| Case Report | ||
Open Vet. J.. 2021; 11(2): 283-288 Open Veterinary Journal, (2021), Vol. 11(2): 283–288 Case Report Use of a pleural access port for the treatment of pyothorax in a catAngel Almendros*Veterinary Medical Center, City University of Hong Kong, Hong Kong SAR, China *Corresponding Author: Angel Almendros. Veterinary Medical Center, City University of Hong Kong, Hong Kong SAR, China. Email: afalm [at] hotmail.com; afalm [at] cityuvmc.com.hk Submitted: 30/03/2021 Accepted: 21/05/2021 Published: 09/06/2021 © 2021 Open Veterinary Journal
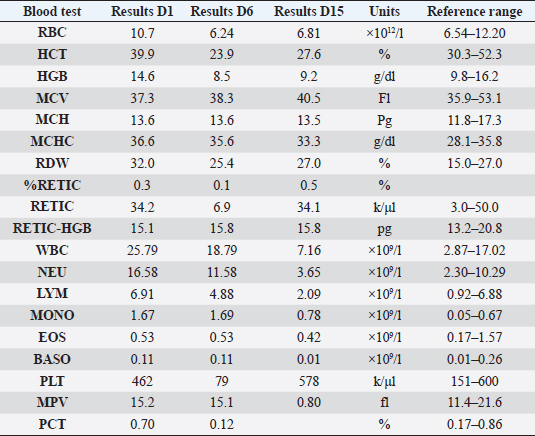
AbstractBackground: Pyothorax in cats is treated with intravenous fluids and antibiotics, and while thoracotomy and debridement are less commonly necessary, thoracostomy tubes are the treatment of choice when repeated drainage of the pleural cavity is needed. Case Description: An 11-month-old British short-haired cat was presented for a sudden onset of lethargy, dyspnea, and tachypnea, following an ovariohysterectomy 10 days prior to the treatment process. Pyrexia and muffled cardiac sounds on the left hemithorax were noted. A hemogram indicated the development of anemia and neutrophilia with a left shift. Radiography and ultrasonography confirmed a pleural effusion, and a CT scan ruled out the presence of any masses or perforating foreign bodies. A PCR on the pleural effusion ruled out feline coronavirus infection, and fluid analysis was confirmed as a septic exudate with Pasteurella multocida infection. A pleural access port was used to treat the pyothorax that successfully reduced hospitalization time and lowered overall financial outlay despite surgical implantation. Conclusion: The present report describes the successful use of a pleural port to treat pyothorax in one cat. This is the first time such a device has been reported for the treatment of pyothorax. Keywords: Pleural effusion, Pleural port, Pyothorax, Thoracocentesis, Thoracostomy tubes. IntroductionPyothorax is reported in both younger and older cats as an accumulation of purulent material in the pleural space. Direct inoculation occurs after trauma, such as perforating bite wounds or surgery. Hematogenous or lymphatic spread and translocation from adjacent spaces such as the mediastinum or esophagus can also be causes of pyothorax. Previous studies have suggested that oral and pharyngeal bacteria and perioperative aspiration can act as contaminants resulting in pleural empyema (Barrs et al., 2005). The treatment for pyothorax includes intravenous fluids and antibiotic therapy in all cases. While thoracotomy and debridement are less often needed in cats than dogs, unilateral or bilateral thoracostomy tube placement has shown a high (95%) success rate and is the preferred treatment when repeat drainage is necessary (Barrs et al., 2005). Newer and narrower thoracostomy tubes are less traumatic and more comfortable for the patients, especially in feline patients where the diameter might cause more discomfort and trocar use is discouraged (Valtolina and Adamantos, 2009). Introduction with a guide-wire reduces trauma and decreases the discomfort and complication rates associated with larger bore catheters. The average time of thoracic drainage via thoracostomy tubes ranges from 3 to 15 days (Barrs et al., 2005). Potential dislodgment of the tube along with bacterial introduction causes potential complications and warrants close monitoring during hospitalization. Postoperative care in an intensive care unit (ICU) is common practice after tube thoracostomy (Valtolina and Adamantos, 2009). Novel surgical approaches for the treatment of pleural effusion include the placement of pleuroperitoneal or pleurovenous shunts and omentalization, but these are not indicated in inflammatory, neoplastic, or infectious effusions due to the risk of spread to another body cavity (Brooks and Hardie, 2011). The use of vascular access ports with drains attached (Cahalane et al., 2007), and more recently of a pleural port device provided favorable outcomes for treating various types of effusions and pneumothorax (Brooks and Hardie, 2011; Cahalane and Flanders, 2012). Case DetailsThis report describes the treatment of pyothorax in a cat with a pleural access port, a controversial although novel approach not yet reported in the literature. An 11-month-old British short-haired cat presented for a sudden onset of dyspnea, lethargy, and hyporexia to the emergency service of the Veterinary Medical Center of the City University of Hong Kong. The cat had been spayed 2 weeks before presentation, and shortly after suture removal, the owner had observed lethargy, hyporexia, and increased respiratory effort. Upon physical examination on day 1, there was tachypnea with a respiratory rate of 80 bpm (RR; 20–30), the heart rate was 190 (RR; 150–200), and pyrexia of 39.5°C (37.7–39.1°C). A hemogram (Table 1) showed neutropenia, lymphocytosis, and monocytosis that after correction on the cytological examination was revealed to be left shift neutrophilia instead. Additionally, there was marginal hyponatremia 148 mmol/l (RR; 150–165). Feline immunodeficiency virus and feline leukemia virus were both negative on an ELISA test (SNAP 4Dx Plus, IDEXX Laboratories, Westbrook, ME). Oxygen saturation was consistent with severe hypoxemia (<90%). Radiographs of the thoracic portion revealed pleural effusion that obscured the cardiac silhouette (Fig. 1). A cardiogenic cause of the effusion was ruled out by echocardiography. Table 1. Hemogram results during hospitalization on day 1 and day 6 and at revisit on day 15.
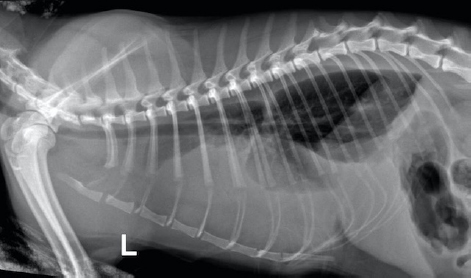
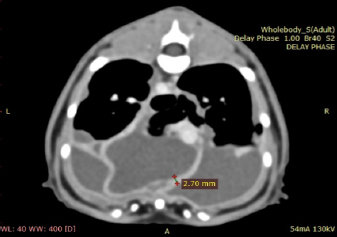
Fig. 1. Day 1: left lateral view of thorax showing pleural effusion. The cat was sedated, and 130 ml of straw brown colored sero-sanguineous exudate was removed via needle thoracentesis using an aseptic technique. Cytology of the fluid revealed mostly neutrophils with toxic changes and a large amount of rods. A sample of the effusion was submitted to an external laboratory for further analysis, including cytological interpretation (Fig. 2), aerobic culture, MIC, and a PCR test for feline infectious peritonitis (FIP). Thoracic radiographs post-thoracocentesis showed residual effusion and nodular radiodensities overlying the cardiac silhouette. Fluid therapy (dextrose 2.5% with NaCl 0.45%, Baxter Healthcare Corp, Deerfield, IL) was given at a maintenance dose of 1.5 ml/kg/hour, and a broad spectrum antibiotic, ampicillin sodium, and sulbactam sodium (Unasyn, Pfizer Ltd, Tadworth, UK) 25 mg/kg given intravenously thrice daily was started. After 2 days, the thoracic effusion reformed, requiring further needle thoracocentesis. The cat remained hyporexic, appeared slightly more lethargic, and had an increased respiratory rate of 42 breaths per minute but was otherwise stable and normothermic (38.5°C). The culture of the effusion reported Pasteurella multocida infection sensitive to ampicillin and doxycycline. The clients declined further treatment, including thoracostomy tube placement, which had been suggested on day 1, preferring to wait for the FIP PCR result before proceeding. The PCR was reported negative on day 4. A blood test (Table 1) on day 6 revealed a mild-to-moderate non-regenerative anemia, neutrophilia, monocytosis, and mild hypoalbuminemia 19 g/l (RR; 22–40). A CT scan (Fig. 3) of the chest was carried out to investigate the radiographic opacities and rule out any potential penetrating foreign body. A large amount of dependent free fluid was noted, with Hounsfield units of about 30, implying a highly cellular effusion. Multiple septations were present with marked contrast enhancement and up to 3-mm-thick walls separating fluid into larger cavities, particularly caudal to the heart (Fig. 3). Fluid took up about 60% of the pleural cavity displacing lungs dorsally and limiting their expansion, and there was mild ventral parietal pleural contrast enhancement. No evidence of inciting pathology, visible foreign bodies, or penetrating traumatic wounds were identified. The radiographic nodular radiopacities were suspected to be previous pockets of effusion.
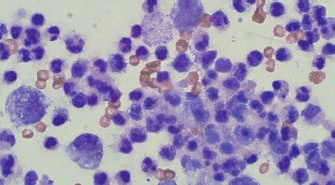
Fig. 2. Day 1: cytology. Large mononuclear cells, neutrophils, and bacterial rods (×40).
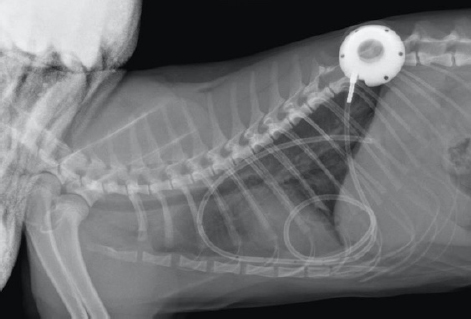
Fig. 3. Day 6: coronal view of a CT scan of the thorax showing contrast-enhancing pleural septations within pyothorax. The clients declined thoracostomy tube placement as the suggested first-line treatment and requested an esophagostomy tube to be placed to ensure enteral nutrition. Upon discussing the advantages and disadvantages of possible treatment options and techniques, the clients decided on a novel approach placing a pleural port device (PleuralPortTM, Norfolk Vet Products, Skokie, IL), limiting hospitalization time and to continue the treatment at home shortly after surgery. Both clients were in the human medical field (nurse and anesthetist). The cat was premedicated with methadone (Methone, Ceva Animal Health, Glenorie, Australia) 0.3 mg/kg IV, induced with alfaxalone (Alfaxan, Jurox Animal health, Crawley, UK) 1 mg/kg IV to effect and maintained under general anesthetic with isoflurane and oxygen. A right-side approach to the thoracic wall was elected based on the ultrasonographic distribution of the pleural effusion. A 4-cm skin incision was made in the dorsal 3rd of the chest wall at approximately the 10th intercostal space, tunneling to a second incision in the 8–9th intercostal space of the central chest area. An 18-gauge catheter and a 0.035″ J-tipped guide wire were then introduced. An eight French expander was used over the J-wire, and a seven French round tip fenestrated silicone tube was placed and secured with suture material in surrounding soft tissue around the thoracostomy tube entry site. The port was secured in the dorsal site with sutures to the fascia of the latissimus dorsi. Port patency was verified using a Huber point needle before and after skin closure. The skin closure was caudal to the port so that the incision line would not interfere with drainage and the healing of the skin would be favorable. Thoracocentesis and lavage of the pleural space were carried out before the surgical site’s closure with 250 ml of warmed sterile saline solution (0.9% NaCl, Baxter Healthcare Corp, Deerfield, IL). An esophagostomy tube was placed following the pleural port placement. Radiographs showed satisfactory positioning of the pleural port (Fig. 4). Lavage and drainage of the pleural space were successfully carried out following surgery using 50 ml of sterile saline solution q12 hours without complications.
Fig. 4. Day 6: left lateral view of thorax showing placement of a pleural port device with partial resolution of pyothorax. The cat was discharged on day 7, after it had started eating. The client was instructed to carry out an aseptic lavage twice daily with 50 ml of sterile saline solution at home. Following lavage and draining of the pleural space, the catheter was injected with 3–5 ml of heparinized (heparin 100 IU/ml) sterile saline after each use to avoid clotting and ensure patency. Doxycycline 5 mg/kg PO q24 hours, (Apo-Doxy, Apotex Inc., North York, ON) for 15 days and buprenorphine (Temgesic, Schering-Plough Limited, Hatfield, UK) 0.02 mg/kg PO q12 hours for 5 days were prescribed on discharge. At a revisit on day 15, the cat was very bright, had been eating well and gaining weight, appeared fully recovered with no fever, had no abnormal breathing effort, and had a normal respiratory (22 breaths per minute) and heart rates (145 beats per minute). The client reported the drain had remained patient when flushed and aspirated. Minimal excess of fluid (5–10 ml) had been collected from day 6 that had decreased and stopped in the last 2 days before the recheck visit. A blood test (Table 1) showed further stabilization of the hematocrit, mild monocytosis, and a normal ALB. Radiographs of the thoracic region showed resolution of the pyothorax. Cytology was repeated in-house after pleural space lavage that showed absence of bacterial infection. The esophagostomy tube was removed, and the lavage of the thorax was stopped after that day. Oral antibiotics were continued until finished, and no other medication was prescribed. At a follow-up visit 1 month later, on day 44, the cat was very bright, had been eating well, gained weight, and had normal physical exam findings. The blood test was unremarkable (Table 1), and radiographs of the chest showed no pleural effusion. The implant was in place, and its removal was suggested to the client if desired or if complications were observed. Ethical approvalThere were no ethical concerns with the collection of data or with the management of the case. All data used in this manuscript were collected in a retrospective manner. The clients and owners of the animal approved the management of the case and this report. DiscussionThe present case describes the treatment of feline pyothorax using a pleural port device, a treatment approach that has not been reported previously for this purpose. Needle thoracocentesis is often a less efficient treatment when the patient is not suitable for general anesthesia or the clients decline more invasive procedures. The placement of a pleural port to manage pleural effusions is similar in principle to the placement of thoracostomy tubes. They both generally require chemical restraint for an appropriate placement, as shown in up to 100% of cats in one study (Valtolina and Adamantos, 2009). The surgical time for placement of this device has been reported to be around 30 minutes (Brooks and Hardie, 2011). A low-profile thoracostomy tube can be placed in a shorter time and under sedation, yet 36% of procedures took longer than 10 minutes in a referral setting (Valtolina and Adamantos, 2009). In the present case, an esophagostomy tube was also requested; therefore, general anesthesia was imperative if the cat’s condition allowed it. Therefore, the placement of the pleural port device for that matter was also agreed upon. Hospitalization times are an advantage when a pleural port device is used compared to the placement of thoracostomy tubes with shorter mean hospitalization times of as little as 24 hours as it occurred in the present case (Valtolina and Adamantos, 2009; Brooks and Hardie, 2011; Cahalane and Flanders, 2012). Hospitalization time and the extensive cost associated with a lengthier stay at an ICU were minimized, which was a deciding factor for the client. Coiling and kinking of the tubing can occur, and the author has experienced that coiling easily occurs even after cutting and shortening the fenestrated part of the tube. Shortening the tube further might limit its draining potential and coiling has not stopped the drainage efficiency in the past in the author’s experience. Kinking, however, can occur at the point of entrance of the tube into the chest and for addressing that problem; in the experience of the author, softening the angles of introduction has given better results. Postoperative complications were not encountered in this case but have been reported elsewhere and include clogging or kinking of the tube, inflammation, iatrogenic infection, and pneumothorax (Cahalane et al., 2007; Brooks and Hardie, 2011). The specific use of pleural ports for pyothorax has not been reported, and there is scarce information with only a single comment about discouraging its use (Culp, 2015). A highly cellular exudative effusion such as the pyothorax reported here might predispose blockage of the drains. However, lavage of the pleural cavity and dilution of the exudate might have facilitated the drainage in this case. Although the author acknowledges the controversy of its use, other studies have reported the efficacy of similar implants where bacterial or fungal infections were also present (Cray et al., 2018; McQuitty and Branter, 2018). Additionally, a potential advantage of the pleural port device is the decreased risk of ascending nosocomial infections into the pleural space compared to needle drainage or thoracostomy tubes since the hub port is placed subcutaneously. More serious complications of pleural port placement include pulmonary parenchymal puncture and pneumothorax which have been reported as a cause for euthanasia (Brooks and Hardie, 2011). The use of newer, narrower tubes made of silicone reduced that risk considerably compared to trocar-induced thoracostomy (Cahalane et al., 2007; Valtolina and Adamantos, 2009). The use of a pleural port eliminated inflammation and discomfort associated with repeated thoracocentesis, reducing the potential damage of pulmonary parenchyma associated with repeated needle punctures. Dislodgement of the port and tube are potential drawbacks. There is a plausible requirement for removal under anesthesia in the future, which would not be necessary with thoracostomy tubes. Although biofilm formation in the indwelling device is a possibility, the hub and tube are made of titanium and silicone. They are relatively inert, preventing any local or systemic reactions. Removal of the port and tube is, therefore, not necessary unless infection occurs. The use of a bactericidal antibiotic at discharge would have been a better choice compared to a bacteriostatic antibiotic such as doxycycline. However, the cat had been treated with an appropriate intravenous bactericidal for 7 days during hospitalization, and doxycycline was chosen based on an antibiogram result guided by the minimum inhibitory concentration of the antimicrobial agent. The author acknowledges that since the clients were both in the human medical profession, the management of the pleural port might have felt less challenging or overwhelming, and that would have been key in their confidence and willingness to treat their pet at home. They were still trained and instructed thoroughly and accordingly, irrespective of their medical background. Other similar conditions where pleural ports are commonly used include chylothorax. In these cases, the training and collaboration of clients is always required even though they rarely are from a medical background. The resolution of the effusion, in this case, occurred within 6 days from the implantation of the port device. However, the port was removed a few months later in a short and unremarkable surgical procedure. Due to the treatment choice, the cat went home 1 day after surgery and recovered rapidly in a favorable environment. The author concurs with previous reports and advocates using thoracostomy tubes as the treatment of choice for feline pyothorax; however, in this study, we contemplated the value of an alternative treatment using a pleural port device in a single case report that resulted in a prompt resolution of the disease. We speculate that for a specific subset of patients, this might be a potentially successful approach. The author, however, acknowledges the need for further studies with larger cohorts before any conclusions are drawn. Conflict of interestThe author declares that there is not conflict of interest. AcknowledgmentsThe author is grateful to Dr. Jonathan Speelman, board certified surgeon at CityU VMC who helped editing the manuscript. He gave general advice, sharing his views on the subject, and pointing out possible shortcomings of this technique that helped to refocus the discussion section of the manuscript. The author also thanks the pathologists at CityU VDL for their input in the interpretation of laboratory data and cytological interpretation. ReferencesBarrs, V.R., Allan, G.S., Martin, P., Beatty, J.A. and Malik, R. 2005. Feline pyothorax: a retrospective study of 27 cases in Australia. J. Feline. Med. Surg. 7, 211–222. Brooks, A.C. and Hardie, R.J. 2011. Use of the PleuralPort device for management of pleural effusion in six dogs and four cats. Vet. Surg. 40, 935–941. Cahalane, A.K. and Flanders, J.A. 2012. Use of pleural access ports for treatment of recurrent pneumothorax in two dogs. J. Am. Vet. Med. Assoc. 241, 467–471. Cahalane, A.K., Flanders, J.A., Steffey, M.A. and Rassnick, K.M. 2007. Use of vascular access ports with intrathoracic drains for treatment of pleural effusion in three dogs. J. Am. Vet. Med. Assoc. 230, 527–531. Cray, M., Berent, A.C., Weisse, C.W. and Bagley, D. 2018. Treatment of pyonephrosis with a subcutaneous ureteral bypass device in four cats. J. Am. Vet. Med. Assoc. 252, 744–753. Culp, W.T.N. 2015. Pleural space disease – thoracic drainage and port placement. In Veterinary-image guided interventions. Eds Weisse, C. and Berent, A. Oxford, UK: John Wiley & Sons, Ltd, pp: 91–99. McQuitty, R.G. and Branter, E.M. 2018. Treatment of fungal pyelonephritis and ureterolithiasis with a subcutaneous ureteral bypass system and systemic antifungal medication in a cat. Vet. Med. 9, 73–78. Valtolina, C. and Adamantos, S. 2009. Evaluation of small-bore wire-guided chest drains for management of pleural space disease. J. Small Anim. Pract. 50, 290–297. | ||
| How to Cite this Article |
| Pubmed Style Angel Almendros|. Use of a pleural access port for the of treatment of pyothorax in a cat. Open Vet. J.. 2021; 11(2): 283-288. doi:10.5455/OVJ.2021.v11.i2.12 Web Style Angel Almendros|. Use of a pleural access port for the of treatment of pyothorax in a cat. https://www.openveterinaryjournal.com/?mno=68940 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i2.12 AMA (American Medical Association) Style Angel Almendros|. Use of a pleural access port for the of treatment of pyothorax in a cat. Open Vet. J.. 2021; 11(2): 283-288. doi:10.5455/OVJ.2021.v11.i2.12 Vancouver/ICMJE Style Angel Almendros|. Use of a pleural access port for the of treatment of pyothorax in a cat. Open Vet. J.. (2021), [cited January 25, 2026]; 11(2): 283-288. doi:10.5455/OVJ.2021.v11.i2.12 Harvard Style Angel Almendros| (2021) Use of a pleural access port for the of treatment of pyothorax in a cat. Open Vet. J., 11 (2), 283-288. doi:10.5455/OVJ.2021.v11.i2.12 Turabian Style Angel Almendros|. 2021. Use of a pleural access port for the of treatment of pyothorax in a cat. Open Veterinary Journal, 11 (2), 283-288. doi:10.5455/OVJ.2021.v11.i2.12 Chicago Style Angel Almendros|. "Use of a pleural access port for the of treatment of pyothorax in a cat." Open Veterinary Journal 11 (2021), 283-288. doi:10.5455/OVJ.2021.v11.i2.12 MLA (The Modern Language Association) Style Angel Almendros|. "Use of a pleural access port for the of treatment of pyothorax in a cat." Open Veterinary Journal 11.2 (2021), 283-288. Print. doi:10.5455/OVJ.2021.v11.i2.12 APA (American Psychological Association) Style Angel Almendros| (2021) Use of a pleural access port for the of treatment of pyothorax in a cat. Open Veterinary Journal, 11 (2), 283-288. doi:10.5455/OVJ.2021.v11.i2.12 |