| Case Report | ||
Open Vet. J.. 2021; 11(4): 525-529 Open Veterinary Journal, (2021), Vol. 11(4): 525–529 Case Report Tricuspid valve chordal rupture in a dog with pulmonary hypertensionTomoki Maeba*Midorigaoka Animal Clinic, 19-1, Midorigaoka, Kashiwa-shi, Chiba 277-0082, Japan *Corresponding Author: Tomoki Maeba. Midorigaoka Animal Clinic, Chiba, Japan. Email: no.g.ambition [at] gmail.com Submitted: 08/06/2021 Accepted: 04/09/2021 Published: 01/10/2021 © 2021 Open Veterinary Journal
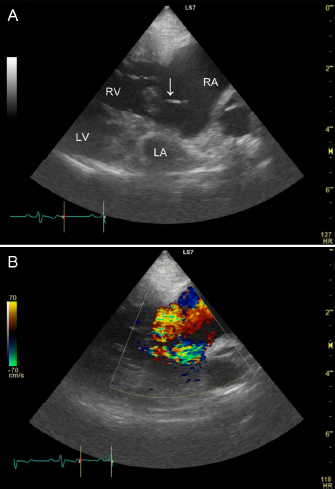
AbstractBackground: Rupture of the chordae tendineae of the tricuspid valve is rare in dogs. We report the case of a dog with tricuspid valve chordal rupture secondary to pulmonary hypertension. Case Description: A 7.7-kg, 13-year-old neutered male Pekingese on annual heartworm prevention presented with respiratory distress. The patient received a diagnosis of pulmonary hypertension and received medical treatment. However, the patient re-presented 405 days post-diagnosis with dyspnea and an increased abdominal circumference. Transthoracic echocardiography showed ruptured chordae tendineae of the tricuspid valve, and abdominal ultrasound identified significant ascites associated with worsening right congestive heart failure. The patient improved after draining ascitic fluid and extra medical treatment, but was released with poor prognosis. Conclusion: Ruptured chordae tendineae of the tricuspid valve should be considered in patients with pulmonary hypertension. Keywords: Canine, Chordal rupture, Congestive heart failure, Pulmonary hypertension. IntroductionRuptured chordae tendineae of the mitral valve frequently occur in small breed dogs with myxomatous mitral valve disease (Serres et al., 2007). On the contrary, tricuspid valve chordal rupture has rarely been reported; thus, diagnostic criteria and optimal treatment for tricuspid valve chordal rupture have not yet been established. A dog had presented with tricuspid valve chordal rupture secondary to pulmonary hypertension, where pulmonary hypertension resulted from an Angiostrongylus vasorum infection (Szatmári, 2020). Here, we report our experience diagnosing and managing the dog with tricuspid valve chordal rupture secondary to chronic, severe pulmonary hypertension. To our knowledge, this is the first case report detailing this condition in a canine patient without Dirofilaria immitis or A. vasorum infection. Case DetailsA 7.7-kg, 13-year-old neutered male Pekingese on annual heartworm prevention presented with respiratory distress. Vital signs included a heart rate of 150 bpm, respiratory rate of 60 breaths/minute, blood pressure of 120/84 mmHg, and rectal temperature of 37.5°C. Pulse oximetry on the right ear using a clip probe revealed a peripheral oxygen saturation of 87%, and excitation provoked tongue cyanosis. A grade 3/6 holosystolic heart murmur was detected in the right cardiac apex. The complete blood count (ProCyte DX; IDEXX, Westbrook) and serum biochemistry (FUJI DRI-CHEM 7000V; FUJIFILM, TOKYO, Japan) profiles were within normal limits except for mild elevations in C-reactive protein of 1.3 mg/l (reference range <1 mg/l) and phosphorus of 5.1 mg/dl (reference range 1.9–5.0 mg/dl). Electrocardiography showed a slightly prolonged P wave duration (0.06 seconds; reference <0.04 seconds) and no arrhythmia. Thoracic radiographs revealed right-sided cardiomegaly, and bilateral pulmonary alveolar infiltrates fissure lines, and a reduction of airway diameters, suggesting bronchomalacia. Abdominal ultrasound identified dilation of the hepatic veins and caudal vena cava without evidence of ascites. Transthoracic echocardiography showed concentric and eccentric hypertrophy of the right ventricle (RV), dilation of the right atrium (RA), and paradoxical interventricular septal movement. Color Doppler showed severe tricuspid regurgitation toward the right atrial free wall, and the peak tricuspid regurgitation velocity determined with continuous-wave Doppler was 4.53 m/second. A systolic right ventricular and right atrial pressure gradient calculated using the simplified Bernoulli equation was 82 mmHg (reference range <31 mmHg; Johnson et al., 1999). The left ventricular end-diastolic diameter normalized for body weight was 0.81 (reference range 1.27–1.85), and the left atrial-to-aortic diameter ratio was normal at 1.56 (reference range ≤1.6). No mitral valve regurgitation was observed; however, a small amount of pleural effusion was identified. The patient was diagnosed with right-sided congestive heart failure due to pulmonary hypertension, and was treated with sildenafil (2 mg/kg q 12 hours PO; HETERO, Telangana, India), torasemide (0.08 mg/kg q 12 hours PO; FUJIFILM, Tokyo, Japan), and benazepril (0.35 mg/kg q 12 hours PO; Fortekor; Elanco Animal Health, Greenfield). Four days after admission, a thoracic radiograph showed improvement in pulmonary infiltrates, and the patient was discharged. After that, he underwent regular blood tests and transthoracic echocardiography about once a month. Clinical signs of dyspnea and coughing increased 87 days after initial presentation. Transthoracic echocardiography showed that the tricuspid regurgitation pressure gradient was 76 mmHg (reference range <31 mmHg) and no pleural effusion. Laboratory testing showed increased blood urea nitrogen, at 44.2 mg/dl (reference range 9.2–29.2 mg/dl), but no other abnormality. Due to worsening symptoms, the sildenafil dosage was increased to 2.8 mg/kg q 12 hours PO, which resulted in clinical improvement. Transthoracic echocardiography at 270 days after the initial presentation showed a tricuspid regurgitation pressure gradient of 57 mmHg and no pleural effusion, and his condition was also satisfactory. Hence, the torasemide dosage was reduced to 0.07 mg/kg q 24 hours PO. Because of financial constraints, the dog’s owner was unable to continue with re-examinations or further laboratory testing but could continue with medications. The patient re-presented with abdominal enlargement and dyspnea 405 days after the initial hospitalization. The owner reported that the abdominal enlargement appeared to have gotten worse in recent days and that respiratory distress had been observed since the previous night. The owner said he was unable to give the patient two doses of medicine because of dyspnea. Vital signs included a heart rate of 120 bpm, respiratory rate of 48 breaths/minute, blood pressure of 81/56 mmHg, and rectal temperature of 35.8°C. Peripheral oxygen saturation was 91%, and the patient had lost weight (to 6.98 kg). A grade 3/6 heart murmur was detected at the right cardiac apex. A SNAP Heartworm RT test (IDEXX) was negative in March of that year, and the patient had been prescribed oral moxidectin (MOXIHEART CHEWABLE; Virbac Japan, Osaka, Japan) every month. Laboratory testing revealed a low packed cell volume, at 27.5% (reference range 37.3%–61.7%), a low red blood cell count, at 4.29 × 106/μl (reference range 5.65–8.87 × 106/μl), a high white blood cell count, at 18.25 × 103/μl (reference range 5.05–16.76 × 103/μl), a high blood urea nitrogen concentration, at 41.9 mg/dl (reference range 9.2–29.2 mg/dl), a high creatinine concentration, at 1.77 mg/dl (reference range 0.40–1.40 mg/dl), a high alanine transaminase concentration, at 278 IU/l (reference range 17–78 IU/l), a normal total protein concentration, at 5.1 g/dl (reference range 5.0–7.2 g/dl), a low albumin concentration, at 2.1 g/dl (reference range 2.6–4.0 g/dl), and a high C-reactive protein concentration, at 2.2 mg/l (reference range <1 mg/l). Ultrasonography of the thorax and abdomen identified slight pleural effusion and severe abdominal effusion. In total, 660 ml of abdominal effusion was removed (660 ml of low-protein effusion, <2.5 g/dl transudates), which resulted in high blood pressure (134/80 mmHg). Transthoracic echocardiography showed dilation of the RA and ventricle, a flattened interventricular septum, and decreased size of the left side of the heart. A ruptured chorda tending of the tricuspid valve was identified in the RA in the right parasternal four-chamber view during systole, which had not been identified in the previous echocardiograms. The chorda tendinea was associated with the septal cusp of the tricuspid valve (Fig. 1). The tricuspid regurgitation pressure gradient was 91 mmHg (reference range <31 mmHg), and no mitral regurgitation was observed. The patient was diagnosed with worsening right congestive heart failure. The patient’s owner signed an informed consent form for treatment, and at the owner’s request, the patient was admitted for additional treatment. He was placed in a 40% oxygen chamber and treated with an increased dosage of sildenafil (30 mg/head q 12 hours PO), furosemide (1 mg/kg q 12 hours IV; Lasix; NICHI-IKO, Toyama, Japan), and carperitide (α-human atrial natriuretic peptide; 0.05 μg/kg/minute CRI; HANP; Daiichi Sankyo, Tokyo, Japan). There were no other findings indicative of infection, but the white blood cell count and C-reactive protein concentration were mildly elevated; hence, the patient was prescribed prophylactic enrofloxacin (5 mg/kg q 24 hours SC; Baytril; Bayer, Tokyo, Japan).
Fig. 1. Two-dimensional echocardiogram tilted from the right, parasternal, long-axis, four-chamber view to optimize identification of the ruptured chordae tendineae (A). The top of the image is the right side of the heart, with the tricuspid valve in the middle, the left chamber being the RV and the right chamber being the RA. The image shows the ruptured chordae tendineae associated with the septal cusp of the tricuspid valve (white arrow). Severe right-sided eccentric hypertrophy of the right heart secondary to volume overload caused decreased preload to the left side of the heart. Severe tricuspid regurgitation is observed (B). A day later, the patient had an appetite and breathed normally in room air. The dog’s body weight was reduced to 6.2 kg because of the removal of ascites and the use of a diuretic drug. The tricuspid regurgitation pressure gradient was reduced to 67 mmHg (reference <31 mmHg). Laboratory testing showed a red blood cell count of 5.19 × 106/μl (reference range 5.65–8.87 × 106/μl), packed cell volume of 34.1% (reference range 37.3%–61.7%), white blood cell count of 13.46 × 103/μl (reference range 5.05–16.76 × 103/l), creatinine concentration of 1.12 mg/dl (reference range 0.40–1.40 mg/dl), and total protein concentration of 5.3 g/dl (reference range 5.0–7.2 g/dl). The patient was discharged with prescriptions for enrofloxacin (5 mg/kg q 24 hours PO; Baytril Flavor Tablets; Bayer, Tokyo, Japan) and torasemide (0.05 mg/kg q 12 hours PO), in addition to continuing sildenafil (4.8 mg/kg q 12 hours PO) and benazepril (0.4 mg/kg q 12 hours PO). After 7 days, the patient died suddenly at home just before re-examination, and the owner did not consent to a necropsy. Ethical approvalNo ethical approval was required for this case. DiscussionRupture of the chordae tendineae of the tricuspid valve is rare in both humans and animals. The etiology of tricuspid chordal rupture, in the absence of trauma or an iatrogenic cause, is unknown. The 2020 American College of Veterinary Internal Medicine consensus guidelines provide information for diagnosing and treating pulmonary hypertension in dogs (Reinero et al., 2020); however, ruptured chordae tendineae of the tricuspid valve have rarely been reported. The pathogenesis of tricuspid valve chordal rupture may be based on clinical observations in a small number of cases. Chordal rupture secondary to pulmonary hypertension has been reported in dogs with A. vasorum infection (Szatmári, 2020). Other animal case reports include a dog with traumatic tricuspid insufficiency, a dog with pulmonary alveolar microlithiasis, a dog with atrioventricular dissociation, a cat with trauma, and a horse with chronic respiratory disease (Closa and Font, 1999; Ettinger and Buergelt, 1968; Liu, 1969; Malik et al., 1991; Torki et al., 2013). In humans, the primary etiology for tricuspid chordae tendineae rupture is chest trauma or iatrogenic trauma as a complication of myocardial biopsy (Baverman et al., 1990; Zhang et al., 2017); however, in our patient, there was no history of trauma. As our patient had chronic pulmonary hypertension, the pathogenesis of chordal rupture may be similar to a reported case of tricuspid papillary muscle rupture in a neonate secondary to hypoxemia (Alkalay et al., 1988). Pekingese may have a breed predisposition for respiratory-related pulmonary hypertension secondary to interstitial lung diseases and bronchomalacia (Koster and Kirberger, 2016; Johnson and Stern, 2020). It is possible that our case had the same pathogenesis. However, other potential causes of pulmonary hypertension, including pulmonary thromboembolism, cannot be eliminated. However, echocardiography showed no findings suggestive of atrioventricular valve degeneration, indicating that myxomatous transformation of the atrioventricular valves, frequently observed in small dogs, may have contributed to the chordal rupture. Infective endocarditis may predispose patients to chordal rupture, as shown in humans (Oliveira et al., 1983). Since we could not rule out infection because the white blood cell count and C-reactive protein concentration were mildly elevated, the patient was treated with antibiotics after admission. In our case, ruptured chordae tendineae of the tricuspid valve has presumed a result of chronic severe pulmonary hypertension. Pulmonary hypertension may have resulted in tricuspid valve pressure overload, right ventricular dilatation, and mechanical stress on the tricuspid valve and subvalvular apparatus, which finally led to the chordal rupture. Although tricuspid valve chordal rupture resulting from pulmonary hypertension secondary to heartworm infection has been reported, it has not been previously described in the absence of heartworm infection. In our case, transthoracic echocardiography confirmed the diagnosis of ruptured chordae tendineae. Tricuspid valve chordal rupture secondary to idiopathic pulmonary hypertension has been diagnosed by transthoracic echocardiography in a human patient (Rodrigues et al., 2016). However, there is a report where real-time transesophageal echocardiography identified tricuspid valve chordal rupture not discernible with transthoracic echocardiography (Hu et al., 2020). The requirement for general anesthesia limits the use of transesophageal echocardiography in animals. In our case, the ruptured chordae tendineae were only clearly identified in the right parasternal four-chamber view and not in the other standard views. Due to the three-dimensional anatomical structure of the tricuspid valve and the site of attachment of the ruptured chordae tendineae, standard echocardiographic views may not allow veterinarians to identify the ruptured chordae. Identifying continuity between the ruptured chordae tendineae and the tricuspid valve required a slightly different focus from the basic four-chamber view. In our case, multiple echocardiographic examinations were carried out until 270 days after the initial presentation, but the chordal rupture could not be confirmed. Since the chordal rupture was discovered simultaneously as the worsening of clinical symptoms, we postulate that it is highly likely that chordal rupture occurred just before the clinical symptoms worsened; however, this could have been an incidental finding. Early surgical intervention before the development of right-sided systolic dysfunction is recommended for traumatic tricuspid insufficiency in humans (Zhang et al., 2017). Surgery has been performed for tricuspid valve dysplasia in dogs; however, there are no reports of surgical intervention for chordal rupture (Arai et al., 2011). In our case, the patient was considered a poor surgical candidate due to severe pulmonary hypertension. Sildenafil, a phosphodiesterase-5 inhibitor that induces pulmonary vasodilation, has been shown to have therapeutic effects on pulmonary hypertension (Reinero et al., 2020). The reported case of pulmonary hypertension due to A. vasorum infection responded to sildenafil treatment after chordal rupture (Szatmári, 2020), and sildenafil reduced pulmonary arterial pressure in a dose-dependent manner in a canine model of chronic embolic pulmonary hypertension (Akabane et al., 2020). Further therapy to lower pulmonary arterial pressure, therefore decreasing pulmonary hypertension, was the primary goal in this case because surgical treatment for chordal rupture was deemed too difficult. After the initial presentation, the patient responded satisfactorily to sildenafil therapy, leading us to increase the dose even after diagnosing the tricuspid valve chordal rupture. Diuretic therapy for ascites due to pulmonary hypertension and tricuspid valve regurgitation should be carried out with caution since diuretics may reduce the left ventricular output because of the reduced preload effect. Carperitide, a recombinant α-human atrial natriuretic peptide, lowers the mean pulmonary arterial pressure/right atrial pressure, induces vasodilation, and increases water-sodium diuresis (Hidaka et al., 1995). Carperitide can lower pulmonary arterial and right atrial pressure, but the vasodilatory and diuretic effects can result in hypotension. We observed no adverse events due to carperitide in this case. In conclusion, ruptured chordae tendineae of the tricuspid valve can follow pulmonary hypertension. Ruptured chordae tendineae may be difficult to detect on transthoracic echocardiography and requires careful examination. More research is warranted to determine whether tricuspid valve chordal rupture affects treatment or prognosis. AcknowledgmentThe author would like to thank Editage (www.editage.com) for English language editing. Conflict of interestThe author declares that there is no conflict of interest. ReferencesAkabane, R., Sakatani, A., Ogawa, M., Nagakawa, M., Miyakawa, H., Miyagawa, Y. and Takemura, N. 2020. The effect of sildenafil on pulmonary haemodynamics in a canine model of chronic embolic pulmonary hypertension. Res. Vet. Sci. 133, 106–110. Alkalay, A.L., Ferry, D.A., Pepkowitz, S.H., Chou, P.J., Oakes, G.K. and Pomerance, J.J. 1988. Critical tricuspid insufficiency due to papillary muscle rupture. A result of prenatal hypoxic insult. Am. J. Dis. Child. 142, 753–755. Arai, S., Griffiths, L.G., Mama, K., Hackett, T.B., Monnet, E., Boon, J.A., Carter, L. and Orton, E.C. 2011. Bioprosthesis valve replacement in dogs with congenital tricuspid valve dysplasia: technique and outcome. J. Vet. Cardiol. 13, 91–99. Baverman, A.C., Coplen, S.E., Mudge, G.H. and Lee, R.T. 1990. Ruptured chordae tendineae of the tricuspid valve as a complication of endomyocardial biopsy in heart transplant patients. Am. J. Cardiol. 66, 111–113. Closa, J.M. and Font, A. 1999. Traumatic tricuspid insufficiency in a kitten. J. Am. Anim. Hosp. Assoc. 35, 21–24. Ettinger, S. and Buergelt, C.D. 1968. Atrioventricular dissociation (incomplete) with accrochage in a dog with ruptured chordae tendineae. Am. J. Vet. Res. 29, 1499–1503. Hidaka, T., Furuya, M., Tani, Y. and Ohno, T. 1995. Hemodynamic and neurohumoral effects of carperitide (alpha-human atrial natriuretic peptide) in dogs with low-output heart failure. Nihon yakurigaku zasshi. 105, 243–261. Hu, W.Y., Zhao, B.W., Li, S.Y. and Wang, B. 2020. A rare case report: tricuspid valve prolapse due to spontaneous chordae rupture in a congenitally corrected transposition of the great arteries patient. J. Cardiothorac. Surg. 15, 152. Johnson, L., Boon, J. and Orton, E.C. 1999. Clinical characteristics of 53 dogs with doppler-derived evidence of pulmonary hypertension: 1992–1996. J. Vet. Intern. Med. 13, 440–744. Johnson, L.R. and Stern, J.A. 2020. Clinical features and outcome in 25 dogs with respiratory- associated pulmonary hypertension treated with sildenafil. J. Vet. Intern. Med. 34, 65–73. Koster, L.S. and Kirberger, R.M. 2016. A syndrome of severe idiopathic pulmonary parenchymal disease with pulmonary hypertension in Pekingese. Vet. Med. (Auck.) 7, 19–31. Liu, S.K. 1969. Pulmonary alveolar microlithiasis with ruptured chordae tendineae in mitral and tricuspid valves in a dog. J. Am. Vet. Med. Assoc. 155, 1692–1703. Malik, R., Hunt, G.B., Porges, W.L. and Wood, A.K.W. 1991. Traumatic tricuspid insufficiency in a dog. J. Am. Anim. Hosp. Assoc. 27, 467–469. Oliveira, D.B., Dawkins, K.D., Kay, P.H. and Peneth, M. 1983. Chordal rupture. I: aetiology and natural history. Br. Heart J. 50, 312–317. Reinero, C., Visser, L.C., Kellihan, H.B., Masseau, I., Rozanski, E., Clercx, C., Williams, Kurt., Abbott, J., Borgarelli, M. and Scansen, B.A. 2020. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 34, 549–573. Rodrigues, A.C.T., Afonso, J.E., Cordovil, A., Monaco, C., Piveta, R., Cordovil, R., Fischer, C.H., Vieira, M., Lira-Filho, E. and Morhy, S.S. 2016. Spontaneous tricuspid valve chordal rupture in idiopathic pulmonary hypertension. Echocardiography 33, 472–475. Serres, F., Chetboul, V., Tissier, R., Sampedrano, C.C., Gouni, V., Nicolle, A.P. and Pouchelon, J.L. 2007. Chordae tendineae rupture in dogs with degenerative mitral valve disease: prevalence, survival, and prognostic factors (114 cases, 2001–2006). Vet. Intern. Med. 21, 258–264. Szatmári, V. 2020. Spontaneous tricuspid valve chordal rupture in a dog with severe, irreversible pulmonary hypertension caused by Angiostrongylus vasorum infection. BMC Vet. Res. 16, 311–317. Torki, E., Dezfouli, M.R.M., Rasekh, M., Abbasi, J., Mirshahi, A. and Janitabar Darzi, S. 2013. Rupture of chorda tendineae of the tricuspid valve in a horse: a case report. Iran J. Vet. Med. 7, 305–309. Zhang, Z., Yin, K., Dong, L., Sun, Y., Guo, C., Lin, Y. and Wang, C. 2017. Surgical management of traumatic tricuspid insufficiency. J. Cardiac Surg. 32, 342–346. | ||
| How to Cite this Article |
| Pubmed Style Tomoki Maeba. Tricuspid valve chordal rupture in a dog with pulmonary hypertension. Open Vet. J.. 2021; 11(4): 525-529. doi:10.5455/OVJ.2021.v11.i4.1 Web Style Tomoki Maeba. Tricuspid valve chordal rupture in a dog with pulmonary hypertension. https://www.openveterinaryjournal.com/?mno=81032 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.1 AMA (American Medical Association) Style Tomoki Maeba. Tricuspid valve chordal rupture in a dog with pulmonary hypertension. Open Vet. J.. 2021; 11(4): 525-529. doi:10.5455/OVJ.2021.v11.i4.1 Vancouver/ICMJE Style Tomoki Maeba. Tricuspid valve chordal rupture in a dog with pulmonary hypertension. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 525-529. doi:10.5455/OVJ.2021.v11.i4.1 Harvard Style Tomoki Maeba (2021) Tricuspid valve chordal rupture in a dog with pulmonary hypertension. Open Vet. J., 11 (4), 525-529. doi:10.5455/OVJ.2021.v11.i4.1 Turabian Style Tomoki Maeba. 2021. Tricuspid valve chordal rupture in a dog with pulmonary hypertension. Open Veterinary Journal, 11 (4), 525-529. doi:10.5455/OVJ.2021.v11.i4.1 Chicago Style Tomoki Maeba. "Tricuspid valve chordal rupture in a dog with pulmonary hypertension." Open Veterinary Journal 11 (2021), 525-529. doi:10.5455/OVJ.2021.v11.i4.1 MLA (The Modern Language Association) Style Tomoki Maeba. "Tricuspid valve chordal rupture in a dog with pulmonary hypertension." Open Veterinary Journal 11.4 (2021), 525-529. Print. doi:10.5455/OVJ.2021.v11.i4.1 APA (American Psychological Association) Style Tomoki Maeba (2021) Tricuspid valve chordal rupture in a dog with pulmonary hypertension. Open Veterinary Journal, 11 (4), 525-529. doi:10.5455/OVJ.2021.v11.i4.1 |