| Case Report | ||
Open Vet. J.. 2021; 11(3): 436-440 Open Veterinary Journal, (2021), Vol. 11(3): 436–440 Case Report Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dogRaffaella Perillo1, Marika Menchetti1, Pasquale A. Giannuzzi1, Angela Marchiori2, Marco Rondena3 and Stefania Gasparini3*1San Marco Veterinary Clinic and Laboratory, Neurology and Neurosurgery Division, Veggiano (PD), Italy 2San Marco Veterinary Clinic and Laboratory, Oncology Division, Veggiano (PD), Italy 3San Marco Veterinary Clinic and Laboratory, Pathology Division Veggiano (PD), Italy *Corresponding Author: Stefania Gasparini. San Marco Veterinary Clinic and Laboratory, Pathology Dvivision Veggiano (PD), Italy. Email: stefania.gasparini [at] sanmarcovet.it Submitted: 15/04/2021 Accepted: 02/08/2021 Published: 22/08/2021 © 2021 Open Veterinary Journal
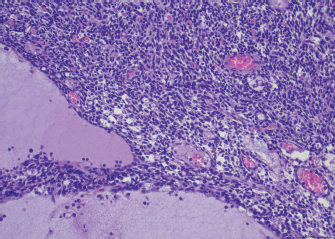
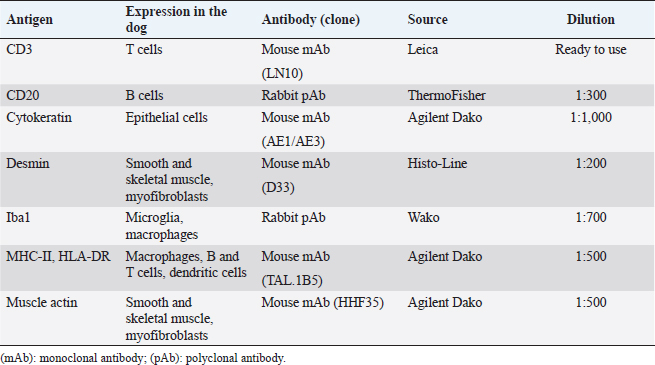
AbstractBackground: Canine thymomas are associated with multiple paraneoplastic syndromes, among which myasthenia gravis (MG) is the most common. Acquired MG is an autoimmune disease characterized by the presence of antibodies against acetylcholine receptors (ACHRs). ACHRs antibodies are the most commonly formed, but the production of antistriational antibodies binding to skeletal and cardiac muscle proteins has also been recorded both in humans and dogs. An association between the occurrence of antistriational antibodies and a severe form of myocarditis, giant cell myocarditis, has been described in humans. Case Description: A 4-year-old mixed-breed dog was referred because of 1 month history of exercise-induced weakness, hypersalivation, and regurgitation. The neurologic examination was indicative of a neuromuscular junction disease, and MG was suspected. A computed tomographic scan examination showed the presence of a megaoesophagus and a thymic mass. Serum antibodies against ACHRs confirmed the diagnosis of MG. Treatment with pyridostigmine was started, and the thymic mass was surgically excised, and a diagnosis of thymoma was confirmed by histology. 24 hours after surgery, the dog developed a third-degree atrioventricular block. Severe arrhythmia and increased troponin serum levels suggested myocarditis which rapidly led to cardiopulmonary arrest. Histopathologic examination of the heart, esophagus and diaphragm revealed a lymphocytic and macrophagic infiltration, consistent with myocarditis and polymyositis. Scattered rare giant multinucleated cells were also detected in the myocardium. Conclusion: To the author’s knowledge, this is the first report of thymoma-associated MG with concurrent polymyositis and giant cell-like myocarditis in a dog. Keywords: Dog, Myasthenia gravis, Myocarditis, Polymyositis, Thymoma. IntroductionThymoma is an uncommon neoplasm occurring in the cranial mediastinum and composed of thymic epithelial cells admixed with a variable degree of benign lymphocytic infiltration (Valli et al., 2016, 2017). Thymoma has been reported in many species, including humans, dogs, cats, rabbits, and ferrets. Although rare, it is one of the most common cranial mediastinal tumors in dogs (Robat et al., 2013). Canine thymomas have been associated with a large spectrum of paraneoplastic syndromes, among which acquired myasthenia gravis (MG) is the most reported (Shelton, 2002; Robat et al., 2013). In dogs affected by thymomas, 30%–50% of the cases carry MG as a paraneoplastic syndrome (Khorzard et al., 2011) with serological evidence of autoantibodies. Paraneoplastic, thymoma-associated MG is an acquired immune mediated disease characterized by alterations of the thymic microenvironment, with impairment of T-cell development and loss of self-tolerance leading to the formation of circulating antibodies against postsynaptic nicotinic acetylcholine receptors (ACHRs) located in the neuromuscular junction (NMJ) (Valli et al., 2016). Acetylcholine is essential for muscle contraction, and autoantibody-mediated destruction of ACHRs results in focal or generalized muscle weakness and exhaustion (Khorzad et al., 2011). In paraneoplastic MG, ACHRs antibodies are the most commonly formed and cause muscle weakness. However, some MG patients also develop antibodies against additional skeletal and cardiac muscle antigens (Suzuki et al., 2011), called antistriational antibodies, which have been recorded both in humans (Priemer et al., 2018; Kufukihara et al., 2019) and dogs (Shelton et al., 2001; Mignan et al., 2020). Antistriational antibodies recognize epitopes on skeletal and cardiac muscle proteins, including actin, myosin, titin, ryanodine receptor (RyR) and muscular voltage-gated potassium channel (Suzuki et al., 2011). The presence of these antibodies is associated with more severe MG symptoms and with the occurrence of other clinical syndromes such as polymyositis and/or myocarditis in MG thymoma associated human patients (Suzuki et al., 2011). In the veterinary literature, an association between MG thymoma associated and polymyositis has been reported (Darke et al., 1975; Aronsohn et al., 1984), but to the author’s knowledge, no associations between MG thymoma associated and myocarditis have been recorded. Here, we describe a case of thymoma in a dog with paraneoplastic associated MG and concurrent polymyositis and myocarditis. Case DetailsA 4-year-old spayed female mixed-breed dog was referred with a 1-month history of regurgitation and progressive generalized weakness. At the time of presentation, the general physical examination was unremarkable. The neurologic examination revealed exercise intolerance, with the development of weakness beginning in the hindlimbs and progressing into a non-ambulatory flaccid tetraparesis with neck ventroflection, which was alleviated by rest and bilateral symmetrical reduction of patellar, tibialis cranialis, and withdrawal of reflexes. The dog showed mild dysphagia characterized by reduced and difficult swallowing and hypersalivation. The neurologic exam was indicative of a generalized lesion of the low motor neuron system. The neurologic examination was followed by an extended laboratory analysis, including blood counts, serum biochemical profile, coagulation and urinary analysis, and chest radiographs. The haematological abnormalities were a mild increase of WBC (13.1; reference ranges (RR): 5.37–12.39 × 103/mcl) characterized by neutrophilia (11,135; RR: 2,778–8,220 × 103/mcl), lymphocytosis (1,179; 1,009–3,471 × 103/mcl), and monocytosis (655; RR: 155–537 × 103/mcl). Biochemistry revealed significant increase in creatine kinase (CK) (7,934; 39–168 U/l) with a less severe increase of aspartate aminotransferase (AST) (389; 16–39 U/l) and alanine aminotransferase (ALT) (302; 15–79 U/l), moderately elevated C reactive protein (4.54; RR: 0.01–0.41 mg/dl), and hyperferritinemia (776; RR: 38–272 ng/ml). Urinalysis was within normal limits. The thoracic radiographs showed a diffusely dilated esophagus and soft tissue opacity in the cranial mediastinum. The diagnostic suspicion was a form of acquired MG associated with a cranial mediastinal mass although clinically polymyositis could not be completely excluded. A 0.05 mg/kg (0.02 mg/lb) of neostigmine (Prostigmina®) was administered intramuscularly to support our first suspicion. After few minutes, the dog showed a positive result, with increased muscular strength. A computed tomographic (CT) scan examination showed a rounded cranial mediastinal neoformation, characterized by heterogeneous appearance due to the presence of cystic intraparenchymal areas and associated with normal cranial sternal and mediastinal lymph nodes. The CT scan also showed an expansion of the entire esophagus and stomach, mainly due to gas. These findings confirmed a moderate megaesophagus and the presence of a cranial mediastinal mass. A cytological examination and, subsequently, a tru-cut biopsy of the mediastinal mass was performed both with non-diagnostic results. Serum antibodies against ACHRs were highly supportive of MG (5.23 nmol/l; normal in dogs < 0.6 nmol/l). The dog was treated with neostigmine therapy (Prostigmina®) at 0.01 mg/kg (0.004 mg/lb) IM q8h, showing mild improvement of muscular weakness. The cranial mediastinal mass was surgically excised by a median sternotomy. Histology of the formalin-fixed specimen revealed a capsulated, well demarcated, not infiltrative neoplasm, composed of sheets and cords of mildly pleomorphic, spindle to oval cells, multifocally lining variably sized cystic spaces, often filled with eosinophilic secretory material. Neoplastic cells were associated with a moderate number of lymphocytes, forming small multifocal aggregates (Fig. 1). To further characterize the neoplasm, immunohistochemistry was performed using the automated immunostainer Bond RX (Leica Biosystem, Nussloch GmbH; Nusloch, Germany). Antibodies manufacturer, source, clone and dilution are listed in Table 1. The antigen unmasking technique was performed as indicated by the manufacturer. Neoplastic cells were found to express cytokeratin, which exhibited strong cytoplasmatic staining within neoplastic cells lining the cysts, while having a weak to moderate intensity in solid areas. Rare aggregates of desmin and muscle actin expressing cells, interpreted as myoid cells, were also detected. According to the WHO classification, based on anatomic location and on morphologic and phenotypic features, a diagnosis of type A thymoma was made. The day after the surgery, dysphagia progressively worsened, and clinical respiratory signs with tachypnea, dyspnea and cough appeared. The patient was hyperthermic [40°C (104°F)]. Reassessment of blood tests showed a worsening of the inflammatory parameters. The blood count showed a severe increase in leukocytes (38; 5.37–12.39 × 103/mcl) characterized by neutrophilia (33,820; 2,778–8,220 × 103/mcl) with the presence of banded neutrophils (1,140 × 103/mcl) and by toxic neutrophils and cytoplasmic foaming detected by blood smear analysis. The serum chemistry profile showed a severe increase of C reactive protein (15.88; 0.01–0.41 mg/dl). An alveolar pattern consistent with aspiration pneumonia was apparent on thoracic radiographs. Oxygen therapy by nasal tube and four quadrant antibiotic therapy with amoxicillin- clavulanic acid [22 mg/kg (10 mg/lb) IV q12h) and enrofloxacin [10 mg/kg (4.5 mg/lb) IV q24h] was promptly started. A few hours later, a third-degree atrioventricular block was observed, and dysphagia and respiratory signs worsened, leading to induction of general anaesthesia to protect the lower airway tracts and to maintain the patient under mechanical ventilation. The appearance of a severe arrhythmia suggested the onset of myocarditis, and the increase in serum troponins (1.34 ng/ml, 0.05–0.24 ng/ml) supported this diagnostic hypothesis. During mechanical ventilation, arrhythmia worsened dramatically up to cardiopulmonary arrest on the second post-surgical day.
Fig. 1. Thymoma, dog. Sheets of neoplastic spindle cells are lining multifocal cystic lacunae. Hematoxylin and eosin. Table 1. Antibodies and immunohistochemical methods.
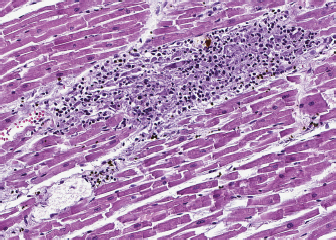
Fig. 2. Heart, dog. Myocardial interstitium is infiltrated by lymphocytes, with myofiber degeneration and necrosis. Hematoxylin and eosin. A necropsy was performed. The main macroscopic finding consisted of a diffused megaesophagus. Low numbers of white, small-sized spots were also visible on the myocardial surface, with random distribution. The subsequent histology of the heart showed that myocardium was infiltrated by a severe multifocal to coalescing inflammatory process, mainly composed of lymphocytes, admixed with a lower number of macrophages, plasma cells and neutrophils, and rare giant multinucleated cells, with up to five haphazardly arranged nuclei. Inflammatory infiltrate was associated with small foci of necrotic cardiomyocytes with hypereosinophilic cytoplasm, loss of cross striations and pyknotic nuclei (Fig. 2). A similar inflammatory process consisting of multiple, variably sized foci of necrotizing myositis also involved esophageal and diaphragmatic skeletal muscle. Immunohistochemistry (IHC) was performed to characterize the inflammation involving the myocardium, esophagus and diaphragmatic skeletal muscle: the inflammatory cell population infiltrating the myocardium was mainly composed of CD3+ lymphocytes (T cells), admixed with a lower number of Iba1 positive macrophages, most of them being MHC-II positive. Rare, scattered B lymphocytes expressed CD20. Interestingly, a low proportion of cardiomyocytes expressed MHC-II in the cytoplasm or on the sarcolemma. Esophageal and diaphragmatic skeletal muscles were also characterized by an infiltration of T lymphocytes and macrophages. The histopathological picture was consistent with lymphocytic and necrotizing myocarditis and polymyositis. Based on this evidence, to exclude possible infectious causes, PCR for the detection of Toxoplasma gondii and Neospora caninum was carried out on myocardial samples and results came out negative. DiscussionMG is an autoimmune disorder that is often the result of a paraneoplastic syndrome related to the presence of thymoma (Valli et al., 2016). The target of the autoantibodies is ACHRs, but autoantibodies can also be directed against muscular proteins, like actin, ryanodine, and titin receptor, and they are called antistriational antibodies. Therefore, antistriational antibodies potentially indicate the presence of a pathological process that, in addition to the ACHR antibody mediated NMJ transmission defect, can influence both striated and cardiac muscle function (Suzuki et al., 2011). Many studies demonstrated that disease tends to be more severe in MG patients found to be positive for antstriational antibodies than in those found to be negative (Suzuki et al., 2013; Kon et al., 2013; Priemer et al., 2018). Moreover, an association between the presence of antistriational antibodies and the occurrence of other clinical syndromes such as polymyositis and/or myocarditis in MG thymoma associated patients is reported (Suzuki et al., 2011). Giant cell myocarditis (GCM) is a rare, lethal, and well-recognized syndrome in these patients (Shivamurthy and Parker, 2014). Although the pathogenesis is uncertain, an autoimmune process, possibly elicited by antistriational antibodies, has been suggested. Histologically GCM is a severe form of myocarditis characterized by myocardial degeneration of varying degrees up to myonecrosis and by widespread interstitial infiltrate of chronic inflammatory cells (Suzuki et al., 2011; Shivamurthy and Parker, 2014). The inflammatory infiltrate is characterized by CD3 and CD8 positive T lymphocytes often admixed with numerous multinucleated giant cells (Kon et al., 2013; Saito et al., 2013), which are reported to express desmin or CD68, therefore suggesting myocytic and histiocytic origins (Kon et al., 2013). Clinically, most human patients deteriorated rapidly with lethal arrhythmias and severe heart failure and died despite intensive treatments (Suzuki et al., 2011; Shivamurthy and Parker, 2014). In veterinary medicine, Shelton et al. (2001) documented antistriational antibodies against titin and RyR in dogs with MG thymoma associated and found an association between the presence of RyR antibodies and more severe disease. An association between MG thymoma associated and polymyositis has also been described in the dog (Darke et al., 1975; Aronshon et al., 1984), while there are no reports of MG with associated GCM or myocarditis. Third-degree atrial ventricular heart block has been described in some dogs with MG thymoma associated, but in these cases, an idiopathic cardiac conduction disturbance was hypothesized (Hackett et al., 1995). In the present report, myocarditis was suspected as a pathological mechanism of the patient's severe arrhythmia, and the marked increase in serum troponins supported this diagnostic hypothesis. Post-mortem myocardial histological examination confirmed the association between third-degree heart block and myocarditis. According to cells morphology, the myocarditis exhibited by the dog is similar to the human GCM type, and the detected histopathological picture could represent a GCM variant in dogs: both entities are characterized by infiltration of CD3 lymphocytes, associated with myofiber damage. Compared to GCM, case a lower number of giant multinucleated cells was detected in the present. Supporting the presence of an immune-mediated mechanism was also the expression of MHC II on the sarcolemma of myocardial cells. The muscle tissue does not usually express MHC II in healthy conditions, and its expression is typical of immune-mediated diseases, even though it is not exclusive (Paciello et al., 2007; Durward-Akhurst and Valberg, 2018). This histological type of inflammatory myopathy, already reported in skeletal muscles of dogs with polymyositis and MG associated thymoma (Darke et al., 1975; Shelton et al., 2001), is similar to that described in polymyositis of human patients with thymoma and MG (Kon et al., 2013). The severe increase in our patient's muscle enzymes, in association with the patient's poor response to traditional MG therapy, could also clinically suggest concomitant polymyositis. Unfortunately, no electrodiagnostic test was performed to confirm the clinical suspicion of polymyositis. However, histopathological findings of esophageal and diaphragmatic muscles and increased muscle enzymes support the presence of an inflammatory process. After the exclusion of the main infectious causes, an immunomediated syndrome is strongly suggested. Although the presence of antistriational antibodies was not investigated in our case, the severity of the clinical signs exhibited by the dog was comparable with the clinical signs generally showed by MG human patients with polymyositis in the presence of antistriational antibodies. To our knowledge, this is the first report of thymoma-associated MG with concurrent myocarditis and polymyositis in a dog. Further studies will demonstrate the correlation between myocarditis and antistriational antibodies in veterinary patients with thymoma associated MG. Conflict of interestThe authors declare that they have no conflict of interest. Author contributionsAll authors contributed to the study conception and design. Material preparation and data collection were performed by Marco Rondena, Stefania Gasparini and Angela Marchiori. Macroscopical, histological and immunohistochemical examination were performed by Stefania Gasparini and Marco Rondena. The clinical case was followed by Angela Marchiori. The first draft of the manuscript was written by Raffaella Perillo, Marika Menchetti and Pasquale Giannuzzi, final version was written by Stefania Gasparini and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. ReferencesAronsohn, M.G., Schunk, K.L., Carpenter, J.L. and King, N.W. 1984. Clinical and pathological features of thymoma in 15 dogs. J. Am. Vet. Med. Assoc. 184, 1355–1362. Darke, P.G., McCullagh, K.G. and Geldart, P.H. 1975. Myasthenia gravis, thymoma and myositis in a dog. Vet. Rec. 97, 392–393. Durward-Akhurst, S.A. and Valberg, S.J. 2018. Immune-mediated muscle diseases of the horse. Vet. Pathol. 55, 68–75. Hackett, T.B., Van Pelt, D.R., Willard, M.D., Martin, L.G., Shelton, G.D. and Wingfield, W.E. 1995. Third degree atrioventricular block and acquired myasthenia gravis in four dogs. J. Am. Vet. Med. Assoc. 206, 1173–1176. Khorzad, R., Whelan, M., Sisson, A. and Shelton, G.D. 2011. Myasthenia gravis in dogs with an emphasis on treatment and critical care management. Vet. Emerg. Crit. Care 21, 193–208. Kon, T., Mori, F., Tanji, K., Miki, Y., Kimura, T. and Wakabayashi, K. 2013. Giant cell polymyositis and myocarditis associated with myasthenia gravis and thymoma. Neuropathology 33, 281–287. Kufukihara, K., Watanabe, Y., Inagaki, T., Takamatsu, K., Nakane, S., Nakahara, J., Ando, Y. and Suzuki, S. 2019. Cytometric cell-based assays for anti-striational antibodies in myasthenia gravis with myositis and/or myocarditis. Sci. Rep. 9, 1–10. Mignan, T., Targett, M. and Lowrie, M. 2020. Classification of myasthenia gravis and congenital myasthenic syndromes in dogs and cats. J. Vet. Intern. Med, 34, 1707–1717. Paciello, O., Shelton, G.D. and Papparella, S. 2007. Expression of major histocompatibility complex class I and class II antigens in canine masticatory muscle myositis. Neuromuscul. Disord. 17, 313–320. Priemer, D.S., Davidson, D.D., Loehrer, P.J. and Badve, S.S. 2018. Giant cell polymyositis and myocarditis in a patient with thymoma and myasthenia gravis: a postviral autoimmune process? J. Neuropathol. Exp. Neurol. 77, 661–664. Robat, C.S., Cesario, L., Gaeta, R., Miller, M., Schrempp, D. and Chun, R. 2013. Clinical features, treatment options, and outcome in dogs with thymoma: 116 cases (1999–2010). J. Am. Vet. Med. Assoc. 243, 1448–1454. Saito, N., Shimizu, K., Kawaishi, M., Araya, J., Nakayama, K. and Kuwano, K. 2013. A survival case of invasive thymoma accompanied by acute fulminant myocarditis. Respirol. Case Rep. 1, 36–38. Shelton, G.D., Skeie, G.O., Kass, P.H. and Aarli, J.A. 2001. Titin and ryanodine receptor autoantibodies in dogs with thymoma and late-onset myasthenia gravis. Vet. Immunol. Immunopathol. 78, 97–105. Shelton, G.D. 2002. Myasthenia gravis and disorders of neuromuscular transmission. Vet. Clin. North Am. Small Anim. Pract. 32, 189–206. Shivamurthy, P. and Parker, M.W. 2014. Cardiac manifestations of myasthenia gravis: a systematic review. IJC Metab. Endocr. 5, 3–6. Suzuki, S., Utsugisawa, K., Nagane, Y. and Suzuki, N. 2011. Three types of striational antibodies in myasthenia gravis. Autoimmune Dis. 2011, 740583; doi:10.4061/2011/740583. Suzuki, S., Utsugisawa, K. and Suzuki, N. 2013. Overlooked non-motor symptoms in myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 84, 989–994. Valli, V.E.O., Bienzle, D., Meuten, D.J. and Linder, K.E. 2017. Tumors of the hemolymphatic System. In Tumor in domestic animals. Ed., Meuten, DJ. Ames, IA: John Wiley & Sons, Inc., pp: 203–321. Valli, V.E.O., Kiupel, M., Bienzle, D. and Wood, R.D. 2016. Hematopoietic system. In Jubb, Kennedy, and Palmer’s pathology of domestic animals, 6th ed. Ed., Maxie, MG. St. Louis, MO: Elsevier, pp: 102–268. | ||
| How to Cite this Article |
| Pubmed Style Perillo R, Menchetti M, Giannuzzi P, Marchiori A, Rondena M, Gasparini S. Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog. Open Vet. J.. 2021; 11(3): 436-440. doi:10.5455/OVJ.2021.v11.i3.16 Web Style Perillo R, Menchetti M, Giannuzzi P, Marchiori A, Rondena M, Gasparini S. Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog. https://www.openveterinaryjournal.com/?mno=72604 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i3.16 AMA (American Medical Association) Style Perillo R, Menchetti M, Giannuzzi P, Marchiori A, Rondena M, Gasparini S. Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog. Open Vet. J.. 2021; 11(3): 436-440. doi:10.5455/OVJ.2021.v11.i3.16 Vancouver/ICMJE Style Perillo R, Menchetti M, Giannuzzi P, Marchiori A, Rondena M, Gasparini S. Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog. Open Vet. J.. (2021), [cited January 25, 2026]; 11(3): 436-440. doi:10.5455/OVJ.2021.v11.i3.16 Harvard Style Perillo, R., Menchetti, . M., Giannuzzi, . P., Marchiori, . A., Rondena, . M. & Gasparini, . S. (2021) Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog. Open Vet. J., 11 (3), 436-440. doi:10.5455/OVJ.2021.v11.i3.16 Turabian Style Perillo, Raffaella, Marika Menchetti, Pasquale Giannuzzi, Angela Marchiori, Marco Rondena, and Stefania Gasparini. 2021. Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog. Open Veterinary Journal, 11 (3), 436-440. doi:10.5455/OVJ.2021.v11.i3.16 Chicago Style Perillo, Raffaella, Marika Menchetti, Pasquale Giannuzzi, Angela Marchiori, Marco Rondena, and Stefania Gasparini. "Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog." Open Veterinary Journal 11 (2021), 436-440. doi:10.5455/OVJ.2021.v11.i3.16 MLA (The Modern Language Association) Style Perillo, Raffaella, Marika Menchetti, Pasquale Giannuzzi, Angela Marchiori, Marco Rondena, and Stefania Gasparini. "Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog." Open Veterinary Journal 11.3 (2021), 436-440. Print. doi:10.5455/OVJ.2021.v11.i3.16 APA (American Psychological Association) Style Perillo, R., Menchetti, . M., Giannuzzi, . P., Marchiori, . A., Rondena, . M. & Gasparini, . S. (2021) Acquired myasthenia gravis with concurrent polymyositis and myocarditis secondary to a thymoma in a dog. Open Veterinary Journal, 11 (3), 436-440. doi:10.5455/OVJ.2021.v11.i3.16 |