| Original Article | ||
Open Vet. J.. 2021; 11(4): 535-543 Open Veterinary Journal, (2021), Vol. 11(4): 535–543 Original Research Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinomaVerônica M. Govoni1, Claudio Pigoli2,3, Felipe Augusto R. Sueiro4, Fernanda Zuliani1, Thayná O. da Silva5, Juliany G. Quitzan1, Renee Laufer-Amorim5, Valeria Grieco3 and Carlos Eduardo Fonseca-Alves1,6*1Department of Veterinary Surgery and Animal Reproduction, School of Veterinary Medicine and Animal Science, Sao Paulo State University—UNESP, Botucatu, Brazil 2Laboratorio di Istologia, Sede Territoriale di Milano, Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna (IZSLER), Milan, Italy 3Department of Veterinary Medicine, University of Milan, Milan, Italy 4VetPat Laboratory, Campinas-SP, Brazil 5Department of Veterinary Clinic, School of Veterinary Medicine and Animal Science, Sao Paulo State University—UNESP, Botucatu, Brazil 6Institute of Health Sciences, Paulista University—UNIP, Bauru-SP, Brazil *Corresponding Author: Carlos Eduardo Fonseca-Alves. School of Veterinary Medicine and Animal Science, Sao Paulo State University—UNESP, Botucatu, Brazil. Email: carlos.e.alves [at] unesp.br Submitted: 20/04/2021 Accepted: 08/09/2021 Published: 04/10/2021 © 2021 Open Veterinary Journal
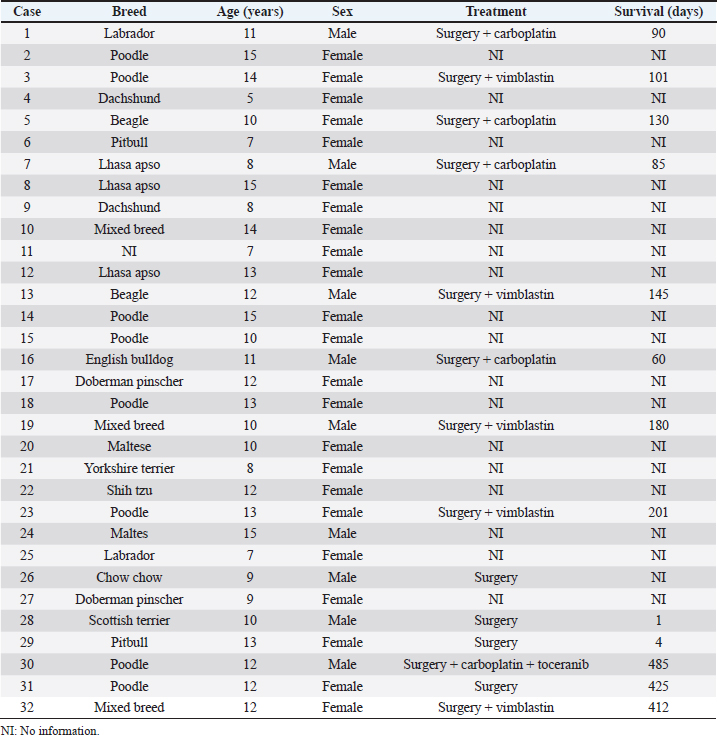
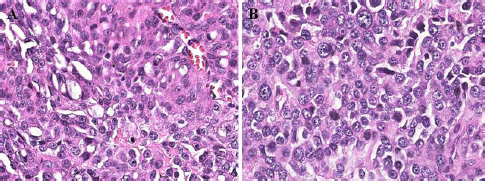
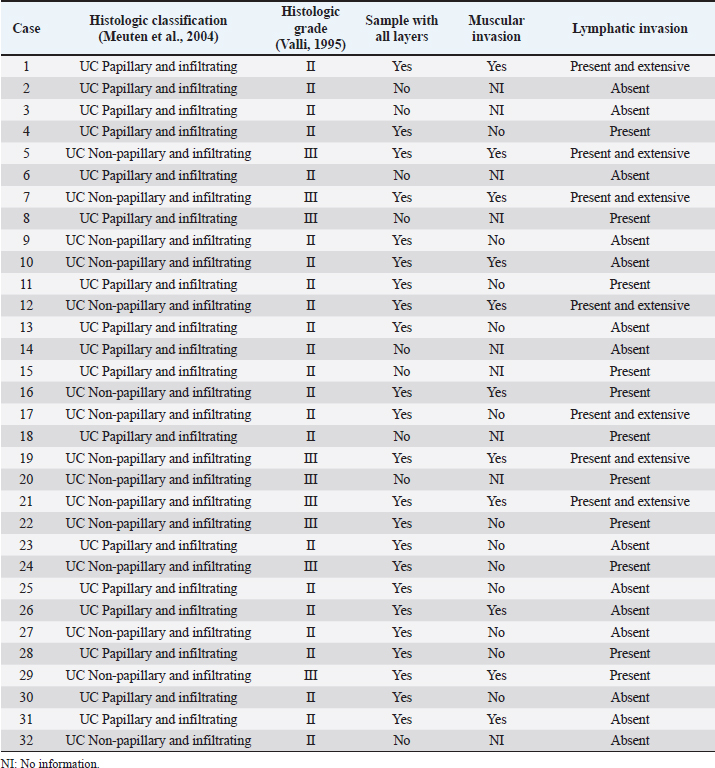
AbstractBackground: Urothelial carcinoma (UC), also known as transitional cell carcinoma, is the most common malignant tumor of the canine urinary bladder and represents a model for studying human bladder cancer. However, the existing literature has limited data on the clinicopathological characteristics of these tumors and their prognostic value. Aim: The aim of this study was to evaluate such factors, correlating them with follow-up, in a group of 32 dogs with bladder UC. Methods: Clinical data of these cases, submitted to São Paulo State University and VetPat Private Laboratory (São Paulo/Brazil), were recorded between January 2000 and November 2019. For each case, formalin-fixed and paraffin-embedded sections were stained with hematoxylin and eosin and histologically evaluated. Survival analysis was carried out, and the prognostic value of the presence of lymphatic invasion and the treatment used was determined. Results: Dogs with neoplastic lymphatic vessel invasion had a lower overall survival compared to those without lymphatic invasion, and dogs that received vinblastine in addition to surgery had higher global survival when compared to animals that received carboplatin in addition to surgery. Conclusion: The results obtained show the importance of further studies regarding the prognostic value of the two factors demonstrated as potential survival predictors, especially the lymphatic vessel invasion. Keywords: Dog, Transitional cell carcinoma, Prognosis, Bladder cancer. IntroductionUrothelial carcinoma (UC) of the bladder represents 1%–2% of all the tumors in dogs (Hayes, 1976; Norris et al., 1992; Knapp et al., 2000a), and is the most common tumor subtype among all the bladder neoplasms (Valli et al., 1995; Knapp et al., 2014). Considering the similarities between human and canine bladder UCs, dogs have been proposed as a model for human bladder UC (Knapp et al., 2014; De Brot et al., 2018; Elbadawy et al., 2019). In humans, however, only invasive bladder cancer has clinical relevance, as the non-invasive-type bladder cancer can be successfully removed by surgery. Thus, dogs can be useful for studying the invasive type of this tumor (Elbadawy et al., 2019). Dogs of different ages can develop bladder UC, but it commonly affects the dogs of 9–11 years of age (Elbadawy et al., 2019). In addition, neutered dogs are more likely to be affected than intact ones (Knapp et al., 2000a; Bryan et al., 2007; Knapp et al., 2014), and female dogs tend to be more affected than male dogs (Knapp et al., 2000a; Griffin et al., 2018). Genetic factors and environmental exposure to chemicals have been reported to be the predisposing factors (Raghavan et al., 2004; Knapp et al., 2014; Fulkerson and Knapp, 2015; Griffin et al., 2018). UC can be classified according to its location, histological type, grade of differentiation, and local invasion (Valli et al., 1995; Fulkerson and Knapp, 2015; Marvel et al., 2017). In the bladder, one of the most common locations of tumor development is the bladder trigone. In several cases, the neoplasm already presents with some degree of infiltration into the wall of the bladder at the time of diagnosis (Fulkerson and Knapp, 2015; Marvel et al., 2017). Previously, the degree of tumor infiltration was evaluated in 33 bladder UC cases, and submucosal, muscular, and transmural infiltrations were observed in 45%, 36%, and 12% of the cases, respectively (Marvel et al., 2017). Because UC tends to be locally invasive, it can lead to partial or total obstruction of the lower urinary tract (Knapp et al., 2000b; Knapp et al., 2014). Approximately 56% of the cases had urethral involvement, and 29% of the affected male dogs had prostatic involvement. In addition, metastases to the regional iliac and pelvic lymph nodes or distant metastases were found in 16% of the patients at the time of diagnosis and in 50% of the patients at necropsy (Knapp et al., 2000b; Knapp et al., 2014). The treatment of bladder tumors in dogs is very challenging, especially in those with advanced disease, while surgery is not always an option for tumors located in the trigone region (Norris et al., 1992; Knapp et al., 2000a; Liptak et al., 2004; Saulnier-Troff et al., 2008; Molnár and Vajdovich, 2012; Fulkerson and Knapp, 2015; Marvel et al., 2017). Considering the importance of bladder UC in veterinary medicine and comparative studies, as well as the lack of prognostic markers for this tumor, we aimed to retrospectively study the natural cases of UC in dogs, correlating the clinical and pathological data with the follow-up data. Materials and MethodsStudy designThis was a retrospective, non-randomized study, using patient samples acquired from São Paulo State University (UNESP) and VetPat Laboratory, Brazil, between January 2000 and November 2019. The inclusion criteria included patients who underwent tissue biopsy or surgical procedures for histopathological diagnosis, availability of formalin-fixed and paraffin-embedded tissue samples and clinical information, and the histological diagnosis of bladder UC. Clinical data were obtained from each animal’s record, including the following parameters: age, breed, sex, and treatment option. Additionally, clinical follow-up was carried out to assess survival time, when available, through the consultation of medical records and contact with the owner or the clinician concerned. Survival time was determined as the period from the day of definitive diagnosis by histopathology until the death of the animal. Histological analysisFor each case, a 4-µm-thick section from the paraffin block was stained with hematoxylin and eosin. The histological subtype was reviewed by three evaluators in a blinded manner (Verônica Mollica Govoni, Valeria Grieco and Renee Laufer-Amorim), according to the criteria proposed by the World Health Organization (Meuten et al., 2004), and histological grading was carried out according to Valli et al.’s (1995) study. In addition, the degree of tumor infiltration into the bladder wall (the different layers involved) and the presence of lymphatic invasion were recorded. Statistical analysisThe clinical and pathological variables were tabulated, and the prevalence was calculated as the percentage of occurrences among the total cases. Animals that died as a consequence of surgery were considered untreated. Animals that underwent surgery, with or without additional chemotherapy, were divided into the following categories: surgery + carboplatin, surgery + vinblastine, surgery + carboplatin + tyrosine kinase inhibitor (toceranib), and surgery only. The results are presented as mean and standard deviation. The available survival information was compared with the clinicopathological parameters using the Kaplan–Meier method, and log-rank test was used to compare the survival curves statistically. Survival analysis was carried out using GraphPad Prism v.5.0 (GraphPad Software Inc., La Jolla, CA). Ethical approvalThis study was approved by the Institutional Ethics Committee on the Use of Animals in Research from UNESP (Protocol 50/2020). All the owners signed an informed consent, allowing the use of the patient’s samples in research. ResultsClinical dataAmong the 32 dogs included in the study, 71.87% were female and 28.13% were male. 47% were elderly animals, aged 9–12 years; 31% were over 13 years old; and only 22% were between 5 and 8 years of age. Among the 14 affected breeds, Poodle (25.81%) was the most frequent, followed by Lhasa apso (9.67%), while the breeds of Dachshund, Labrador, Beagle, Pitbull, Doberman pinscher, and Maltese accounted for 6.45% each. The breeds English bulldog, Yorkshire, Shih Tzu, Scottish terrier, and Chow Chow accounted for only one case each (3.23%). Mixed-breed dogs accounted for 9.67% of the cases. Information on treatment was obtained from the cases of 14 animals: 10 underwent surgery and received chemotherapy, while 4 received only surgical treatment. Of the 10 animals that received chemotherapy, the most used drugs were vinblastine (50%) and carboplatin (40%), followed by carboplatin + tyrosine kinase inhibitor (toceranib) (10%). The only animal that received toceranib also received carboplatin. The corresponding clinical information is reported in Table 1. Pathological dataOf the 32 tumors evaluated, the most prevalent histological subtype was the papillary and infiltrating UC (53.13%) (Fig. 1), followed by the non-papillary and infiltrating subtypes (46.87%) (Fig. 1). All the tumors (n=32) showed an invasive pattern. The predominant histological grade was grade II (71.88%), followed by grade III (28.12%) (Fig. 2), while no grade I tumors were identified. In 23 out of the 32 samples, all the layers of the bladder wall were represented in the histological section, and of these samples, 47.83% (11/23) showed tumor invasion up to the muscle layer. Additionally, in 18 out of the 32 cases (56.25%), lymphatic vessel invasion was detected, which was striking and extensive in some samples (Fig. 3) (Table 2). Table 1. Clinical information from the 32 UC affected dogs.
Survival analysisSurvival analysis was carried out in association with the following parameters: type of treatment, histological subtype, histological grade, presence of muscle invasion, and presence of lymphatic invasion. Of the 32 animals included in the study, survival data (in days) of 13 animals were obtained. Of the 13 animals with bladder UC, 1 died due to post-surgical complications (4-day survival), and another 1 underwent euthanasia during surgery, due to the finding of an advanced stage disease, incompatible with less radical techniques of surgical tumor removal (1-day survival). The highest survival was 485 days for an animal that is still alive (treated with surgery + carboplatin + tyrosine kinase inhibitor), and the mean overall survival was 178.38 ± 161.05 days. The histological subtype (papillary or non-papillary), histological grade, and presence of muscle invasion were not significantly correlated with the survival time (Fig. 4). On the contrary, the type of treatment influenced survival time ( p=0.0497). Considering the treatment groups, the most prominent were those who received vinblastine and carboplatin as single adjuvant therapy, respectively. It was observed that the dogs with bladder UC who received vinblastine as adjuvant treatment had a longer survival when compared to the dogs that received carboplatin. Of the three animals that are still alive, each one received treatment according to different protocols and with different chemotherapeutic agents, having in common, only the surgical procedure. The presence of lymphatic invasion also had a prognostic value for survival: animals without tumor lymphatic invasion showed longer survival than those with it.
Fig. 1. Histological type photomicrograph of canine UC. (A) UC papillary (case 4) (H&E, 5× magnification). (B) The tumor “A,” focusing on tumor infiltration (H&E, 10× magnification). (C) UC non-papillary and infiltrating (case 21) (H&E, 5× magnification). (D) The tumor “C,” focusing on tumor infiltration (H&E, 10× magnification).
Fig. 2. Histological grading photomicrograph of canine bladder UC. (A) UC with histological grade II (case 11) (H&E, 40× magnification). (B) UC with histological grade III (case 5) (H&E, 40× magnification). Enlarged nucleus, anisokaryosis, and multiple evident nucleoli in grade III are more evident when compared with grade II. DiscussionUC is the most common canine bladder tumor and represents a challenge in terms of treatment due to its location and frequent invasive behavior. Considering the resembling clinical features, the canine bladder UC has been proposed as a model for studying the muscle-invasive subtype of human bladder UC (Knapp et al., 2000a; Mutsaers et al., 2003). Keeping in view the growing importance canine bladder UC as a model for research in human medicine and the lack of information about this tumor in veterinary medicine, the present study aimed to describe the clinicopathological features of canine bladder UC cases, investigate the prognostic value of some of these features, and emphasize the importance of epidemiological studies for greater knowledge about the behavior of this tumor.
Fig. 3. Histological photomicrograph of invasive canine bladder UC. (A) UC with muscular infiltration (case 21) (H&E, 10× magnification). (B) UC with lymphatic infiltration (case 12) (H&E, 40× magnification). According to the literature (Knapp and McMillan, 2013; De Brot et al., 2018), most of the animals considered in the present study were female and elderly animals, aged 9–12 years. The most commonly affected breed was the Poodle, which is not one of the breeds mentioned to be more prevalent in other countries. Among all the animals included in this study, the breeds that have already been reported to have the highest probability of developing a bladder tumor (Fulkerson and Knapp, 2015) are Beagle and Scottish Terrier. This is probably due to differences in breed prevalence among the world’s different regions and countries, derived from cultural preferences and differences in environmental conditions such as climate. A recent meta-analysis reviewed several studies related to bladder UC in both human and veterinary medicine, revealing that most studies of canine bladder UC do not assess pathological features such as tumor muscle invasion and do not provide survival data, but considered only unique markers without investigating their prognostic value (Gambim et al., 2020). This fact reveals a low degree of standardization among veterinary practitioners and highlights an important obstacle in searching for information in retrospective studies. Furthermore, a large number of biopsies collected by cystoscopy make it impossible to assess the deeper layers of the bladder wall to investigate tumor invasion in most cases (Dhawan et al., 2015, 2018). Similar difficulties were encountered in the present study, where 13 out of the 32 cases had information on survival time, and 23 out of the 32 samples had all the layers sampled for histological evaluation of muscle invasion. In addition, the assessment of tumor invasion in dogs with UC has not been standardized. For this reason, in the present study, the muscle layer and lymphatic vessel invasion by the tumor were both classified as present or absent. In humans, the muscle-invasive bladder UC represents only 30% of the cases (Grzegółkowski et al., 2017). On the contrary, in veterinary medicine, most bladder UCs show invasive behavior, reaching the muscle layer, entering the lymphatic vessels, or even affecting adjacent organs such as the urethra and prostate. This is probably due to the evolution of the disease at the time of diagnosis (Priester and McKay, 1980; Valli et al., 1995, Knapp et al., 2000a; Mutsaers et al., 2003). On confirmation of the invasive characteristic of the samples in the present study that allowed such assessments, 47.83% had muscle layer invasion and 56.25% had lymphatic invasion. Muscle invasion did not show prognostic value on the Kaplan–Meier curve. On the other hand, lymphatic invasion was evaluated in all the samples and showed prognostic value ( p=0.0139): animals with lymphatic vessel invasion had lower survival compared to those without it. This result emphasizes the prognostic importance of considering this feature, which should always be evaluated and reported by the pathologists. Other pathological characteristics, such as UC subtype and histological grade, did not show a significant impact in survival analysis, but we need to consider that the histological graduation described by Valli et al. (1995) evaluated only the nuclear features of tumor cells, introducing a possible bias in the application of such grading. For this reason, further studies comparing the different grading systems applied in both human and veterinary medicine are needed to identify the one with the greatest prognostic value for bladder UC. Several conventional or emerging therapies have been used for the treatment of canine bladder UC. Many of them have been transported from human medicine due to the similarity between human and canine diseases (Knollman et al., 2015; Fulkerson et al., 2017). The dogs considered in this study were subdivided on the basis of the treatment received to identify the treatment modality that resulted in a longer survival time. Although the “surgery” and “surgery + carboplatin + toceranib” treatment groups were represented by only one animal each, there was a statistically significant difference between the other types of therapy ( p=0.0497). Table 2. Pathological data of the 32 canine bladder UC cases.
Allstadt et al. (2015) compared the use of piroxicam in association with carboplatin or mitoxantrone. Although they did not find a significant difference in patient survival, they observed a difference in the disease-free interval, where animals receiving carboplatin showed a shorter disease-free period (73.5 days) than those receiving mitoxantrone (106 days). Arnold et al. (2011) conducted a clinical trial with vinblastine in dogs with bladder UC and observed a median survival of 147 days and a disease-free period of 122 days. Although the two studies mentioned above considered different animals and conditions, the disease-free period results were higher for the animals that received vinblastine than those that received carboplatin or mitoxantrone associated with piroxicam. In addition, a clinical trial by Chun et al. (1997) demonstrated that carboplatin was not beneficial in the treatment of 14 dogs with bladder UC. In the present study, the dogs that received vinblastine as adjuvant therapy had a longer survival time than those that received carboplatin as adjuvant therapy. This shows that vinblastine has a better therapeutic potential than carboplatin. Among the treatment options, surgery is the first option and offers good results in terms of survival. However, as stated above, its execution is not always possible or compatible with the animals’ quality of life (Molnár and Vajdovich, 2012). The tumors located on the bladder trigone pose a challenge for the feasibility of surgical procedures. On the contrary, when the tumor is not located on the bladder trigone, good results are achieved by partial cystectomy in association with medical treatment (Marvel et al., 2017). In this study’s therapeutic groups, only one animal received surgery as the sole treatment modality, making it difficult to arrive at a conclusion about this method alone.
Fig. 4. Survival curves (Kaplan–Meier) in relation to different parameters. (A) Survival curve for types of treatment. (B) Survival curve for histological types. (C) Survival curve for muscle invasion. (D) Survival curve for lymphatic invasion. Another predictor of prognosis, which also interferes with the treatment to be carried out, is the tumor location within the bladder and the involvement of other structures. Allstadt et al. (2015) noted that men with prostatic involvement had a lower median survival. Animals with bladder trigone involvement had lower survival rates than animals with urethral involvement or apical tumors. Patients with tumors at the bladder apex had a higher median survival (Allstadt et al., 2015). For the cases included in the present study, no information was obtained regarding the tumor’s location, which hampered the evaluation of its prognostic importance. This study evaluated the prognostic value of different treatment options and histological features of canine bladder UC. In particular, lymphatic invasion, a feature rarely considered in the available literature, proved to be a significant negative prognostic factor. The results reported above encourage further studies on the canine bladder UC, not only for application in translational research with human disease, but also for developing new therapies that enable better prognosis and quality of life in veterinary medicine. Conflict of interestThe authors declare that they have no conflict of interest. Authors’ contributionsConceptualization: V.M.G., C.E.F-A., and J.G.Q.; methodology: V.M.G., C.E.F-A., V.G., R.L-A., and J.G.Q.; software: V.M.G., C.P., and C.E.F-A.; validation: V.M.G, C.E.F-A., V.G., and R.L-A.; formal analysis: V.M.G and C.E.F-A.; investigation: V.M.G., C.P., F.A.R.S., C.E.F-A., R.L-A. V.G., T.O.daS., and F.Z.; resources: C.E.F-A. and R.L-A; data curation: V.M.G., C.P., C.E.F-A, V.G., R.L-A, T.O.daS., and F.Z. ; writing—original draft preparation: V.M.G..; writing—review and editing: V.M.G., C.E.F-A., C.P., R.L-A., and V.G.; visualization: V.M.G., C.E.F-A., R.L-A., C.P., and V.G.; supervision: C.E.F-A.; project administration: C.E.F-A., J.G.Q., and V.G. All authors have read and agreed to the published version of the manuscript. ReferencesAllstadt, S.D., Rodriguez, C.O., Boostrom, B., Rebhun, R.B. and Skorupski, K.A. 2015. Randomized phase III trial of piroxicam in combination with mitoxantrone or carboplatin for first-line treatment of urogenital tract transitional cell carcinoma in dogs. J. Vet. Intern. Med. 29, 261–267. Arnold, E.J., Childress, M.O., Fourez, L.M., Tan, K.M., Stewart, J.C., Bonney, P.L. and Knapp, D.W. 2011. Clinical trial of vinblastine in dogs with transitional cell carcinoma of the urinary bladder. J. Vet. Intern. Med. 25, 1385–1390. Bryan, J.N., Keeler, M.R., Henry, C.J., Bryan, M.E., Hahn, A.W. and Caldwell, C.W. 2007. A population study of neutering status as a risk factor for canine prostate cancer. Prostate 67, 1174–1181. Chun, R., Knapp, D.W., Widmer, W.R., DeNicola, D.B., Glickman, N.W., Kuczek, T., Degortari, A. and Han, C.M. 1997. Phase II clinical trial of carboplatin in canine transitional cell carcinoma of the urinary bladder. J. Vet. Intern. Med. 11, 279–283. De Brot, S., Robinson, B.D., Scase, T., Grau-Roma, L., Wilkinson, E., Boorjian, S.A., Gardner, D. and Mongan, N.P. 2018. The dog as an animal model for bladder and urethral urothelial carcinoma: comparative epidemiology and histology. Oncol. Lett. 16, 1641–1649. Dhawan, D., Hahn, N.M., Ramos-Vara, J.A. and Knapp, D.W. 2018. Naturally-occurring canine invasive urothelial carcinoma harbors luminal and basal transcriptional subtypes found in human muscle invasive bladder cancer. PLoS Genet. 14, e1007571. Dhawan, D., Paoloni, M., Shukradas, S., Choudhury, D.R., Craig, B.A., Ramos-Vara, J.A., Hahn, N., Bonney, P.L., Khanna, C. and Knapp, D.W. 2015. Comparative gene expression analyses identify luminal and basal subtypes of canine invasive urothelial carcinoma that mimic patterns in human invasive bladder cancer. PLoS One 10, e0136688. Elbadawy, M., Usui, T., Mori, T., Tsunedomi, R., Hazama, S., Nabeta, R., Uchide, T., Fukushima, R., Yoshida, T., Shibutani, M., Tanaka, T., Masuda, S., Okada, R., Ichikawa, R., Omatsu, T., Mizutani, T., Katayama, Y., Noguchi, S., Iwai, S., Nakagawa, T., Shinohara, Y., Kaneda, M., Yamawaki, H. and Sasaki, K. 2019. Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 110, 2806–2821. Fulkerson, C.M., Dhawan, D., Ratliff, T.L., Hahn, N.M. and Knapp, D.W. 2017. Naturally occurring canine invasive urinary bladder cancer: a complementary animal model to improve the success rate in human clinical trials of new cancer drugs. Int. J. Genomics 2017, 6589529. Fulkerson, C.M. and Knapp, D.W. 2015. Management of transitional cell carcinoma of the urinary bladder in dogs: a review. Vet. J. 205, 217–225. Gambim, V.V., Laufer-Amorim, R., Fonseca-Alves, R.H., Grieco, V. and Fonseca-Alves, C.E. 2020. A comparative meta-analysis and in silico analysis of differentially expressed genes and proteins in canine and human bladder cancer. Front. Vet. Sci. 7, 558978. Griffin, M.A., Culp, W.T.N. and Rebhun, R.B. 2018. Lower urinary tract neoplasia. Vet. Sci. 5, 96. Grzegółkowski, P., Kaczmarek, A., Lemiński, A., Soczawa, M., Gołąb, A. and Słojewski, M. 2017. Assessment of the infiltrative character of bladder cancer at the time of transurethral resection: a single center study. Cent. Eur. J. Urol. 70, 22–26. Hayes, H.M. 1976. Canine bladder cancer: epidemiologic features. Am. J. Epidemiol. 104, 673–677. Knapp, D.W., Glickman, N.W., Denicola, D.B., Bonney, P.L., Lin, T.L. and Glickman, L.T. 2000a. Naturally-occurring canine transitional cell carcinoma of the urinary bladder A relevant model of human invasive bladder cancer. Urol. Oncol. 5, 47–59. Knapp, D.W., Glickman, N.W., Widmer, W.R., DeNicola, D.B., Adams, L.G., Kuczek, T., Bonney, P.L., DeGortari, A.E., Han, C. and Glickman, L.T. 2000b. Cisplatin versus cisplatin combined with piroxicam in a canine model of human invasive urinary bladder cancer. Cancer Chemother. Pharmacol. 46, 221–226. Knapp, D.W. and McMillan, S.K. 2013. 29—Tumors of the urinary system. In Withrow, Stephen J. Withrow and MacEwen's small animal clinical oncology, 5th ed. Eds., Vail, D.M. and Page, R.L. Saint Louis, MO: Saunders, pp: 572–582. Knapp, D.W., Ramos-Vara, J.A., Moore, G.E., Dhawan, D., Bonney, P.L. and Young, K.E. 2014. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 55, 100–118. Knollman, H., Godwin, J.L., Jain, R., Wong, Y.N., Plimack, E.R. and Geynisman, D.M. 2015. Muscle-invasive urothelial bladder cancer: an update on systemic therapy. Ther. Adv. Urol. 7, 312–330. Liptak, J.M., Dernell, W.S. and Withrow, S.J. 2004. Haemangiosarcoma of the urinary bladder in a dog. Aust. Vet. J. 82, 215–217. Marvel, S.J., Séguin, B., Dailey, D.D. and Thamm, D.H. 2017. Clinical outcome of partial cystectomy for transitional cell carcinoma of the canine bladder. Vet. Comp. Oncol. 15, 1417–1427. Meuten, D.J., Everitt, J., Inskeep, W., Jacobs, R.M., Peleteiro, M. and Thompson, K.G. 2004. Histological classification of urinary system of domestic animals. WHO Int. Classif. Tumors Domest. Anim. 2nd Ser. 11, 1–70. Molnár, T. and Vajdovich, P. 2012. Clinical factors determining the efficacy of urinary bladder tumour treatments in dogs: surgery, chemotherapy or both? Acta Vet. Hung. 60, 55–68. Mutsaers, A.J., Widmer, W.R. and Knapp, D.W. 2003. Canine transitional cell carcinoma. J. Vet. Intern. Med. 17, 136–144. Norris, A.M., Laing, E.J., Valli, V.E., Withrow, S.J., Macy, D.W., Ogilvie, G.K., Tomlinson, J., McCaw, D., Pidgeon, G. and Jacobs, R.M. 1992. Canine bladder and urethral tumors: a retrospective study of 115 cases (1980–1985). J. Vet. Intern. Med. 6, 145–153. Priester, W.A. and McKay, F.W. 1980. The occurrence of tumors in domestic animals. Natl. Cancer Inst. Monogr. 54, 1–210. Raghavan, M., Knapp, D.W., Dawson, M.H., Bonney, P.L. and Glickman, L.T. 2004. Topical flea and tick pesticides and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J. Am. Vet. Med. Assoc. 225, 389–394. Saulnier-Troff, F.G., Busoni, V. and Hamaide, A. 2008. A technique for resection of invasive tumors involving the trigone area of the bladder in dogs: preliminary results in two dogs. Vet. Surg. 37, 427–437. Valli, V.E., Norris, A., Jacobs, R.M., Laing, E., Withrow, S., Macy, D., Tomlinson, J., McCaw, D., Ogilvie, G.K. and Pidgeon, G. 1995. Pathology of canine bladder and urethral cancer and correlation with tumour progression and survival. J. Comp. Pathol. 113, 113–130. | ||
| How to Cite this Article |
| Pubmed Style Govoni VM, Pigoli C, Sueiro FAR, Zuliani F, Silva TOD, Quitzan JG, Laufer-amorim R, Grieco V, Fonseca-alves CE. Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. Open Vet. J.. 2021; 11(4): 535-543. doi:10.5455/OVJ.2021.v11.i4.3 Web Style Govoni VM, Pigoli C, Sueiro FAR, Zuliani F, Silva TOD, Quitzan JG, Laufer-amorim R, Grieco V, Fonseca-alves CE. Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. https://www.openveterinaryjournal.com/?mno=71029 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.3 AMA (American Medical Association) Style Govoni VM, Pigoli C, Sueiro FAR, Zuliani F, Silva TOD, Quitzan JG, Laufer-amorim R, Grieco V, Fonseca-alves CE. Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. Open Vet. J.. 2021; 11(4): 535-543. doi:10.5455/OVJ.2021.v11.i4.3 Vancouver/ICMJE Style Govoni VM, Pigoli C, Sueiro FAR, Zuliani F, Silva TOD, Quitzan JG, Laufer-amorim R, Grieco V, Fonseca-alves CE. Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 535-543. doi:10.5455/OVJ.2021.v11.i4.3 Harvard Style Govoni, V. M., Pigoli, . C., Sueiro, . F. A. R., Zuliani, . F., Silva, . T. O. D., Quitzan, . J. G., Laufer-amorim, . R., Grieco, . V. & Fonseca-alves, . C. E. (2021) Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. Open Vet. J., 11 (4), 535-543. doi:10.5455/OVJ.2021.v11.i4.3 Turabian Style Govoni, Veronica Mollica, Claudio Pigoli, Felipe Augusto Ruiz Sueiro, Fernanda Zuliani, Thayna Oliveira Da Silva, Juliany Gomes Quitzan, Renee Laufer-amorim, Valeria Grieco, and Carlos Eduardo Fonseca-alves. 2021. Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. Open Veterinary Journal, 11 (4), 535-543. doi:10.5455/OVJ.2021.v11.i4.3 Chicago Style Govoni, Veronica Mollica, Claudio Pigoli, Felipe Augusto Ruiz Sueiro, Fernanda Zuliani, Thayna Oliveira Da Silva, Juliany Gomes Quitzan, Renee Laufer-amorim, Valeria Grieco, and Carlos Eduardo Fonseca-alves. "Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma." Open Veterinary Journal 11 (2021), 535-543. doi:10.5455/OVJ.2021.v11.i4.3 MLA (The Modern Language Association) Style Govoni, Veronica Mollica, Claudio Pigoli, Felipe Augusto Ruiz Sueiro, Fernanda Zuliani, Thayna Oliveira Da Silva, Juliany Gomes Quitzan, Renee Laufer-amorim, Valeria Grieco, and Carlos Eduardo Fonseca-alves. "Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma." Open Veterinary Journal 11.4 (2021), 535-543. Print. doi:10.5455/OVJ.2021.v11.i4.3 APA (American Psychological Association) Style Govoni, V. M., Pigoli, . C., Sueiro, . F. A. R., Zuliani, . F., Silva, . T. O. D., Quitzan, . J. G., Laufer-amorim, . R., Grieco, . V. & Fonseca-alves, . C. E. (2021) Lymphatic invasion is a significant indicator of poor patient outcome in canine bladder urothelial carcinoma. Open Veterinary Journal, 11 (4), 535-543. doi:10.5455/OVJ.2021.v11.i4.3 |