| Case Report | ||
Open Vet. J.. 2023; 13(3): 376-381 Open Veterinary Journal, (2023), Vol. 13(3): 376–381 Case Report Perforated duodenal ulcer in a dog with gallbladder agenesisLuca Ciammaichella, Armando Foglia, Sara Del Magno*, Veronica Cola, Stefano Zanardi, Francesca Del Baldo, Marco Pietra, Maria Morini and Luciano PisoniDipartimento di Scienze Mediche Veterinarie, Alma Mater Studiorum, University of Bologna, Bologna, Italy *Corresponding Author: Sara Del Magno. Dipartimento di Scienze Mediche Veterinarie, Alma Mater Studiorum, University of Bologna, Bologna, Italy. Email: sara.delmagno [at] unibo.it. Submitted: 28/06/2022 Accepted: 14/02/2023 Published: 21/03/2023 © 2023 Open Veterinary Journal
AbstractBackground: Duodenal ulceration (DU) in dogs derives from different causes but has never previously been related to gallbladder agenesis (GA). GA is a rare congenital disorder in dogs and is considered a predisposing factor for DU in humans. Case Description: A 5-month-old intact female Maltese was presented for acute vomiting and diarrhea. Abdominal ultrasound suggested duodenal perforation and absence of the gallbladder. Exploratory laparotomy was performed to treat the perforation and confirmed GA. Hepatic ductal plate malformation (DPM) was histologically diagnosed in liver biopsy, but no signs of liver dysfunction were detected by blood work at first admission. Two months later, the dog developed signs of portal hypertension and medical treatment was started. However, the clinical condition gradually worsened until liver failure and the dog was euthanized 8 months after surgery. Necropsy confirmed hepatic abnormalities. Conclusion: This report describes a case of DU associated with GA and DPM in a dog. As in humans, GA may represent a hepatobiliary disease predisposing to gastroduodenal ulcerations. Keywords: Agenesis, Dog, Duodenum, Gallbladder, Ulceration. IntroductionDuodenal ulceration (DU) is a severe and potentially life-threatening condition, affecting 0.2% of dogs (Saravanan et al., 2012; Daure et al., 2017). In both humans and dogs, risk factors for the development of this condition are glucocorticoid or non-steroid anti-inflammatory drug administration, extensive trauma, severe gastroenteritis, pancreatitis, sepsis, liver disease, foreign bodies, hypoadrenocorticism, or neoplasia (Cariou et al., 2009). Treatment includes the resolution of underlying causes and medical management, but surgical intervention is required if the ulcer is perforated. In general, the prognosis is fair to good (53%–71%) with prompt and adequate treatment (Cariou et al., 2009; Fitzgerald et al., 2017). In dogs, DU has never been associated with gallbladder agenesis (GA), a rare congenital anomaly resulting from a developmental failure of the hepatic diverticulum (Liptak et al., 2000; Kamishina et al., 2010; Sato et al., 2018). A relationship between gallbladder absence and DU has been hypothesized and reported in human medicine (Haoues et al., 2014; Tsai et al., 2016). In healthy animals, the gallbladder regulates biliary salt excretion and counteracts post-prandial gastric acid secretion, thanks to biliary salts’ buffering power. When the gallbladder is absent, biliary salts are excreted continuously and the bile buffering capacity is decreased, predisposing the patient to various consequences, from enteritis to DU (Argenzio, 1993). Based on a literature search performed on online databases (e.g.: PubMed, Google Scholar) with different keywords (e.g.: GA, DU, dog, cat) on February 2022, this is the first report presenting a dog with DU and concomitant GA. Case DetailsA 5-month-old intact female Maltese dog, 2.7 kg bodyweight, was presented with acute onset of vomiting, diarrhea, inappetence, and depression. The dog was regularly vaccinated and dewormed for ectoparasites and endoparasites. Physical examination revealed a poor body condition score (3/9), depression, approximately 10% dehydration, pale mucosal membranes, tachycardia (180 ppm), and abdominal pain. A complete blood count showed mild anemia and moderate neutrophilic leukocytosis, with band and toxic granulation neutrophils. Biochemistry revealed a moderate increase in hepatocellular and cholestatic enzymes, with hypoproteinemia and hypoalbuminemia (Table 1, T0). Radiographs of the abdomen, interpreted by a board-certified radiologist, revealed multiple spot-like calcific areas in the ventral epigastric region and loss of serosal details, suggestive of peritoneal effusion and/or peritonitis. Abdominal ultrasonography (US) showed focal thickening of the proximal part of the descending duodenal wall, associated with reduced layering details, and multiple spot-like hyperechoic reverberant interfaces crossing the whole duodenal wall, consistent with gas. The mesenteric fat surrounding the duodenum appeared focally thickened and hyperechoic; tributary lymph nodes were enlarged, and there was mild peritoneal effusion between the proximal duodenum and the liver. Furthermore, the gallbladder was not identified. The remaining organs were within normal limits. On the basis of clinical, laboratory, and imaging findings, duodenal wall perforation and focal peritonitis were suspected, and an exploratory laparotomy was scheduled. Table 1. Significant clinicopathological findings over time.
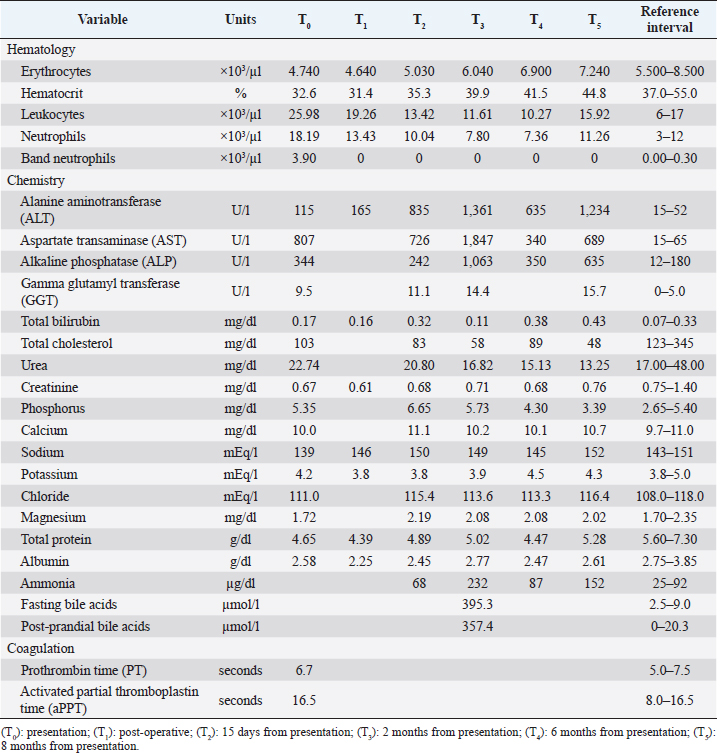
During abdominal exploration, a small amount of free turbid peritoneal fluid and severe hyperemia of the greater omentum was observed. The peritoneal exudate was collected and underwent cultural examination, which did not yield any bacterial growth. The liver appeared subjectively small with diffusely irregular margins; however, obvious focal lesions were not detected. The gallbladder was not identified either in the classic or ectopic sites. Moreover, the lesser omentum was severely thickened and hyperemic, with numerous adherences to the liver capsule, the pylorus, and the proximal portion of the duodenum. These adherences were gently solved by blunt and sharp dissection, revealing a duodenal perforation of 4 mm in diameter on the antimesenteric side, 3 cm distal to the pylorus (Fig. 1A). The edges of the duodenal lesion were extremely friable and bleeding slightly, and surgical findings were consistent with perforated DU. Ulcer debridement was performed and tissue from the marginal surface of the ulcerative lesion was sent for histopathological analysis. The defect was sutured transversely with a full-thickness simple interrupted pattern with 4-0 USP absorbable monofilament (Glycomer 631) (ByosinTM; Medtronic, Dublin, D02, Ireland) (Fig. 1B). A serosal patching, using a nearby jejunal loop, was also performed to reinforce the duodenal repair and multiple liver biopsies were collected for histological evaluation. After peritoneal lavages with sterile warm saline, the celiotomy was closed routinely.
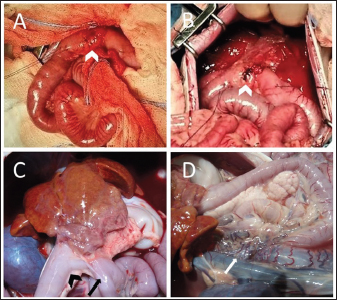
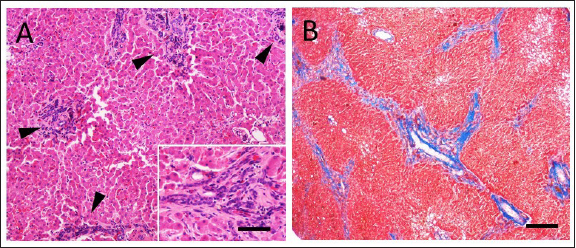
Fig. 1. (A) Cranial: above; Caudal: below. Intraoperative view of the ulcer (white arrowhead) on the antimesenteric side of the proximal duodenum, after debridement has been performed. Stay sutures were placed proximally and distally to the ulcer. (B) Cranial: above; Caudal: below. Intraoperative view of the DU (white arrowhead) closed transversely with full-thickness approximating simple interrupted sutures, prior to perform the serosal patching. Cranially, note the liver with diffusely irregular margins and the absence of the gallbladder. (C) Cranial: above; Caudal: below. Necropsy view of the previous site of DU, covered by adherence between proximal duodenum (black arrowhead), the jejunal loop used for serosal patching (black arrow) and the visceral aspect of the right medial liver lobe. Note the fibrotic liver cranially and the right kidney on the left side. (D) Cranial: left side; Caudal: right side. Necropsy view of the multiple shunts (white arrow) deriving from the portal vein. Note the duodenum and the pancreas above and the liver on the left side. The dog recovered uneventfully from surgery and anesthesia and was hospitalized in the intensive care unit for post-operative monitoring. Buprenorphine (10 µg/kg IV q 8 hours), ampicillin-sulbactam (20 mg/kg IV q 8 hours), maropitant (1 mg/kg IV q 24 hours), and omeprazole (1 mg/kg IV q 12 hours) were administered. The clinical condition and blood work gradually improved during post-operative hospitalization (Table 1, T1). The dog started eating a canned gastrointestinal diet 24 hours postoperatively and was discharged 4 days after surgery with antimicrobial therapy (amoxicillin-clavulanic acid 12.5 mg/kg orally q 12 hours) and proton-pump inhibitor (omeprazole 1 mg/kg orally q 12 hours) to be administered for 7 days. Histopathology on formalin-fixed paraffin-embedded samples collected during explorative laparotomy confirmed a severe, diffuse subacute fibrinous peritonitis, with steatonecrosis and granulation tissue corresponding to transmural intestinal ulceration, and the liver showed a moderate, multifocal, bridging portal fibrosis with moderate to marked bile ductule proliferation and moderate hyperplasia of juvenile intrahepatic arterioles (Fig. 2). These findings were suggestive of a congenital hepatic disorder, such as ductal plate malformation (DPM), with histopathologic features of proliferative-like phenotype (Seibert et al., 2021). At 15-days follow up, the dog was in good clinical condition, but occasional post-prandial depression was reported by the owner. Biochemistry showed a persistent increase in liver enzymes with normal blood ammonia levels (Table 1, T2), and no signs of DU relapse were observed at the abdominal US. Two months after surgery the patient was rechecked for vomiting and diarrhea, associated with inappetence and depression. Physical examination revealed tachycardia (160 ppm), polypnea (60 bpm), and enlargement of the abdomen. Abdominal US reassessment highlighted different tortuous vessels directed from the splenic vein to the left renal vein, consistent with acquired nephro-splenic shunts. Portal blood flow velocity, measured by micro-convex probe (5–8 Mhz) (Epiq5G Ultrasound System; Philips Healthcare, Monza, 20900, Italy) with color flow Doppler, was 7.43 cm/second. Furthermore, decreasing liver size, pancreatic edema, and severe peritoneal effusion were also detected, without any sign of DU recurrence. The abdominal fluid was cytologically and biochemically consistent with low-protein transudate, and these findings were suggestive of pre-sinusoidal portal hypertension, likely secondary to the ductal plate abnormalities (Buob et al., 2011). Results of serum biochemistry were suggestive of liver failure (Table 1, T3). Specific treatment with omeprazole (1 mg/kg orally q 12 hours), famotidine (1 mg/kg q 24 hours), ursodeoxycholic acid (20 mg/kg orally q 24 hours), s-adenosylmethionine (18 mg/kg orally q 24 hours), metronidazole (7.5 mg/kg orally q 12 hours), spironolactone (5 mg/kg orally q 12 hours), lactulose (0.5 ml/kg orally q 8 hours), and a hepatic diet were introduced. Clinical signs gradually improved and 1 month later (3 months after surgery) the dog was bright, alert, and responsive with a good appetite. The physical exam was unremarkable and a decrease in liver enzymes and ammonia was noted (Table 1, T4). Ursodeoxycholic acid, s-adenosylmethionine, spironolactone, and hepatic diet were continued, while other therapies were discontinued, and the patient maintained a good clinical condition over the next 5 months.
Fig. 2. Histopathologic characteristics of a liver with DPM and GA. (A) There are numerous bile duct profiles in fibrotic portal space, and entrapment of normal-appearing hepatic parenchyma (Hematoxylin and Eosin stain, bar 100 µ). INSET: detail of a portal space (bar 50 µ). (B) Mild amounts of fibrillar collagen and portal-to-portal bridging can be observed (Masson’s trichrome stain, bar 300 µ). Eight months after surgery, the dog was reexamined for disorientation, circling, vomiting, and diarrhea. Icteric mucous membranes, severe dehydration, enlargement of the abdomen, neurologic signs (postural reaction deficits and prosencephalic signs) were detected on physical examination, and hepatic encephalopathy was confirmed by blood work (Table 1, T5). Additionally, severe peritoneal effusion and worsening of portal hypertension (portal blood flow velocity of 5.74 cm/second) were found in abdominal US. Due to the poor clinical condition and unfavorable prognosis, the owner elected euthanasia. Severe peritoneal effusion, decreased liver size, gallbladder absence, and multiple acquired portosystemic shunts were found on necropsy examination (Fig. 1C and D). Signs of bowel ulceration or perforation were not detected. DiscussionBased on the literature search, this is the first report of a dog with a perforated DU, concomitant and presumably related to GA. GA is a rare congenital condition both in humans and dogs and can originate from different mechanisms, including failure of vacuolization of the pars cistica and cystic duct, alterations of gallbladder recanalization, or failure of development of the hepatic diverticulum (Liptak et al., 2000; Haoues et al., 2014). Dogs affected by GA are frequently young and small pure breed (e.g.: Maltese, Chihuahua) and clinical signs vary from none to non-specific ones, such as vomiting, anorexia, and lethargy, which are also reported in the case of gastrointestinal ulceration (Daure et al., 2017; Sato et al., 2018). Although GA has never previously been described in association with DU, the authors suppose that the initial clinical presentation of the dog was mainly related to DU, since the clinical signs promptly disappeared after the initial surgical treatment of the perforated DU. Clinicopathological abnormalities of DU, as already reported, are non-specific, including neutrophilia, left shift, normocytic and normochromic anemia, and hypoalbuminemia (Daure et al., 2017). Conversely, dogs with GA often manifest only an increase in hepatocellular enzyme concentrations (Sato et al., 2018). In this case, DU was suspected on the basis of ultrasonographic findings and confirmed during abdominal surgical exploration. Abdominal US cannot differentiate GA from other gallbladder abnormalities, such as shrinkage, rupture, or ectopia, and computed tomography (CT) scans are therefore suggested (Kamishina et al., 2010; Kelly et al., 2019). A CT scan was not performed in the described case because of the need for emergency surgery, and GA was then confirmed at the time of exploratory laparotomy. A jejunal loop serosal patch was preferred to reinforce the duodenal suture since the omentum was severely reactive due to the peritonitis and therefore might not have guaranteed the same support for ulcer healing. In human medicine, gallbladder absence (i.e., cholecystectomy) appears to predispose to DU, because of the continuous excretion of biliary salt and decreased bile buffering power, leading to persistent irritation, inflammation, and eventual ulceration (Haoues et al., 2014; Tsai et al., 2016). The same pathophysiological mechanism which relates GA and DU in dogs is based on the awareness that bile acts as a buffer in the duodenum, neutralizing hydrogen ions derived from the stomach (Argenzio, 1993). In the case of the absence of a gallbladder, as happens in patients that have undergone cholecystectomy, bile excretion is continuous, but biliary salt concentrations are decreased due to the missing storage and absorption in the gallbladder (Tsai et al., 2016; Kelly et al., 2019). Diluted bile seems to have few consequences on fat digestion and absorption. Conversely, a continuous flow of biliary salts might predispose to enteritis and DU due to the inadequate buffering power of the bile (Argenzio, 1993). Hepatic histopathologic findings typical of DPM are often encountered in dogs with GA (94%), suggesting a close relationship between these congenital abnormalities (Sato et al., 2018). Although the DPM histopathology resembles that of the primary hypoplasia of the portal vein, based on information from previous reports, a final diagnosis of DPM was made (Pillai et al., 2016; Sato et al., 2018). This congenital hepatobiliary malformation results from an incomplete remodeling of embryonal liver tissue, with consequent permanence of excessive embryonal biliary ductal structures (Pillai et al., 2016). The etiology of DPM and its consequences appear to have consistent similarities between humans and dogs (Pillai et al., 2016). Progressive congenital fibrosis induces portal hypertension, leading to increased liver enzyme activity, mild gastroenteric signs, ascites, and seizures, with concurrent development of acquired portosystemic shunts and hepatic encephalopathy (Pillai et al., 2016). DU has also been associated with liver disease in humans and dogs, although the pathophysiology remains unknown and it is unclear which specific liver disorder can be related to this condition (Daure et al., 2017). Moreover, duodenal perforation has been described as a co-morbidity in dogs with non-cirrhotic portal hypertension (Bunch et al., 2001). Nevertheless, our patient was very young and did not show clinicopathological signs of liver failure or signs of portal hypertension at the time of DU diagnosis. Even if many other factors may predispose to DU development, in the authors’ opinion, GA is one probable cause in this case (Argenzio, 1993; Hinton et al., 2002; Haoues et al., 2014; Tsai et al., 2016). Indeed, the dog described in the present report was young, and no drug had been administered prior to the first presentation. Moreover, infectious gastroenteritis, pancreatitis, or foreign bodies were considered less likely due to history, blood exams, imaging, surgical, and histological findings. Lastly, at the time of the first presentation, based on clinic-pathological findings, the dog had not yet developed liver failure; thus, it is unlikely that liver disease was responsible for the DU. It can therefore be assumed that the dog developed DU first due to GA even if the two conditions (GA and DU) might be concurrent and unrelated. The prognosis of dogs with DU is strictly related to prompt diagnosis and intervention and treatment of the underlying causes. Generally, prognosis and quality of life are good in adequately managed dogs (Fitzgerald et al., 2017). On the contrary, the prognosis of GA in dogs is unknown, and it seems to be related to concurrent congenital liver disease (Sato et al., 2018). Life expectation in canine DPM is quite long (7.5–8.8 years) with early diagnosis and treatment, but consequences, including portal hypertension, hepatic encephalopathy, and biliary dyskinesia, can severely impact survival, as in the present case (Pillai et al., 2016; Sato et al., 2018). Moreover, acute clinical signs and severe clinicopathological findings in dogs with DU may conceal the more subtle and chronic presentation of liver disease, perhaps delaying liver failure treatment. In conclusion, GA might be considered an underlying cause of DU and can be associated with concurrent liver abnormalities, potentially leading to liver failure, which negatively impacts the clinical condition and quality of life. Moreover, the progression of congenital liver disease and portal hypertension makes it necessary to strictly monitor the patient, aiming to prevent clinical deterioration by medical therapy modulation. Conflict of interestThe Authors declare that there is no conflict of interest. Author contributionsLC collected the data, wrote, and reviewed the manuscript. AF managed the case and wrote the manuscript. SDM conceptualized the case, supported the drafting, and reviewed the manuscript. VC collected the data, supported the drafting, and reviewed the manuscript. SZ collected the data and reviewed the manuscript. FDB managed the case, supported the drafting, and reviewed the manuscript. MP managed the case and reviewed the manuscript. MM managed the case, reviewed the manuscript, and edited the figures. LP managed the case and reviewed the manuscript. All Authors read and approved the final manuscript. ReferencesArgenzio, R.A. 1993. Secretory functions of the gastrointestinal tract. In Dukes’ physiology of domestic animals. Eds., Dukes, H.H., Swenson, M.J. and Reece, W.O. Minneapolis, MN: University of Minnesota: Comstock, pp: 349–361. Bunch, S.E., Johnson, S.E. and Cullen, J.M. 2001. Idiopathic noncirrhotic portal hypertension in dogs: 33 cases (1982–1998). J. Am. Vet. Med. Assoc. 218(3), 392–399. Buob, S., Johnston, A.N. and Webster, C.R.L. 2011. Portal hypertension: pathophysiology, diagnosis, and treatment. J. Vet. Int. Med. 25(2), 169–186. Cariou, M., Lipscomb, V.J., Brockman, D.J., Gregory, S.P. and Baines, S.J. 2009. Spontaneous gastroduodenal perforations in dogs – a retrospective study of 15 cases. Vet. Rec. 165(15), 436–441. Daure, E., Ross, L. and Webster, C.R. 2017. Gastroduodenal ulceration in small animals: part 1. Pathophysiology and epidemiology. J. Am. Anim. Hosp. Assoc. 53(1), 1–10. Fitzgerald, E., Barfield, D., Lee, K.C.L. and Lamb, C.R. 2017. Clinical findings and results of diagnostic imaging in 82 dogs with gastrointestinal ulceration. J. Small. Anim. Pract. 58(4), 211–218. Haoues, N., Zairi, S., Zaafouri, H., Maamer, A.B., Noomene, R., Oueslati, A., Bouhafa, A. and Cherif, A. 2014. Gallbladder agenesis intraoperatively diagnosed: a case report. Tunis. Med. 92(2), 168–169. Hinton, L.E., McLoughlin, M.A., Johnson, S.E. and Weisbrode, S.E. 2002. Spontaneous gastroduodenal perforation in 16 dogs and seven cats (1982–1999). J. Am. Anim. Hosp. Assoc. 38(2), 176–187. Kamishina, H., Katayama, M., Okamura, Y., Sasaki, J., Chiba, S., Goryo, M., Sato, R. and Yasuda, J. 2010. Gallbladder agenesis in a Chihuahua. J. Vet. Med. Sci. 72(7), 959–962. Kelly, D., Moreno-Aguado, B. and Lamb, V. 2019. Gallbladder agenesis in a dog: clinicopathological, histopathology, and computed tomography findings. J. Am. Anim. Hosp. Assoc. 55(6), e55602. Liptak, J.M., Swinney, G.R., Rothwell, T.L.W. and Hunt, G.B. 2000. Aplasia of the gallbladder in a dog. J. Small. Anim. Pract. 41(4), 175–177. Pillai, S., Center, S.A., McDonough, S.P., Demarco, J., Pintar, J., Henderson, A.K., Cooper, J., Bolton, T., Sharpe, K., Hill, S., Benedict, A.G. and Haviland, R. 2016. Ductal plate malformation in the liver of boxer dogs: clinical and histological features. Vet. Pathol. 53(3), 602–613. Saravanan, M., Nagarajan, B., Kavitha, S., Balachandaran, C. and Srinivasan, S.R. 2012. Duodenoscopic appraisal of duodenal ulcer in dogs. Vet. World. 5(7), 420. Sato, K., Sakai, M., Hayakawa, S., Sakamoto, Y., Kagawa, Y., Kutara, K., Teshima, K., Asano, K. and Watari, T. 2018. Gallbladder agenesis in 17 dogs: 2006–2016. J. Vet. Intern. Med. 32(1), 188–194. Seibert, L.M., Center, S.A., Randolph, J.F., Miller, M.L., Miller, A.D., Choi, E., Flanders, J.A. and Harvey, H.J. 2021. Relationships between congenital peritoneopericardial diaphragmatic hernia or congenital central diaphragmatic hernia and ductal plate malformations in dogs and cats. J. Am. Vet. Med. Assoc. 259(9), 1009–1024. Tsai, M.C., Huang, C.C., Kao, L.T., Lin, H.C. and Lee, C.Z. 2016. Increased risk of peptic ulcers following a cholecystectomy for gallstones. Sci. Rep. 6(1), 1–6. | ||
| How to Cite this Article |
| Pubmed Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Baldo FD, Pietra M, Morini M, Pisoni L. Perforated duodenal ulcer in a dog with gallbladder agenesis. Open Vet. J.. 2023; 13(3): 376-381. doi:10.5455/OVJ.2023.v13.i3.15 Web Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Baldo FD, Pietra M, Morini M, Pisoni L. Perforated duodenal ulcer in a dog with gallbladder agenesis. https://www.openveterinaryjournal.com/?mno=70062 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i3.15 AMA (American Medical Association) Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Baldo FD, Pietra M, Morini M, Pisoni L. Perforated duodenal ulcer in a dog with gallbladder agenesis. Open Vet. J.. 2023; 13(3): 376-381. doi:10.5455/OVJ.2023.v13.i3.15 Vancouver/ICMJE Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Baldo FD, Pietra M, Morini M, Pisoni L. Perforated duodenal ulcer in a dog with gallbladder agenesis. Open Vet. J.. (2023), [cited January 25, 2026]; 13(3): 376-381. doi:10.5455/OVJ.2023.v13.i3.15 Harvard Style Ciammaichella, L., Foglia, . A., Magno, . S. D., Cola, . V., Zanardi, . S., Baldo, . F. D., Pietra, . M., Morini, . M. & Pisoni, . L. (2023) Perforated duodenal ulcer in a dog with gallbladder agenesis. Open Vet. J., 13 (3), 376-381. doi:10.5455/OVJ.2023.v13.i3.15 Turabian Style Ciammaichella, Luca, Armando Foglia, Sara Del Magno, Veronica Cola, Stefano Zanardi, Francesca Del Baldo, Marco Pietra, Maria Morini, and Luciano Pisoni. 2023. Perforated duodenal ulcer in a dog with gallbladder agenesis. Open Veterinary Journal, 13 (3), 376-381. doi:10.5455/OVJ.2023.v13.i3.15 Chicago Style Ciammaichella, Luca, Armando Foglia, Sara Del Magno, Veronica Cola, Stefano Zanardi, Francesca Del Baldo, Marco Pietra, Maria Morini, and Luciano Pisoni. "Perforated duodenal ulcer in a dog with gallbladder agenesis." Open Veterinary Journal 13 (2023), 376-381. doi:10.5455/OVJ.2023.v13.i3.15 MLA (The Modern Language Association) Style Ciammaichella, Luca, Armando Foglia, Sara Del Magno, Veronica Cola, Stefano Zanardi, Francesca Del Baldo, Marco Pietra, Maria Morini, and Luciano Pisoni. "Perforated duodenal ulcer in a dog with gallbladder agenesis." Open Veterinary Journal 13.3 (2023), 376-381. Print. doi:10.5455/OVJ.2023.v13.i3.15 APA (American Psychological Association) Style Ciammaichella, L., Foglia, . A., Magno, . S. D., Cola, . V., Zanardi, . S., Baldo, . F. D., Pietra, . M., Morini, . M. & Pisoni, . L. (2023) Perforated duodenal ulcer in a dog with gallbladder agenesis. Open Veterinary Journal, 13 (3), 376-381. doi:10.5455/OVJ.2023.v13.i3.15 |