| Original Article | ||
Open Vet. J.. 2022; 12(5): 735-743 Open Veterinary Journal, (2022), Vol. 12(5): 735–743 Original Research Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluidEiji Naito, Roka Shimada and Masashi Yuki*Yuki Animal Hospital, 2-99, Kiba-cho, Minato-ku, Nagoya, Aichi, Japan *Corresponding Author: Masashi Yuki. Yuki Animal Hospital, Nagoya, Japan. Email: yuki-masashi [at] mvf.biglobe.ne.jp Submitted: 14/07/2022 Accepted: 09/09/2022 Published: 05/10/2022 © 2022 Open Veterinary Journal
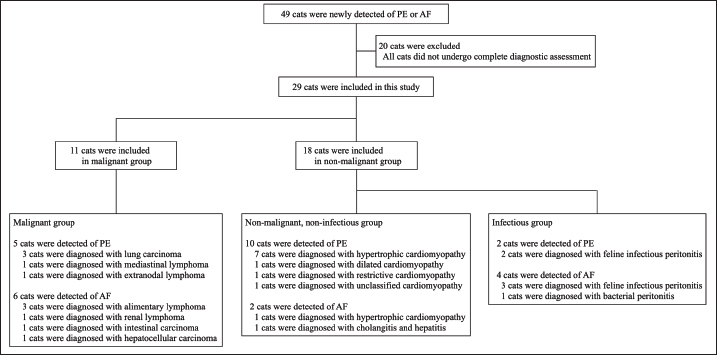
AbstractBackground: Lactate dehydrogenase (LDH) isoenzymes may be useful in the differential diagnosis of pleural effusion (PE) and ascitic fluid (AF) etiologies in cats since tissue damage induces their release, changing the pattern of their activity. Aim: This study aimed to determine the diagnostic utility of measuring LDH levels and isoenzyme activities in PE or AF in cats with malignancy. Methods: LDH levels and isoenzyme activities in the serum, PE, and AF were compared among cats in the malignant, infectious, and non-malignant, non-infectious groups. A receiver operating characteristic (ROC) analysis was performed to assess the accuracy in diagnosing feline malignancy. Results: Significant differences in LDH level and LDH isoenzyme activities in the PE and AF were observed among the three groups. The combination of LDH level and LDH-1 activity in PE or AF had the highest area under the ROC (AUC) values for discriminating malignant effusion from non-malignant effusion. The AUC of the combination of LDH level and LDH-1 activity in PE or AF was 0.874. The sensitivity and specificity of using the combination of LDH level (cut-off: <2,269 U/l) and LDH-1 activity (cut-off: <4.8%) in PE or AF for predicting malignancy with the highest AUC value were 94.4% and 72.7%, respectively. Conclusion: Our results suggest that the combination of LDH level and LDH-1 activity in PE or AF is a potential factor for diagnosing malignancy. Considering that LDH isoenzymes can be measured inexpensively and easily, LDH tests can be readily accommodated in veterinary clinical practice. Keywords: Ascitic fluid, Cat, Lactate dehydrogenase isoenzymes, Malignancy, Pleural effusion. IntroductionPleural effusion (PE) and ascitic fluid (AF) have diagnostic challenges in cats. Differential diagnoses related to PE and AF in cats include a variety of diseases, such as congestive heart failure (CHF), chronic liver failure (CLF), feline infectious peritonitis (FIP), bacterial peritonitis, and malignancy (Ettinger et al., 2017). However, the classification of PE and AF into malignant or non-malignant diseases remains challenging, despite using various tests. Cytological tests for malignant cells in PE and AF demonstrate high specificity (100%) but low sensitivity (50%–61%) (Hirschberger et al., 1999; Spangler et al., 2000). Therefore, it is necessary to identify a new method for detecting malignancy in PE and AF. In humans, lactate dehydrogenase (LDH), which is involved in the glycolytic pathway, has been the focus of the differential diagnosis of various diseases because of its release from cells after cell damage or death (Dawson et al., 1964). The distribution of five LDH isoenzymes differs in various tissues (Wroblewski and Gregory, 1961; Lott and Stang, 1980). Several studies on LDH have revealed that LDH isoenzymes exhibit atypical patterns (Wroblewski and Gregory, 1961; Dawson et al., 1964). LDH isoenzyme activities in the PE and AF of humans are useful biomarkers for distinguishing between malignancy and non-malignancy (Paavonen et al., 1991; Cobben et al., 1997; Lossos et al., 1999; Sevinc et al., 2005). Interestingly, LDH-1 activity, which works efficiently under aerobic conditions, is significantly lower in malignant diseases than in non-malignant diseases, whereas LDH-4 activity, which works efficiently under anaerobic conditions, is significantly higher in malignant diseases than in non-malignant diseases (Paavonen et al., 1991; Sevinc et al., 2005). In veterinary medicine, LDH levels have limited use in differentiating dogs with cancer from dogs with non-malignant diseases and healthy dogs (Marconato et al., 2009). A study on cats with lymphoma reported that serum LDH levels served as a negative prognostic indicator for survival time (Hadden et al., 2008). Another study reported that LDH levels in PE in cats with FIP and pyothorax were higher than those in other diseases (Zoia et al., 2009; Zoia and Drigo, 2016). Regarding LDH isoenzymes, it has been reported that LDH-5 is dominant and LDH-1 is very scant in feline leukocytes, suggesting that the leukocytes in cats tend to produce more energy under anaerobic conditions than those in dogs or rabbits (Washizu et al., 2002). However, the diagnostic values of LDH isoenzyme patterns in serum and body cavity effusions are unknown in veterinary practice. There have been no reports regarding feline LDH analyses in PE and AF. The purpose of this study was to evaluate LDH levels and LDH isoenzyme activities in serum, PE, and AF in cats with malignant and non-malignant diseases. We hypothesized that the LDH level in PE and AF with malignant diseases would be higher than that in PE and AF with non-malignant, non-infectious (NMNI) diseases and lower than that in PE and AF with infectious diseases because of the difference in the number of exudative cells in PE and AF. We also hypothesized that aerobic LDH isoenzyme activities in PE and AF with malignant and infectious diseases would be lower than those in PE and AF with NMNI diseases, and anaerobic LDH isoenzyme activities in PE and AF with malignant and infectious diseases would be higher than those in PE and AF with NMNI diseases because PE and AF with malignant and infectious diseases would include more inflammatory cells or tumor cells, which work efficiently under anaerobic conditions, than PE and AF with NMNI diseases. Materials and MethodsCat selectionThis retrospective cross-sectional study analyzed cats in which PE or AF was newly detected at Yuki Animal Hospital from December 2014 to 2018. All owners signed an informed consent form. Each cat was evaluated using the following tests: physical examination, complete blood count, serum biochemistry profile, radiography, and ultrasonography. All the PE and AF samples were newly collected immediately after diagnosis and tested for total nucleated cell counts (TNCC), specific gravity (SG), total protein (TP), cytological examination, LDH, and LDH isoenzyme measurements. These cats were divided into two groups based on the diagnosis. The malignant group included cats with tumors that were diagnosed through fine needle aspiration, clonality analysis, core biopsy, and surgical biopsy. The non-malignant group included cats with diseases that cause PE and AF without tumors. The non-malignant group included two subgroups: NMNI diseases such as idiopathic chylothorax, CHF, and liver disease without the malignant or infectious disease (NMNI group), and infectious diseases such as FIP, pyothorax, and bacterial peritonitis (infectious group). CHF was confirmed using cardiac ultrasonography or on detection of serum N-terminal-pro B-type natriuretic peptide (NT-pro BNP; IDEXX laboratories Inc., Tokyo, Japan). CLF was diagnosed by histopathological examination of the liver. FIP was diagnosed when supportive history, signalment, clinical, and clinicopathological data including lymphopenia, serum, or fluid albumin to globulin ratio (<0.45 or <1.0, respectively), serum and fluid feline coronavirus antibody titers (>1:1,280), positive polymerase chain reaction analysis and increased serum alpha-1 acid-glycoprotein were present and no other causes of PE and AF could be identified. Pyothorax and bacterial peritonitis were diagnosed based on the results of the cytological examination and positive bacterial culture. Cats with an incomplete diagnosis and cats with co-existing diseases that cause PE and AF were excluded from this study. LDH assayAll serum, PE, and AF samples were centrifuged and separated within 30 minutes and processed within 24 hours of collection. All samples were stored at 4°C during the experiment. LDH levels and LDH isoenzyme subgroup activities were measured in all samples (LSI Medience Co., Tokyo, Japan). Statistical analysisThe results of each laboratory test and the ages of the cats were compared among the malignant group, NMNI group, and infectious group using Kruskal–Wallis test. Post hoc comparisons were performed using Mann–Whitney U test with Bonferroni correction. Fisher’s exact test was used for comparing breeds, sex, and types of effusion. The accuracy of the tests indicating significant differences in the parameters between the malignant and non-malignant groups was analyzed by performing a receiver operating characteristic (ROC) analysis. The area under the ROC curve (AUC) as well as sensitivity and specificity with 95% confidence intervals (CIs) were calculated. To evaluate the diagnostic accuracy of malignancy with the combination of the LDH level and LDH isoenzyme activities used for ROC analysis, logistic regression was performed. p < 0.05 was considered statistically significant, and p < 0.01 was considered highly significant. The statistical analysis was performed using Easy R software (Kanda, 2013). Ethical approvalIn Japan, there is no ethics committee available for private-practice animal hospitals. Nevertheless, this research was conducted according to the ethical codes of the Japan Veterinary Medical Association. The samples obtained in this study were used after obtaining written consent from each cat owner. ResultsClinical characteristicsThe case selection flowchart is presented in Figure 1. A total of 49 cats were newly diagnosed with PE or AF. Of those, 20 cats were excluded because the complete diagnostic assessment was not achieved, leaving a total of 29 cats enrolled in the study. The malignant group included 11 cats, of which 9 were diagnosed with lymphoma or lung carcinoma via fine needle aspiration, and 2 were diagnosed with intestinal carcinoma and hepatocellular carcinoma based on the results of histopathological examination. The NMNI group included 12 cats, of which 9 were diagnosed with cardiomyopathy through ultrasonography, 2 were diagnosed with cardiomyopathy based on the results of an NT-pro BNP test at enrollment, followed by a subsequent confirmation through ultrasonography, and 1 was diagnosed with cholangitis and hepatitis based on the results of histopathological examination of the liver. The infectious group included six cats.
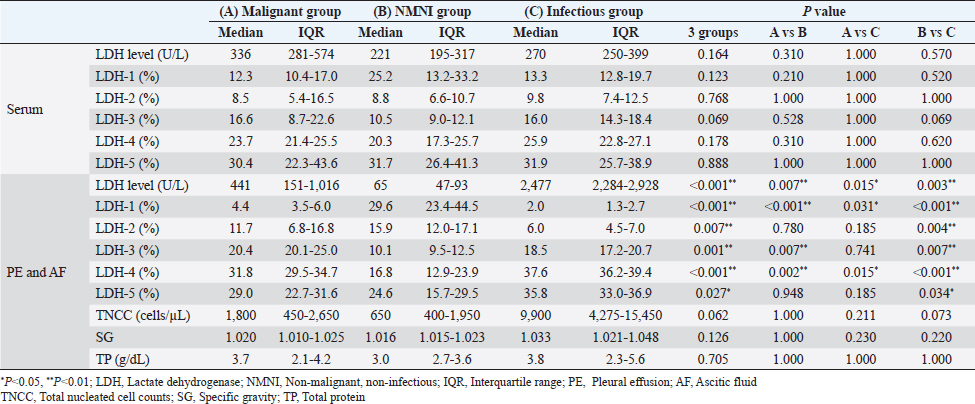
Fig. 1. Flowchart of the case selection process. (PE): pleural effusion; (AF): ascitic fluid. The cat breeds included 25 crossbreeds, 2 Scottish Folds, and 2 Ragdolls. The malignant group consisted of six males (two castrated) and five females (three spayed). The median age of these cats was 12.2 years (range, 3.0–17.4 years). The NMNI group consisted of six males (four castrated) and six females (four spayed). The median age of these cats was 16 years (range, 6.8–21.4. years). The infectious group consisted of five males (two castrated) and one female. The median age of these cats was 3.8 years (range, 0.4–17.0 years). There were no significant differences in breed, sex, and age between the cats in the malignant and non-malignant groups. Comparison of LDH levelsLDH levels, LDH isoenzyme activities, TNCC, SP, and TP are shown in Table 1 and Figure 2. There were no significant differences in the serum LDH level among the malignant (median, 336 U/l; interquartile range, 281–574 U/l), NMNI (221 U/l; 195–317 U/l), and infectious groups (270 U/l; 250–399 U/l) (p.≥ 0.05). In PE and AF, the LDH level in the malignant group (441 U/l; 151–1,016 U/l) was significantly higher than that in the NMNI group (65 U/l; 47–93 U/l) (p < 0.01). The LDH level in the infectious group (2,477 U/l; 2,284–2,928 U/l) was significantly higher than that in the malignant (p < 0.05) and NMNI groups (p < 0.01). Table 1. Comparison of LDH levels and LDH isoenzymes activities among the malignant group, the NMNI group, and the infectious group
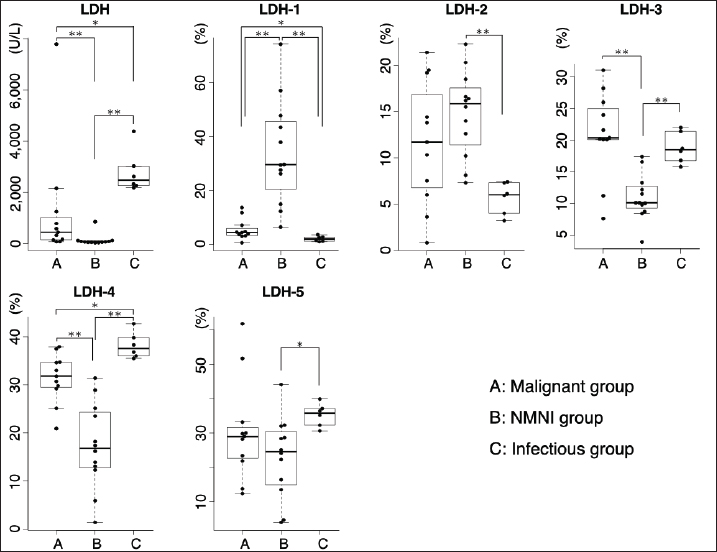
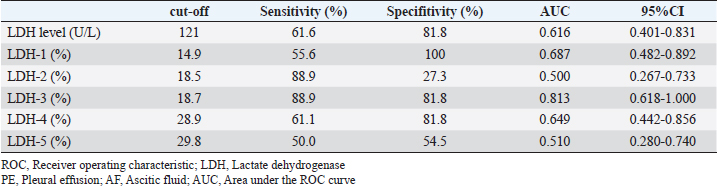
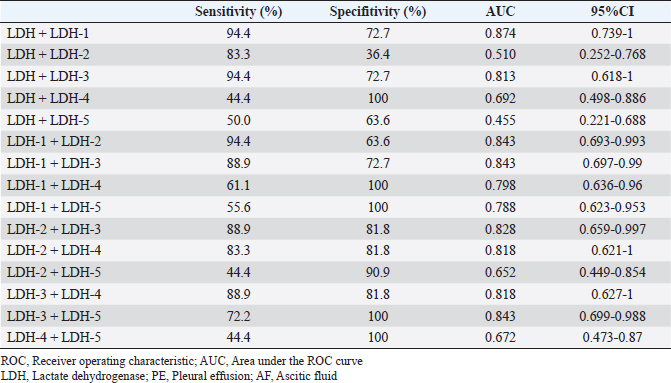
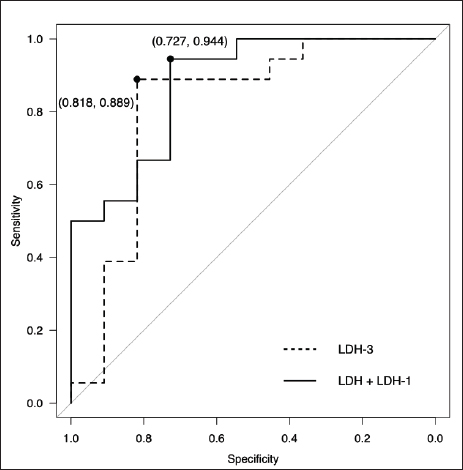
Fig. 2. Comparison of LDH isoenzyme activities in PE and AF among the malignant, NMNI, and infectious groups. Box plots show the median, range, and 25th and 75th quartiles within the groups. LDH level, LDH-3, and LDH-4 activities were significantly higher in the malignant group than those in the NMNI group (p=0.007, 0.007, and 0.002, respectively). LDH-1 activity was significantly lower in the malignant group than that in the NMNI group (p < 0.001). LDH-1 activity was significantly lower in the malignant group than that in the infectious group (p=0.031). LDH level and LDH-4 activity were lower in the malignant group than those in the infectious group (p=0.015, respectively). Statistical analyses were performed using the Kruskal–Wallis test. Post hoc comparisons were conducted using the Mann–Whitney U test with Bonferroni correction. *p < 0.05, **p < 0.01; LDH, lactate dehydrogenase; PE, pleural effusion; AF, ascitic fluid; NMNI, non-malignant, non-infectious. Comparison of LDH-1 activitiesThere were no significant differences in the serum LDH-1 activity among the malignant (12.3%; 10.4%–17.0%), NMNI (25.2%; 13.2%–33.2%), and infectious groups (13.3%; 12.8%–19.7%) (p ≥ 0.05). In PE and AF, the LDH-1 activity in the malignant group (4.4%; 3.5%–6.0%) was significantly higher than that in the infectious group (2.0%; 1.3%–2.7%) (p < 0.05). The LDH-1 activity in the NMNI group (29.6%; 23.4%–44.5%) was significantly higher than that in the malignant (p < 0.01) and infectious groups (p < 0.01). Comparison of LDH-2 activitiesThere were no significant differences in the serum LDH-2 activity among the malignant (8.5%; 5.4%–16.5%), NMNI (8.8%; 6.6%–10.7%), and infectious groups (9.8%; 7.4%–12.5%) (p ≥ 0.05). In PE and AF, the LDH-2 activity in the NMNI group (15.9%; 12.0%–17.1%) was significantly higher than that in the infectious group (6.0%; 4.5%–7.0%) (p < 0.01). There was no significant difference in the LDH-2 activity between the malignant (11.7%; 6.8%–16.8%) and NMNI (p ≥ 0.05) or infectious groups (p ≥ 0.05). Comparison of LDH-3 activitiesThere were no significant differences in the serum LDH-3 activity among the malignant (16.6%; 8.7%–22.6%), NMNI (10.5%; 9.0%–12.1%), and infectious groups (16.0%; 14.3%–18.4%) (p ≥ 0.05). In PE and AF, the LDH-3 activity in the NMNI group (10.1%; 9.5%–12.5%) was significantly lower than that in the malignant (20.4%; 20.1%–25.0%) (p < 0.01) and infectious groups (18.5%; 17.2%–20.7%) (p < 0.01). There was no significant difference in the LDH-3 activity between the malignant and NMNI groups (p ≥ 0.05). Comparison of LDH-4 activitiesThere were no significant differences in the LDH-4 activity in serum among the malignant (23.7%; 21.4%–25.5%), NMNI (20.3%; 17.3%–25.7%), and infectious groups (25.9%; 22.8%–27.1%) (p ≥ 0.05). In PE and AF, the LDH-4 activity in PE or AF in the NMNI group (16.8%; 12.9%–23.9%) was significantly lower than that in the malignant (31.8%; 29.5%–34.7%) (p < 0.01) and infectious groups (37.6%; 36.2%–39.4%) (p < 0.01). The LDH-4 activity in the malignant group was significantly lower than that in the infectious group (p < 0.05). Comparison of LDH-5 activitiesThere were no significant differences in the LDH-5 activity in serum among the malignant (30.4%; 22.3%–43.6%), NMNI (31.7%; 26.4%–41.3%), and infectious groups (31.9%; 25.7%–38.9%) (p ≥ 0.05). In PE and AF, the LDH-5 activity in the NMNI group (24.6%; 15.7%–29.5%) was significantly lower than that in the infectious group (35.8%; 33.0%–36.9%) (p < 0.05). There was no significant difference in the LDH-5 activity between the malignant group (29.0%; 22.7%–31.6%) and NMNI (p ≥ 0.05) or infectious groups (p ≥ 0.05). Comparison of TNCC, SG, and TPIn PE and AF, there were no significant differences in the TNCC among the malignant (1,800 cells/µl; 450–2,650 cells/µl), NMNI (650 cells/µl; 400–1,950 cells/µl), and infectious groups (9,900 cells/µl; 4,275–15,450 cells/µl) (p ≥ 0.05). There were no significant differences in the SG among the malignant (1.020; 1.010–1.025), NMNI (1.016; 1.015–1.023), and infectious groups (1.033; 1.021–1.048) (p ≥ 0.05). There were no significant differences in the TP level among the malignant (3.7 g/dl; 2.1–4.2 g/dl), NMNI (3.0 g/dl; 2.7–3.6 g/dl), and infectious groups (3.8 g/dl; 2.3–5.6 g/dl) (p ≥ 0.05). ROC curve analysisThe results of the ROC curve analysis are shown in Tables 2 and 3. When comparing the AUC value, the ROC curve analysis revealed the activity of LDH-3 in PE and AF had the highest AUC values (0.81; 95% CI, 0.618–1) for discriminating malignant effusion from non-malignant effusion. The sensitivity and specificity of using the LDH-3 activity in PE and AF for predicting malignancy (cut-off: >18.7%) with the highest AUC value were 88.9% and 81.8%, respectively (Fig. 3). When evaluating the diagnostic accuracy of malignancy with the combination of LDH levels and LDH isoenzyme activities, the combination of LDH level and LDH-1 activity in PE and AF had the highest AUC values (0.874; 95% CI, 0.739–1) for discriminating malignant effusion from non-malignant effusion. The sensitivity and specificity of using the combination of LDH level (cut-off: <2,269 U/l) and LDH-1 activity (cut-off: <4.8%) in PE and AF for predicting malignancy with the highest AUC value were 94.4% and 72.7%, respectively (Fig. 3). There was no significant difference between the AUC values of LDH-3 and the combination of LDH and LDH-1 (p=0.641). Table 2. ROC curve analysis of LDH level and LDH isoenzymes activities in PE and AF
Table 3. ROC curve analysis of the combination of LDH level and/or LDH isoenzymes activities in PE and AF
Fig. 3. ROC curve for LDH-3 activity in PE or AF and the combination of LDH level and LDH-1 activity in PE or AF plotted for diagnosing malignancy. The AUC values of LDH-3 activity in PE or AF and the combination of LDH level and LDH-1 activity in PE or AF were 0.813 (95% CI: 0.618–1.000) and 0.874 (95% CI: 0.739–1.000), respectively. The sensitivity and specificity of using LDH-3 activity (cut-off: >18.7%) in predicting malignancy were 88.9% and 81.8%, respectively. The sensitivity and specificity of using the combination of LDH level (cut-off: <2,269 U/l) and LDH-1 activity (cut-off: <4.8%) in predicting malignancy were 94.4% and 72.7%, respectively. ROC, receiver operating characteristic; LDH, lactate dehydrogenase; PE, pleural effusion; AF, ascitic fluid; CI, confidence intervals. DiscussionOur study revealed the diagnostic utility of measuring the LDH level and LDH isoenzyme activities in feline PE and AF. Especially, the combination of the LDH level and LDH-1 activity had the highest diagnostic potential with moderate specificity (72.7%) and high sensitivity (94.4%) to distinguish between malignancy and non-malignancy. In contrast, cytological tests for malignant cells in PE and AF demonstrate high specificity but low sensitivity (Spangler et al., 2000). Therefore, the combination of the LDH analyses and cytological tests in PE and AF may be more useful for diagnosing malignancy. However, the diagnostic accuracy of cytological tests for malignant cells in PE and AF was unknown in previous studies (Hirschberger et al., 1999; Spangler et al., 2000). In the future, it is necessary to compare the diagnostic accuracy of LDH and LDH isoenzyme activities with that of cytological tests. Although there was no significant difference in LDH levels and LDH isoenzyme activities in serum, the LDH levels and LDH-1 and LDH-4 activities in PE and AF were significantly different among malignant, NMNI, and infectious diseases in this study. These differences in LDH levels and LDH isoenzyme activities were caused due to differences in body cavity effusions and blood; however, the underlying mechanism is not completely understood. A previous study reported that LDH levels and LDH isoenzyme activities might be affected by the release of enzymes from inflammatory cells and erythrocytes present in body cavity effusions (Saint-Rémy et al., 1986). Another study reported that visceral or parietal pleural cells are rich in LDH (Paavonen et al., 1991). For these reasons, LDH isoenzymes might be released from cells that infiltrate body cavity effusions other than serum (Sevinc et al., 2005). Therefore, we considered that LDH levels in body cavity effusions may be higher than those in serum in cats, which are similar to human beings, and the differences in these levels between body cavity effusions and serum appear to occur easily. We suggest that the LDH levels and LDH isoenzymes activities in PE and AF be measured immediately upon encountering a case involving PE and AF, considering that special techniques are not required. LDH is present in essentially all organ systems and is released from cells only after cell damage or death (Glick, 1969; Drent et al., 1996). Elevated LDH levels in PE and AF were caused by activated, injured, or dead leucocytes, tumor cells, or mesothelial cells (Washizu et al., 2002; Sevinc et al., 2005). Our study revealed that LDH level and TNCC in PE and AF tended to increase in the order of infectious, malignant, and NMNI diseases. LDH levels depend on the number of cells because LDH is released from cells in PE and AF. However, LDH is also released from dead cells regardless of the number of cells in PE and AF. Therefore, evaluation of LDH levels in PE and AF is important for diagnosing malignancy. In humans, a tissue composed of LDH-1, such as that in the brain and heart, works efficiently under aerobic conditions (Firth et al., 1995). In fact, in humans, CHF shows a high percentile of LDH-1 activity in PE and AF (Cobben et al., 1997; Sevinc et al., 2005). Moreover, an increase in the LDH-1 activity in AF was also observed in patients with CLF (Sevinc et al., 2005). In this study, 11 cats were diagnosed with CHF, and 1 cat was diagnosed with cholangitis and hepatitis in the NMNI group. Consequently, we considered that there was a larger difference in the LDH-1 activity than in other LDH isoenzyme activities. Generally, there is an increase in hypoxic areas in malignant tumors and their metastases (Fantin et al., 2006). Tumor cells need LDH, which works efficiently under anaerobic conditions, to maintain and proliferate (Fantin et al., 2006). Therefore, human LDH-4 activity in PE and AF has been reported to be significantly higher in malignant effusions than in non-malignant effusions (Cobben et al., 1997; Sevinc et al., 2005). However, these studies included no patients with hematologic cancers. Moreover, LDH-3 has been reported to be the most abundant isoenzyme in normal lymphoid tissue (Dumontet et al., 1999). The malignant group in this study included 6/11 cats with lymphoma. Furthermore, the infectious group in this study included five cats with FIP and one cat with bacterial peritonitis. In the infectious group, cells in PE and AF mainly contained neutrophils. Leukocytes have more anaerobic LDH isoenzymes in cats (Washizu et al., 2002). Therefore, the activity of the LDH isoenzymes in malignant and infectious diseases might have been higher under aerobic conditions when compared with that in previous studies on humans. This study had several limitations. This study investigated PE and AF together. Generally, the content of LDH isoenzymes varies across different organs. However, in our research, there were no significant differences in LDH levels and LDH isoenzyme activities between PE and AF in each of the three groups (data not shown). Therefore, we included both cats with PE and AF. The malignant group included various malignant diseases. It may be necessary to investigate each malignant disease individually in the future. We checked feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) only in 16/29 cats; 3 of them were FIV-positive and 2 were FeLV-positive. It may be necessary to investigate the association between LDH and FIV/FeLV in the future. The age, weight, and breed in each group were not consistent. Given that only examined cats were selected, our findings may not be reflective of all cats with malignant effusion. ConclusionThis study suggests that the combination of LDH level and LDH-1 activity will improve diagnostic accuracy in cats with malignant effusions. An examination of LDH isoenzyme activities in PE and AF can be readily accommodated in primary veterinary clinical care, considering that they can be measured inexpensively and easily. AcknowledgmentsThe authors wish to thank all staff at the Yuki Animal Hospital for their support. The authors also wish to thank Mr. Hosoda (LSI Medience Corp.) for his technical assistance in the acquisition of LDH data. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsE. Naito and M. Yuki conceived the study design. E. Naito and R. Shimada collected data. E. Naito drafted the article and approved and revised the final version of the manuscript on behalf of all authors. M. Yuki reviewed the final manuscript. ReferencesCobben, N.A., van Belle, A.F., Pennings, H.J., Mulder, P.G., van Dieijen-Visser, M.P., Wouters, E.F. and Drent, M. 1997. Diagnostic value of lactate dehydrogenase isoenzyme pattern in pleural effusions. Eur. J. Clin. Chem. Clin. Biochem. 35, 523–528. Dawson, D.M., Goodfriend, T.L. and Kaplan. 1964. Lactic dehydrogenases: functions of the two types rates of synthesis of the two major forms can be correlated with metabolic differentiation. Science 143, 929–933. Drent, M., Cobben, N.A., Henderson, R.F., Wouters, E.F. and van Dieijen-Visser, M. 1996. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur. Respir. J. 9, 1736–1742. Dumontet, C., Drai, J., Bienvenu, J., Berard, E.N., Thieblemont, C., Bouafia, F., Bayle, F., Moullet, I., Salles, G. and Coiffier, B. 1999. Profiles and prognostic values of LDH isoenzymes in patients with non-hodgkin’s lymphoma. Leukemia 13, 811–817. Ettinger, S.J., Feldman, E.C. and Cote, E. 2017. Textbook of veterinary internal medicine, 8th ed. St. Louis, MO: Elsevier Saunders, pp: 439–448. Fantin, V.R., St-Pierre, J. and Leder, P. 2006. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer. Cell. 9, 425–434. Firth, J.D., Ebert, B.L. and Ratcliffe, P.J. 1995. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J. Biol. Chem. 270, 21021–21027. Glick, J.H. 1969. Serum lactate dehydrogenase isoenzyme and total lactate dehydrogenase values in health and disease, and clinical evaluation of these tests by means of discriminant analysis. Am. J. Clin. Pathol. 52, 320–328. Hadden, A.G., Cotter, S.M., Rand, W., Moore, A.S., Davis, R.M. and Morrissey, P. 2008. Efficacy and toxicosis of VELCAP-C treatment of lymphoma in cats. J. Vet. Intern. Med. 22, 153–157. Hirschberger, J., DeNicola, D.B., Hermanns, W. and Kraft, W. 1999. Sensitivity and specificity of cytologic evaluation in the diagnosis of neoplasia in body fluids from dogs and cats. Vet. Clin. Pathol. 28, 142–146. Kanda, Y. 2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone. Marrow. Transplant. 48, 452–458. Lossos, I.S., Intrator, O., Berkman, N. and Breuer, R. 1999. Lactate dehydrogenase isoenzyme analysis for the diagnosis of pleural effusion in haemato-oncological patients. Respir. Med. 93, 338–341. Lott, J.A. and Stang, J.M. 1980. Serum enzymes and isoenzymes in the diagnosis and differential diagnosis of myocardial ischemia and necrosis. Clin. Chem. 26, 1241–1250. Marconato, L., Crispino, G., Finotello, R., Mazzotti, S., Salerni, F. and Zini, E. 2009. Serum lactate dehydrogenase activity in canine malignancies. Vet. Comp. Oncol. 7, 236–243. Paavonen, T., Liippo, K., Aronen, H. and Kiistala, U. 1991. Lactate dehydrogenase, creatine kinase, and their isoenzymes in pleural effusions. Clin. Chem. 37, 1909–1912. Saint-Rémy, P., Buret, J. and Radermecker, M. 1986. Significance of lactate dehydrogenases in pleural effusions. Rev. Pneumol. Clin. 42, 74–81. Sevinc, A., Sari, R. and Fadillioglu, E. 2005. The utility of lactate dehydrogenase isoenzyme pattern in the diagnostic evaluation of malignant and nonmalignant ascites. J. Natl. Med. Assoc. 97, 79–84. Spangler, E.A., Rogers, K.S., Thomas, J.S., Pustejovsky, D., Boyd, S.L. and Shippen, D.E. 2000. Telomerase enzyme activity as a diagnostic tool to distinguish effusions of malignant and benign origin. J. Vet. Intern. Med. 14, 146–150. Washizu, T., Nakamura, M., Izawa, N., Suzuki, E., Tsuruno, S., Washizu, M., Nakajo, S. and Arai, T. 2002. The activity ratio of the cytosolic MDH/LDH and the isoenzyme pattern of LDH in the peripheral leukocytes of dogs, cats and rabbits. Vet. Res. Commun. 26, 341–346. Wroblewski, F. and Gregory, K.F. 1961. Lactic dehydrogenase isozymes and their distribution in normal tissues and plasma and in disease states. Ann. N. Y. Acad. Sci. 94, 912–932. Zoia, A. and Drigo, M. 2016. Diagnostic value of light’s criteria and albumin gradient in classifying the pathophysiology of pleural effusion formation in cats. J. Feline. Med. Surg. 18, 666–672. Zoia, A., Slater, L.A., Heller, J., Connolly, D.J. and Church, D.B. 2009. A new approach to pleural effusion in cats: markers for distinguishing transudates from exudates. J. Feline. Med. Surg. 11, 847–855. | ||
| How to Cite this Article |
| Pubmed Style Naito E, Shimada R, Yuki M. Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid. Open Vet. J.. 2022; 12(5): 735-743. doi:10.5455/OVJ.2022.v12.i5.19 Web Style Naito E, Shimada R, Yuki M. Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid. https://www.openveterinaryjournal.com/?mno=71636 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i5.19 AMA (American Medical Association) Style Naito E, Shimada R, Yuki M. Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid. Open Vet. J.. 2022; 12(5): 735-743. doi:10.5455/OVJ.2022.v12.i5.19 Vancouver/ICMJE Style Naito E, Shimada R, Yuki M. Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid. Open Vet. J.. (2022), [cited January 25, 2026]; 12(5): 735-743. doi:10.5455/OVJ.2022.v12.i5.19 Harvard Style Naito, E., Shimada, . R. & Yuki, . M. (2022) Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid. Open Vet. J., 12 (5), 735-743. doi:10.5455/OVJ.2022.v12.i5.19 Turabian Style Naito, Eiji, Roka Shimada, and Masashi Yuki. 2022. Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid. Open Veterinary Journal, 12 (5), 735-743. doi:10.5455/OVJ.2022.v12.i5.19 Chicago Style Naito, Eiji, Roka Shimada, and Masashi Yuki. "Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid." Open Veterinary Journal 12 (2022), 735-743. doi:10.5455/OVJ.2022.v12.i5.19 MLA (The Modern Language Association) Style Naito, Eiji, Roka Shimada, and Masashi Yuki. "Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid." Open Veterinary Journal 12.5 (2022), 735-743. Print. doi:10.5455/OVJ.2022.v12.i5.19 APA (American Psychological Association) Style Naito, E., Shimada, . R. & Yuki, . M. (2022) Diagnostic utility of measuring lactate dehydrogenase levels and its isoenzyme activities for the evaluation of malignancy in feline pleural effusion and ascitic fluid. Open Veterinary Journal, 12 (5), 735-743. doi:10.5455/OVJ.2022.v12.i5.19 |