfahani et al. (2002) used Ham’s F10 plus 25mM HEPES to prepare the MTT stock solution. Then, the MTT solution (0.5 mg/ml, pH=7.4–7.45) was filtered (0.22 μm) and added with sterile HSA, to attain 10% concentration. The solution was then stored at 4°C in the dark for a maximum of 1 week and kept warm at 37°C, 30 minutes before use. For MTT assay, 50 μl of washed sperms (5 × 106–1 × 107 sperms in Ham’s F10 plus 10% HSA) was added to 450 μl of MTT solution. The sperm-MTT mixture was incubated at 37°C for 2 hours to obtain the maximum percentage of live sperms. The observation of MTT formation in the midpiece region of sperm was carried out by using a microscope. Results showed high significant correlations between sperm MTT viability assay, eosin-nigrosin (r=0.73), and hypo-osmotic swelling test (r=0.75) (Nasr-Esfahani et al., 2002). Furthermore, sperms experienced with the MTT test could fertilize normally and produce healthy embryos after intracytoplasmic sperm injection (ICSI), suggesting that the MTT test has no adverse effect on and is useful for ICSI in patients with absolute or severe asthenospermia especially in cases with tail abnormality (Aboutorabi et al., 2006). The cytotoxic effect of zinc oxide nanoparticles (ZnO NPs) on human sperms was evaluated using the MTT assay (Barkhordari et al., 2013). Fresh extended sperms exposed to ZnO NPs at different concentrations and times were washed by Hanks’ balanced salt solution (HBSS). The washed sperms in a 96-well plate were added with 10 μl of 5 mg/ml MTT solution and incubated at 37°C for 3 hours. To dissolve formazan crystals, 50 μl of 70% isopropanol was added; OD of each well was read at 490 nm using a microplate reader. Instead of the percentage of viable sperms, this study reported results as cell death percentage compared with the values of unexposed samples in the control group. The MTT methods used for detecting human sperm viability are concluded in Table 2. Table 2. The MTT assay protocol for determining viability of human sperm.
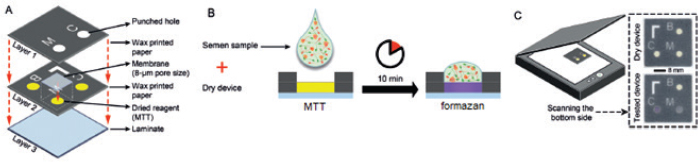
Paper-based MTT assayApart from conventional MTT assay performed in tubes or plates described above, sperm viability can also be evaluated through the MTT assay undertaken on the paper (known as a paper-based MTT assay) (Nosrati et al., 2016; Zhou et al., 2019). The principle of paper-based MTT assay is similar to that of its counterpart, namely yellowish tetrazolium salt applied on the device is converted to purple MTT formazan, in part, by the action of dehydrogenase enzyme present in metabolically active sperms. Change in color intensity relates to the amount of formazan formed. It is used to quantify the concentration of viable sperm. In a proof-of-concept study, an MTT solution (5 mg/ml of distilled water, 3 μl) was first pipetted onto a paper-based device and was subsequently dried at room temperature. Later on, 3 μl of semen samples diluted with HEPES-buffered salt solution (135 mM NaCl, 5 mM KCl, 12 mM D-glucose, 25 mM HEPES, and 0.75 mM Na(2)HPO(4) 2H(2)O) supplemented with 1 g/l polyvinyl alcohol and 0.5% methylcellulose was placed onto this MTT-treated paper. After a 10-minute reaction time, the image was recorded and further converted into grayscale. The grayscale image intensity was obtained in ImageJ software. Simultaneously, the same semen samples were analyzed for vitality with a dye exclusion assay (eosin-nigrosin staining). Results from the paper-based semen analysis device (i.e., color change intensity) strongly correlated with clinical data from a dye exclusion vitality test (i.e., viable sperm concentration), with r2=0.84 (Nosrati et al., 2016). The concentration of viable sperm in unknown samples was determined through the calibration curve plotted between values of a dye exclusion assay (X-axis) and those of the paper-based device (Y-axis) through a regression equation (Y=a + bX). Schematics of the paper-based sperm viability diagnostic device and paper-based MTT assay protocol are demonstrated in Figures 3 and Table 2, respectively. Correlation between MTT assay, ATP production, and sperm motilityIn addition to sperm viability, the MTT assay relationship with energy production and sperm motility has been addressed. Molecular mechanisms of sperm motility are regulated by motor protein reaction coupled with ATP hydrolysis. In sperm, ATP can be synthesized in mitochondria through oxidative phosphorylation and in the cytosol of flagellum through glycolysis (Tourmente et al., 2015). The contribution of ATP production for sperm motility is different across animal species. For example, boar, ram, and stallion sperm motility relies mainly on mitochondrial oxidative phosphorylation (Windsor, 1997; Storey, 2008). This metabolic pathway in the laboratory mouse, rabbit, and human sperms is, on the other hand, less efficient than glycolysis (Storey and Kayne, 1980; Nascimento et al., 2008; Tourmente et al., 2015). Sperms from bull and guinea pig are able to use the energy deriving from both glycolysis and oxidative phosphorylation to sustain their motility (Storey, 2008; Tourmente et al., 2015). Regardless of energy-producing catabolic pathways, the coenzyme NADH with a high reduction potential is also produced besides ATP (Bertram et al., 2006). This coenzyme is capable of reducing MTT to MTT formazan by direct or indirect electron transfer. According to the above theory, the ATP generation rate in sperm is directly associated with sperm motility and the quantity of formazan formed in the MTT assay. This relationship brings about the development of a paper-based MTT assay that can be used to estimate motile, live sperm concentration; the experimental results suggested that MTT color intensity changes measured by this device were correlated to human (Matsuura et al., 2014, 2015; Nosrati et al., 2016) and porcine (Matsuura et al., 2017) sperm motility determined with standard approaches. Correlation between MTT assay and other assays designed for the assessment of mitochondrial metabolismBesides measuring the activity of mitochondrial enzymes by MTT assay, mitochondria function in viable cells can also be assessed through detection of dynamic changes in mitochondrial membrane potential (ΔΨm) using fluorescent dyes such as tetramethylrhodamine ethyl ester (TMRE), 3,3′-Dihexyloxacarbocyanine iodide [DiOC6(3)], and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1). The fluorescence intensity of such cationic lipophilic dyes accumulated in mitochondria corresponds directly to ΔΨm. However, binding of TMRE and JC-1 to the outer surface of the inner mitochondrial membrane and hence the emission of light was interfered with the presence of MTT in the solution (Bernas and Dobrucki, 2002). This implies that measurements of mitochondrial transmembrane electric potential by fluorochromes and of mitochondrial enzymatic activity by MTT are not identical and should not be performed concurrently. In human sperm, a positive correlation was found between the percentage of ΔΨm high cells and progressive motility and also fertilization rates after in vitro fertilization (Wang et al., 2003; Marchetti et al., 2004). The positive relationship with sperm motility is similar to that seen in the MTT assay.
Fig. 3. Schematics of the paper-based sperm viability diagnostic device and protocol. (A) an exploded view of the device. (B) The semen sample is pipetted directly on the dried MTT in paper to generate colorimetric signal. (C) a schematic view of the assembled device and images of devices before (top) and 10 minutes after (bottom) applying a semen sample; Modified from Nosrati et al. (2016). Advantages and disadvantages of MTT viability assayThe following sections summarize the advantages and disadvantages of MTT reduction assay, compared with other viability tests. Understanding its pros and cons can help researchers decide whether to select this method for determining cell viability. AdvantagesCan be used for various eukaryotic cells: MTT can be used for viability assay of various eukaryotic cells other than sperm. For example, it has been employed for bacteria (Abate et al., 1998), protozoa (Dias et al., 1999), fungi (Freimoser et al., 1999), and mammalian cell lines (Mosmann, 1983; Campling et al., 1988; Diaz et al., 2007). Allows objective evaluation and assesses many specimens simultaneously: The MTT viability assay allows semi-automated and objective evaluation using a multi-well plate and a spectrophotometer which can be used to assess many specimens, with the maximum of 10, at the same time and many replications of each sample simultaneously (Aziz et al., 2005; Yurdakok-Dikmen et al., 2017). Only needs a simple tool: Instead of expensive and complex flow cytometer used in fluorescence staining method, the MTT reduction assay only needs a plate reading spectrophotometer which is a simple and practical tool for estimating sperm viability in a simply-equipped laboratory or a breeding farm (Mosmann, 1983; Byun et al., 2008; Lin et al., 2019). Time-stability of the product: The reaction product of the MTT assay, i.e., formazan crystals, offers better time-stability than that of the fluorescence dyes (Grela et al., 2015). The color is stable for a few hours at room temperature (Mosmann, 1983). Cost savings: The paper-based MTT assay requires minimal reagents and equipment, with a total material cost of approximately US$0.05 per device, and can quantitatively measure live sperm concentration in only 10 minutes. These approaches apply to current clinical practices and self-diagnostic applications (Nosrati et al., 2016; Zhou et al., 2019). DisadvantagesAlthough the MTT assay is more advantageous than other viability evaluation methods, particularly in the aspect of the number of samples evaluated simultaneously, there are some drawbacks, limitations, and precautions of this viability test or the reagent itself that must be concerned. Avoid contamination and light exposure while keeping: MTT solution must be prepared and kept under aseptic and dark conditions. Contamination with cells or microbes and light exposure may cause alteration in the color of the solution and hence erroneous results when included in the reduction test (Barnabe, 2017). Can cause skin and eye irritation: The appropriate personal protection equipment must always be worn while performing the MTT assay, as MTT can cause skin and eye irritation (Barnabe, 2017). Container type affects the accuracy of results: Some types of containers in which the MTT assay is carried out are not suitable, for instance, single-wall carbon nanotubes. These vessels were found to adsorb MTT formazan, interfere with absorbance measurements even under cell-free conditions, decrease in the peak absorbance and therefore cause false results for the MTT viability assay (Worle-Knirsch et al., 2006). The medium pH affects the absorbance: The type and pH of culture medium and solubilizing agent influence optical absorption values and hence the viability of cells estimated (Vistica et al., 1991; Nasr-Esfahani et al., 2002). In strongly acidic media, the cationic MTT formazan presence resulted in a complete disappearance of the absorption at 575 nm (Wang et al., 2014). Acid isopropanol originally used for dissolving formazan caused precipitation of protein from some serum-supplemented culture media and may deviate OD values (Twentyman and Luscombe, 1987). Also, the color produced by MTT formazan was quenched when 10% SDS in 0.1 M HCl was used as a solubilizing agent for chicken sperm (Hazary and Wishart, 2001). All are methodologically misleading and should be avoided. Nevertheless, an acidified solubilizing solution is suitable for culture medium containing phenol red. It has the benefit of changing the color of phenol red to yellow, which has less interference with absorbance readings (Riss et al., 2013). This may be a reason why acidified isopropanol keeps on being in use, even if DMSO and other solvents such as DMF are better alternatives for formazan solubilization (van Meerloo et al., 2011). More complicated than other enzyme-based cell viability assays: The conventional MTT assay requires solubilizing agent to dissolve formazan crystals before measuring the absorbance. On the other hand, other enzyme-based methods for determining cell viability such as the 2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium, monosodium salt (WST-8), 3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), and 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assays do not need any solubilizing agents. Their products are water-soluble and we can measure the absorbance directly after completing incubation. Therefore when compared with WST-8, MTS, and XTT tests, the MTT assay is less convenient and may offer lower sensitivity (Riss et al., 2013; Lin et al., 2019). Requires longer incubation periods than other viability tests: As MTT formazan formation is an enzyme-dependent reaction, there is a need for some incubation time between a substrate and enzyme to develop the reaction products (Nasr-Esfahani et al., 2002). The application of the conventional MTT assay for sperm viability assessment generally requires an incubation time of approximately 1–2 hours, despite being faster than other cells. Therefore, using this technique, it is impossible to obtain the number of viable sperms in just a very short period, such as less than 15 minutes per sample as evaluated by supravital staining methods (Aziz, 2007; Byun et al., 2008; Iqbal et al., 2009). This is an important drawback of this viability assay in the author viewpoint. Requires a reference method: MTT reduction assay cannot be used as a sole method to determine the percentage of sperm viability (i.e., absolute viability) in the test semen sample. It is necessary to have reference method such as eosin-nigrosin and SYBR-14/PI staining and the different, predetermined ratios of viable and killed sperms. The absolute viability of sperm in the test sample is subsequently determined by the relationship between the reference method (% viability) and those of the MTT assay (OD). There are no standard protocols: As there are a variety of MTT assay protocols, it seems difficult for researchers to select the appropriate protocol that fits their experiment conditions and at the same time yields the most accurate results. This means that optimizing the assay protocol is highly recommended before utilizing this method to determine the viability of sperm. Also, as no standard MTT reduction assay protocols have been given so far, even in the same animal species, comparison of the sperm viability determined by this method among different studies is an impediment and should be done with caution. The amount of formazan generated depends on many factors: MTT reduction and the amount of formazan generated are dependent on several parameters such as the MTT concentration, the D-glucose concentration of the culture medium, the duration of incubation, and solubilization as well as the metabolic cell density (Vistica et al., 1991; Abate et al., 1998; Dias et al., 1999; Hazary et al., 2001; Byun et al., 2008; Iqbal et al., 2009). All of these parameters should be considered when optimizing the assay conditions to generate a sufficient amount of product that can be detected (Riss et al., 2013). Formazan can be generated even in cell-free conditions: MTT can be reduced by some particular compounds present in culture media such as polyphenols (Han et al., 2010), pyruvate analog (van Tonder et al., 2015), nanomaterials, and superoxide (Wang et al., 2011) to form extracellular formazan; this may result in inaccurate results when determining cell viability. Therefore in each measurement, a cell-free well should be used as a background in the analysis. This well contains the same contents as the tested wells except that there are no cells in it. Alternatively, other cell viability detection methods measuring different endpoints, e.g., cell membrane damage assays, should also be used to verify the MTT reduction results. The conversion of MTT is not specific to the mitochondria: MTT reduction seems not to be an ideal assay for measuring mitochondrial activity in living cells since the localization of MTT formazan imaged using phase-contrast and confocal microscopy is not specific to the mitochondria (Bernas and Dobrucki, 2002; van den Berg, 2015). Other cell organelles such as the plasma membranes, cytoplasm, endoplasmic reticulum, and lysosomes also participate as the reduction sites of the MTT in mammalian cells (Bernas and Dobrucki, 2000; Tourmente et al., 2015). However, this extra-mitochondrial reduction activity can be suppressed by the presence of superoxide dismutase mimetic and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitors such as co-enzyme Q, diphenylene iodonium, zinc, and 2-deoxyglucose (Aitken et al., 2020). The formazan product causes adverse changes to cellular morphology: In general, cells that get exposed to MTT will be morphologically damaged, largely due to exocytosis of MTT formazan crystals (Lu et al., 2012; Riss et al., 2013). For this reason, it is recommended not to subsequently use such samples to evaluate cell morphology. ConclusionDetermination of sperm viability by the MTT reduction assay is based on a principle that dehydrogenase enzymes in active mitochondria of viable sperm reduce MTT to MTT formazan. Color intensity of the formazan product after solubilization is then measured by the spectrophotometer. The absorbance of a solution, also known as OD, is positively correlated with the percentage of viable sperms in the samples. Nevertheless, there are several confounding factors influencing the OD values and thus the percent viable sperm estimated, for example, sperm and MTT concentrations, type and acidity of media, length and conditions of incubation, solubilizing agents, and so on. All of these variables must be taken into consideration and controlled while conducting and optimizing the MTT assay to get reliable viability results. Researchers, including me, may be faced with a difficult decision and are probably reluctant to use this colorimetric assay for their sperm samples after considering a wide variety of the existing protocols and weighing the positives and negatives of this method. However, with limited time and resources and a large number of sperm samples to be measured, the MTT assay may be implemented. Researchers are suggested to commence selecting the available protocols and validating assay procedures at least by comparing the results of the assay with the value of a well-developed viability test such as supravital staining methods. Conflict of interestThe author declares that there is no conflict of interest. ReferencesAbate, G., Mshana, R.N. and Miorner, H. 1998. Evaluation of a colorimetric assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2(12), 1011–1016. Aboutorabi, R., Nasr-Esfahani, M.H. and Baharvand, H. 2006. Effect of human sperm MTT viability test on outcome of intracytoplasmic sperm injection. Yakhteh Med. J. 7(4), 254‒257. Aitken, R.J., Gregoratos, D., Kutzera, L., Towney, E., Lin, M., Wilkins, A. and Gibb, Z. 2020. Patterns of MTT reduction in mammalian spermatozoa. Reproduction 160(3), 431‒445. Al Naib, A., Hanrahan, J.P., Lonergan, P. and Fair, S. 2011. In vitro assessment of sperm from bulls of high and low field fertility. Theriogenology 76, 161–167. Altman, F.P. 1976. Tetrazolium salts and formazans. Prog. Histochem. Cytochem. 9(3), 1‒56. Aziz, D.M. 2006. Assessment of bovine sperm viability by MTT reduction assay. Anim. Reprod. Sci. 92, 1‒8. Aziz, D.M. 2007. Evaluation of the percentage of live sperm in ram semen by using the MTT reduction assay. Iraqi J. Vet. Sci. 21(1), 147‒154. Aziz, D.M., Ahlswede, L. and Enbergs, H. 2005. Application of MTT reduction assay to evaluate equine sperm viability. Theriogenology 64, 1350–1356. Barkhordari, A., Hekmatimoghaddam, S., Jebali, A., Khalili, M.A., Talebi, A. and Noorani, M. 2013. Effect of zinc oxide nanoparticles on viability of human spermatozoa. Iran J. Reprod. Med. 11(9), 767‒771. Barnabe, M. 2017. Cell viability assays: MTT assay application and protocol. Available via https://blog.quartzy.com/2017/05/01/cell-viability-assays-mtt-protocol (Accessed 12 May 2020). Bernas, T. and Dobrucki, J. 2002. Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry 47, 236–242. Bernas, T. and Dobrucki, J.W. 2000. The role of plasma membrane in bioreduction of two tetrazolium salts, MTT, and CTC. Arch. Biochem. Biophys. 380, 108–116. Bertram, R., Gram Pedersen, M., Luciani, D.S. and Sherman, A. 2006. A simplified model for mitochondrial ATP production. J. Theor. Biol. 243(4), 575‒586. Byun, J.W., Choo, S.H., Kim, H.H., Kim, Y.J., Hwang, Y.J. and Kim, D.Y. 2008. Evaluation of boar sperm viability by MTT reduction assay in Beltsville thawing solution extender. Asian-Aust. J. Anim. Sci. 21, 494–498. Campling, B.G., Pym, J., Galbraith, P.R. and Cole, S.P. 1988. Use of the MTT assay for rapid determination of chemosensitivity of human leukemic blast cells. Leuk. Res. 12, 823‒831. Dias, N., Nicolau, A., Carvalho, G.S., Mota, M. and Lima, N. 1999. Miniaturization and application of the MTT assay to evaluate metabolic activity of protozoa in the presence of toxicants. J. Basic Microbiol. 39, 103–108.V Diaz, G., Melis, M., Musin, A., Piludu, M., Piras, M. and Falchi, A.M. 2007. Localization of MTT formazan in lipid droplets. An alternative hypothesis about the nature of formazan granules and aggregates. Eur. J. Histochem. 51, 213‒218. Duracka, M., Galovicova, L., Slavik, M., Arvay, J. and Tvrda, E. 2019. The in vitro effect of the Origanum vulgare extract on semen. J. Microbiol. Biotech. Food Sci. 8(4), 1089‒1092. El-Badry, D.A., Anwar, A.M. and El-Bakhmy, A.S.H. 2008. Assessment of mitochondrial activity of fresh and frozen-thawed buffalo sperm in relation to sperm viability and in vitro fertilizing ability. Vet. Med. J. Giza. 56(1), 169‒184. El-Badry, D.A., Scholkamy, T.H., Abeer, M. Anwer and Karima, Gh.M. Mahmoud. 2015. Assessment of freezability and functional integrity of dromedary camel spermatozoa harvested from caput, corpus and cauda epididymides. Alex. J. Vet. Sci. 44, 147‒158. Eskandari, F. and Momeni, H.R. 2016. Protective effect of silymarin on viability, motility and mitochondrial membrane potential of ram sperm treated with sodium arsenite. Int. J. Reprod. BioMed. 14(6), 397‒402. Freimoser, F.M., Jakob, C.A., Aebi, M. and Tuor U. 1999. The MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl. Environ. Microbiol. 65(8), 3727–3729. "https://europepmc.org/search?query=AUTH:%22Hana%20Greifov%C3%A1%22" Greifova, H., Tvrda, E., Jambor, T. and Lukac, N. 2017. Dose- and time-dependent effects of epicatechin on bovine spermatoza in vitro. J. Microbiol. Biotech. Food Sci. 7(3), 235‒239. Grela, E., Zabek, A. and Grabowiecka, A. 2015. Interferences in the optimization of the MTT assay for viability estimation of Proteus mirabilis. Avicenna J. Med. Biotech. 7(4), 159‒167. Han, M., Li, J.F., Tan, Q., Sun, Y.Y. and Wang, Y.Y. 2010. Limitations of the use of MTT assay for screening in drug discovery. J. Chin. Pharm. Sci. 19(3), 195–200. Hara, H., Takai, R., Tanaka, N., Mizoguchi, K., Tsuno, T., Igarashi, S. and Usami, M. 1995. Simple methods for objective assessment of sperm viability and motility with MTT assay and sperm quality analyzer (SQA) in rats. Cong. Anom. 35, 477‒480. Hazary, R.C. and Wishart, G.J. 2001. Assay of sperm quality in the domestic fowl by MTT reduction. Br. Poult. Sci. 42, 111–114. Hazary, R.C., Chaudhuri, D. and Wishart, G.J. 2001. Application of an MTT reduction assay for assessing sperm quality and predicting fertilizing ability of domestic fowl semen. Br. Poult. Sci. 42, 115–117. Iqbal, M., Ijaz, A., Aleem, M., Rehman, H. and Yousaf, M.S. 2009. Assessment of Nili-Ravi buffalo (Bubalus bubalis) semen by MTT reduction Assay. South African J. Anim. Sci. 39(Suppl. 1), 294‒300. Jang, H.Y, Lee, H.Y, Cheong, H.T, Kim, J.T, Park, I.C, Park, C.K. and Yang, B.K. 2010. Development of sperm MTT assay for its application in boar semen. J. Embryo Transf. 25(4), 229‒235. Jofre, I., Cuevas, M., de Castro, L.S., de Agostini Losano, J.D., Torres, M.A., Alvear, M., Scheuermann, E., Cesar Andrade, A.F., Nichi, M., Ortiz Assumpcao, M.E. and Romero, F. 2019. Antioxidant effect of a polyphenol-rich Murtilla (Ugni molinae Turcz.) extract and its effect on the regulation of metabolism in refrigerated boar sperm. Oxid. Med. Cell Longev. 2019, 2917513. Johnson, A.R., Craciunescu, C.N., Guo, Z., Teng, Y., Thresher, R.J., Blusztajn, J.K. and Zeisel, S.H. 2010. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J. 24(8), 2752–2761. Kalina, M. and Palmer, J.M. 1968. The reduction of tetrazolium salts by plant mitochondria. Histochemie 14, 366‒374. Knazicka, Z., Lukac, N., Gren, A., Formicki, G. and Massanyi, P. 2012a. In vitro effects of copper on the motility and viability of spermatozoa. J. Microbiol. Biotechnol. Food Sci. 1(6), 1529‒1539. Knazicka, Z., Tvrda, E., Bardos, L. and Lukac, N. 2012b. Dose- and time-dependent effect of copper ions on the viability of bull spermatozoa in different media. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 47(9), 1294‒1300. Kumar, P., Srivastava, N., Pande, M., Prasad, J.K. and Sirohi, A.S. 2017. Evaluating sperm cell viability and membrane integrity. In Protocols in Semen Biology (Comparing Assays), Eds., Srivastava, N. and Pande, M. Singapore, Singapore: Springer. Lin, H.L., Liaw, R.B., Chen, Y.H., Kang, T.C., Lin, D.Y., Chen, L.R. and Wu, M.C. 2019. Evaluation of cockerel spermatozoa viability and motility by a novel enzyme based cell viability assay. Br. Poult. Sci. 60(4), 467‒471. Liu, Y., Peterson, D.A., Kimura, H. and Schubert, D. 1997. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 69, 581–593. Lu, L., Zhang, L., Wai, M.S., Yew, D.T. and Xu, J. 2012. Exocytosis of MTT formazan could exacerbate cell injury. Toxicol. In Vitro. 26(4), 636–644. Marchetti, C., Jouy, N., Leroy-Martin, B., Defossez, A., Formstecher, P. and Marchetti, P. 2004. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod. 19(10), 2267–2276. Matsuura, K., Chen, K.H., Tsai, C.H., Li, W., Asano, Y., Naruse, K. and Cheng, C.M. 2014. Paper-based diagnostic devices for evaluating the quality of human sperm. Microfluid Nanofluid 16, 857–867. Matsuura, K., Huang, H.W., Chen, M.C., Chen, Y. and Cheng, C.M. 2017. Relationship between porcine sperm motility and sperm enzymatic activity using paper-based devices. Sci. Rep. 7, 46213. Matsuura, K., Komiyama, J. and Okitsu, O. 2015. Relationship between human spermatozoa motility and enzymatic reactivity. Fertil. Steril. 104, e239. Molinari, B.L., Tasat, D.R., Palmieri, M.A. and Cabrini, R.L. 2005. Kinetics of MTT-formazan exocytosis in phagocytic and non-phagocytic cells. Micron 36, 177‒183. Morgan, D.M. 1998. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol. Biol. 79, 179–183. Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 65, 55–63. Nascimento, J.M., Shi, L.Z., Tam, J., Chandsawangbhuwana, C., Durrant, B., Botvinick, E.L. and Berns, M.W. 2008. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell Physiol. 217, 745–751. Nasr-Esfahani, M.H., Aboutorabi, R., Esfandiari, E. and Mardani, M. 2002. Sperm MTT viability assay: a new method for evaluation of human sperm viability. J. Assoc. Reprod. Genet. 19, 477–482. Nosrati, R., Gong, M.M., San Gabriel, M.C., Pedraza, C.E., Zini, A. and Sinton, D. 2016. Paper-based quantification of male fertility potential. Clin. Chem. 62(3), 458–465. Resgalla, Jr. C., Marcus Vinicius, M., do Nascimento Brasil, M. and Marcos Luiz, P. 2018. Colorimetric method for determining viability of sea urchin sperm applied in toxicity tests. Ecotoxicology 27, 499–504. Riss, T.L., Moravec, R.A., Niles, A.L., Duellman, S., Benink, H.A., Worzella, T.J. and Minor, L. 2013. Cell viability assays. May 1 [Updated 2016 Jul 1]. In Assay Guidance Manual [Internet], Eds., Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld. D., Austin C.P., Baell, J., Caaveiro, J.M.M., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., Devanaryan, V., Foley, T.L., Glicksman, M., Hall, M.D., Haas, J.V., Hoare, S.R.J., Inglese, J., Iversen, P.W., Kahl, S.D., Kales, S.C., Kirshner, S., Lal-Nag, M., Li, Z., McGee, J., McManus, O., Riss, T., Saradjian, P., Trask, O.J., Weidner, J.R., Wildey, M.J., Xia, M., Xu, X. Bethesda, MD: Eli Lilly & Company and the National Center for Advancing Translational Sciences, 2004. Roy, S., Tamang, S. and Chaudhuri, T.K. 2013. Sperm viability assessment using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction assay of Swiss albino mice treated with Diplazium esculentum. Asian J. Pharm. Hea. Sci. 3(2), 684‒689. Storey, B.T. 2008. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int. J. Dev. Biol. 52, 427–437. Storey, B.T. and Kayne, F.J. 1980. Properties of pyruvate kinase and flagellar ATPase in rabbit spermatozoa: relation to metabolic strategy of the sperm cell. J. Exp. Zool. 211, 361–367. Tourmente, M., Villar-Moya, P., Rial, E. and Roldan, E.R.S. 2015. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 290, 20613–20626. Trentin, J.M., Rodrigues, M.F., Pessoa, G.A., Fiorenza, M.F., Schenatto, R.O., de Araujo, L.B., Aires, K.V. and Bubin, M. 2017. Viability of pony stallion semen in different temperature and dilution. Acta Sci. Vet. 45, 1‒8. Tvrda, E., Botman, B., Halenar, M., Slanina, T. and Lukac, N. 2017a. In vitro effects of Salvia officinalis on bovine spermatozoa. World Academy of Science, Engineering and Technology Inter. J. Anim. Vet. Sci.11(2), 101‒107. "https://europepmc.org/authors/0000-0003-2895-1249" Tvrda, E., Greifova, H., Mackovich, A., Hashim, S. and Lukac, N. 2018. Curcumin offers antioxidant protection to cryopreserved bovine semen. Czech J. Anim. Sci. 63, 247–255. Tvrda, E., Lukac, N., Jambor, T., Lukacova, J., Hashim, S. and Massanyi, P. 2016c. In vitro supplementation of lycopene to bovine spermatozoa: effects on motility, viability and superoxide production. Anim. Sci. Pap. Rep. 34(4), 319‒328. Tvrda, E., Lukac, N., Lukacova, J., Jambor, T. and Massanyi, P. 2015. Dose- and time-dependent in vitro effects of divalent and trivalent iron on the activity of bovine spermatozoa. Biol. Trace Elem. Res.167, 36–47. Tvrda, E., Mackovich, A., Greifova, H., Hashim, S. and Lukac, N. 2017b. Antioxidant effects of lycopene on bovine sperm survival and oxidative profile following cryopreservation. Vet. Med. (Praha) 62, 429–436. Tvrda, E., Straka, P., Galbavy, D. and Ivanic, P. 2019. Epicatechin provides antioxidant protection to bovine spermatozoa subjected to induced oxidative stress. Molecules 24(18), 3226. Tvrda, E., Tusimova, E., Kovacik, A., Paal, D., Greifova, H., Abdramanov, A. and Lukac, N. 2016a. Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Anim. Reprod. Sci. 172, 10–20. Tvrda, E., Tusimova, E., Kovacik, A., Paal, D., Libova, L. and Lukac, N. 2016b. Protective effects of quercetin on selected oxidative biomarkers in bovine spermatozoa subjected to ferrous ascorbate. Reprod. Dom. Anim. 51, 524–537. Twentyman, P.R. and Luscombe, M. 1987. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 56, 279‒285. van den Berg, B.M. 2015. Microscopic analysis of MTT stained boar sperm cells. Open Vet. J. 5(1), 58‒63. van Meerloo, J., Kaspers, G.J. and Cloos, J. 2011. Cell sensitivity assays: the MTT assay. Methods Mol. Biol. 731, 237–245. van Tonder, A., Joubert, A.M. and Cromarty, A.D. 2015. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes. 8, 47. Vistica, D.T., Skehan, P., Scudiero, D., Monks, A., Pittman, A. and Boyd, M.R. 1991. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 51, 2515‒2520. Wang, S., Yu, H. and Wickliffe, J.K. 2011. Limitation of the MTT and XTT assays for measuring cell viability due to superoxide formation induced by nano-scale TiO2. Toxicol. In Vitro. 25, 2147–2151. Wang, X., Sharma, R.K., Gupta, A., George, V., Thomas, A.J., Falcone, T. and Agarwal, A. 2003. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 80(Suppl 2), 844–850. Wang, X.D., Deng, R.C., Liu, Y., LI, B., Shen, H., Ouyang, H. and Xiao, Z.P. 2014. Modification of MTT assay for precision and repeatability and its mechanistic implication. Asian J. Chem. 26(23), 8015‒8018. Whelly, S., Serobian, G., Borchardt, C., Powell, J., Johnson, S., Hakansson, K., Lindstrom, V., Abrahamson, M., Grubb, A. and Cornwall, G.A. 2014. Fertility defects in mice expressing the L68Q variant of human cystatin C: a role for amyloid in male infertility. J. Biol. Chem. 289(11), 7718–7729. Windsor, D.P. 1997. Mitochondrial function and ram sperm fertility. Reprod. Fertil. Dev. 9, 279–284. Worle-Knirsch, J.M., Pulskamp, K. and Krug, H.F. 2006. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 6, 1261–1268. Yu, J.F., Lai, Y.H., Wang, T.E., Wei, Y.S., Chang, Y.J., Li, S.H., Chin, S.C., Joshi, R., Chang, H.W. and Tsai, P.S. 2018. The effects of type I collagenase on the degelification of chimpanzee (Pan troglodytes) semen plug and sperm quality. BMC Vet. Res.14, 58. Yurdakok, B., Tekin, K., Daskin, A. and Filazi, A. 2015. Effects of polychlorinated biphenyls 28, 30 and 118 on bovine spermatozoa in vitro. Reprod. Dom. Anim. 50, 41–47. Yurdakok-Dikmen, B., Tekin, K., Tirpan, M.B., Daskin, A. and Filazi, A. 2017. In vitro toxicity of some pesticides on goat and dog spermatozoa. Kafkas. Univ. Vet. Fak. Derg. 23(2), 297‒303. Zhou, Z., Kang, Y., Xu, Z. and Xue, P. 2019. Development and prospects of microfluidic platforms for sperm inspection. Anal. Methods 11, 4547‒4560. Zobeiri, F., Salami, S., Sadrkhanlou, R. and Peirouvi, T. 2013. Role of mitochondria in ciprofloxacin-induced apoptosis in murine sperm cells. Reprod. Sci. 20(9), 1090‒1095. | ||
| How to Cite this Article |
| Pubmed Style Kakanang Buranaamnuay|. The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Vet. J.. 2021; 11(2): 251-269. doi:10.5455/OVJ.2021.v11.i2.9 Web Style Kakanang Buranaamnuay|. The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. https://www.openveterinaryjournal.com/?mno=50208 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i2.9 AMA (American Medical Association) Style Kakanang Buranaamnuay|. The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Vet. J.. 2021; 11(2): 251-269. doi:10.5455/OVJ.2021.v11.i2.9 Vancouver/ICMJE Style Kakanang Buranaamnuay|. The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Vet. J.. (2021), [cited January 25, 2026]; 11(2): 251-269. doi:10.5455/OVJ.2021.v11.i2.9 Harvard Style Kakanang Buranaamnuay| (2021) The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Vet. J., 11 (2), 251-269. doi:10.5455/OVJ.2021.v11.i2.9 Turabian Style Kakanang Buranaamnuay|. 2021. The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Veterinary Journal, 11 (2), 251-269. doi:10.5455/OVJ.2021.v11.i2.9 Chicago Style Kakanang Buranaamnuay|. "The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols." Open Veterinary Journal 11 (2021), 251-269. doi:10.5455/OVJ.2021.v11.i2.9 MLA (The Modern Language Association) Style Kakanang Buranaamnuay|. "The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols." Open Veterinary Journal 11.2 (2021), 251-269. Print. doi:10.5455/OVJ.2021.v11.i2.9 APA (American Psychological Association) Style Kakanang Buranaamnuay| (2021) The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Veterinary Journal, 11 (2), 251-269. doi:10.5455/OVJ.2021.v11.i2.9 |