| Original Article | ||
Open Vet. J.. 2021; 11(4): 700-706 Open Veterinary Journal, (2021), Vol. 11(4): 700–706 Original Research Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladderTomohiro Nishimori1, Kiwamu Hanazono1* , Kazuya Matsuda1, Yoshio Kawamura1, Tsuyoshi Kadosawa1, Yoshifumi Endo1 and Tsuyoshi Uchide21School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Japan 2Laboratory of Veterinary Molecular Pathology and Therapeutics, Tokyo University of Agriculture and Technology, Fuchu, Japan *Corresponding Author: Kiwamu Hanazono. School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Japan. Email: k-hanazono [at] rakuno.ac.jp Submitted: 06/06/2021 Accepted: 19/11/2021 Published: 15/12/2021 © 2021 Open Veterinary Journal
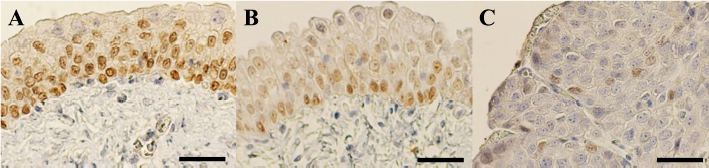
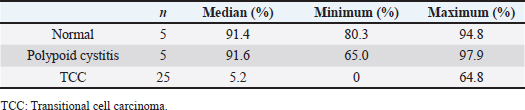
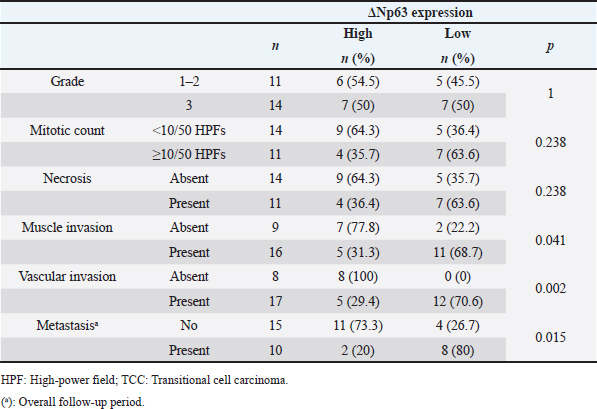
AbstractBackground: Decreased p63 protein expression in canine transitional cell carcinoma (TCC) of the urinary bladder is associated with vascular invasion of the tumor, metastasis, and shortened survival. ΔNp63, an isoform of p63, is downregulated in high-grade invasive urothelial carcinoma in humans. However, the clinical significance of ΔNp63 expression in canine urinary bladder tumors is unknown. Therefore, it is essential to investigate ΔNp63 expression patterns in TCC, the most common urinary bladder tumor in dogs. Aim: This study aimed to evaluate the expression and role of ΔNp63 in canine TCC of the urinary bladder. Methods: ΔNp63 expression was compared between the normal canine urinary bladder, polypoid cystitis, and TCC. The correlation of ΔNp63 expression with histopathological and clinical findings were further evaluated, and its usefulness as a prognostic factor was examined. Results: We observed that ΔNp63 was highly expressed in dogs’ normal urinary bladder and polypoid cystitis, and its expression levels were low in TCC. Furthermore, low levels of ΔNp63 expression were associated with vascular invasion, metastasis, and shortened survival in dogs with TCC. Conclusion: These results indicate that ΔNp63 expression could serve as a valuable biomarker for invasion, metastasis, and prognosis of canine TCC of the urinary bladder. Keywords: ΔNp63, Metastasis, Survival, Transitional cell carcinoma, Urinary bladder. IntroductionTransitional cell carcinoma (TCC) is the most common malignant tumor arising from the urinary bladder of dogs and accounts for 50%–75% of all canine urinary bladder tumors (Knapp et al., 2000). TCC may invade the urinary bladder wall at diagnosis, with distant metastasis to the lungs and liver (Mutsaers et al., 2003). There are several treatment options for canine TCC of the urinary bladder, including surgery, chemotherapy, and radiation therapy; however, long-term survival of dogs with TCC remains low. Factors affecting survival include secondary complications due to the urinary system’s obstruction and other biological characteristics, such as tumor proliferation, invasion, and metastasis. Hence, there is a need for biomarkers that can assess these biological characteristics at an early stage. In recent years, the p63 protein has attracted attention as a molecule associated with tumor proliferation, invasion, and metastasis in human urinary bladder cancer (BC). The tumor–node–metastasis (TNM) stage progression and shortened survival have been associated with decreased expression of the p63 protein, especially in high-grade invasive BC (Urist et al., 2002; Koga et al., 2003b). The TP63 gene belongs to the same gene family as the TP53 gene and encodes the tumor suppressor protein, p63, that has the same apoptosis-inducing effect as the p53 protein. Therefore, a decrease in the expression of or a loss-of-function mutation in the TP63 gene may lead to uncontrolled cell growth. Abnormal expression of p63 may be a factor associated with cancer progression (Urist et al., 2002; Koga et al., 2003b). The p63 protein has two isoforms: TAp63, which has a transactivation domain at the N-terminal, and ΔNp63, which does not have an activation domain. Different promoters regulate the expression of these two isoforms. However, the functions of TAp63 and ΔNp63 remain unknown. TAp63 may be responsible for the transactivation of p53 target genes, such as p21, and the initiation of apoptosis during excessive cell proliferation (Osada et al., 1998; Yang et al., 1998), while ΔNp63 is assumed to inhibit transactivation by p53 and TAp63 (Yang et al., 1998). Based on these functions, it has been suggested that ΔNp63 is overexpressed in human squamous cell carcinoma and other malignant tumors and is involved in carcinogenesis (Parsa et al., 1999; Hibi et al., 2000). During immunohistochemistry, antibodies against p63 can recognize the core domains of both ΔNp63 and TAp63. On the contrary, antibodies against ΔNp63 are particular as they recognize the ΔN transactivation domain, thus making ΔNp63 a more specific diagnostic marker than p63 for human lung squamous cell carcinoma (Bishop et al., 2012). However, some reports suggest that the decreased expression of ΔNp63 is linked to malignant behavior in human urinary bladder tumors. The relationship between ΔNp63 expression and tumorigenicity is currently controversial (Koga et al., 2003a,b; Fukushima et al., 2009). In addition, the decreased expression of ΔNp63 may be associated with tumor invasion in high-grade invasive urothelial carcinoma in humans (Koga et al., 2003a; Fukushima et al., 2009). In a study that assessed p63 expression in canine TCC of the urinary bladder using immunohistochemistry, p63 expression was found to be significantly lower in the TCC tissue than in the normal bladder tissue and polypoid cystitis. This low expression was associated with tumor tissue necrosis, vascular invasion, tumor growth, metastasis, and shortened survival (Hanazono et al., 2016). On the contrary, although there have been reports on ΔNp63 expression in dogs with mammary tumors (Bertagnolli et al., 2009), none of the studies have assessed ΔNp63 expression in dogs with urinary bladder tumors. Therefore, we hypothesized that as in the case of human invasive urothelial carcinoma, the expression of ΔNp63 is downregulated in canine TCC of the urinary bladder, which is associated with the prognosis of TCC. This study aimed to evaluate the clinical significance of ΔNp63 expression in canine urinary bladder tumors. The level of ΔNp63 expression in the normal canine urinary bladder, polypoid cystitis, and TCC was investigated along with the relationship between ΔNp63 expression and histopathological and clinical findings. Materials and MethodsParaffin-embedded urinary bladder tissues from 25 dogs that underwent cystectomy at the Animal Medical Center of Rakuno Gakuen University were evaluated between 2004 and 2011. The diagnosis of TCC was made based on postoperative histopathological examination. Five specimens diagnosed with polypoid cystitis and five normal canine urinary bladder tissues were used as controls. For dogs diagnosed with TCC, clinical information was obtained until 2012. The TNM classification for canine urinary BC was used according to the criteria set by the World Health Organization (Owen, 1980). Primary tumors (T) were classified as Tis: carcinoma in situ; T0: no evidence of primary tumor; T1: superficial papillary tumor; T2: tumor invading the bladder wall with induration; or T3: tumor invading the neighboring organs (prostate, uterus, vagina, and pelvic cavity). The regional lymph nodes (N) were classified as N0: no regional lymph node (internal or external iliac lymph node) involved; N1: regional lymph node involved; or N2: regional and juxtaregional lymph nodes involved. Distant metastasis (M) was classified as M0: no evidence of metastasis or M1: the presence of distant metastasis. Metastasis was evaluated based on imaging, biopsy, lymphadenectomy, or necropsy findings. In addition, hematoxylin and eosin staining was made on tissue specimens to assess the grade (Valli et al., 1995), mitotic count, and the presence of necrosis and vascular invasion. The mitotic count was determined using a microscope and expressed as the number of mitoses per 50 high-power fields (HPFs). Additionally, the presence of tissue necrosis and vascular invasion was evaluated. The presence or absence of metastasis during the observation period and survival time was also investigated. Immunohistochemical staining was carried out to determine the expression of ΔNp63 in the urinary bladder tissues. The rabbit anti-p40 (ΔNp63) polyclonal antibody (dilution factor: 2000×, manufacturer: calbiochem, immunized animals: rabbit, catalog number: PC373), which has been used in previous studies on dogs, was used as the primary antibody (Bertagnolli et al., 2009). Tissue sections were deparaffinized, immersed in Tris ethylenediaminetetraacetic acid buffer (pH 9.0) for antigen activation, and heat-treated in an autoclave for 15 minutes at 121°C. The sections were then immersed in 0.5% periodate solution for 15 minutes to remove endogenous peroxidase. After thoroughly rinsing with water, the sections were immersed in a blocking agent (Block Ace, Dainippon Sumitomo Pharma, Osaka, Japan) at 37°C for 30 minutes to suppress non-specific reactions. Tissue sections were incubated with the primary antibody diluted in phosphate-buffered saline (PBS) at 23°C–25°C for 1 hour. After incubation with the primary antibody, the tissue sections were washed with PBS and incubated with the biotin-labeled secondary antibody (anti-rabbit IgG antibody, Vecter Laboratories, Burlingame, CA) diluted 400-fold in PBS for 30 minutes at room temperature. After incubation with the secondary antibody, the tissue sections were washed with PBS, and labeled avidin-biotin staining (Vectestain ABC Kit; Vecter Laboratories, Burlingame, CA) was carried out for 30 minutes at room temperature. The tissue sections were washed with PBS and stained with a 0.03% aqueous hydrogen peroxide 3,3-diaminobenzidine solution, followed by rinsing the tissue sections and nuclear staining with hematoxylin (Tissue-Tek, Osaka, Japan). For evaluating the expression of ΔNp63, cells were observed under the microscope, and those with stained nuclei were considered positive; the number of positive cells per 1,000 cells was calculated. The dogs were classified into high- and low-ΔNp63-expression groups with the median expression rate as the cut-off, and the survival time for dogs with TCC was compared between the two groups. The software, Excel Tokei (SSRI, Tokyo, Japan) was used for statistical analysis. The Shapiro–Wilk normality test was employed to assess the normality of the ΔNp63 expression rate. The ΔNp63 expression rate in TCC was compared with that in the normal bladder and polypoid cystitis tissues using the Kruskal–Wallis test. The association of ΔNp63 expression with the grade, mitotic count, vascular invasion, necrosis, and metastasis during the observation period was assessed using Fisher’s exact test. The log-rank test was used to compare the survival time between the high- and low-ΔNp63-expression groups in dogs with TCC. A p-value < 0.05 was considered statistically significant. Ethical approvalAll experiments in this study were conducted with the approval of the Ethics Committee of the Rakuno Gakuen University (No. VH24A13). ResultsAt the time of the first surgery, the mean age for dogs with polypoid cystitis and TCC was 9.6 ± 3.7 and 9.7 ± 2.4 years, respectively. The breeds of dogs with polypoid cystitis were Shih Tzu (n=2), Miniature Schnauzer (n=1), Papillon (n=1), and Siberian Husky (n=1). The breeds of dogs with TCC were Shetland Sheepdog (n=4), mongrel (n=3), Shiba Inu (n=2), Maltese (n=2), Beagle (n=2), Dalmatian (n=1), French Bulldog (n=1), Great Pyrenees (n=1), Miniature Dachshund (n=1), Miniature Pinscher (n=1), Pomeranian (n=1), Samoyed (n=1), Scottish Terrier (n=1), Shih Tzu (n=1), Siberian Husky (n=1), Japanese Spitz (n=1), and West Highland White Terrier (n=1). All polypoid cystitis and TCC tissues were surgically obtained from the dogs. Normal bladder tissues were obtained by necropsy from five beagles (two males and three females) in our university. At the time of surgery, there were 9 cases with T1, 10 with T2, and 6 with T3 stage tumors; 22 cases had N0 and 3 cases had N1 stage tumors; none of the TCCs showed any evidence of metastasis. With regard to the site of occurrence of TCC in the urinary bladder, 1 occurred in the apex, 12 in the body, and 13 in the trigone. Postoperative chemotherapy was administered as follows: piroxicam alone (n=6), mitoxantrone alone (n=1), carboplatin alone (n=1), and a combination of piroxicam and mitoxantrone (n=3). Dogs with TCC were followed-up from the time of surgery until death. The mean follow-up period for estimating the overall survival was 17 months (range, 1–60 months), regardless of the cause of death. At the end of the follow-up period, 19 dogs with TCC died due to metastasis (n=5), secondary renal failure (n=2), infection (n=2), other tumors (n=1, lymphoma), or unknown causes (n=9). Histopathological examination revealed 1 case of grade-1 TCC, 10 cases of grade-2 TCC, and 14 cases of grade-3 TCC. The mitotic count was less than 10/50 HPF in 14 cases and more than 10/50 HPF in 11 cases. Necrosis was absent in 14 and evident in 11 cases; the vascular invasion was absent in 8 and present in 17 cases. Metastatic lesions were present in 15 cases, while no metastasis was observed in 10 cases during the entire follow-up period. The images of immunohistochemical staining for ΔNp63 are shown in Figure 1. In the normal urinary bladder tissue, ΔNp63 expression was observed in the nuclei in all layers of the urinary bladder mucosa, especially in the cells forming the basal layer. In polypoid cystitis, ΔNp63 expression was observed in the nuclei of cells in all layers of the urinary bladder mucosa, especially in the basal layer and normal tissue; however, in TCC, ΔNp63 expression was observed in the nuclei of a small number of cells scattered throughout the tumor tissue. Table 1 and Figure 2 show the comparison of ΔNp63 expression among the three groups: normal urinary bladder, polypoid cystitis, and TCC. Dogs with TCC were classified into high- (≥5.2%) and low- (<5.2%) ΔNp63-expression groups, based on the median ΔNp63 expression rate (5.2%). The relationship of ΔNp63 expression with grade, mitotic count, vascular invasion, necrosis, and metastasis was investigated during the observation period. Low expression of ΔNp63 was significantly associated with vascular invasion and metastasis. On the contrary, ΔNp63 expression level was not significantly associated with grade, mitotic count, or necrosis (Table 2).
Fig. 1. Immunohistochemical staining for ΔNp63. (A) ΔNp63 expression is observed in the nuclei of cells in all layers of the normal urinary bladder mucosa, especially in the basal layer. (B) ΔNp63 expression is observed in the nuclei of cells in all layers of the urinary bladder mucosa in polypoid cystitis, especially in the nuclei of cells in the basal layer. (C) In TCC, the expression of ΔNp63 is found in the nuclei of a small number of cells (scale bar=50 μm). Table 1. ΔNp63 expression rate in normal urinary bladder tissue, polypoid cystitis, and TCC.
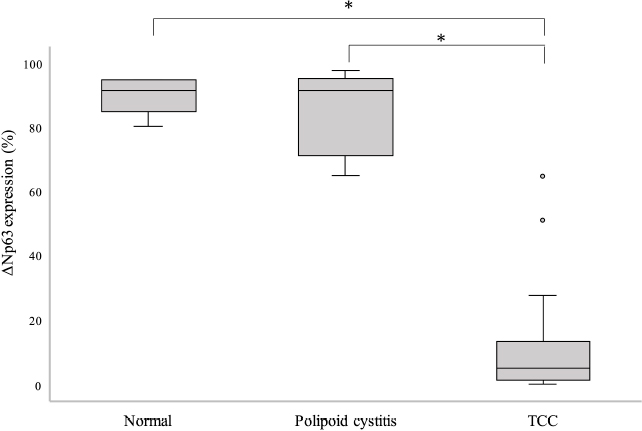
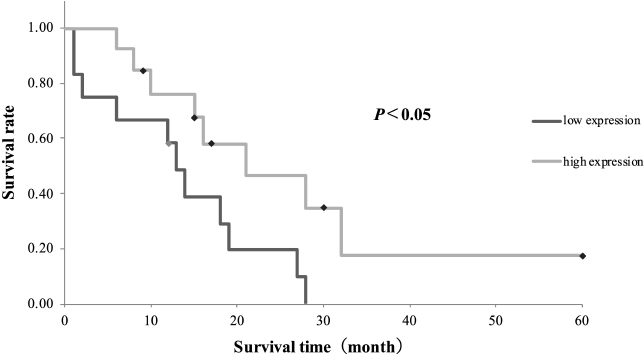
Fig. 2. Comparison of ΔNp63 expression rate between the normal urinary bladder tissue, polypoid cystitis, and TCC. TCC shows a significantly lower ΔNp63 expression rate than the normal urinary bladder tissue and polypoid cystitis (* p < 0.001). Kaplan–Meier survival curves based on ΔNp63 expression rate are shown in Figure 3. The median survival time was 13 months in the low-ΔNp63-expression group and 21 months in the high-ΔNp63-expression group, and the survival time was significantly shorter in the low-ΔNp63-expression group (p < 0.05). DiscussionStudies on ΔNp63 in human urinary BC have not yielded consistent results. Karni et al. (2011) reported a higher expression of ΔNp63 in invasive urinary BC than in noninvasive urinary BC. On the contrary, Koga et al. (2003a) reported that ΔNp63 showed an expression trend similar to that of p63 using immunohistochemical staining, and its expression was maintained in the normal urinary bladder tissue and low-grade urinary BC. ΔNp63 expression was, however, lost in invasive BC (Koga et al., 2003a). Bertagnolli et al. (2009) also found a strong positive correlation (r=0.832; p < 0.0001) between p63 and ΔNp63 expression in canine mammary tumors. Similar to the results shown in the present study, another study found that p63 expression in canine TCC of the urinary bladder was significantly lower than that in the normal urinary bladder tissue, and benign lesions of polypoid cystitis (Hanazono et al., 2016). This supports the reports of Koga et al. (2003a) and Bertagnolli et al. (2009). In this study, we observed an association of reduced ΔNp63 expression with vascular invasion, metastasis, and shorter survival, which corroborates a previous report suggesting that reduced p63 expression in canine TCC of the urinary bladder is also associated with vascular invasion and metastasis, as well as shorter survival (Hanazono et al., 2016).
Fig. 3. Comparison between survival times based on ΔNp63 expression. The median survival time is 21 months in the high-ΔNp63-expression group and 13 months in the low-ΔNp63-expression group. The difference is statistically significant (p < 0.05). TAp63 has a transactivation domain at its N-terminus, similar to that of p53, and is thought to exert the same tumor-suppressive effect as that of p53. However, ΔNp63 lacks this transactivation domain and, therefore, does not feature the tumor suppressor effect of p53. In addition, since ΔNp63 has the same binding domain as p53 and TAp63, it antagonizes the tumor-suppressive effect of p53 and TAp63 by competing with these proteins for the same binding site at the promoter region (Yang et al., 1998). Based on this molecular background and the known overexpression of ΔNp63 in squamous cell carcinoma, the association between ΔNp63 overexpression and carcinogenesis has attracted much attention (Parsa et al., 1999; Hibi et al., 2000). Although the present results are inconsistent with the concepts mentioned above, it has been reported that the loss of ΔNp63α, an isoform of ΔNp63, can cause malignant behavior in human urinary BC (Koga et al., 2003a, b). Decreased expression of ΔNp63α has been implicated in tumor invasion and metastasis by upregulating N-cadherin and increasing the production of matrix metalloproteinase 9, which degrades intercellular matrix proteins (Fukushima et al., 2009). Gaya et al. (2015) reported that 21.1% of the patients with high-grade T1 urinary bladder tumors in their study lacking ΔNp63 expression progressed to muscle-invasive disease, while those with ΔNp63 expression did not exhibit the same progression. In addition, decreased expression of ΔNp63 transcripts in the muscle layer of non-invasive BC has been associated with recurrence and progression (Papadimitriou et al., 2019). These results suggest that reduced expression of ΔNp63 may also promote tumor invasion and metastasis in canine TCC of the urinary bladder. In this study, ΔNp63 expression was not associated with grade, mitotic count, or necrosis. ΔNp63 expression is generally low in high-grade urinary BC in humans. On the contrary, low-grade papillary non-invasive tumors with low expression of ΔNp63 may later develop into muscle-invasive BC (Fukushima et al., 2009). Furthermore, p63 expression has not been associated with grade and mitotic count (Stefansson et al., 2006). In the past, p63 expression in canine bladder tumors did not correlate with the mitotic count. Necrosis tends to occur in biologically aggressive tumors that grow rapidly (Portillo et al., 1991) and is associated with prognosis in canine TCC of the urinary bladder (Hanazono et al., 2014). Necrosis in canine urothelial carcinoma is recognized as comedones necrosis (de Brot et al., 2019), which is often associated with the tumor grade (Epstein et al., 2017). In this study, it is unclear why necrosis was not associated with ΔNp63 expression; however, the authors suggested that it is similar to the lack of association with grade. Thus, low expression of ΔNp63 may not be associated with the histological grade or invasiveness at the time of tumor removal but may indicate subsequent behavior. Table 2. Correlation between ΔNp63 expression and histopathological findings and metastasis in TCC.
A limitation of this study is that we only investigated the immunohistological expression of the ΔNp63 protein in canine urinary bladder tissue and did not analyze the corresponding gene or its function. These analyses need to be carried out to prove the true pathological significance of ΔNp63. In addition, in this study, the dogs were followed-up until death due to any cause; the number of deaths due to TCC could not be recorded, because in some cases, necropsy was not performed; hence, it was not possible to establish whether the cause of death was tumor-related. ConclusionIn conclusion, our results suggest that expressions of ΔNp63 and p63 are reduced in canine TCC of the urinary bladder. This is similar to human invasive urothelial carcinoma. This decreased expression is associated with tumor invasion, metastasis, and shortened survival. Thus, ΔNp63 expression could serve as a valuable biomarker for invasion, metastasis, and prognosis of canine TCC of the urinary bladder. AcknowledgmentThe authors thank Rie Kumazawa and Daiji Endoh (Rakuno Gakuen University) for their cooperation and support. Conflict of interestThe authors declare that there are no conflicts of interest. Authors’ contributionsTomohiro Nishimori: Immunohistochemical staining, investigation, and writing original draft preparation. Kiwamu Hanazono: Conceptualization and editing. Kazuya Matsuda: Pathologic diagnosis, sample supply, and methodology. Yoshio Kawamura: Hematoxylin and eosin staining and methodology. Tsuyoshi Kadosawa: Surgery and investigation. Yoshifumi Endo: Surgery and investigation. Tsuyoshi Uchide: Supervision. ReferencesBertagnolli, A.C., Cassali, G.D., Genelhu, M.C., Costa, F.A., Oliveira, J.F. and Gonçalves, P.B. 2009. Immunohistochemical expression of p63 and ΔNp63 in mixed tumors of canine mammary glands and its relation with p53 expression. Vet. Pathol. 46, 407–415. Bishop, J.A., Teruya-Feldstein, J., Westra, W.H., Pelosi, G., Travis, W.D. and Rekhtman, N. 2012. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod. Pathol. 25, 405–415. de Brot, S., Grau-Roma, L., Stirling-Stainsby, C., Dettwiler, M., Guscetti, F., Meier, D., Scase, T., Robinson, B. D., Gardner, D. and Mongan, N.P. 2019. A fibromyxoid stromal response is associated with muscle invasion in canine urothelial carcinoma. J. Comp. Pathol. 69, 35–46. Epstein, J.I., Amin, M.B., Reuter, V.E. and Humphrey, P.A. 2017. Contemporary Gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 41, e1–e7. Fukushima, H., Koga, F., Kawakami, S., Fujii, Y., Yoshida, S., Ratovitski, E., Trink, B. and Kihara, K. 2009. Loss of ΔNp63α promotes invasion of urothelial carcinomas via N-cadherin/Src homology and collagen/extracellular signal-regulated kinase pathway. Cancer. Res. 69, 9263–9270. Gaya, J.M., López-Martínez, J.M., Karni-Schmidt, O., Bonal, D.M., Algaba, F., Palou, J., Villavicencio, H., Benson, M.C., Cordon-Cardo, C. and Castillo-Martin, M. 2015. deltaNp63 expression is a protective factor of progression in clinical high grade T1 bladder cancer. J. Urol. 193, 1144–1150. Hanazono, K., Fukumoto, S., Endo, Y., Ueno, H., Kadosawa, T. and Uchide, T. 2014. Ultrasonographic findings related to prognosis in canine transitional cell carcinoma. Vet. Radiol. Ultrasound. 55, 79–84. Hanazono, K., Nishimori, T., Fukumoto, S., Kawamura, Y., Endo, Y., Kadosawa, T. and Uchide T. 2016. Immunohistochemical expression of p63, Ki67 and β-catenin in canine transitional cell carcinoma and polypoid cystitis of the urinary bladder. Vet. Comp. Oncol. 14, 263–269. Hibi, K., Trink, B., Patturajan, M., Westra, W.H., Caballero, O.L., Hill, D.E., Ratovitski, E.A., Jen, J. and Sidransky, D. 2000. AIS is an oncogene amplified in squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A. 97, 5462–5467. Karni, S.O., Castillo, M.M., Shen, T.H., Gladoun, N., Domingo, D.J., Sanchez, C.M., Li, Y., Lowe, S., Prives, C. and Cordon, C.C. 2011. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am. J. Pathol. 178, 1350–1360. Knapp, D.W., Glickman, N.W., Denicola, D.B., Bonney, P.L., Lin, T.L. and Glickman, L.T. 2000. Naturally-occurring canine transitional cell carcinoma of the urinary bladder a relevant model of human invasive bladder cancer. Urol. Oncol. 5, 47–59. Koga, F., Kawakami, S., Kumagai, J., Ando, N., Takizawa, T., Kageyama, Y. and Kihara, K. 2003a. Impaired ΔNp63 Expression Associates with reduced β-catenin and aggressive phenotypes of Urothelial neoplasms. Br. J. Cancer. 88, 740–747. Koga, F., Kawakami, S., Fujii, S., Saito, K., Ohtsuka, Y., Iwai, A., Ando, N., Takizawa, T., Kageyama, Y. and Kihara, K. 2003b. Impaired p63 expression associates with poor prognosis and uroplakin iii expression in invasive urothelial carcinoma of the bladder. Clin. Cancer. Res. 9, 5501–5507. Mutsaers, A.J., Widmer, W.R. and Knapp, D.W. 2003. Canine transitional cell carcinoma. J. Vet. Intern. Med. 17, 136–144. Osada, M., Ohba, M., Kawahara, C., Ishioka, C., Kanamaru, R., Katoh, I., Ikawa, Y., Nimura, Y., Nakagawara, A., Obinata, M. and Ikawa, S. 1998. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat. Med. 4, 839–843. Owen, L.N. 1980. TNM classification of tumours in domestic animals, 1st ed. Geneva. Switzerland: World Health Organization. Papadimitriou, M.A., Avgeris, M., Levis, P.K., Tokas, T., Stravodimos, K. and Scorilas, A. 2019. deltaNp63 transcript loss in bladder cancer constitutes an independent molecular predictor of TaT1 patients post-treatment relapse and progression. J. Cancer Res. Clin. Oncol. 145, 3075–3087. Parsa, R., Yang, A., McKeon, F. and Green, H. 1999. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J. Invest. Dermatol. 113, 1099–1105 Portillo, M.J.A., Val Bernal, F., Garijo Ayenza, F., Buelta Carrillo, L., Martín García, B., Hernández Rodríguez, R., Gutiérrez Baños, J.L., Correas Gómez, M.A., Concepción Masip, T. and Fernández Gómez, J.M. 1991. Prognostic factors in 243 transitional carcinomas of the bladder (II): microscopic parameters of the tumor and staging. Arch. Esp. Urol. 44, 161–168. Stefansson, I.M., Salvesen, H.B. and Akslen, L.A. 2006. Loss of p63 and cytokeratin 5/6 expression is associated with more aggressive tumors in endometrial carcinoma patients. Int. J. Cancer. 118, 1227–1233. Urist, M.J., Di, C.C., Lu, M.L., Charytonowicz, E., Verbel, D., Crum, C.P., Ince, T.A., McKeon, F.D. and Cordon, C.C. 2002. Loss of p63 expression is associated with tumor progression in bladder cancer. Am. J. Pathol. 161, 1199–1206. Valli, V.E., Norris, A., Jacobs, R.M., Laing, E., Withrow, S., Macy, D., Tomlinson, J., McCaw, D., Ogilvie, G.K. and Pidgeon, G. 1995. Pathology of canine bladder and urethral cancer and correlation with tumour progression and survival. J. Comp. Pathol. 113, 113–130. Yang, A., Kaghad, M., Wang, Y., Gillett, E., Fleming, M.D., Dotsch, V., Andrews, N.C., Caput, D. and McKeon, F. 1998. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell. 2, 305–316. | ||
| How to Cite this Article |
| Pubmed Style Nishimori T, Hanazono K, Matsuda K, Kawamura Y, Kadosawa T, Endo Y, Uchide T. Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder. Open Vet. J.. 2021; 11(4): 700-706. doi:10.5455/OVJ.2021.v11.i4.22 Web Style Nishimori T, Hanazono K, Matsuda K, Kawamura Y, Kadosawa T, Endo Y, Uchide T. Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder. https://www.openveterinaryjournal.com/?mno=86227 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.22 AMA (American Medical Association) Style Nishimori T, Hanazono K, Matsuda K, Kawamura Y, Kadosawa T, Endo Y, Uchide T. Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder. Open Vet. J.. 2021; 11(4): 700-706. doi:10.5455/OVJ.2021.v11.i4.22 Vancouver/ICMJE Style Nishimori T, Hanazono K, Matsuda K, Kawamura Y, Kadosawa T, Endo Y, Uchide T. Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 700-706. doi:10.5455/OVJ.2021.v11.i4.22 Harvard Style Nishimori, T., Hanazono, . K., Matsuda, . K., Kawamura, . Y., Kadosawa, . T., Endo, . Y. & Uchide, . T. (2021) Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder. Open Vet. J., 11 (4), 700-706. doi:10.5455/OVJ.2021.v11.i4.22 Turabian Style Nishimori, Tomohiro, Kiwamu Hanazono, Kazuya Matsuda, Yoshio Kawamura, Tsuyoshi Kadosawa, Yoshifumi Endo, and Tsuyoshi Uchide. 2021. Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder. Open Veterinary Journal, 11 (4), 700-706. doi:10.5455/OVJ.2021.v11.i4.22 Chicago Style Nishimori, Tomohiro, Kiwamu Hanazono, Kazuya Matsuda, Yoshio Kawamura, Tsuyoshi Kadosawa, Yoshifumi Endo, and Tsuyoshi Uchide. "Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder." Open Veterinary Journal 11 (2021), 700-706. doi:10.5455/OVJ.2021.v11.i4.22 MLA (The Modern Language Association) Style Nishimori, Tomohiro, Kiwamu Hanazono, Kazuya Matsuda, Yoshio Kawamura, Tsuyoshi Kadosawa, Yoshifumi Endo, and Tsuyoshi Uchide. "Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder." Open Veterinary Journal 11.4 (2021), 700-706. Print. doi:10.5455/OVJ.2021.v11.i4.22 APA (American Psychological Association) Style Nishimori, T., Hanazono, . K., Matsuda, . K., Kawamura, . Y., Kadosawa, . T., Endo, . Y. & Uchide, . T. (2021) Prognostic role of ΔNp63 expression in canine transitional cell carcinoma of the urinary bladder. Open Veterinary Journal, 11 (4), 700-706. doi:10.5455/OVJ.2021.v11.i4.22 |