| Original Article | ||
Open Vet. J.. 2022; 12(4): 489-494 Open Veterinary Journal, (2022), Vol. 12(4): 489–494 Original Research A pilot study of patch Holter electrocardiograph recordings in healthy catsMizuki Ogawa*, Saran Fatim Kaba, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa and Naoyuki TakemuraLaboratory of Veterinary Internal Medicine II, School of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan *Corresponding Author: Mizuki Ogawa. Laboratory of Veterinary Internal Medicine II, School of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan. Email: d1804 [at] nvlu.ac.jp Submitted: 24/02/2022 Accepted: 24/06/2022 Published: 16/07/2022 © 2022 Open Veterinary Journal
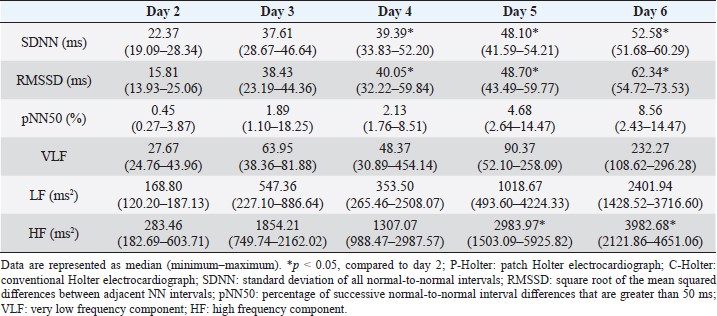
AbstractBackground: A patch Holter electrocardiograph (P-Holter) is cordless, making it lightweight, unlike the conventional Holter electrocardiograph (C-Holter). A P-Holter can also take continuous measurements for up to 14 days without replacing the battery or SD card. Aim: To compare the performance of the P-Holter and the C-Holter in healthy cats. Additionally, we aimed to investigate whether multiday recordings with the P-Holter decrease sympathetic nerve activity or improve the accuracy of arrhythmia detection. Methods: Five healthy domestic short-haired cats were used for this study. Both a P-Holter and C-Holter were used on the first day, but only the P-Holter was used on days 2–6. The evaluated variables were the analyzable time of both Holter types, heart rate (HR), HR variability (HRV), and the number of arrhythmia occurrences. Results: For two out of the five cats, measurement of P-Holter was interrupted. Eventually, continuous recordings using the P-Holters were able to be collected from all individuals for 6 days. The 24 hours analyzable time from the P-Holter and C-Holter was almost identical (p=0.94). The 24 hours mean HR did not differ across Holter types (p=0.67). In addition, the timing of the occurrences of arrhythmias was almost identical to the P-Holter and C-Holter. Results of HRV suggested that sympathetic nerve activity was likely to decrease and vagal nerve activity was likely to increase after 4–5 days of measurement, compared to the second day of measurement (p < 0.05). When only the P-Holter was installed, the number of arrhythmia occurrences was similar on days 2–6. Conclusion: In this study, the P-Holter may be as useful as the C-Holter in cats with suspected intermittent arrhythmias, although the P-Holters were placed on cats without a clinical indication. However, cats may have individual differences in their adaptation to the device. P-Holter recordings taken for more than 4–5 days may allow the cat to acclimate to the device and reduce sympathetic nerve activity. The accuracy of arrhythmia detection across multiday P-Holter recordings requires further investigation using clinical cases. Keywords: Electrocardiograph, Heart rate variability, Heart rate, Arrhythmias. IntroductionIn cats, arrhythmias are one of the most common causes of syncope. Arrhythmias that can cause syncope include sinus arrest, ventricular tachycardia, high-grade, second-degree atrioventricular block, and third-degree, atrioventricular block (Willis et al., 2018). In particular, sinus arrest and ventricular tachycardia occur intermittently, so these arrhythmias may not be detected by electrocardiography performed in the hospital alone. Therefore, in cats where syncope due to arrhythmia is suspected, a Holter electrocardiograph, which allows continuous electrocardiography recording over 24 hours, should be performed to confirm the arrhythmia at the time of the syncope event. Recently, a cordless patch Holter electrocardiograph (P-Holter) has been shown to be successful for use in humans (Barrett et al., 2014; Cheung et al., 2014; Bolourchi and Batra, 2015). Unlike the conventional Holter electrocardiograph (C-Holter), the P-Holter is cordless, making it lightweight (P-Holter: 25 g; C-Holter: 40 g). The P-Holter can also take continuous measurements for 14 days without replacing the battery or SD card. In cats, wearing a C-Holter can sometimes decrease the cat’s activity (Jackson et al., 2014). It has been reported that stress increases sympathetic nerve activity, leading to changes in heart rate (HR) and HR variability (HRV) compared to resting state in cats (Ogawa et al., 2022). If a cat is able to acclimatize to the P-Holter by wearing it for several days, sympathetic nerve activity may decrease, compared to the elevated level on the first day of measurement, such that the recorded data is closer to that at rest. In dogs, Holter electrocardiographs should be performed across several consecutive days rather than on a single day because of the diurnal variation in the frequency of arrhythmias (Gunasekaran et al., 2020). Although there is no such evidence in cats, P-Holters may more accurately detect arrhythmias in cats if electrocardiograph testing is performed on consecutive days. The purpose of this study was to compare the performance of the P-Holter and C-Holter in healthy cats. Additionally, we aimed to investigate whether multiday recordings with the P-Holter decrease sympathetic nerve activity or improve the accuracy of arrhythmia detection. Materials and MethodsFive healthy domestic short-haired cats (two intact males and three neutered males; 9.2–11.3 years of age; 4.20–4.55 kg) were used for this study. The cats were clinically healthy based upon physical examinations, blood tests, electrocardiography, blood pressure, thoracic radiography, and echocardiography. These cats had unrestricted movement in individual stainless steel cages. Food was provided at 08:00 and 20:00. Drinking water was provided ad libitum. We followed the Guidelines for Institutional Laboratory Animal Care and Use at the Nippon Veterinary and Life Science University (Approval 2021s-29). For each animal, Holter electrocardiograph recordings were taken for six consecutive days. Both a P-Holter (WR-100, Fukuda Denshi, Tokyo, Japan) and C-Holter (QR2500, Fukuda M-E Kogyo, Tokyo, Japan) were used on the first day, but only the P-Holter was used on days 2–6. The sampling frequencies of the P-Holter and C-Holter were 250 and 150 Hz, respectively, and the effective analogue/digital bit resolutions were 0.01 and 0.50 mV for both Holter types. The P-Holter was placed on the left side of the cat’s thorax, at the fifth intercostal space, slightly dorsal to the costochondral junction, and oriented diagonally at a 45° angle from vertical, parallel to the long axis of the heart (Lichtenberger et al., 2018). The P-Holter provided a one-lead electrocardiograph. For the C-Holter, five electrodes were fixed to a shaved area, which provided a two-lead electrocardiograph (Ogawa et al., 2022). The installation of P-Holter and C-Holter is shown in Figure 1a and b. The evaluated variables were the analyzable time of both Holter types, HR, HRV [the time-domain analysis included the standard deviation of all normal-to-normal intervals (SDNN); the square root of the mean squared differences between adjacent NN intervals (RMSSD); the percentage of successive normal-to-normal interval differences that were greater than 50 ms (pNN50); and the very low frequency (VLF)], low frequency (LF), and high-frequency components (HF)], and the number arrhythmia occurrences. For this mean power spectrum, the VLF, LF, and HF powers were calculated by integrating the power spectral density into defined frequency bands (< 0.04 Hz, 0.04–0.15 Hz, and 0.15–0.70 Hz, respectively) (Rienzo et al., 1991; Abbott, 2005; Khor et al., 2014; Ogawa et al., 2022). The analysis of HRV was performed as a time series of the last 5 minutes of the 20 minutes that the cats were at rest during monitoring (Soares-Miranda et al., 2012; Verma et al., 2018). The time and frequency domain variables of HRV were analyzed using R wave detection. The HRV analyses were performed using an automatic electrocardiogram analyzer (Kubios HRV software ver. 2.1, Kubios Oy, Kuopio, Finland) (Tarvainen et al., 2014). All data acquisition and post-acquisition analyses were performed with reference to human guidelines (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Both P-Holter and C-Holter analyses were performed by a third party, and the diagnosis of arrhythmia was made by visual confirmation rather than by machine. The statistical analysis was performed using commercial software (SPSS Statistics version 24.0, IBM, New York). The normality of the data was assessed using the Shapiro–Wilk test. Wilcoxon’s rank sum test was used to compare the analyzable time and mean HR over 24 hours according to P-Holter and C-Holter data. A repeated analysis of variance was used to compare the HRV and the number of arrhythmia occurrences on days 2–6. A p-value < 0.05 was considered statistically significant. Ethical approvalThis work involved the use of experimental animals and the study therefore had ethical approval from an established committee as stated earlier. We followed the Guidelines for Institutional Laboratory Animal Care and Use at the Nippon Veterinary and Life Science University (Approval 2021s-29). ResultsOne of the five cats chewed on the device such that measurement was interrupted; as such, a leather cover was attached to the device (Fig. 1c). In another cat, the electrode came off the body surface such that recording became difficult, so we shaved the cat extensively and fixed the electrode with a fixative (Ortex, Alcare, Tokyo, Japan; Fig. 1d). After these modifications, continuous recordings using the P-Holter were able to be collected from all individuals for 6 days. The 24 hours analyzable time from the P-Holter and C-Holter was 99.90% [median; interquartile range (IQR): 99.85%–99.90%] and 99.90% (IQR: 99.80%–99.90%) of the total wear time (p=0.94). The 24 hours mean HR according to the P-Holter and C-Holter was 138.38 (128.84–153.13) bpm and 134.75 (125.53–151.62) bpm, respectively; these mean HRs did not differ across Holter types (p=0.67). In addition, the timing of the occurrences of arrhythmias appeared to be identical with the P-Holter and the C-Holter (Fig. 2). When the P-Holter was used for multiday recordings, the SDNN and RMSSD increased on days 4, 5, and 6 compared with day 2 (SDNN; p =0.02, p < 0.01, p < 0.01, respectively; RMSSD; p=0.02, p < 0.01, p < 0.01, respectively), whereas the HF increased on days 5 and 6 compared with day 2 (p=0.02, p=0.03, respectively; Table 1). Additionally, starting from day 5, pNN50 and LF were likely to increase, although this difference was not significant.
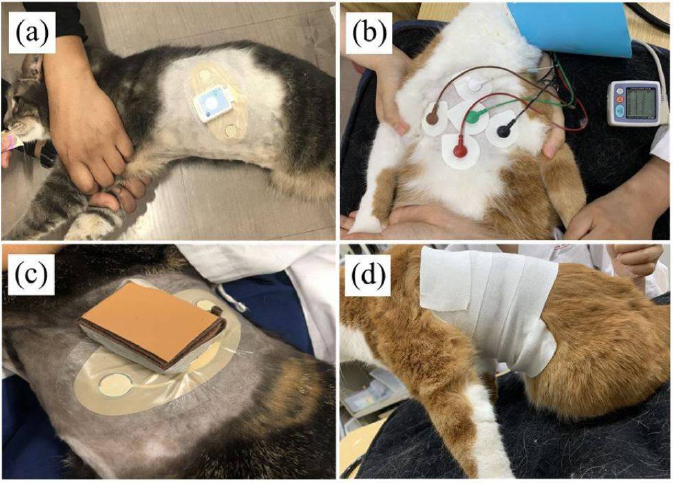
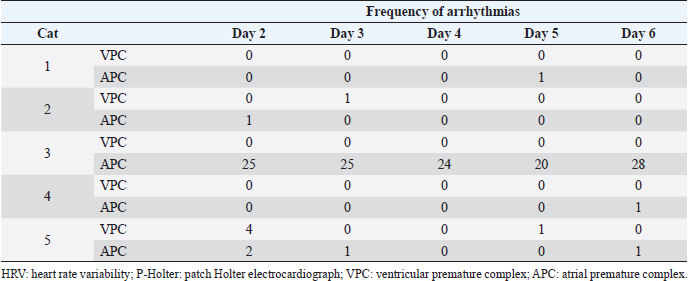
Fig. 1. Installation of the Holter electrocardiographs (a, b) and cats that needed adjustments after P-Holter installation (c, d). (a) Cat with P-Holter attached. The P-Holter was placed on the left side of the cat’s thorax, at the fifth intercostal space, slightly dorsal to the costochondral junction, and oriented diagonally at a 45° angle from vertical, parallel to the long axis of the heart. This installation provided a one-lead electrocardiograph. (b) Cat with C-Holter attached. Five electrodes were fixed to a shaved area, which provided a two-lead electrocardiograph. (c) One of the five cats chewed on the device such that measurement was interrupted, so a protective leather cover was attached to the device. (d) For one of the five cats, the electrode detached from the body surface and recording became difficult, so we shaved the cat extensively and used a fixative to affix the electrode. P-Holter: patch Holter electrocardiograph; C-Holter: conventional Holter electrocardiograph. When only the P-Holter was installed, the number of arrhythmia occurrences was similar on days 2–6. At least once during the P-Holter measurement period, all cats had arrhythmias, although this was not necessarily every day (Table 2). DiscussionIn this study, there was no difference in the analyzable time and HR measured using P-Holter and C-Holter. Additionally, the number of occurrences of arrhythmias was consistent across both Holter types. These results are similar to those reported in humans (Barrett et al., 2014; Cheung et al., 2014; Murali et al., 2020). In this study of cats, the analysis rate of the P-Holter was higher than that reported for dogs (>93.00%) (Lichtenberger et al., 2018), suggesting that the performance of the P-Holter is not inferior to that of the C-Holter and that it may be useful in cats. Incidentally, similar P-Holter types to previous human and canine studies were used in this study. Nonetheless, for two out of the five cats, device protection or extensive shaving and a fixative were required; these measures can be stressful. Therefore, although the P-Holter is as useful as the C-Holter in cats, it may be prone to individual differences in the cats’ adaptation to the device. Time-domain analysis uses the variation in RR intervals resulting from changes in sympathetic and vagal nerve activity (Ogawa et al., 2020). SDNN and RMSSD are indicators of the degree of variation in HRV, and pNN50 is an indicator of vagal nerve activity (Sayers, 1973). Unlike time-domain analysis, frequency-domain analysis can quantify sympathetic and vagal nerve activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). The HF is an index of vagal nerve activity and LF is an index of sympathetic and vagal nerve activity (Noda et al., 2019; Raue et al., 2019). In this study, sympathetic nerve activity was likely to decrease and vagal nerve activity was likely to increase after 4 days of measurement, compared to the second day of measurement. It is possible that the cats acclimatized to the P-Holter after 4 days, and their stress was reduced.
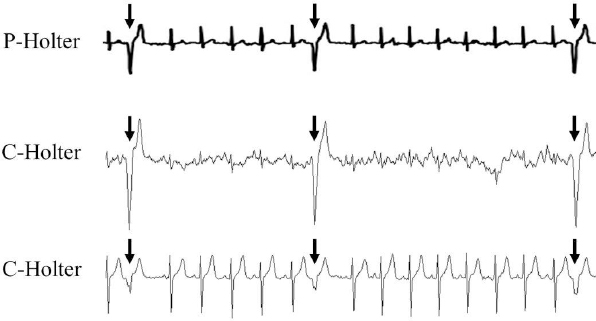
Fig. 2. Excerpt of an electrocardiograph recording. The timing of the occurrence of arrhythmias was consistent between the P-Holter and C-Holter devices. The pictured arrhythmias are ventricular premature complexes (arrows). P-Holter: patch Holter electrocardiograph; C-Holter: conventional Holter electrocardiograph. Table 1. HRV measurements from the P-Holter used for multiday recordings.
In this study, unlike previous studies, the frequency of arrhythmias varied, but the number of arrhythmias did not differ during the measurements (Pastor-Perez et al., 2010; Gunasekaran et al., 2020). Arrhythmias such as ventricular premature complex and atrial premature complex are commonly observed in healthy cats (Hanås et al., 2009). Therefore, we expected that multiday Holter electrocardiograph recordings would increase the incidence of arrhythmias and improve the accuracy of arrhythmia detection, compared to single day recordings. In the present study, if cats with cardiomyopathy or arrhythmias such as ventricular tachycardia had been included, then there may have been a higher incidence of arrhythmias in multiday compared with single-day Holter electrocardiograph recordings. This study has some limitations. First, the sample size was small. In this study, concerning animal welfare, a minimal number of cats that can be statistically analyzed were included. However, this study identified significant differences in some evaluation parameters even with the small number of cats. Secondly, clinically healthy cats were used in the present study rather than clinical cases. The reason is that there is a difference in the degree of autonomic nerve system activity and the breeding environment among them (Abbott, 2005). Since this study aimed to investigate whether multiday recordings with the P-Holter decrease sympathetic nerve activity, the husbandry condition, light/dark cycle, and feeding time should have been unified. Thirdly, all the cats included in this study were male. In humans, there is a sex difference in autonomic activity (von Holzen et al., 2016). Since this study compared changes in autonomic functioning on a daily basis rather than individual basis, we believe that individual differences in HRV at baseline would not have a significant impact on the results. Nonetheless, the HRV values from this study may differ slightly if females were incorporated. Table 2. Frequency of arrhythmias from the P-Holter used for multiday recordings.
In conclusion, the P-Holter is as useful as the C-Holter, but cats may have individual differences in their adaptation to the device in healthy cats. P-Holter recordings taken for more than 4–5 days may allow the cat to acclimate to the device and reduce sympathetic nerve activity. The accuracy of arrhythmia detection across multiday P-Holter recordings requires further investigation using clinical cases. This study included healthy cats for whom the Holter is not clinically indicated. However, the P-Holter may be indicated in cats in which syncope occurs intermittently rather than daily, or in which the C-Holter would reduce activity, given that multiple-day measurements were possible and cat activity was unaffected. AcknowledgmentsThe authors would like to thank the students of the Laboratory of Veterinary Internal Medicine II at Nippon Veterinary and Life Science University for supporting our study. In addition, the authors would also like to thank Enago (www.enago.jp) for the English language editing. Conflicts of interestThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Authors’ contributionsMizuki Ogawa: conception and design of the study, acquisition, analysis, interpretation of data, and drafting of the article. Saran Fatim Kaba: acquisition, analysis, and interpretation of data. Hirosumi Miyakawa: review of the article. Huai-hsun Hsu: review of the article. Yuichi Miyagawa: review of the article. Naoyuki Takemura: review of the article. FundingThe authors received no financial support for the research, authorship, and/or publication of this article. ReferencesAbbott, J.A. 2005. Heart rate and heart rate variability of healthy cats in home and hospital environments. J. Feline. Med. Surg. 7, 195–202. Barrett, P.M., Komatireddy, R., Haaser, S., Topol, S., Sheard, J., Encinas, J., Fought, A.J. and Topol, E.J. 2014. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 127, 95.e11–e17. Bolourchi, M. and Batra, A.S. 2015. Diagnostic yield of patch ambulatory electrocardiogram monitoring in children (from a national registry). Am. J. Cardiol. 115, 630–634. Cheung, C.C., Kerr, C.R. and Krahn, A.D. 2014. Comparing 14-day adhesive patch with 24-h Holter monitoring. Future. Cardiol. 10, 319–322. Gunasekaran, T., Olivier, N.B. and Sanders, R.A. 2020. Comparison of single- versus seven-day Holter analysis for the identification of dilated cardiomyopathy predictive criteria in apparently healthy Doberman Pinscher dogs. J. Vet. Cardiol. 27, 78–87. Hanås, S., Tidholm, A., Egenvall, A. and Holst, B.S. 2009. Twenty-four hour Holter monitoring of unsedated healthy cats in the home environment. J. Vet. Cardiol. 11, 17–22. Jackson, B. L., Lehmkuhl, L. B. and Adin, D. B. 2014. Heart rate and arrhythmia frequency of normal cats compared to cats with asymptomatic hypertrophic cardiomyopathy. J. Vet. Cardiol. 16, 215–225. Khor, K.H., Shiels, I.A., Campbell, F.E., Greer, R.M., Rose, A. and Mills, P.C. 2014. Evaluation of a technique to measure heart rate variability in anaesthetised cats. Vet. J. 199, 229–235. Lichtenberger, J., Meurs, K.M. and Côté, E. 2018. Preliminary assessment of a novel 14-day electrocardiographic adhesive patch monitor in dogs. J. Am. Anim. Hosp. Assoc. 54, 138–143. Murali, S., Brugger, N., Rincon, F., Mashru, M., Cook, S. and Goy, J.J. 2020. Cardiac ambulatory monitoring: new wireless device validated against conventional Holter monitoring in a case series. Front. Cardiovasc. Med. 7, 587945. Noda, A., Hayano, J., Ito, N., Miyata, S., Yasuma, F. and Yasuda, Y. 2019. Very low frequency component of heart rate variability as a marker for therapeutic efficacy in patients with obstructive sleep apnea: preliminary study. J. Res. Med. Sci. 24, 84. Ogawa, M., Ishizaka, M., Akabane, R., Sakatani, A., Nagakawa, M., Miyakawa, H., Miyagawa, Y. and Takemura, N. 2020. Evaluation of the autonomic nervous system in a canine model of chronic embolic pulmonary hypertension. Vet. Res. Commun. 44, 73–81. Ogawa, M., Kawamura, A., Akabane, R., Sakatani, A., Miyakawa, H., Hsu, H.H., Miyagawa, Y. and Takemura, N. 2022. Effects of ivabradine and atenolol on heart rate and heart rate variability in healthy cats over a 24 h period: A pilot study. Vet. Rec. Open. 9, e28. Pastor-Perez FJ, Fernández SM, Esteban RG, Pascual-Figal, D.A., Barquero-Pérez, O., Rojo-Alvarez, J.L., Martinez-Espejo, M.D.M., Chavarri, M.D. and García-Alberola, A. 2010. Comparison of detection of arrhythmias in patients with chronic heart failure secondary to non-ischemic versus ischemic cardiomyopathy by 1 versus 7-day Holter monitoring. Am. J. Cardiol. 106, 677–681. Raue, J.F., Tarvainen, M.P. and Kästner, S.B.R. 2019. Experimental study on the effects of isoflurane with and without remifentanil or dexmedetomidine on heart rate variability before and after nociceptive stimulation at different MAC multiples in cats. BMC. Vet. Res. 15, 258. Rienzo MD, Parati G, Castiglioni P, Omboni, S., Ferrari, A.U., Ramirez, A.J., Pedotti, A. and Mancia, G. 1991. Role of sinoaortic afferents in modulating BP and pulse-interval spectral characteristics in unanesthetized cats. Am. J. Physiol. 261, 1811–1818. Sayers, B.M. 1973. Analysis of heart rate variability. Ergonomics. 16, 17–32. Soares-Miranda, L., Sandercock, G., Vale, S., Santos, R., Abreu, S., Moreira, C. and Mota, J. 2012. Metabolic syndrome, physical activity and cardiac autonomic function. Diabetes. Metab. Res. Rev. 28, 363–369. Tarvainen, M.P., Niskanen, J.P., Lipponen, J.A., Ranta-Aho, P.O. and Karjalainen, P.A. 2014. Kubios HRV heart rate variability analysis software. Comput. Methods. Programs. Biomed. 113, 210–220. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. 1996. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 93, 1043–1065. Verma, S., Moiz, J.A., Answer, S., Alghadir, A.H. and Hussain, M.E. 2018. A doseresponse study of aerobic training for oxygen uptake, oxidative stress and cardiac autonomic function in type 2 diabetes mellitus: study protocol for a randomized controlled trial. Trials. 19, 289. von Holzen, J.J., Capaldo, G., Wilhelm, M. and Stute, P. 2016. Impact of endo- and exogenous estrogens on heart rate variability in women: a review. Climacteric. 19, 222–228. Willis, R., Oliveira, P. and Mavropoulou, A. 2018. Guide to canine and feline electrocardiography. Wiley-Blackwell, pp: 301–330. | ||
| How to Cite this Article |
| Pubmed Style Ogawa M, Kaba SF, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. A pilot study of patch Holter electrocardiograph recordings in healthy cats. Open Vet. J.. 2022; 12(4): 489-494. doi:10.5455/OVJ.2022.v12.i4.10 Web Style Ogawa M, Kaba SF, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. A pilot study of patch Holter electrocardiograph recordings in healthy cats. https://www.openveterinaryjournal.com/?mno=90065 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i4.10 AMA (American Medical Association) Style Ogawa M, Kaba SF, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. A pilot study of patch Holter electrocardiograph recordings in healthy cats. Open Vet. J.. 2022; 12(4): 489-494. doi:10.5455/OVJ.2022.v12.i4.10 Vancouver/ICMJE Style Ogawa M, Kaba SF, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. A pilot study of patch Holter electrocardiograph recordings in healthy cats. Open Vet. J.. (2022), [cited January 25, 2026]; 12(4): 489-494. doi:10.5455/OVJ.2022.v12.i4.10 Harvard Style Ogawa, M., Kaba, . S. F., Miyakawa, . H., Hsu, . H., Miyagawa, . Y. & Takemura, . N. (2022) A pilot study of patch Holter electrocardiograph recordings in healthy cats. Open Vet. J., 12 (4), 489-494. doi:10.5455/OVJ.2022.v12.i4.10 Turabian Style Ogawa, Mizuki, Saran Fatim Kaba, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. 2022. A pilot study of patch Holter electrocardiograph recordings in healthy cats. Open Veterinary Journal, 12 (4), 489-494. doi:10.5455/OVJ.2022.v12.i4.10 Chicago Style Ogawa, Mizuki, Saran Fatim Kaba, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. "A pilot study of patch Holter electrocardiograph recordings in healthy cats." Open Veterinary Journal 12 (2022), 489-494. doi:10.5455/OVJ.2022.v12.i4.10 MLA (The Modern Language Association) Style Ogawa, Mizuki, Saran Fatim Kaba, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. "A pilot study of patch Holter electrocardiograph recordings in healthy cats." Open Veterinary Journal 12.4 (2022), 489-494. Print. doi:10.5455/OVJ.2022.v12.i4.10 APA (American Psychological Association) Style Ogawa, M., Kaba, . S. F., Miyakawa, . H., Hsu, . H., Miyagawa, . Y. & Takemura, . N. (2022) A pilot study of patch Holter electrocardiograph recordings in healthy cats. Open Veterinary Journal, 12 (4), 489-494. doi:10.5455/OVJ.2022.v12.i4.10 |