| Original Article | ||
Open Vet. J.. 2022; 12(4): 463-468 Open Veterinary Journal, (2022), Vol. 12(4): 463–468 Original Research Bilateral asymptomatic common carotid artery stenosis: Mouse model for stroke researchAchmad Firdaus Sani1,2, Widjiati Widjiati3*, Paulus Sugianto2, Muhammad Hamdan2 and Jovian Philip Swatan21Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Neurology, Faculty of Medicine, Universitas Airlangga /Dr. Soetomo General Hospital, Surabaya, Indonesia 3Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia Submitted: 11/04/2022 Accepted: 16/06/2022 Published: 13/07/2022 *Corresponding Author: Widjiati Widjiati. Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: widjiati [at] fkh.unair.ac.id © 2022 Open Veterinary Journal
AbstractBackground: Asymptomatic carotid artery stenosis has become more prevalent worldwide and is often associated with a poor prognosis. Numerous guidelines highlighted surgical interventions as treatment for carotid artery stenosis, but only a few recommendations were made regarding non-surgical interventions due to its limited data. Aims: This study aims to develop a mice model for research in non-surgical interventions of asymptomatic carotid artery stenosis. Methods: Adult male Rattus norvegicus, Wistar strain models with bilateral asymptomatic common carotid artery stenosis (BACAS) were created by ligating the common carotid artery with a 0.6 mm diameter needle and then removing the needle. The mice’s body weight, clinical signs and symptoms, and post-mortem brain analysis were compared between the sham-operated group and the BACAS group. Results: The mortality rate among the BACAS group is 11.11%. There is no significant difference in mean body weight before surgery, after the observation period, and percentage of weight decrease between sham-operated and BACAS groups (p=0.710, 0.632, and 0.806, respectively). None of the surviving mice in this study exhibit signs of motor paralysis. Gross examination of the brain reveals no signs of infarction or hemorrhage. Conclusion: We have established a novel BACAS mouse model which is cost-efficient, easy to produce, and with no significant alteration in body weight, clinical parameters, and brain morphology. Keywords: Animal models of disease, Carotid arteries, Mouse models, Non-communicable disease, Scientific research. IntroductionThe number of asymptomatic carotid artery stenosis is steadily increasing as metabolic disorders become more prevalent worldwide. Previous studies have demonstrated that the prevalence of asymptomatic carotid artery stenosis among the general population varies from 0% to 7.5% for moderate degree and 0% to 3.1% for the severe degree (de Weerd et al., 2010); however, the prevalence of bilateral carotid artery stenosis is largely unknown. One study identifies bilateral carotid artery stenosis in 10% of patients undergoing carotid ultrasonography screening before coronary artery bypass surgery (Wanamaker et al., 2012). Despite its low prevalence, patients with bilateral carotid artery stenosis are usually associated with poor outcomes due to a high risk of developing stroke (9.6% in 1 year) and high mortality rate (>50% in 6 years) (AbuRahma and Copeland, 1998; Rijbroek et al., 2006). The majority of the guidelines recommend surgical interventional procedures reserved only for severe asymptomatic or high-risk symptomatic carotid artery stenosis (Abbott et al., 2015). For stenosis to a lesser degree, non-surgical interventions such as lifestyle modification and medical treatment are endorsed (Abbott, 2009). Currently, there is limited evidence to suggest which non-surgical interventions are the most beneficial for asymptomatic carotid artery stenosis. Several animal models for bilateral carotid artery stenosis have been identified including models for cerebral hypoperfusion to mimic vascular dementia (Hainsworth et al., 2017; Washida et al., 2019; Wang et al., 2020) and ischemic carotid artery disease (Hattori et al., 2014). However, none of the current established animal models are asymptomatic. Therefore, we propose a novel mouse model that successfully mimics bilateral asymptomatic common carotid artery stenosis (BACAS) for stroke research, specifically to determine the most beneficial non-surgical interventions for asymptomatic carotid artery stenosis. Materials and MethodsExperimental protocolWe used healthy male Rattus norvegicus mice of Wistar strain aged 10–12 weeks (weight 150–350 g) obtained from the Integrated Research and Testing Laboratory Universitas Gadjah Mada, Yogyakarta, Indonesia. The mice were allocated into two groups using a simple random sampling technique, namely a sham-operated group (n=10) and a BACAS group (n=9). We utilized a pre-experimental group (n=14) to evaluate whether the intervention was successful in creating an asymptomatic mice model with no significant brain morphological alteration. All mice were housed with food and water ad libitum under a 12-hours light/dark cycle (lights on at 6:00 am) at a temperature of 25°C ± 2°C and relative humidity of 35%–50%. Surgical procedureBefore the surgery, all mice were examined for any signs of paralysis and external injuries. The mice were put on a weighing scale and anesthetized using an intramuscular Ketamine/Xylazine anesthetic cocktail with a dose of 45 mg/kg Ketamine (Ket-A-100, Agrovet Market S.A, Lima, Peru) and 10 mg/kg Xylazine (Xyla, Interchemie, Venray, Netherlands). The anesthetic procedure was considered effective when the mice were fully immobilized and unresponsive to a toe pinch. Following disinfection of the operation site using iodine tincture and 70% ethanol, a median incision was made in the neck. We separated each layer of the skin and muscle carefully until the common carotid artery was exposed. It was separated from surrounding tissue using an aseptic technique. We used a 0.6 mm-diameter needle (Needle 23G, Terumo Indonesia, Jakarta, Indonesia) to mimic a moderate to severe stenosis (Zhou et al., 2012). The artery was ligated with the needle in two circles using a 5–0 monofilament polypropylene suture (Premilene, B-Braun Indonesia, Jakarta, Indonesia) at 1.5 cm proximal to the internal-external carotid bifurcation (Fig. 1). After a careful ligation of the artery, the needle was cautiously removed and the steps were repeated for the contralateral side so that both common carotid arteries were ligated during the same surgical session. The surgical incision was sutured using a 3–0 non-absorbable silk suture (Silk Braided USP 3/0, GEA, Jiangsu, China) and covered using sterile gauze. Aspirin (Aspilets, Medifarma Laboratories, Depok, Indonesia) was given as an anticoagulant (30 mg/l) in the mice’s drinking waters for 3 days after surgery. In the sham-exposed group, the bilateral common carotid artery was exposed, but no ligature was made. Observation periodFollowing the surgery, the mice were placed in individual cages and monitored daily for signs of paralysis. The pre-experimental group was observed for 3 days, while the sham-exposed and BCAS group were observed for 10 days. After the observation period concluded, the mice were sent to the experimental animal laboratory in the Faculty of Veterinary Medicine, Universitas Airlangga to be humanely euthanized. Mice that presented signs of paralysis before the observation period concluded were immediately sent to be euthanized. The euthanasia procedure was conducted using intramuscular injection of Ketamine 150 mg/kg (Ket-A-100, Agrovet Market S.A, Lima, Peru) and 15 mg/kg Xylazine (Xyla, Interchemie, Venray, Netherlands) mixture. As one study pointed out that Ketamine may impact the brain mitogen-activated protein kinase (MAPK) activity, we decided to wait for 45 minutes before conducting the decapitation procedure to collect the mice’s brain (Ko et al., 2019). A standardized decapitation procedure was carried out after the mice were fully immobilized and unresponsive to a toe pinch. The mice’s brain was collected for post-mortem analysis.
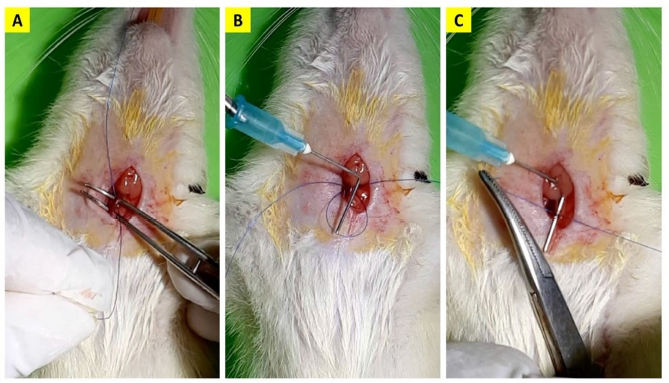
Fig. 1. The common carotid artery ligation procedure. A 5–0 monofilament polypropylene suture was inserted beneath the artery (A), the 0.6 mm-diameter needle was placed besides the artery (B) and ligated in two circles with a knot (C). Afterwards, the needle was carefully removed. Outcome variablesThe mice’s body weight was measured using a scale with an accuracy of 0.1 g. The measurement was conducted on the first day before surgery and the final day of the observation. The mice’s clinical condition was monitored twice daily. For each mouse, we examined the movement of all four extremities when walking and held at a height of 1 m (Fig. 2). Paralysis was defined as inactivity or decreased movement in one of the extremities during observation. Following the euthanasia procedure, the mice’s brain was examined macroscopically for signs of infarction. Post-mortem analysis of the brain was only conducted for mice that completed the observation period. Data analysisThe data obtained were analyzed using IBM SPSS Statistics for Windows ver. 23.0 (IBM Corp, Armonk, NY). Descriptive data were expressed as mean ± standard deviation unless stated otherwise. The comparison of mean and decrease in body weight between groups was analyzed using an independent t-test. A p-value of <0.05 was considered statistically significant. Ethical approvalThis study had received ethical clearance from Animal Care and Use Committee Faculty of Veterinary Medicine Universitas Airlangga, Surabaya, Indonesia (number 2.KE.002.01.2022). All procedures done in this study were by the United Kingdom Animal Act 1986. All surgeries were conducted under anesthesia and all efforts were made to minimize the suffering. ResultsPre-experimental groupA total of five mice (35.71%) in the pre-experimental group died during the observation period. One mouse did not regain consciousness after the surgery, while four mice died within the next 2 days. We found the mean body weight of the surviving mice is higher than the non-surviving mice (201.22 ± 28.15 vs. 190.20 ± 6.53 g). Although this difference is not statistically significant (p =0.413), we decided to enroll mice weighted 200 g and above for the rest of this study. None of the mice that completed the observation period showed any signs of paralysis. Morphological analysis of the surviving mice’s brains also revealed no signs of infarction or hemorrhage (Fig. 3A). Sham and BACAS groupSurvival rateA total of 18 mice completed the observation period. One mouse from the BACAS group died 1 day after the surgery. The survival rates for sham and BACAS groups are 100% and 88.89%, respectively. Body weightThe comparison of body weight among surviving mice is documented in Table 1. One mouse from the BACAS group that died had a body weight of 208 g, which is lower than the mean body weight of the group. We observe no significant difference in the mean body weight before surgery, after the observation period, and percentage of weight decrease between the sham and BACAS groups (p=0.710, 0.632, and 0.806, respectively).
Fig. 2. Examination of mouse extremities muscle when (A) walking and (B) held at a height of 1 m.
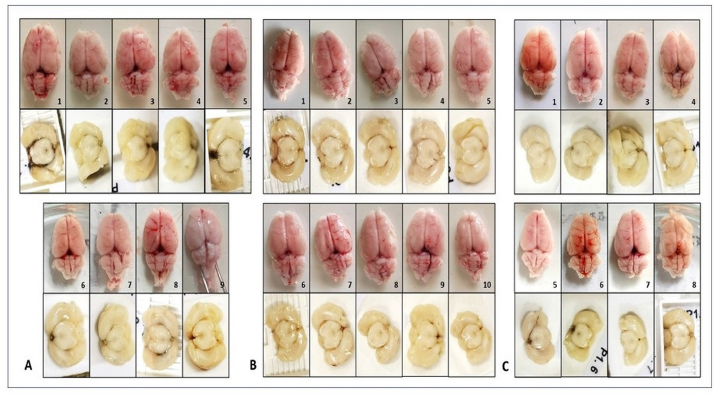
Fig. 3. Gross morphological evaluation of mice’s brain in (A) Pre-experimental, (B) Sham-operated, and (C) BACAS group revealed no signs of infarction or hemorrhage. Table 1. Body weight comparison of surviving mice between groups.
Clinical outcomeRegarding the clinical outcome, none of the mice that complete the observation period suffered paralysis. We also observe no signs of paralysis in the non-surviving BACAS mouse before its death. All of the surviving mice regained consciousness and exhibit active motor movements 1 hour after induction of anesthesia. The non-surviving BACAS mouse can regain consciousness despite exhibiting a sluggish movement. It did not eat and drink during the first 24-hour period after the operation and died the next day. Brain morphological changesAfter completing the observation period, all of the surviving mice are euthanized and we found that all ligatures in the pre-experimental and BACAS group were still in place. The mice’s brain is extracted using a standardized procedure. Gross examination of the brain revealed no visible signs of infarction and hemorrhage in all surviving mice (Fig. 3B and C). DiscussionWe propose a novel BACAS model which is cost-efficient, easy to reproduce, demonstrated no clinical evidence of cerebral infarction or hemorrhage, and has a low mortality rate (11.11%) during the observation period. This model may demonstrate its usability in research involving non-surgical intervention for carotid artery stenosis as there was an increasing prevalence of asymptomatic carotid artery stenosis, especially among the Asia population (Lee, 2021). We consider the use of Wistar mice in this study for having the highest resistance to cerebral ischemia compared to other strains of mice as it is commonly used as a model of chronic cerebral hypoperfusion and vascular dementia (Washida et al., 2019; Wang et al., 2020; Kim and Kim, 2021). A meta-analysis reported that Wistar mice have the lowest infarct size compared to Sprague Dawley and Wistar-Kyoto mice (Ström et al., 2013). Another study suggests that Wistar mice have a functioning posterior communicating artery, which may explain why Wistar mice do not develop cerebral infarction even after bilateral occlusion of the common carotid artery (Hattori et al., 2014). Due to this trait, we consider this model appropriate to replicate BACAS. The surgical procedure conducted in this study is conducted similarly to the previous study. We use a 0.6 mm-diameter needle to mimic a moderate to severe degree of stenosis. The calculation is based on reports from a previous study, where using a 0.8 mm-diameter needle cause a moderate degree of stenosis (55% ± 6.9%) while using a 0.45 mm-diameter needle causes a severe degree of stenosis (80% ± 7.3%) (Zhou et al., 2012). In addition, we consider the use of 5.0 monofilament polypropylene suture rather than 3.0 or 2.0 silk suture as reported in previous studies—due to the smaller size of mice used in this study and the high consistency and reliability as reported in a previous study (Takano et al., 1997). During the surgery, there may be a significant disruption in the cerebral blood flow primarily due to the ligation procedure. This is why we administer aspirin in the mice’s drinking water for 3 days after the surgery to prevent clotting (Jiang et al., 2020). We advise cautious handling during the ligation procedure because a sudden or excessive force given on the mouse’s common carotid artery may disrupt the cerebral hemodynamic which may lead to a fatal outcome. We observe that surviving mice become fully awake and active in 1 hour after the induction. However, non-surviving mice remain sluggish despite regaining consciousness. This finding is similar to a previous study where mice will fully recover from Ketamine/Xylazine anesthesia between 45 and 60 minutes after induction (Tammam et al., 2019). We argue that induction of anesthesia may not be the cause of non-survival because the dose was adjusted according to the mouse body weight and most of the mice in the non-surviving group (pre-experimental and BACAS) were able to fully regain consciousness. The possible cause for the non-survival of mice in this study is small bodyweight—causing a smaller common carotid artery diameter—which will exaggerate the degree of hypoperfusion caused by the procedure compared to larger mice (David et al., 2018). Cerebral edema may develop within the first 24–48 hours after the procedure, resulting in death (Chen et al., 2021). This finding is consistent with our study where we observe a decline in mortality rate after increasing the lower limit of mice’s body weight in this study. Most of the non-surviving mice died within 2 days after the procedure and were unable to eat or drink within the first 24 hours after surgery. Our study found no significant difference in body weight, clinical parameters, and brain morphological changes between sham and BACAS groups. This result shows that the surgical procedure conducted still provides adequate cerebral blood flow, thus replicating an asymptomatic stenosis. This study has several limitations. Firstly, the wide distribution of mice body weight used in this study may cause variation in the degree of stenosis. Secondly, we did not conduct angiographic analysis for the degree of stenosis in this study due to limitations in diagnostic equipment availability. Therefore, we were only able to estimate it based on replication from a previous study. Thirdly, the brain morphological evaluation was conducted solely through macroscopic examination and among surviving mice. This is because the brain of non-surviving mice is unable to be timely harvested, which caused the pathological findings found may not reflect the actual condition. ConclusionWe have successfully established a novel BACAS mouse model which is cost-efficient, easy to produce, and with no significant alteration of body weight, clinical parameters, and brain morphology. Further studies with angiographical confirmation are needed to accurately measure the degree of stenosis and assess the replicability of this model. Authors’ contributionAFS, WW, and PS designed this research. AFS, MH, and JPS conducted a survey and took samples at the samples field. All authors examined samples in the research laboratory. All authors compiled, read, revised, and approved the final manuscript. Conflict of interestAll authors declare that there is no conflict of interest. FundingThe authors received no financial support for the research, authorship, and/or publication of this article. ReferencesAbbott, A.L. 2009. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 40(10), e573–e583. Abbott, A.L., Paraskevas, K.I., Kakkos, S.K., Golledge, J., Eckstein, H., Diaz-Sandoval, L.J., Cao, L., Fu, Q., Wijeratne, T., Leung, T.W., Montero-Baker, M., Lee, B.C., Pircher, S., Bosch, M., Dennekamp, M. and Ringleb, P. 2015. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke 46(11), 3288–3301. AbuRahma, A.F. and Copeland, S.E. 1998. Bilateral internal carotid artery occlusion: natural history and surgical alternatives. Cardiovasc. Surg. 6(6), 579–583. Chen, S., Shao, L. and Ma, L. 2021 Cerebral edema formation after stroke: emphasis on blood–brain barrier and the lymphatic drainage system of the brain. Front. Cell Neurosci. 15, 716825. David, A., Prim, D.A., Mohamed, M.A., Brooks, A., Lane, B.A., Poblete, K., Wierzbicki, M.A., Lessner, S.M., Tarek Shazly, T. and Eberth, J.F. 2018. Comparative mechanics of diverse mammalian carotid arteries. PLoS One 13(8), e0202123. de Weerd, M., Greving, J.P., Hedbald, B., Lorenz, M.W., Mathiesen, E.B., O’Leary, D.H., Rosvall, M., Sitzer, M., Buskens, E. and Bots, M.L. 2010. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke 41(6), 1294–1297. Hainsworth, A.H., Allan, S.M., Boltze J, Cunningham, C., Farris, C., Head, E., Ihara, M., Isaacs, J.D., Kalaria, R.N., Saskia, A.M., Oberstein, J.L., Moss, M.B., Nitzsche, B., Rosenberg, G.A., Rutten, J.W, Salkovic-Petrisic, M. and Troen, A.M. 2017. Translational models for vascular cognitive impairment: a review including larger species. BMC Med. 15(1), 16–23. Hattori, Y., Kitamura, A. and Nagatsuka, K. 2014. A novel mouse model of ischemic carotid artery disease. PLoS One 9(6), e100257. Jiang, X., Liu, X., Liu, X., Wu, X., Jose, P. A., Liu, M. and Yang, Z. 2020. Low-dose aspirin treatment attenuates male rat salt-sensitive hypertension via platelet cyclooxygenase1 and complement cascade pathway. Am. Heart Assoc. 9(1), e013470. Ko, M.J., Mulia, G.E. and van Rjin, R.M. 2019. Commonly used anesthesia/euthanasia methods for brain collection differentially impact MAPK activity in male and female C57BL/6 mice. Front. Cell Neurosci. 13, 96–101. Kim, Y. and Kim Y.J. 2021 Effect of obesity on cognitive impairment in vascular dementia rat model via BDNF-ERK-CREB pathway. Biol. Res. Nurs. 23(2), 248–257. Lee, T.H. 2021. Management of carotid artery stenosis. Acta Neurol. Taiwan 30(4), 123–127. Rijbroek, A., Wisselink, W., Vriens, E.M., Barkhof, F., Lammertsma, A.A. and Rauwerda, J.A. 2006. Asymptomatic carotid artery stenosis: past, present and future. Eur. Neurol. 56(3), 139–154. Ström, J.O., Ingberg, E., Theodorsson, A. and Theodorsson, E. 2013. Method parameters’ impact on mortality and variability in rat stroke experiments: a meta-analysis. BMC Neurosci. 14(41), 1–24. Takano, K., Tatlisumak, T., Bergmann, A.G., Gibson, D.G. and Fisher, M. 1997. Reproducibility and reliability of middle cerebral artery occlusion using a silicone-coated suture (Koizumi) in rats. J. Neurol. Sci. 153(1), 8–11. Tammam, O.Y., Taha, A.A. and El-Sherif M.W. 2019. Optimization of xylazine-ketamine anesthetic dose in mice suffering chronic liver injury. J. Anesth. Crit. Care 11(1), 6–8. Wanamaker, K.M., Moraca, R.J., Nitzberg, D. and Magovern, G.J. 2012. Contemporary incidence and risk factors for carotid artery disease in patients referred for coronary artery bypass surgery. J. Cardiothorac. Surg. 28(7), 78–82. Wang, J., Yang, C., Wang, H., Li, D., Li, T., Sun, Y., Zhao, M., Ma, J., Hua, W., Zhuo, Y. and Yang, Z.A. 2020. New rat model of chronic cerebral hypoperfusion resulting in early-stage vascular cognitive impairment. Front. Aging. Neurosci. 12, 86–101. Washida, K., Hattori, Y. and Ihara, M. 2019. Animal models of chronic cerebral hypoperfusion: from mouse to primate. Int. J. Mol. Sci. 20(24), 6176–684. Zhou, Z., Zhang, Y., Zhu, C., Sui, J., Wu, G., Meng, Z., Huang, H. and Chen, K. 2012. Cognitive functions of carotid artery stenosis in the aged rat. Neuroscience 219, 137–144. | ||
| How to Cite this Article |
| Pubmed Style Sani AF, Widjiati W, Sugianto P, Hamdan M, Swatan JP. Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research. Open Vet. J.. 2022; 12(4): 463-468. doi:10.5455/OVJ.2022.v12.i4.7 Web Style Sani AF, Widjiati W, Sugianto P, Hamdan M, Swatan JP. Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research. https://www.openveterinaryjournal.com/?mno=5523 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i4.7 AMA (American Medical Association) Style Sani AF, Widjiati W, Sugianto P, Hamdan M, Swatan JP. Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research. Open Vet. J.. 2022; 12(4): 463-468. doi:10.5455/OVJ.2022.v12.i4.7 Vancouver/ICMJE Style Sani AF, Widjiati W, Sugianto P, Hamdan M, Swatan JP. Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research. Open Vet. J.. (2022), [cited January 25, 2026]; 12(4): 463-468. doi:10.5455/OVJ.2022.v12.i4.7 Harvard Style Sani, A. F., Widjiati, . W., Sugianto, . P., Hamdan, . M. & Swatan, . J. P. (2022) Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research. Open Vet. J., 12 (4), 463-468. doi:10.5455/OVJ.2022.v12.i4.7 Turabian Style Sani, Achmad Firdaus, Widjiati Widjiati, Paulus Sugianto, Muhammad Hamdan, and Jovian Philip Swatan. 2022. Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research. Open Veterinary Journal, 12 (4), 463-468. doi:10.5455/OVJ.2022.v12.i4.7 Chicago Style Sani, Achmad Firdaus, Widjiati Widjiati, Paulus Sugianto, Muhammad Hamdan, and Jovian Philip Swatan. "Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research." Open Veterinary Journal 12 (2022), 463-468. doi:10.5455/OVJ.2022.v12.i4.7 MLA (The Modern Language Association) Style Sani, Achmad Firdaus, Widjiati Widjiati, Paulus Sugianto, Muhammad Hamdan, and Jovian Philip Swatan. "Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research." Open Veterinary Journal 12.4 (2022), 463-468. Print. doi:10.5455/OVJ.2022.v12.i4.7 APA (American Psychological Association) Style Sani, A. F., Widjiati, . W., Sugianto, . P., Hamdan, . M. & Swatan, . J. P. (2022) Bilateral Asymptomatic Common Carotid Artery Stenosis (BACAS) Mouse Model for Stroke Research. Open Veterinary Journal, 12 (4), 463-468. doi:10.5455/OVJ.2022.v12.i4.7 |