| Original Article | ||
Open Vet. J.. 2021; 11(3): 407-417 Open Veterinary Journal, (2021), Vol. 11(3): 407–417 Original Research Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPAMohamed A. Al-Griw1, Zohour M. Marwan2, Ismail M. Hdud3 and Taher Shaibi2*1Department of Histology and Genetics, Faculty of Medicine, University of Tripoli, Tripoli, Libya 2Department of Zoology, Faculty of Sciences, University of Tripoli, Tripoli, Libya 3Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Taher Shaibi. Department of Zoology, Faculty of Sciences, University of Tripoli, Tripoli, Libya. Email: t.shaibi [at] uot.edu.ly Submitted: 20/04/2021 Accepted: 17/07/2021 Published: 12/08/2021 © 2021 Open Veterinary Journal
AbstractBackground: During early development, environmental compounds can induce adult onset diseases and disrupt the circulating vitamin D (VitD) levels. Aim: This study aimed to examine the protective role of VitD against the adverse effects of BPA on male and female mice. Methods: A total of 60 male and female Swiss Albino mice (3 weeks old) were randomly divided into 5 groups; each consisted of 12 mice (6 males and 6 females) and was treated as follows: Group I received no treatment (sham control); Group II, sterile corn oil only (vehicle control); Group III, BPA (400 μg/kg); Group IV, VitD (2,195 IU/kg); and Group V, BPA + VitD. At 10.5 weeks, the animals were sacrificed to conduct histological examinations. Results: BPA-exposed mice were found to have neurobehavioral abnormalities, heart, kidney, and lung diseases with increased apoptotic indices in both sexes. On the other hand, the treatment of BPA mice with VitD altered this scenario with modulated motor activity, enhanced body and organ weights, and preserved the heart, kidney, and lung architecture, alongside a decreased percent apoptotic index. Conclusion: Our findings illustrate that VitD protects mice against BPA-induced heart, kidney, and lung abnormalities. Keywords: Bisphenol A, Heart, Kidney, Lung, Vitamin D. IntroductionStress, nutritional anomalies, and toxicants are all examples of environmental insults that can trigger phenotypic changes and diseases (Waterland, 2009; Nilsson, 2018). Exposure to environmental toxicants has become a major health concern. They interfere with many metabolic processes and cause widespread damage to body tissues and organs (Humblet et al., 2008). This interference may result in changes in appetite, food efficiency, and fat, carbohydrate, and protein metabolism. As a result of apparent pervasiveness in today’s environment, the effects of endocrine-disrupting chemicals can manifest primarily in populations, with less regard for inter-individual differences within populations. Evidence indicates that endocrine-disrupting chemicals, such as halogenated aromatic hydrocarbons, may disrupt hormonal regulation and modify other body functions (Elobeid and Allison, 2008). Exposure to environmental toxicants during germ cell, intrauterine life, postnatal life, and/or early life plays a major role in determining mature phenotypes and susceptibility/resistance to abnormalities/diseases later in life (Skinner et al., 2010, 2013; Al-Gubory, 2014; Al-Griw et al., 2017). Bisphenol A (BPA), an endocrine disruptor chemical, has been extensively used to produce polycarbonate plastics and epoxy resins for many years (Willhite et al., 2008; Camarca et al., 2016; Jalal et al., 2018). People of all ages are unwittingly exposed to BPA in everyday life due to its frequent use in the manufacture of plastic containers of food and beverage and the coating of food cans (Gassman, 2017; Gear and Belcher, 2017). Many studies have detected that BPA is widely present in food, the environment, and even human body fluids (Mouneimne et al., 2017). BPA can be found at concentrations of 0.2–106 ng/g in food samples, 2–208 ng/m3 in the air, and 54–79 μg/cm2 in thermal paper. In addition, BPA concentrations can reach 10 μg/l in human blood, 4.76 μg/l in the placenta, and 59.72 μg/l in the urine. The ubiquitous BPA exposure (Vandenberg et al., 2007) and the toxic potential raise concerns about their adverse impacts on different biological systems (Kim and Hong, 2017). The toxic effects of BPA have been discovered (Crain et al., 2007), and in vivo and in vitro studies suggest that it may cause endocrine disorders as an estrogenic compound in organs (Korkmaz et al., 2010). Despite long-standing concerns about its safety, BPA is still used in various food packaging products and medical devices because developing a chemical replacement that is both cost-effective and safe is difficult (Warner and Flaws, 2018). More than 90% of the general population had detectable levels of BPA, as revealed from epidemiological data (Trasande et al., 2013), while those of occupational exposure had higher BPA levels than the general population by about 70 times (Hines et al., 2017). These data indicate that humans are vulnerable to BPA exposure, and this condition may be unavoidable. Emerging research has shown that certain tissues/organs are highly vulnerable to the toxic BPA effects, which can cause abnormalities/diseases (Fenichel et al., 2013; Rochester, 2013). Therefore, the impact of BPA on human health has drawn widespread attention to the field of public health. BPA shows potential acute, short-term, and subchronic toxicity (Tyl et al., 2002; Tyl, 2008). A study established BPA effects on the liver, kidneys, and body weight at doses of 50 mg/kg body weight (bw) and higher (Tyl, 2008). Several studies have determined the BPA effect on the reproductive system with inadequate reports on other tissues/organs. A report (Stump et al., 2010) used different BPA doses, which was confirmed by a similar finding (Tyl, 2008), with a lowest no observed adverse effect level (NOAEL) of 5 mg/kg bw. There are no chronic organ toxicity studies on BPA. Vitamin D (VitD) is a protein associated with bone metabolism, but recently a wide range of activities was identified. VitD, especially 1,25 (OH)2D3, (1,25 dihydroxy vitamin D3), which is the active form of VitD, plays an important role in human metabolism (Walentowicz-Sadłecka et al., 2013). VitD treatment decreases ischemia-reperfusion injury after myocardial infarction with anti-inflammatory and anti-apoptotic actions (Bae et al., 2013). Moreover, VitD contributes to the regeneration of injured muscles (Stratos et al., 2013). The deficiency of VitD is associated with the increase in oxidative stress and the apoptosis process (Assalin et al., 2013). Pretreatment with VitD3 has protective effects on ischemia-reperfusion injury of the kidneys (Sinanoglu et al., 2012) and hepatic tissues (Seif and Abdelwahed, 2014). The current study investigated the protective effects of VitD against adverse effects of BPA on male and female mice as no earlier study has been conducted till now. Materials and MethodsAnimals and housing conditionsA total of 60 adult male Swiss albino mice, aged 3 weeks old, and weighing 13.5 ± 1.48 g, were used in this study. Animals were bred and housed under standard conditions of a 12-hour light/dark cycle and temperature (26°C ± 2°C) in the animal facility at the Faculty of Sciences, University of Tripoli. All animals had free access to food and water ad libitum. Experimental designThe animals were randomly divided into 5 groups; each group consisted of 12 mice (6 males and 6 females) and was treated as follows: Group I received no treatment (sham control); Group II, sterile corn oil only (vehicle control); Group III, BPA (400 μg/kg); Group IV, VitD (2,195 IU/kg); and Group V, BPA + VitD. Corn oil, BPA, and VitD were administrated intraperitoneally (i.p.) twice per week for 6 weeks (Fig. 1). The BPA and VitD were dissolved in sterile corn oil, and therefore the sterile corn oil was used as the vehicle. The BPA dose was chosen based on prior studies regarding BPA adverse effects and their environmental relevance (Vom-Saal and Hughes, 2005; Tyl, 2008; Sadowski et al., 2014; Khodayar et al., 2020), whereas the VitD dose was selected based on previous work (Tesic et al., 2015). At 10.5 weeks, the animals were sacrificed to conduct histological examinations. Clinical assessmentClinical observations were made daily. Any abnormal clinical signs or behavior that may have resulted from toxicity were recorded. The survival rate was recorded during the entire study. Body and organ weightBody weight was recorded at the onset and at the end of the experiment to calculate body weight change (%). After the experimental duration, the organs (hearts, kidneys, and lungs) were excised, and their relative weight (%) was determined in different experimental groups. Motor activity measurementMotor performance was assessed throughout the study course described previously (Ferrante et al., 2003; Al-Griw et al., 2015a). Briefly, training sessions were given from week 4 to week 10 to acclimate mice to the rotarod apparatus. Motor performance was measured twice weekly for 6 weeks in all studied groups. Three 60-second trials were given during each session and averaged. Values are recorded as the mean rotation number during the 5 counting periods per 60 minutes. Histopathological studies and microscopyThe heart, lung, and kidney tissues were prepared for histological preparation as described elsewhere (Ginsberg et al., 1981; Al-Griw et al., 2015b). Histopathological tissue injury scores were determined and compared between groups. The scoring scale was set from 0 to 4, with the following criteria: (0) no pathological changes, (1) very few pathological changes, (2) mild pathological changes, (3) moderate pathological changes, and (4) severe pathological changes. For each tissue section, the four scoring criteria were averaged, and this average was considered a replicate. The tissue architecture from each animal was assessed blindly by a histopathologist for structural changes. The tissue sections were examined and imaged using low- and high-power objectives under light microscopy (Leica, Germany). Statistical analysisThe findings were analyzed using Statistical Package for the Social Science (SPSS, version 20.0) (SPSS Inc., Chicago, IL, USA). Normal distribution was assessed using skewness and kurtosis detection test. Homogeneity of variances was examined by Livene’s test. A two-way analysis of variance test was carried out for data that were normally distributed. The data that were not normally distributed were analyzed using nonparametric analysis, and statistical significance was determined by the Kruskal–Wallis test, followed by the Bonferroni-corrected Mann–Whitney U test. Data were expressed as means ± SEM. A p-value of 0.05 was considered statistically significant.
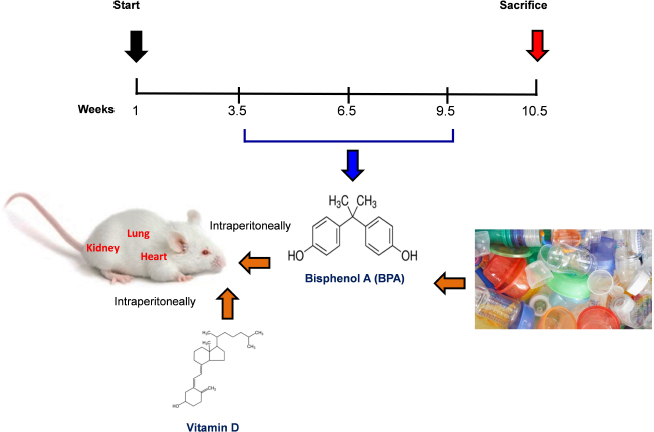
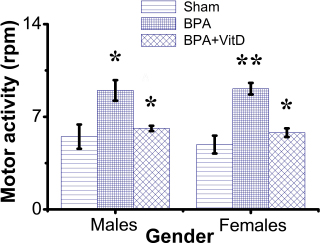
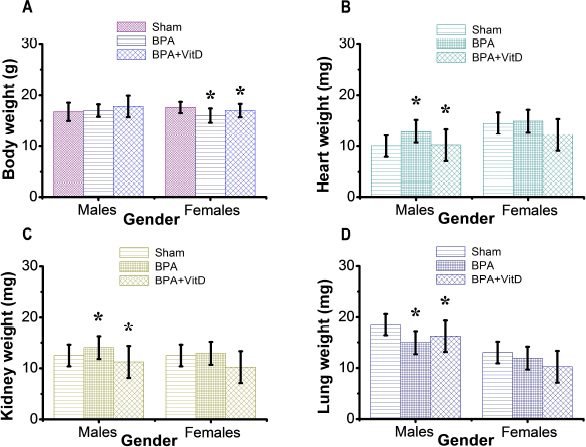
Fig. 1. Schematic of the animal treatment procedure. Animals were exposed to conditions of sham, vehicle, VitD, BPA, or BPA+VitD. BPA and VitD were administered intraperitoneally (i.p.) at doses of 400 μg/kg and 2,195 IU/kg VitD, respectively, for 6 weeks. At 10.5 weeks, the animals were sacrificed for histological examinations. Ethical approvalEthical approval for animal work was obtained from the Research Ethics Committee at the Biotechnology Research Center, Tripoli, Libya (Reference BEC-BTRC 10-2019). ResultsEffects of VitD on animal survival upon BPA exposureFrom the daily clinical follow-up of the mice in this study, no mortality has been recorded in all groups along the course of the experiment and no signs of acute toxicity were noticed. VitD attenuates motor activity upon BPA exposureThere were significant treatment effects on motor activity between the control groups and BPA groups in male and female rats (p < 0.05 and p < 0.01, respectively; Fig. 2), but no difference between control group was evident. Regarding motor activity, there were significant treatment effects between BPA groups and BPA + VitD groups in males and females (p < 0.05; Fig. 2). No significant differences were detected between the sexes when comparing the male and female experimental groups. VitD modulates body and organ weights upon BPA exposureThe body weights of all animals in the study were monitored from birth until study completion, with final body, heart, kidney, and lung weights recorded for analysis at the time of necropsy (Fig. 3). For body weights (Fig. 3A), there were significant treatment effects between control groups and BPA groups in females but not males (p < 0.05 and p > 0.05, respectively), and that treatment with VitD preserved the body weights in females (p < 0.05). No significant sex differences in the body weights were detected when male and female experimental groups were compared. For heart, kidney, and lung weights (Fig. 3B–D), there were significant treatment effects between control groups and BPA groups in males but not females (p < 0.05 and p > 0.05, respectively), and that treatment with VitD preserved the heart, kidney, and lung weights in males (p < 0.05). No significant sex differences in the heart, kidney, and lung weights were detected when male and female experimental groups were compared.
Fig. 2. VitD modulates motor activity after BPA exposure. Mice were exposed to conditions of sham, vehicle, VitD, BPA, or BPA + VitD. Measurement of motor activity in adult male and female mice. Data are mean ± SEM (n=12 per group). *p < 0.05; **p < 0.01.
Fig. 3. VitD preserves the animal body and organ weights. Mice were exposed to conditions of sham, vehicle, VitD, BPA, or BPA + VitD. (A) Quantification of body weight. (B) Quantification of heart weight. (C) Quantification of kidney weight. (D) Quantification of lung weight. Data are mean ± SEM (n=12 per group). *p < 0.05.
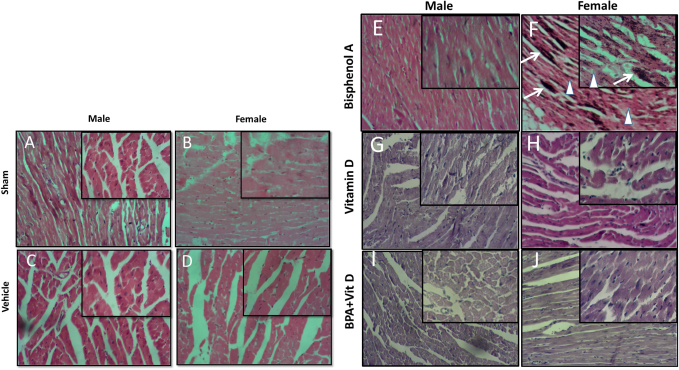
Fig. 4. VitD preserves heart architecture after BPA exposure. Representative photomicrographs of H&E-stained heart tissues of adult male and female sham controls (A and B), vehicle controls (C and D), BPA (E and F), VitD (G and H), and BPA+VitD (I and J). Histopathological features of heart tissues of control-exposed mice showed a normal heart structure. Heart tissues of BPA-exposed mice showed marked alterations in the heart structure. These changes include necrosis (white triangle) and calcification (white arrow). Heart tissues of BPA + VitD-exposed mice showed regeneration of the heart structure (100×, H&E). Table 1. Comparison of the study groups according to the histopathological heart tissue injury scores.
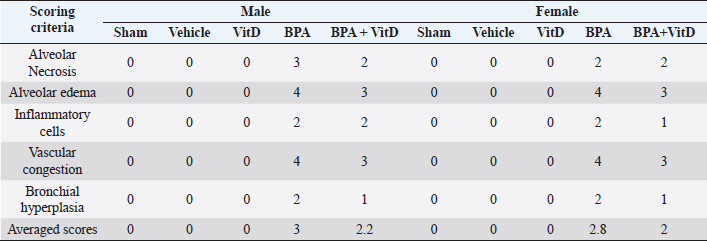
VitD reduces heart pathology induced by BPA exposureHistology of the heart sections of control and corn oil-treated mice for both sexes (males and females) showed normal histological features with preserved cardiac structures (Fig. 4 A–D; Table 1). Cardiac muscles showed longitudinal striation with anastomosing muscle fibers and normal oval nuclei. Heart sections of male mice treated with 50 mg of BPA did not show histological changes (p > 0.05) (Fig. 4E), whereas female mice treated with the same dose had shown foci of necrotic changes with dystrophic calcifications (Fig. 4F); the sections also showed infiltration of inflammatory cells. The administration of males and females with VitD did not show any pathological alteration (Fig. 4G and H). The male group which was exposed to BPA alone (Fig. 4E) or top combination of BPA and VitD (Fig. 4I) did not show any significant histological changes (p > 0.05). The changes in the female group exposed to the BPA showed significant cardiac damage associated with mononuclear inflammatory infiltration and fibrosis comparing to sham group (p < 0.001). Myocardiocytes in this group also showed focal areas of necrosis accompanied by deposition of calcium (dystrophic calcification) (p < 0.001) (Fig. 4F). These changes in this group were significantly prevented by combining the BPA with VitD (p < 0.001) (Fig. 4J). VitD reduces kidney pathology induced by BPA exposureThe histological examination of kidney tissues of the control and vehicle-treated mice showed normal uriniferous tubules and glomeruli (Fig. 5A–D; Table 2). Moreover, VitD-treated mice did not show any pathological alteration (Fig. 5G and H). Examination of renal tissue of male and female mice treated with 50 mg/kg of BPA showed significant alterations in glomeruli as well as all kidney tubules when compared to the BPA group (p < 0.001) (Fig. 5E and F). Several glomeruli were segmented and atrophied with the increase of Bowman’s space (urine space). The renal tubules showed necrosis of several endothelial lining cells, vacuolation, and sloughing of others with infiltration of protein casts inside the tubular lumen. Hydropic degeneration is also seen in some tubular lining epithelial cells. These alterations induced by exposure to BPA in renal tissues did not protect by VitD neither in male nor female mice (p > 0.05) (Fig. 5I and J). VitD reduces lung pathology induced by BPA exposureThe histological examination of photomicrographs of lung tissue sections taken from the control group (Fig. 6A and B) and corn oil-treated group (Fig. 6C and D) showed normal histological architecture for alveoli sac, alveolar duct, bronchioles, bronchi, and blood vessels (Table 3). Mice treated with VitD alone did not show any significant histological changes comparing to control groups (p > 0.05) (Fig. 6G and F). Nevertheless, administration of BPA showed significant histopathological changes in the lung of treated animals in both sexes (males and females) comparing to sham groups (p < 0.001). Changes included thickening of alveolar septal, congestion of capillaries of the alveolar septum, localized focal areas infiltration of inflammatory cells, necrosis in alveolar lining epithelium with moderate to severe edema, and emphysema. It also showed hyperplasia of bronchial lining epithelium cells with congestion of blood vessels (Fig. 6E and F). Administration of VitD to animals (males and females) treated with BPA minimized the damaged effect induced by BPA (Fig. 6I and J; Table 3).
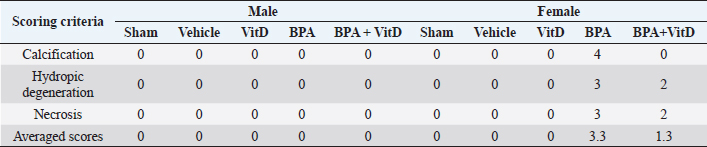
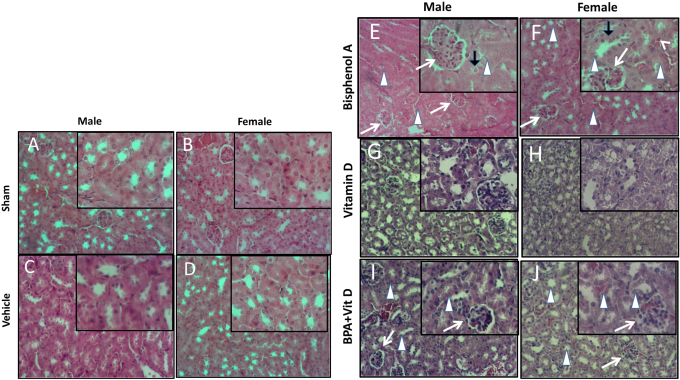
Fig. 5. VitD preserves kidney architecture after BPA exposure. Representative photomicrographs of H&E-stained kidney tissues of adult male and female sham controls (A and B), vehicle controls (C and D), BPA (E and F), VitD (G and H), and BPA+VitD (I and J). Histopathological features of kidney tissues of control mice showed normal renal structure with regulated nuclear arrangement of uriniferous tubules and collecting tubules with glomerulus and blood vessel. Kidney tissues of BPA-exposed mice showed marked tubular hydropic degeneration (whit triangle) with atrophic and segmented glomeruli (white arrow). It also showed necrotic and sloughing endothelial cells (black arrow) with infiltration of hyaline casts inside the tubular lumen (white arrow head). Kidney tissues of BPA + VitD-exposed mice showed regeneration of the lubulie lining with mild congested glomeruli (100×, H&E). Table 2. Comparison of the study groups according to the histopathological kidney tissue injury scores.
DiscussionOur findings illustrated that BPA triggered a significant decrease in body and organ (heart, kidney, and lungs) weights, ultimately leading to heart, kidney, and lung pathologies. Specifically, BPA increased apoptosis and histopathological tissue injury scores, as revealed by histopathological findings. These effects were significantly attenuated by VitD treatment. To our knowledge, this is the first work to demonstrate the protective impacts of VitD on heart, kidney, and lung pathologies. We found significant VitD effects in terms of some histopathological parameters, although not all parameters were improved significantly in the treated group compared to the untreated group. Our findings suggest that a therapeutic opportunity exists for the use of VitD in preventing or reversing heart, kidney, and lung pathologies mediated by BPA toxicity.
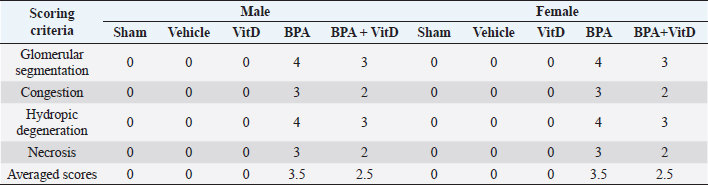
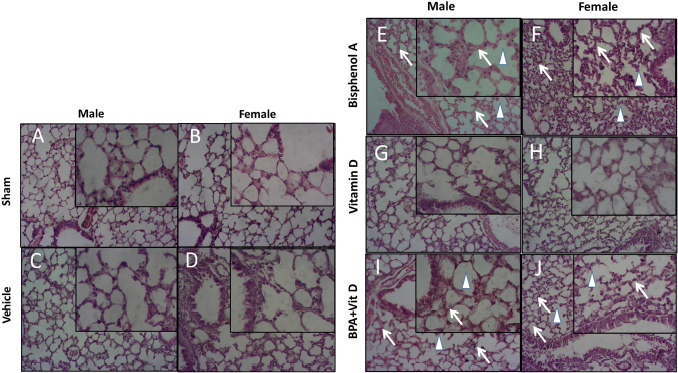
Fig. 6. VitD preserves lung architecture after BPA exposure. Representative photomicrographs of H&E-stained lung tissues of adult male and female sham controls (A and B), vehicle controls (C and D), BPA (E and F), VitD (G and H), and BPA + VitD (I and J). Histopathological features of lung tissues of control-exposed mice showed normal lung structure. Lung tissues of BPA-exposed mice showed marked alterations in the lung tissue architecture including rupture of alveolar walls (imphysema) (white triangle) with swelling of alveolar walls (white arrows). Lung tissues of BPA + VitD-exposed mice showed minimal damage compared to BPA-treated mice (100×, H&E). Table 3. Comparison of the study groups according to the histopathological lung tissue injury scores.
Environmental factors can promote diseases/phenotypic variations (NTP, 2000; EPA, 2003; Manikkam et al., 2012; Al-Griw et al., 2016; Nilsson, 2018). Numerous studies suggested a link between impacts observed following developmental exposures to environmental toxicants and worldwide public health (Aranow, 2011; Camarca et al., 2016; Gassman, 2017). BPA disrupts the biological system even at lower doses (Rogers et al., 2013; Schug et al., 2013; Camarca et al., 2016; Koike et al., 2018; Özaydın et al., 2018). The hormonally active form of VitD plays a significant role in clinical medicine, mainly due to its potent effects on Ca2+ homeostasis and bone metabolism (Piotrowska et al., 2016; Parva et al., 2018). VitD has a beneficial impact on the functions of many biological systems (Aranow, 2011). Recently, it was reported that VitD and VitD receptor activators have protective effects on ischemia and reperfusion injury (Tokgoz et al., 2018). It was also established that VitD3 protects the kidney from reperfusion injury (Tan et al., 2008). Similarly, it was determined that VitD receptor activator paricalcitol has beneficial effects on renal ischemia-reperfusion damage (Azak et al., 2013). Accumulating evidence reported that BPA exposure plays a role in developing motor abnormalities (Kulig, 1987; Mousain-Bosc et al., 2006; Sathyanarayana et al., 2011; Inadera, 2015). Similarly, we found that BPA induced development motor abnormalities in male and female mice. It was reported that the status of VitD was negatively correlated with the risk of motor abnormalities (Fu et al., 2017). In the present study, we found that co-treatment with VitD has beneficial effects on the motor activity induced by BPA toxicity. Exposure to environmental toxicant BPA is linked to an increased risk of being overweight (Braun, 2017). Precisely, it was found that prenatal and early postnatal BPA exposure was correlated with increased body weight (Akingbemi et al., 2004; Suttie, 2006; Magliano and Lyons, 2013; Picard and Turnbull, 2013; Gear and Belcher, 2017). BPA actions on body weight were found in both sexes, without sex-specific effects (Suttie, 2006; Gear and Belcher, 2017). It was reported that a statistically significant decrease in body weight in the group treated with 0.1 mg/kg BPA (Hassan et al., 2012) and with ≥ 466 mg/kg/day BPA (Razzoli et al., 2005). Statistically significant and dose-related decreases in absolute (>22%) and relative (>10%) liver weights were observed at ≥466 mg/kg/day, compared with controls (Yamasaki et al., 2002a, 2002b). Similarly, the findings of this study showed that BPA promoted a significant decrease in the body and organ (heart, kidney, and lung) weights of adult male and female mice. The role of VitD in body weight control is controversial. Precisely, there was no significant difference in body weight variation between VitD-treated mice and control during the supplementation period (Bella et al., 2017). On the contrary, we found that co-treatment with VitDfor 6 weeks was positively associated with the mean body and organ (heart, kidney, and lung) weights without reaching statistical significance. Increasing evidence documents show that the BPA toxic effects depend on its doses and route of administration. For example, exposing rats aged 4.5 weeks to 125 mg BPA/kg/day via oral route for 13 weeks was found to damage livers and kidneys (Tyl, 2008; Dong et al., 2013; Yildiz and Barlas, 2013). There were no BPA impacts on the liver and kidney when mice were exposed to 5 mg BPA/kg/day in dietary, while the toxic effects were found in 50 or 600 mg BPA/kg/day exposed (Tyl, 2008; Dong et al., 2013). In the present study, we found that BPA promoted heart, kidney, and lung pathologies in male and female mice. Precisely, our histopathological findings of heart, kidney, and lung in the BPA-treated mice showed serious cellular and microstructural alterations. However, such alterations have not been noticed in control-treated mice. Many studies reported that treatment with VitD significantly prevented the detrimental impacts of chemical substances on the development of morphological abnormalities in the rats (Guareschi et al., 2019) and on ischemia-reperfusion injury of the ovary in a rat model (Tokgoz et al., 2018). Interestingly, we found that co-treatment with VitD significantly attenuated the detrimental impacts of BPA on mouse heart, kidney, and lung. Specifically, we found that the heart, kidney, and lung histology of the VitD-treated mice exhibited improvement with the regeneration of heart, kidney, and lung architecture restoring to normal. ConclusionThis work demonstrated that BPA promoted heart, kidney, and lung pathologies as revealed by histological alterations. These findings shed light on the possible underlying mechanisms for developing a range of abnormalities induced by BPA. VitD seems an effective molecule for protecting BPA toxicity/injury of the heart, kidney, and lung. We determined significant beneficial effects in BPA-induced toxicity/injury of the heart, kidney, and lung with concurrent co-treatment with VitD, especially tissue architecture. Although some improvement is obtained, there are no statistically significant differences in other histopathological findings. We thought that it might be related to the long elimination and action time of VitD. However, we were incapable of finding the precise mechanism by which VitD exerts its actions. Nevertheless, our findings suggest that VitD provides effective treatments for BPA-induced heart, kidney, and lung injuries. Further studies should provide greater knowledge for a better understanding of the protective mechanism of VitD and assess the clinical applications, effects, and doses of VitD. We hope that this study and its outcomes will inspire others to carry out further studies. AcknowledgmentThe authors express their deepest appreciation to the University of Tripoli for supporting this work. Conflict of interestAll authors have no conflicts of interest to declare. FundingThis work was supported by the University of Tripoli, Tripoli, Libya. Authors’ contributionsConceptualization: MAA and ZMM; methodology: MAA and ZMM; formal analysis and data curation: ZMM and IMH.; writing and original draft preparation: MAA.; writing, review and editing: TS; supervision: MAA and TS; project administration: MAA; funding acquisition: ZMM and MAA. ReferencesAkingbemi, B.T., Sottas, C.M., Koulova, A.I., Klinefelter, G.R. and Hardy, M.P. 2004. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145, 592–603. Al-Griw, A.M., Treesh, S.A., Alghazeer, R.O. and Regeai, S.O. 2017. Environmentally toxicant exposures induced intragenerational transmission of liver abnormalities in mice. Open Vet. J. 7, 244–253. Al-Griw, M.A., Al-Ghazeer, R.O., Al-Azreg, S.A. and Bennour, E.M. 2016. Cellular and molecular etiology of hepatocyte injury in a murine model of environmentally induced liver abnormality. Open Vet. J. 6, 150–157. Al-Griw, M.A., Elnfati, A.H., Salama, N.M., Maamar, M.S., Treesh, S.A. and Shaibi, T. 2015a. Mode of cell death in mouse brain following early exposure to low-dose trichloroethane: apoptosis or necrosis. Am. J. Life Sci. 3, 232–240. Al-Griw, M.A., Salama, N.M., Treesh, S.A. and Elnfati, A.H. 2015b. Transgenerational genetic effect of trichloroethane (TCE) on phenotypic variation of acrosomal proteolytic enzyme and male infertility risk. Int. J. Genet. Genom. 3, 43–49. Al-Gubory, K.H. 2014. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 29, 17–31. Aranow, C. 2011. Vitamin D and the immune system. J. Investig. Med. 59, 881–886. Assalin, H.B., Rafacho, B.P., dos-Santos, P.P., Ardisso, N.L.P., Roscani, M.G., Chiuso-Minicucci, F., Barbisan, L.F., Fernandes, A.A., Azevedo, P.S., Minicucci, M.F., Zornoff, L.A. and de-Paiva, S.A. 2013. Impact of the length of vitamin D deficiency on cardiac remodeling. Circ. Heart Fail. 6, 809–816. Azak, A., Huddam, B., Haberal, N., Kocak, G., Ortabozkoyun, L. and Senes, M. 2013. Effect of novel vitamin D receptor activator paricalcitol on renal ischaemia/reperfusion injury in rats. Ann. R. Coll. Surg. Engl. 95, 489–494. Bae, S., Singh, S.S., Yu, H., Lee, J.Y., Cho, B.R. and Kang, P.M. 2013. Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J. Appl. Physiol. 114, 979–987. Bella, L.M., Fieri, I., Tessaro, F.H.G., Nolasco, E.L., Nunes, F.P.B., Ferreira, S.S., Azevedo, C.B. and Martin, J.O. 2017. Vitamin D modulates hematological parameters and cell migration into peritoneal and pulmonary cavities in alloxan-diabetic mice. Biomed. Res. Int. 2017, 7651815. Braun, J.M. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 13, 161–173. Camarca, A., Gianfrani, C., CimminoI, A.F., Bruzzese, D. and Scerbo, R. 2016. Human peripheral blood mononuclear cell function and dendritic cell differentiation are affected by bisphenol-aexposure. PLoS One 11, e0161122. Crain, D.A., Eriksen, M. and Iguchi, T. 2007. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 24, 225–239. Dong, Y., Zhai, L., Zhang, L., Jia, L. and Wang, X. 2013. Bisphenol A impairs mitochondrial function in spleens of mice via oxidative stress. Mol. Cell. Toxicol. 9, 401–406. Elobeid, M.A. and Allison, D.B. 2008. Putative environmental-endocrine disruptors and obesity: a review. Curr. Opin. Endocrinol. 15, 403–408. EPA, 2003. Interpretation of body weight data; Health Effects Division (HED) Guidance Document #G2003.01. Prepared by the HED Toxicology Science Advisory Council, Health Effects Division, Office of Pesticide Programs (OPP). Fenichel, P., Chevalier, N. and Brucker-Davis, F. 2013. Bisphenol A: an endocrine and metabolic disruptor. Ann. Endocrinol. (Paris) 74, 211–220. Ferrante, R.J., Kubilus, J.K., Lee, J., Ryu, H., Beesen, A., Zucker, B., Smith, K., Kowall, N.W., Ratan, R.R., Luthi-Carter, R. and Hersch, S.M. 2003. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J. Neurosci. 23, 9418–9427. Fu, L., Chen, Y.H., Chen, X., Xu, S., Yu, Z. and Xu, D.X. 2017. Vitamin D deficiency impairs neurobehavioral development in male mice. Physiol. Behav. 179, 333–339. Gassman, N. R. 2017. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 58, 60–71. Gear, R.B. and Belcher, S.M. 2017. Impacts of bisphenol A and ethinyl estradiol on male and female cd-1 mouse spleen. Sci. Rep. 7, 856. Ginsberg, L.C., Johnson, S.C., Salama, N.M. and Ficsor, G. 1981. Acrosomal proteolytic assay for detection of mutagens in mammals. Mutat. Res. 91, 413–418. Guareschi, Z.M., Ceglarek, V.M., Rodrigues, P.F., Huning, L.P. and Festinalli, C. 2019. Exercise and vitamin D supplementation modify spleen morphology in lean, but not, in monosodium-glutamate-obese rats. J. Spleen Liver Res. 1, 1-14. Hassan, Z.K., Elobeid, M.A., Virk, P., Omer, S.A., ElAmin, M., Daghestani, M.H. and AlOlayan, E.M. 2012. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid. Med. Cell. Longev. 2012, 194829. Hines, C.J., Jackson, M.V., Deddens, J.A., Clark, J.C., Ye, X., Christianson, A.L., Meadows, J.W. and Calafat, A.M. 2017. Urinary bisphenol A (BPA) concentrations among workers in industries that manufacture and use BPA in the USA. Ann. Work. Expo. Health. 61, 164–182. Humblet, O., Birnbaum, L., Rimm, E., Mittleman, M.A. and Hauser, R. 2008. Dioxins and cardiovascular disease mortality. Environ. Health Perspect. 116, 1443–1448. Inadera, H. 2015. Neurological effects of bisphenol A and its analogues. Int. J. Med. Sci. 12, 926–936. Jalal, N., Surendranath, A.R., Pathak, J.L., Yu, S. and Chung, C.Y. 2018. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 5, 76–84. Khodayar, M.J., Kalantari, H., Mahdavinia, M., Khorsandi, L., Alboghobeish, S., Samimi, A., Alizadeh, S. and Zeidooni, L. 2020. Protective effect of naringin against BPA-induced cardiotoxicity through prevention of oxidative stress in male Wistar rats. Drug Chem. Toxicol. 43, 85–95. Kim, J.H. and Hong, Y. 2017. Increase of urinary malondialdehyde level by bisphenol A exposure: a longitudinal panel study. Environ. Health 16, 8. Koike, E., Yanagisawa, R., Win-Shwe, T.T. and Takano, H. 2018. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice. Int. J. Immunopathol. Pharmacol. 32, 1–14. Korkmaz, A., Ahbab, M.A., Kolankaya, D. and Barlas, N. 2010. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 48, 2865–2871. Kulig, B.M. 1987. The effects of chronic trichloroethylene exposure on neurobehavioral functioning in the rat. Neurobehav. Toxicol. 9, 171–178. Magliano, D.J. and Lyons, J.G. 2013. Bisphenol A and diabetes, insulin resistance, cardiovascular disease and obesity: controversy in a (plastic) cup? J. Clin. Endocrinol. Metab. 98, 502–504. Manikkam, M, Guerrero-Bosagna, C., Tracey, R., Haque, M.M. and Skinner, M.K. 2012. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One 7, e31901. Mouneimne, Y., Nasrallah, M., Khoueiry-Zgheib, N., Nasreddine, L., Nakhoul, N., Ismail, H., Abiad, M., Koleilat, L. and Tamim, H. 2017. Bisphenol A urinary level, its correlates, and association with cardiometabolic risks in Lebanese urban adults. Environ. Monit. Assess. 189, 517–529. Mousain-Bosc, M., Roche, M., Polge, A., Pradal-Prat, D., Rapin, J. and Bali, J.P. 2006. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. II. Pervasive developmental disorder-autism. Magnes. Res. 19, 53–62. Nilsson, E.E., Sadler-Riggleman, I. and Skinner, M.K. 2018. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 4, 1–13. NTP. (2000). NTP technical report on the toxicity studies of 1,1,1-trichloroethane (CAS no.71-55-6) administered in microcapsules in feed to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser. 41, 1–E20. Özaydın, T., Öznurlu, Y., Sur, E., Çelik, İ. and Uluışık, D. 2018. The effects of bisphenol A on some plasma cytokine levels and distribution of CD8+ and CD4+ T lymphocytes in spleen, ileal Peyer's patch and bronchus associated lymphoid tissue in rats. Acta Histochem. 120, 728–733. Parva, N.R., Tadepalli, T., Singh, P., Qian, A., Joshi, R., Kandala, H., Nookala, V.K. and Cheriyath, P. 2018. Prevalence of Vitamin D deficiency and associated risk factors in the US population (2011–2012). Cureus 10, e2741. Picard, M. and Turnbull, D.M. 2013. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62, 672–678. Piotrowska, A., Wierzbicka, J., Ślebioda, T., Woźniak, M., Tuckey, R.C., Slominski, A.T. and Żmijewski, M.A. 2016. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids 110, 49–61. Razzoli, M., Valsecchi, P. and Palanza, P. 2005. Chronic exposure to low doses bisphenol a interferes with pair-bonding and exploration in female Mongolian gerbils. Brain Res. Bull. 65, 249–254. Rochester, J.R. 2013. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 42, 132–155. Rogers, J.A., Metz, L. and Yong, V.W. 2013. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and potential mechanisms. Mol. Immunol. 53, 421–430. Sadowski, R.N., Wise, L.M., Park, P.Y., Schantz, S.L. and Jurask, J.M. 2014. Early exposure to bisphenol a alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience 279, 122–131. Sathyanarayana, S., Braun, J.M., Yolton, K., Liddy, S. and Lanphear, B.P. 2011. Case report: High prenatal bisphenol a exposure and infant neonatal neurobehavior. Environ. Health Perspect. 119, 1170–1175. Schug, T.T., Heindel, J.J., Camacho, L., Delclos, K.B., Howard, P., Johnson, A.F. and Bucher, J.R. 2013. A new approach to synergize academic and guideline-compliant research: The CLARITY-BPA research program. Reprod. Toxicol. 40, 35–40. Seif, A.A. and Abdelwahed, D.M. 2014. Vitamin D ameliorates hepatic ischemic/reperfusion injury in rats. J. Physiol. Biochem. 70, 659–666. Sinanoglu, O., Sezgin, G., Ozturk, G., Tuncdemir, M., Guney, S., Aksungar, F.B. and Yener, N. 2012. Melatonin with 1,25-dihydroxyvitamin D3 protects against apoptotic ischemia-reperfusion injury in the rat kidney. Ren. Fail. 34, 1021–1026. Skinner, M.K., Manikkam, M. and Guerrero-Bosagna, C. 2010. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 21, 214–222. Skinner, M.K., Manikkam, M., Tracey, R., Guerrero-Bosagna, C., Haque, M. and Nilsson, E.E. 2013. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 11, 228–243. Stratos, I., Li, Z., Herlyn, P., Rotter, R., Behrendt, A.K., Mittlmeier, T. and Vollmar, B. 2013. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am. J. Pathol. 18, 895-904. Stump, D.G., Beck, M.J. and Radovsky, A. 2010. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol. Sci. 115, 167–182. Suttie, A.W. 2006. Histopathology of the spleen. Toxicol. Pathol. 34, 466–503. Tan, X., Wen, X. and Liu, Y. 2008. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J. Am. Soc. Nephrol. 19, 1741–1752. Tesic, D., Hawes, J.E., Zosky, G.R. and Wyrwoll, C.S. 2015. Vitamin D deficiency in BALB/c mouse pregnancy increases placental transfer of glucocorticoids. Endocrinology 156, 3673–3679. Tokgoz, V.Y., Sipahi, M., Keskin, O., Guvendi, G.F. and Takir, S. 2018. Protective effects of vitamin D on ischemia-reperfusion injury of the ovary in a rat model. Iran. J. Basic Med. Sci. 21, 593–59. Trasande, L., Attina, T.M. and Trachtman, H. 2013. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 83, 741–748. Tyl, R.W. 2008. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 104, 362–384. Tyl, R.W., Myers, C.B. and Marr, M.C. 2002. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 68, 121–146. Vandenberg, L.N., Hauser, R., Marcus, M., Olea, N. and Welshons, W.V. 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177. Vom-Saal, F.S. and Hughes, C. 2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113, 926–933. Walentowicz-Sadłecka, M., Sadłecki, P., Walentowicz, P. and Grabiec, M. 2013. The role of vitamin D in the carcinogenesis of breast and ovarian cancer. Ginekol. Pol. 84, 305–308. Warner, G.R. and Flaws, J.A. 2018. Bisphenol a and phthalates: How environmental chemicals are reshaping toxicology. Toxicol. Sci. 166, 246–249. Waterland, R.A. 2009. Is epigenetics an important link between early life events and adult disease? Horm. Res. 71, 13–16. Willhite, C.C., Ball, L. and McLellan, C.J. 2008. Derivation of a bisphenol a oral reference dose (RfD) and drinking-water equivalent concentration. J. Toxicol. Environ. Health 11, 69–146. Yamasaki, K., Sawaki, M., Noda, S., Imatanaka, N. and Takatsuki, M. 2002a. Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch. Toxicol. 76, 65–74. Yamasaki, K., Takeyoshi, M., Noda, S. and Takatsuki, M. 2002b. Changes of serum alpha2u-globulin in the subacute oral toxicity study of ethynyl estradiol and bisphenol A based on the draft protocol for the ‘enhanced OECD test guideline. Toxicology 176, 101–112. Yildiz, N. and Barlas, N. 2013. Hepatic and renal functions in growing male rats after bisphenol A and octylphenol exposure. Hum. Exp. Toxicol. 32, 675–786. | ||
| How to Cite this Article |
| Pubmed Style Al-griw MA, Marwan ZM, Hdud IM, Shaibi T. Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. Open Vet. J.. 2021; 11(3): 407-417. doi:10.5455/OVJ.2021.v11.i3.12 Web Style Al-griw MA, Marwan ZM, Hdud IM, Shaibi T. Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. https://www.openveterinaryjournal.com/?mno=73996 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i3.12 AMA (American Medical Association) Style Al-griw MA, Marwan ZM, Hdud IM, Shaibi T. Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. Open Vet. J.. 2021; 11(3): 407-417. doi:10.5455/OVJ.2021.v11.i3.12 Vancouver/ICMJE Style Al-griw MA, Marwan ZM, Hdud IM, Shaibi T. Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. Open Vet. J.. (2021), [cited January 25, 2026]; 11(3): 407-417. doi:10.5455/OVJ.2021.v11.i3.12 Harvard Style Al-griw, M. A., Marwan, . Z. M., Hdud, . I. M. & Shaibi, . T. (2021) Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. Open Vet. J., 11 (3), 407-417. doi:10.5455/OVJ.2021.v11.i3.12 Turabian Style Al-griw, Mohamed A, Zohour M Marwan, Ismail M Hdud, and Taher Shaibi. 2021. Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. Open Veterinary Journal, 11 (3), 407-417. doi:10.5455/OVJ.2021.v11.i3.12 Chicago Style Al-griw, Mohamed A, Zohour M Marwan, Ismail M Hdud, and Taher Shaibi. "Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA." Open Veterinary Journal 11 (2021), 407-417. doi:10.5455/OVJ.2021.v11.i3.12 MLA (The Modern Language Association) Style Al-griw, Mohamed A, Zohour M Marwan, Ismail M Hdud, and Taher Shaibi. "Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA." Open Veterinary Journal 11.3 (2021), 407-417. Print. doi:10.5455/OVJ.2021.v11.i3.12 APA (American Psychological Association) Style Al-griw, M. A., Marwan, . Z. M., Hdud, . I. M. & Shaibi, . T. (2021) Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. Open Veterinary Journal, 11 (3), 407-417. doi:10.5455/OVJ.2021.v11.i3.12 |