| Original Article | ||
Open Vet. J.. 2023; 13(4): 451-465 Open Veterinary Journal, (2023), Vol. 13(4): 451–465 Original Research Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickensEssam S. Soliman1*, Rania A. Hassan2, and Doaa S. Farid31Department of Veterinary Public Health, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 2Animal Production Division, Department of Animal Wealth Development, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 3Department of Environmental Protection, Faculty of Environmental Agricultural Sciences, Arish University, El-Arish, Egypt *Corresponding Author: Essam S. Soliman. Department of Veterinary Public Health, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. Email: soliman.essam [at] vet.suez.edu.eg Submitted: 10/12/2022 Accepted: 19/03/2023 Published: 15/04/2023 © 2023 Open Veterinary Journal
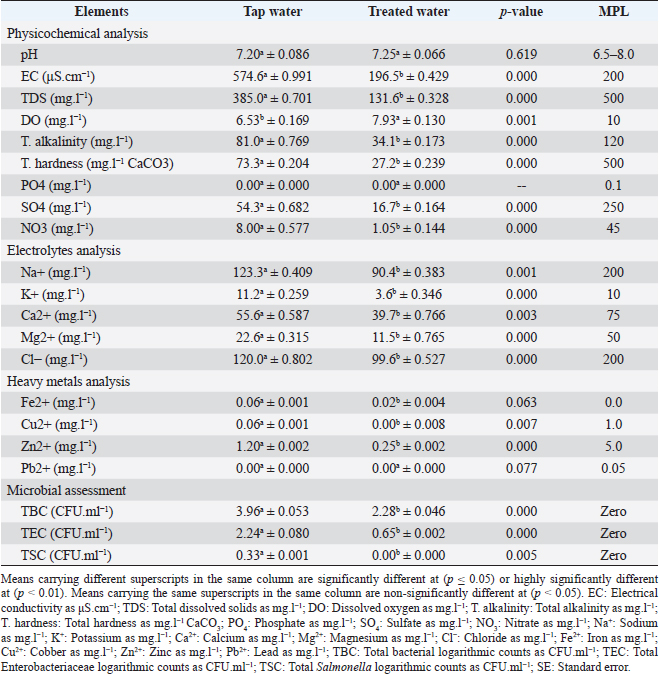
AbstractBackground: The implementation of green technologies is continually gaining attention worldwide and was considered for removing water pollutants and treating municipal water before its disposal. Aim: Evaluation of the laboratory antimicrobial actions and chelating activities, and the field influence of Eichhornia crassipes on performance, biochemical and immunoglobulin concentrations, and intestinal microbiota in overwhelmed broiler chickens. Methods: We assessed the laboratory antimicrobial actions of E. crassipes 1% suspension against bacterial (Escherichia coli O157: H7 and Salmonella Typhimurium) and fungal (Aspergillus niger and Candida albicans) microorganisms using a 96-well minimal inhibitory concentration, and the chelating activities of E. crassipes against calcium sulfate and copper sulfate. Also, we designed randomly four equal groups out of 200 1–day-old Ross® 308 chicks on a deep litter system. Three groups (G1, G2, and G3) were supplied daily with E. crassipes suspension of 1% from the third day until the end of the experiment, while the fourth group (G4) received non-treated tap water. Broilers of G1–3 were challenged with calcium sulfate (75 mg.l-1), copper sulfate (200 mg.l-1), and S. Typhimurium (1.5 × 106 CFU.ml-1) polluted water respectively on the 7th, 14th, 21st, 28th, 35th days of age. We collected 1,914 samples by the end of the study, these samples included 90 E. crassipes pollutants and 480 E. crassipes microbial mixes, 192 sera, 192 intestinal swabs, and 960 tissues. Results: Eichhornia crassipes treated water reveals highly significant (p < 0.01) improvements in water quality assessments and a highly significant (p < 0.01) increase in dissolved oxygen levels compared to tap water. Eichhornia crassipes 1% achieved a 100% adsorption capability for calcium and copper sulfate after 1-hour and 100% bactericidal (E. coli O157: H7 and S. Typhimurium) and fungicidal (A. niger and C. albicans) actions after 1, 2, 2, and 2-hours, respectively. Broilers treated with 1% E. crassipes revealed highly significant (p < 0.01) improvements in performance indices, carcasses characteristics, biochemical and immunological parameters, and highly significant (p < 0.01) decreases of cortisol hormone and bacteriological parameters in all treated broiler groups compared to the control. Conclusion: Eichhornia crassipes 1% reveals a significant improvement in drinking water quality, as well as produces high adsorptive and antimicrobial actions. Eichhornia crassipes 1% improved performance traits, carcass quality, and intestinal microbiota in overwhelmed broilers. Keywords: Antimicrobial, Broiler chickens, Eichhornia crassipes, Immunity, Performance. IntroductionWater pollution is a major worldwide reproducible problem with urbanization and industrial growth. Water quality has been used to point out the physicochemical characteristics to meet suitability for various uses (Li and Qian, 2018a). Water quality is influenced by numerous factors including source geology, meteorological conditions, topography, seasonal variations, and biological considerations (Li et al., 2017a). Clean and palatable water with freedom from disease-producing agents has been the main demand for livestock production (Tian and Wu, 2019). Water is negatively affected by populations' activities and becomes polluted with chemicals and infectious/zoonotic pathogens including bacteria (Soliman et al., 2009a) such as Escherichia coli, Salmonella, Leptospira, and Listeria, protozoa (Soliman et al., 2009b) such as Giardia and Eimeria, and parasitic agents such as Echinococcus, Schistosoma, and Faschiola. Biosecurity pillars have included water quality to provide birds with excellent/satisfactory water (Su et al., 2019). Water purification has been performed using various methods such as photocatalysis (Raval et al., 2016), biochar (Palansooriya et al., 2020), sludge materials (Anastopoulos et al., 2017), ion exchange (Szczepanik, 2017), and eco-friendly byproducts as clay (Soliman et al., 2021d) and Oreochromis niloticus bone (Soliman et al., 2022). Developing eco-friendly, economical, and less destructive alternatives (Phytoremediation) for the handling of water pollutants is a worldwide interest (Shah, 2010). These eco-friendly means must possess higher absorption capabilities in short contact time and accumulation actions for longer periods (Mudgal et al., 2010). Aquatic plants such as Eichhornia crassipes are characterized by high invasiveness, wide spreading (tropical and sub-tropical countries), floating freely, and can grow up to 1 m above the water's surface (Pellegrini et al., 2018). It is known as a fast-growing runner plant and its seeds can remain viable for 28 years (Sarika et al., 2014). Eichhornia crassipes usually survive in an ambient temperature of 25°C–30°C (12°C: 35°C), pH of 5.0: 7.5, and salinity of low than 15%, which above contributes to chlorosis and death (Kong et al., 2014). It has been recognized as an ecological plague in Egypt from 1879 to 1892 and contributed to biomass suffocation, diminishing fish reservoirs, and negatively influencing the economy (Twongo, 2019). Eichhornia crassipes has high absorbing power and thus has been used in wastewater purification. Eichhornia crassipes have been used in numerous fields such as braiding material and fiber source in India, Thailand, and Vietnam, biogas production, sludge field, adsorbent (Abdel Shafy et al., 2016), bio-cleaning (Ansari et al., 2014), food source, small scale paper production, carotene-based table vegetable, dermatological medicinal applications in horses, sustainable source of oxygen, and bio-herbicidal agent through the production of hydrogen peroxide and inhibition of the soluble peroxidase activities (Chai et al., 2013). Here, in this study, we evaluated the laboratory antimicrobial actions of the E. crassipes 1% suspension against bacterial (E. coli O1527: H7 1.7 × 1011 CFU.ml-1 and Salmonella Typhimurium 1.5 × 106 CFU.ml-1) and mycotic (Aspergillus niger 2.5 × 108 CFU.ml-1 and Candida albicans 2.5 × 108 CFU. ml-1) microorganisms, and the chelating activity against inorganic (calcium sulfate, 75 mg.l-1) and heavy metals (Copper sulfate, 200 mg.l-1) water pollutants. We also evaluated the field influence of the E. crassipes 1% suspension on growth traits, some biochemical parameters, immunoglobulin concentrations, some antioxidant enzymatic activity, cortisol hormone levels, and intestinal microbiota in broiler chickens challenged once weekly with polluted/contaminated (calcium sulfate; 75 mg.l-1, copper sulfate; 200 mg.l-1, and S. Typhimurium; 1.5 × 106 CFU.ml-1) water. Materials and MethodsStudy location and durationThe laboratory (antimicrobial and chelating activities) trials were run in the Veterinary Public Health Department Laboratory from June to July 2022 and the field experiment was run in the broilers' units at Suez Canal University from August to September 2022. Water quality, production traits, carcass quality, and biochemical and bacteriological examinations were run in the Veterinary Public Health Department Laboratory. Water electrolytes, antioxidant enzymes, and cortisol and immunoglobulin concentrations were run in the University Hospital Laboratories. Heavy metal analysis was run in the Toxicology Laboratory at Suez Canal University. Eichhornia crassipes preparationThe procedures were run after Sarkar et al. (2017) with some modifications. Eichhornia crassipes leaves were obtained from the Ismailia canal in polyethylene bags. The leaves were washed with de-ionized water four to five times, cut into pieces, sun-dried for 24–72 hours, and minced in a mortar (Granite-Rock). The minced powder was extra-dried in a hot air oven (Daihan®, Indonesia) at 80°C/24–48 hours. The powder was stored in a dark bottle. Pollutant suspensions preparationInorganic calcium sulfate dihydrate (Spectrum®, United States) and organic copper sulfate (HiMedia®, India) powders were purchased. Calcium sulfate was dissolved at a rate of 75 mg.l-1 in distilled water (Total hardness=2,100 mg.l-1 CaCO3; MPL < 75 mg.l-1 and calcium hardness=1,600 mg.l-1 CaCO3; MPL < 60 mg.l-1). Copper sulfate was dissolved at a rate of 200 mg.l-1 in distilled water (Copper=5 mg.l-1; MPL=1.3 mg.l-1). Propagation of bacterial and fungal isolatesThe bacterial isolates were propagated after Soliman et al. (2021b, 2021c). Escherichia coli O157: H7 suspension (2.0 × 104 CFU.ml-1) was propagated into Mac-Conkey broth (Oxoid™) at 44°C/24 hours, eosin methylene blue agar (OxoidTM) at 37°C/24 hours, and tryptone soya broth (Oxoid™) providing 1.7 × 1011 CFU. ml-1 suspension. Lyophilized S. Typhimurium (3.4 × 102 CFU) was propagated into Rappaport-Vassiliadis broth (Oxoid™) at 37°C/24 hours, xylose lysine deoxycholate agar (Oxoid™) at 37°C/24 hours, and tryptone soya broth providing 1.5 × 106 CFU.ml-1 suspension. The fungal isolates were propagated following Soliman et al. (2021b). Aspergillus niger and C. albicans clinical isolates were propagated into sabouraud dextrose broth (SDB; HIMEDIA®) at 37°C/24–72 hours, sabouraud dextrose agar (SDA; Oxoid™) at 37°C/24 hours, identified with lactophenol cotton blue stain (Hardy Diagnostics®), and resuspended in SDB broth, providing 2.5 × 108 CFU.ml-1 suspensions. In-vitro antimicrobial activities of E. crassipesA 96-well minimal inhibitory concentration technique was run to evaluate the antimicrobial efficacy of E. crassipes 1% on E. coli O157: H7 (1.7 × 1011 CFU. ml-1), S. Typhimurium (1.5 × 106 CFU.ml-1), A. niger (2.5 × 108 CFU. ml-1), and C. albicans (2.5 × 108 CFU. ml-1) after Elshikh et al. (2016) with a few modifications. The 96 wells were inoculated with 100 µl of E. crassipes 1% suspension. Wells were inoculated with 10 µl from microbial suspensions as follows: E. coli O157: H7 into C1–3 (n =24 wells), S. Typhimurium into C4–6 (n =24 wells), A. niger into C7–9 (n=24 wells), and C. albicans into C10–12 (n =24 wells). After contact times (0.25, 0.5, 1.0, 2.0, and 4.0 hours), 10 µl of the mixes were added into 10 ml of buffered peptone water (Oxoid™). The tubes were mixed at 37°C/2 hours (Elshikh et al., 2016). 10 µl were dropped onto eosin methylene blue, XLD, and SDA agar at 37°C/24 hours. The specific colonies were counted (Quebec Darkfield Colony Counter). The antimicrobial efficacies (%) of E. crassipes 1% were calculated by proportionating the surviving colonies to the initial counts as follows: In-vitro chelating activities of E. crassipes1 ml of E. crassipes 1% suspension (1 g.l-1) was dispensed into each of three triplets of conical flasks containing either 1 l of calcium sulfate or copper sulfate. The mixes were harvested after contact times (0.25, 0.5, 1.0, 2.0, and 4.0 hours) for determining total and calcium hardness levels and copper concentrations. Total hardness levels were quantified using the titrimetric method against ethylene diamine tetra-acetic acid; EDTA (0.01 mol.l-1) with aerochrom black-T indicator (American Public Health Association, 2012), calcium hardness levels were quantified using titrimetric method against EDTA with murexide powder indicator (American Public Health Association, 2012), and copper concentrations were quantified using atomic absorption spectrophotometer (American Public Health Association, 2012). The chelating efficacies (%) of E. crassipes 1% were calculated by proportionating the final to the initial concentrations. In-vivo effectiveness of E. crassipesExperimental birds’ housing and biosecurityWe used 200 1-day-old Ross®308 broiler chicks from El-Helal Company—Egypt. We weighed the chicks on their arrival (W0) and randomly divided them into 4 groups (50 birds each, 5 replicates of 10 birds) into units with a hay-deep litter system after Soliman and Hassan (2020b). The units were optimized with biosecurity measures following Soliman and Abdallah (2020a). These measures were foot dips of crude carbolic acid 5%, fly-proof nets, wild bird-proof entrances, secured food storage areas, proper interior arrangements to facilitate daily observation; feeding; and watering act, wire baited traps to discourage rodents, dry cleaning using brooms, wet cleaning using glutaraldehyde, disinfection using povidone-iodine 7.5%; sodium hydroxide 5%; and formaldehyde spraying respectively. The units were supplied after Soliman and Hassan (2019) with a continuous lighting program (23 hours L and 1 hour D) using a white light-emitting diode of 20 W. The ventilation act was served by negative cross-ventilation using side-wall V-shaped windows (air inlets), ceiling fans for proper air distribution, and side-wall suction fans across the rooms (air outlets). The units' floors were covered with a thin film of a chemical litter treatment (superphosphate) at a rate of 0.5 g.m-2 after Soliman et al. (2018) to maintain litter abiotic conditions. Experimental birds' microclimate and managementThe room's microclimatic temperature was optimized at 35°C by turning on indirect heat sources (halogen and oil heaters) following Soliman et al. (2021a). This temperature was sufficient for brooding. Starting from the eighth day of the rearing trial, the temperature was reduced by 0.5°C daily (3.5°C/week) until achieving 25°C–28°C/third week of the trial. Chicks were given ad libitum access to the experimented treated or non-treated drinking water. The dietary requirements of the broilers were met by corn-soybean ration (El-Eman company, Egypt) following the National Research Council (1994), and Applegate and Angel (2014) modifications. The nutritive ingredients of the used starter (1–14 days) and the grower (15–38 days) rations were protein (22.5% and 21%), energy (2,930 and 3,150 kcal), crude fiber (3.79% and 3.35%), and fat (5.4% and 2.67%), respectively. Broilers were massively immunized via dechlorinated drinking water vaccination act that was served after 2–4 hours of water deprivation. Birds were vaccinated against infectious bronchitis (7th day), infectious bursal disease (14th and 21st days), and Newcastle virus disease (16th and 26th days). Close observation was followed for the early detection of any abnormalities and mortalities. The experiment was designed to last for 38 days during which the daily prevailing microclimatic conditions were recorded. Eichhornia crassipes treated water and challenges actBroilers were supplied daily with E. crassipes (1 g.l-1) treated water for 4–6 hours, starting from the third-day-old throughout the experiment (38 days). Meanwhile, polluted water was supplied to groups as follows: G1 with calcium sulfate (75 mg.l-1) polluted water, G2 with copper sulfate (200 mg.l-1) polluted water, G3 with S. Typhimurium (1.5 × 106 CFU.ml-1) contaminated water, and G4 with non-polluted tap water (control negative). Polluted/contaminated water was supplied to birds weekly on the 7th, 14th, 21st, 28th, and 35th days. The repetition of the challenge was designed to evaluate the effectiveness of the E. crassipes to neutralize the impact of overwhelming challenges in broiler farms. On the challenge day, water deprivation was run for 2–4 hours to ensure the weekly challenge act, and the E. crassipes treated water was supplied following the challenge. Performance indices (PIs)Representative simple random samples were harvested from broilers' groups (44 birds/ group) on their arrival and weekly for weighing the live body weight (LBW.g-1) with a digital scale (WONHENG®, China). We calculated the sample size at a 95% confidence level following Thrusfield and Christley (2018) as follows: n=(Z1- α/2)2.p (1-p)/d2 where (n) is the samples' number, (Z1-α/2) is the normal standard, (p) is the probable population proportions, and (d) is the absolute precision. The PIs were calculated following Soliman and Hassan (2017). The weight gains (WG.g-1) are the differences between week-ending weights (Wx) to initial weights (W0). Feed (FI.g-1) and water (WI.ml-1) intakes are the proportions between the amounts consumed in a group to the number of surviving birds in such a group. The feed conversion ratios (FCR) are the proportions of FI.g-1 to WG.g-1, while the PI is the proportion of LBW.kg-1 to FCR. SamplingWe collected 1,914 samples divided into 570 including 90 E. crassipes pollutants mixes (3 replicates × triplets × 2 pollutants × 5 sampling times) and 480 E. crassipes microbial mixes (96 well × 5 sampling times) from the in-vitro study, and 1,344 including 192 sera, 192 duodenal swabs, and 960 tissues (breast muscles, liver, spleen, heart, and bursa of Fabricius) from 5 bird groups after the in-vivo study. Nine ml buffered peptone water (Oxoid™) were used as a vehicle for the duodenal swabs. We sacrificed 192 broilers (48/group) by the end of the clinical trial (38th day) via slaughtering with decapitation for harvesting blood samples on serum tubes (BD Vacutainer®). Samples were warmed in a water bath (Thermo®, Germany) at 28°C/20 minutes and centrifuged (Fisher® CL10) at 3,500 rpm/15 minute. Sera were pipetted (Thermo Scientific™) into Eppendorfs (2.5 ml) and stored at -20°C (Soliman et al., 2017). The carcasses were weighed (CW.g-1) after de-feathering and evisceration. Breast muscles were incised for the bacteriological assessment. Edible organs (liver and heart) and immune organs (spleen, and bursa of Fabricius) were harvested and weighed (g). Carcasses were disposed of by burial with lime. Biochemical profile and immunoglobulin concentrationsWe analyzed sera (n =192) calorimetrically using ROCHE® COBAS Integra 400 Plus chemical analyzer for some biochemical parameters [total protein (TP); g. dl-1, alanine aminotransferase (ALT); IU.l-1, urea; mg.dl-1, creatinine (CREAT); mg.dl-1, Glucose (GLUCO); mg.dl-1, total cholesterol (TC); mg.dl-1, and triglycerides (TGs); mg.dl-1] and some antioxidant enzymatic activity [total antioxidant capacity (TAC); mM.ml-1, malondialdehyde (MDA); nmol.ml-1, and superoxide dismutase (SOD); U.ml-1]. We analyzed sera also using ROCHE® ELECSYS 1010 Immunoassay Analyzer for hormonal and immunological profiles (Cortisol hormone; mcg.dl-1 and immunoglobulin G and M; mg.dl-1). Bacteriological examinationsFrozen breast muscle samples (n=192) were thawed, smashed in the stomacher (Seward®, UK), and added to 9 ml of buffered peptone water. Breast muscles (n=192) and duodenal swabs (n=192) solutions were diluted up to (10-8) after American Public Health Association (2017) to cover the contamination range. We analyzed the dilutions for aerobic total bacterial (TBC) onto standard plate count agar (Oxoid™), Enterobacteriaceae (TEC) onto eosin methylene blue agar, and Salmonella counts (TSC) onto CHROMagarTM Salmonella (BD BBLei) using the drop plate (Kim and Lee, 2016) at 37°C/24–48 hours. Ideal colonies were counted on the Dark-field colony counter (Murray et al., 2015). Statistical analysisWe analyzed the data using the Statistical Package for Social Sciences (SPSS version 27.0, IBM Corp, NY, USA) software package (SPSS, 2020). The multifactorial two-way analysis of variance was employed to determine the overall influence of the variables (water pollutants and broiler's age) and their interactions. The statistical model is as follows: Yijk=µ + αi + βj + (αβ)ij + Ɛijk where Yijk is the final measurement of the dependent variables; µ is the overall mean; αi is the fixed effect of the pollutants, βj is the fixed effect of the broiler's age, (αβ)ij is the interactions, and Ɛijk is the random error. We expressed microbial counts as logarithms (Log) using Microsoft Excel 2016. We expressed the results as highly significant at (p < 0.01), significant at (p ≤ 0.05), and non-significant at (p > 0.05). Ethical approvalThe laboratory and field clinical trials' designs and materials were approved by the Scientific Research Ethics Committee, Faculty of Veterinary Medicine, Suez Canal University–Ismailia, Egypt with approval number (2022025). Broilers were housed and approached in comfortable microclimatic conditions as much as possible to prevent the development of any overwhelming challenges other than the induced (Polluted and/or contaminated water). We minimized the number of birds as an understudy as possible and followed the laboratory animal rights (3Rs). ResultsWater analysisEichhornia crassipes treated water in Table 1 revealed highly significant (p < 0.01) decreases in physicochemical such as electrical conductivity (df=17, F=442.2, and p=0.000), total dissolved solids (df=17, F=442.24, and p =0.000), total alkalinity (df=17, F=66.9, and p=0.000), sulfates (df=17, F=164.9, and p=0.000), nitrates (df=17, F=136.1, and p=0.000), and total hardness (df=17, F=138.4, and p=0.000), electrolyte such as sodium (df=17, F=17.9, and p =0.001), potassium (df=17, F=306.4, and p =0.000), calcium (df=17, F=12.0, and p =0.003), magnesium (df=17, F=20.7, and p=0.000), and chloride (df=17, F=42.8, and p=0.000), heavy metal such as iron (df=17, F=4.0, and p =0.063), copper (df=17, F=9.3, and p=0.007), and zinc (df=17, F=30.2, and p=0.000), and microbial assessments such as aerobic TBC (df=17, F=552.3, and p=0.000), TEC (df=17, F=110.5, and p=0.000), and TSC (df=17, F=10.8, and p=0.005) compared to tap water. Eichhornia crassipes treated water also revealed highly significant (p < 0.01) increases in the dissolved oxygen levels (df=17, F=43.0, and p =0.000). pH levels reveal no-significant differences (df=17, F=0.258, and p=0.619) in E. crassipes treated and tap water. Table 1. Water analysis (Mean ± SE) in tap and E. crassipes treated water.
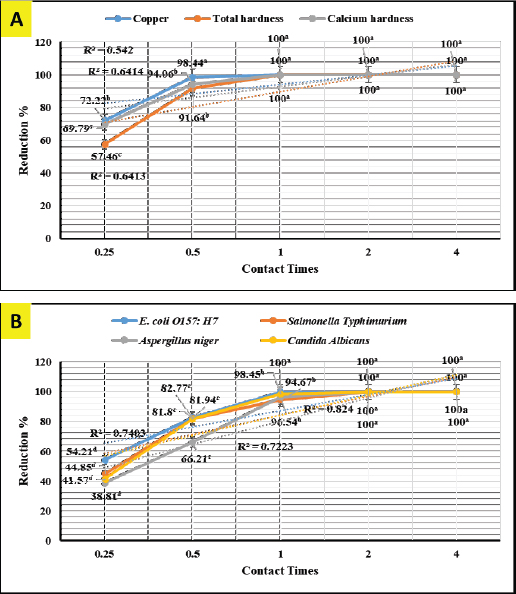
In-vitro adsorption and antimicrobial efficaciesEichhornia crassipes 1% suspension reveals in Figure 1A 100% adsorption (chelating) activity (p < 0.01) for copper (Cu+2, df=44, F=444.8, and p=0.000) and total (df=44, F=6,124.5, and p=0.000) and calcium (df=44, F=5,549.7, and p=0.000) hardness after 1-hour. Eichhornia crassipes 1% suspension also reveals in Figure 1B 100% killing (antimicrobial) efficacy (p < 0.01) against E. coli O157: H7 (df=119, F=13,843.9, and p =0.000), S. Typhimurium (df=119, F=2,595.2, and p =0.000), A. niger (df=119, F=7,738.1, and p=0.000), and C. albicans (df=119, F=8,773.4, and p=0.000) after 1, 2, 2, and 2-hour respectively.
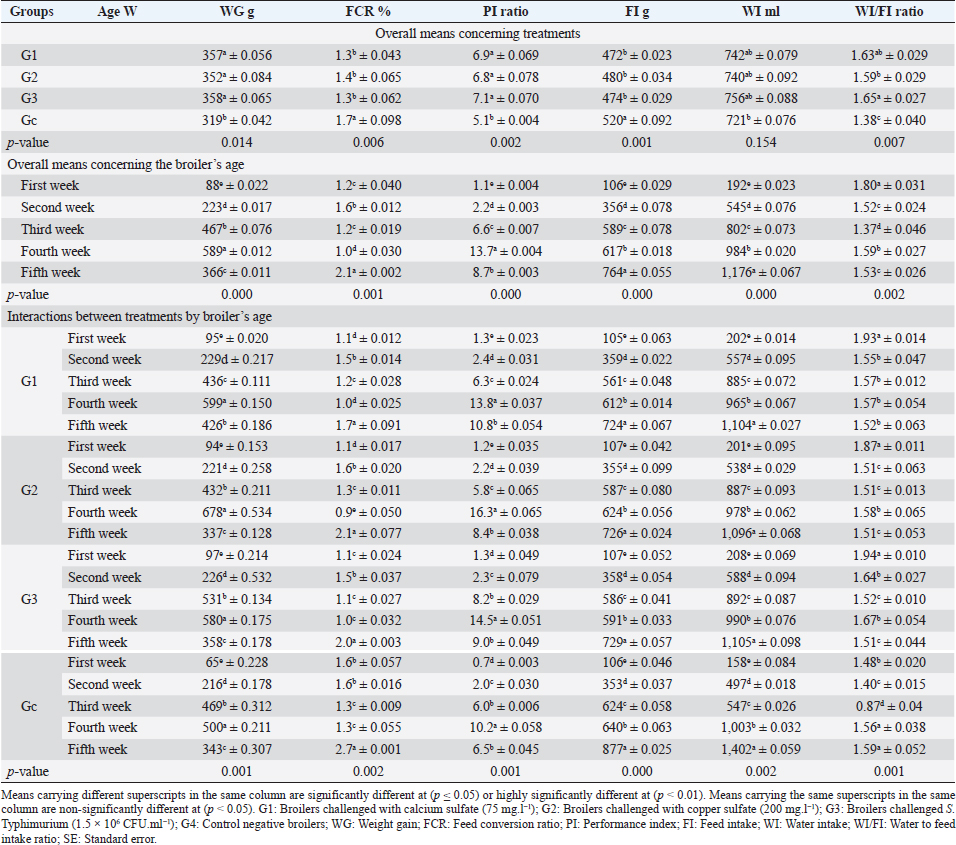
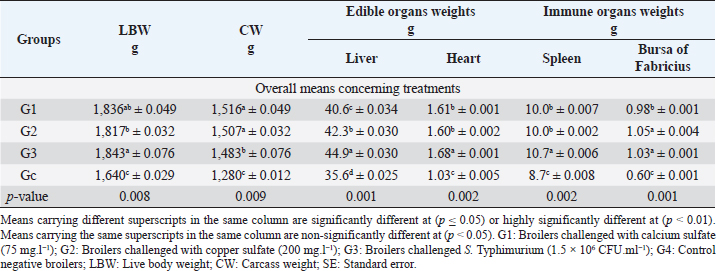
Fig. 1. In-vitro adsorption and antimicrobial efficacy (Reduction percentage Mean ± SE) of E. crassipes 1% suspension. (A) Chemical adsorption action. Initial levels of total hardness=2,100 mg.l-1 CaCO3, calcium hardness=1,600 mg.l-1 CaCO3, and copper=5 mg/l. (B) Microbial survival. Initial levels of E. coli O157: H7=1.7 × 1011 CFU.ml-1, Salmonella Typhimurium=1.5 × 106 CFU.ml-1, A. niger =2.5 × 108 CFU.ml-1, and C. albicans =2.5 × 108 CFU.ml-1 Growth traitsEichhornia crassipes 1% treated water reveals in Table 2 highly significant (p < 0.01) improvements of WG (df=3, F=11.1, and p=0.000), PI (df=3, F=35.8, and p=0.000), WI interactions (df=12, F=22.8, and p =0.000), WI/FI ratio (df=3, F=48.9, and p=0.000), FCR (df=3, F=24.5, and p =0.000), and FI (df=3, F=47.1, and p=0.000) in all treated broiler groups compared to the control. The overall means concerning age reveal in Table 2 highly significant (p < 0.01) improvements of WG (df=4, F=987.3, and p =0.000), FCR (df=4, F=120.6, and p =0.000), and PI (df=4, F=843.2, and p=0.000) in the fourth week, an d WI (df=4, F=1,021.8, and p=0.000) in the second week, as well as, highly significant (p < 0.01) decreases in FI (df=4, F=4,914.4, and p =0.000) in the second week. Live birds’ weights and carcasses traitsEichhornia crassipes 1% treated water reveals in Table 3 highly significant (p < 0.01) increases in LBW (df=3, F=191.1, and p=0.000) in G3 (broilers challenged with S. Typhimurium) compared to other challenged groups and the control. Carcass weights (CW, Table 3) reveal highly significant (p < 0.01) increases (df=3, F=256.2, and p=0.000) in G1 (broilers challenged with calcium sulfate) and G2 (broilers challenged with copper sulfate) with no significant differences between the two groups. Liver (df=3, F=126.0, and p=0.000), heart (df=3, F=89.8, and p=0.000), and spleen (df=3, F=355.3, and p=0.000) weights reveal highly significant (p < 0.01) increases in G3 compared to all other groups (Table 3). Bursa's weights (Table 3) reveal highly significant (p < 0.01) increases (df=3, F=348.0, and p=0.000) in G3 and G2 with no significant differences between the two groups. Table 2. PIs (Mean ± SE) in different groups supplied with E. crassipes treated water (1 g.l-1).
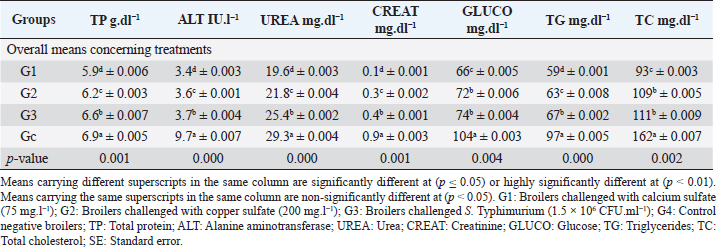
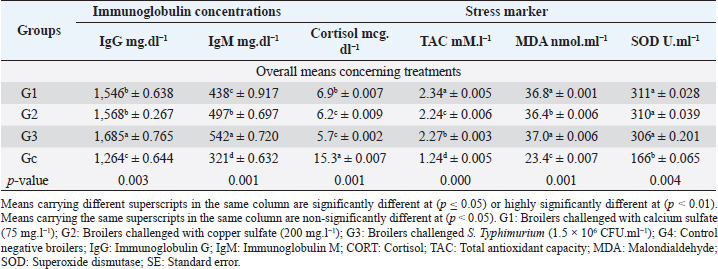
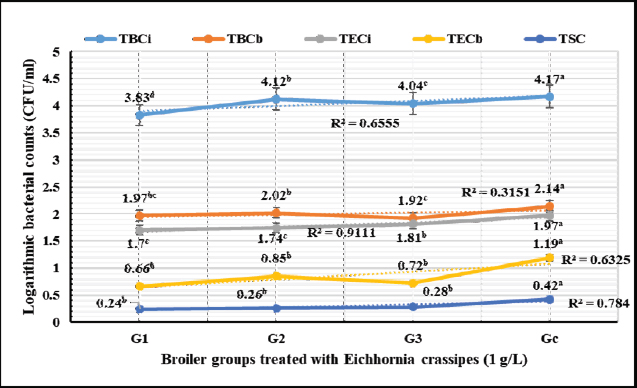
Biochemical, immunological, and hormonal profilesEichhornia crassipes 1% treated water reveals highly significant (p < 0.01, Table 4) improvements of the measured biochemical such as TP (df=3, F=60.6, and p=0.000), ALT (df=3, F=4,329.5, and p =0.000), urea (df=3, F=142.4, and p =0.000), CREAT (df=3, F=762.1, and p=0.000), GLUCO (df=3, F=1,197.9, and p=0.000), TG (df=3, F=17,063.2, and p=0.000), and TC (df=3, F=557.1, and p=0.000), and antioxidant parameters such as TAC (df=3, F=4,491.8, and p=0.000), MDA (df=3, F=1,625.8, and p=0.000), and SOD (df=3, F=640.0, and p =0.000) in all treated broiler groups compared to the control. We reported that E. crassipes 1% treated water reveals in Table 5 highly significant (p < 0.01, Table 5) increases in immunoglobulins G (df=3, F=112.5, and p=0.000) and M (df=3, F=36.3, and p=0.000) of G3 compared to the other treated groups and the control. Meanwhile, the cortisol hormone (Table 5) reveals highly significant (p < 0.01) decreases (df=3, F=358.0, and p=0.000) in all treated broiler groups compared to the control. Bacteriological assessmentsEichhornia crassipes 1% treated water in Figure 2 reveals highly significant (p < 0.01) decreases in the TBC of intestinal swabs (df=3, F=29.9, and p =0.000) and breast muscles (df=3, F=13.2, and p =0.000), TEC of intestinal swabs (df=3, F=24.0, and p=0.000) and breast muscles (df=3, F=7.6, and p=0.000), and TSC (duodenal swabs) (df=3, F=5.0, and p=0.002) in all treated broiler groups compared to the control. DiscussionWater quality in broiler farms contributes to the general conditions of production, performance, and reproduction (Maharjan et al., 2016). Broilers hold an important position among food animals, thus requiring a healthy environment and water of high quality to contribute to good health and production (Magor et al., 2013). Drinking water in broiler farms can be easily polluted/contaminated with a variety of micro-organisms through the birds themselves or the surrounding sources contributing to a potential risk to the broilers and humans (Van der et al., 2016). According to Maes et al. (2019), the arriving pollutants concerning some micro-organisms such as Stenotrophomonas maltophilia, Pseudomonas geniculata, and Pseudomonas aeruginosa contribute to a lowering in the water quality and characterization. Table 3. Live, carcass, and organ weights (Mean ± SE) in different groups supplied with E. crassipes (1 g.l-1) treated water.
Table 4. Biochemical profile (Mean ± SE) in different groups supplied with E. crassipes (1 g.l-1) treated water.
Table 5. Immunoglobulin concentrations and stress markers (Mean ± SE) in different groups supplied with E. crassipes (1 g.l-1) treated water.
Conventional water treatments (coagulation, sedimentation, flocculation, membrane filter, irradiation, and adsorption) influence the water's physicochemical characteristics as recorded by Robinson et al. (2001) to achieve water of good quality at a high cost, and the treatment process wastes require special treatment or disposal and thus increase the cost. On the other hand, Mishra and Maiti (2017) revealed that the adsorption and phytoremediation processes are waste-free and provide higher advantageous water conditions compared to other treatments as they use natural and biological products such as rice husk, bark, coal, wood dust, tree bark powder, lignin, seaweeds, orange peel, banana peel, coconut pulp, and E. crassipes. Eichhornia crassipes is advantageous by higher adsorption capabilities in shorter time and accumulation (leaves, roots, and bulb) for long periods against chemical and biological pollutants (Thapa et al., 2016), and can be used in biogas production, feed product for fish and animals, microbial metabolism because of its high carbon contents, vermicomposting field, and in medicinal uses (Sharma et al., 2016).
Fig. 2. Logarithmic bacterial counts (Mean) in different groups supplied with E. crassipes (1 g.l-1) treated water. G1=Broilers challenged with calcium sulfate (75 mg.l-1), G2=Broilers challenged with copper sulfate (200 mg.l-1), G3=Broilers challenged Salmonella Typhimurium (1.5 × 106 CFU.ml-1), G4=control broilers. TBCi=Total bacterial counts in intestinal swabs, TBCm=Total bacterial counts in breast muscles, TECi=Total Enterobacteriaceae counts in intestinal swabs, TECm=Total Enterobacteriaceae counts in breast muscles, TSC=Total Salmonella count in intestinal swabs. Eichhornia crassipes reveals some capabilities in different studies such as enhancing the water quality characteristics, minimizing the pollutants' (heavy metals, and dyes) concentrations (Priya and Selvan, 2017), minimizing the biological oxygen demands (Wei et al., 2019), extract palm oil from mill waste in Malaysia, bio-indication for heavy metal pollution of rivers in Pakistan (Srivastava et al., 2019), Phyto-purification of wastewater (Victor et al., 2016), reduction of chromium levels (99.5%) in wastewater 15 days post-treatment (Saha et al., 2017), and extreme reduction of nitrates in wastewater combined with eutrophication (Wenwei et al., 2016). Eichhornia crassipes treated water in the current study reveals highly significant declines in physicochemical, electrolytes, heavy metals, and microbial assessments and a highly significant increase in dissolved oxygen levels compared to tap water. The results agreed with those of Wuijts et al. (2018) who recorded that E. crassipes can actively minimize heavy metals and coloring agent concentrations from the water through bio-sorption, precipitation, and accumulation. Hartmann et al. (2018) also reported the high capabilities of E. crassipes to absorb dyes and heavy metals using electrostatic forces and improve water quality characteristics. Mahmood et al. (2010) recorded that E. crassipes water treatment contributed to a significant reduction of the biological and chemical oxygen demand (70.0%), the total solids (50.6%), and pH to a level that counteracts microbial survival. Also in agreement with our results, Dar et al. (2011) evaluated the capability of E. crassipes to treat different dilutions of wastewater (25%, 50%, 75%, and 100%) and recorded significant improvements in the physicochemical and biological parameters with significant increases of the dissolved oxygen levels. They recommended E. crassipes as an adsorbent to treat wastewater from agricultural, industrial, and municipal sources. Eichhornia crassipes 1% suspension in the current results achieved 100% reductions of cobber and total and calcium hardness after 1-hour and 100% antimicrobial efficacies against E. coli O157: H7, S. Typhimurium, A. niger, and C. albicans after 1, 2, 2, and 2-hours, respectively. The results were synchronized with those of Vishwakarma et al. (2018) who reported that E. crassipes contributed to a shift in the pH up to 8.6 which is unfavorable to microbial growth and survival, and a significant reduction in the levels of total dissolved solids, conductivity, total hardness, biological and chemical oxygen demands, nitrate-nitrogen, and ammonium nitrogen in the treated water. Our results were also synchronized with those of Long et al. (2018) who revealed the strong abilities of E. crassipes for minimizing copper, cadmium, lead, zinc, chromium, and iron up to 97.3%, 94.8%, 94.7%, 96.8%, 94.3%, and 93.5%, respectively. Deng et al. (2018) recorded in agreement with the current results the strong antimicrobial activity of E. crassipes against Mycobacterium phlei, Candida parapsilosis, and Rhizopus oryzae. The field clinical trial reveals highly significant improvements in PIs, live weights, carcasses quality, biochemical parameters, immunoglobulin concentrations, cortisol hormone, and bacteriological assessments in broilers supplemented with E. crassipes treated water compared to the control group. The results agreed with those of Che et al. (2020) who recorded similar capabilities of Eichhornia purpurea (100, 200, and 600 mg.l-1) treated water which enhanced weight acquires and carcasses yield and they recommended E. purpurea (200 mg.l-1) as a natural effective eco-friendly water treatment. Abdel Shafy et al. (2016) reported that E. crassipes collected from the Nile River in Egypt contain some nutritive ingredients such as protein (10.7%), fat (4.94%), crude fiber (17.9%), nitrogen (44.3%), calcium (1.42%), and phosphorus (0.58%) making it suitable as a feed supplement with enhanced weight gains and improved feed conversions, and potential adsorbent of heavy metals polluting the drinking water. Jianbo et al. (2008) found an increased performance, FI, and egg production in ducks supplied with E. crassipes treated wastewater, and they attributed these improvements to the higher crude protein contents of the supplied E. crassipes. Rufchaei et al. (2021) documented an improvement in the marketing weights, weight gains, and growth rates in fish fed with 1.5% of E. crassipes than those fed 0%, 0.5%, and 1% of E. crassipes leaves powder. Dumaup et al. (2020) from another perspective revealed no significant effect on the broiler's performance except for FI when supplied with E. crassipes. Abdulganiyu et al. (2013) also recorded no significant differences in initial and final body weights, FIs, and FCR among day-old Isa Brown pullets that received E. crassipes (0%, 5%, 10%, and 15%) for 8 weeks. The high recorded improvements in broilers supplemented with E. crassipes treated water in our study might be attributed to the highly digestible protein contents. Wimalarathane and Perera (2019) recommended the use of E. crassipes as a dietary supplement in animals and poultry for its nutritive value and low cost. Indulekha et al. (2019) also recommended the use of E. crassipes as a dietary supplement to increase the production in animals and poultry. The recorded prominent immune-stimulant, antioxidant activities, and the biochemical enhancements of E. crassipes treated water in our study could be attributed to its chemical composition that exhibits anti-inflammatory, antifungal, antibacterial, and anticancer functions. The results were synchronized with those of Guna et al. (2017) who showed an increase in DNA inhibition and DPPH radical scavenging properties of E. crassipes. Eichhornia crassipes in our study could act as a phytoremediation agent and sanitize water from all the chemical pollutants and microbial contaminants used (challenges) contributing to the production of high-quality water, enhanced production, performance, and immunity. The same concept was concluded by Maharjan (2016) who reported that water entering the broiler farms usually is contaminated with a microbial load of up to 300 CFU.ml-1, but hence the breeders disinfect water using chemical or eco-friendly alternatives contributing to enhanced performance. Jacobs et al. (2020) also reported that sanitized and/or treated water enhanced significant growth traits. ConclusionEichhornia crassipes 1% contributed to significant enhancements in the water's physicochemical characteristics such as declines of conductivity, total dissolved solids, total alkalinity, hardness, sulfates, nitrates, electrolyte (sodium, potassium, calcium, magnesium, and chloride), heavy metals (iron, copper, and zinc), and microbial counts (TBC, TEC, and TEC). Eichhornia crassipes 1% was also able to significantly increase the levels of dissolved oxygen. Eichhornia crassipes 1% suspension exhibited 100% adsorption activities for copper (5 mg.l-1) and total (2,100 mg.l-1 CaCO3) and calcium (1,600 mg.l-1 CaCO3) hardness after 1-hour, and 100% antimicrobial activities against E. coli O157: H7 (1.7 × 1011 CFU.ml-1), S. Typhimurium (1.5 × 106 CFU.ml-1), A. niger (2.5 × 108 CFU.ml-1), and C. albicans (2.5 × 108 CFU.ml-1) after 1, 2, 2, and 2-hours respectively. The clinical trial showed that E. crassipes 1% suspension significantly improved PIs (LBW, WG, FI, WI, FCR, and PI), carcasses quality, biochemical parameters, antioxidant enzymatic activities, immunoglobulin concentrations, cortisol hormone, and intestinal microbiota in all treated broilers. Thus, E. crassipes 1% could be used with great potential as an eco-friendly alternative for the water and wastewater treatment processes to reduce waste and treatment costs and provide broilers with water of good quality. Authors’ contributionsESS deliberated and executed the laboratory and field trails' design, run water physicochemical analysis, water, and broiler samples' microbial examination, participated in sera samples analysis for biochemical and antioxidant profiles, engaged in writing the manuscript, and reviewed the submitted version. RHA calculated and judged the growth traits, determined carcass characteristics, analyzed sera for biochemical and antioxidant profiles, and collaborated in the manuscript writing. DSF took a part in the water physicochemical analysis, biochemical analysis, and microbial assessments, and collaborated in the manuscript writing. AcknowledgmentWe would like to thank Prof. MA. Sobieh for his directions during the study and OF Mohamed for her help in the laboratory examinations. FundingThe authors declare that the study was self-funded without receiving financial aid from the affiliating institute or any funding agency. Conflict of interestThe authors declare that they have no competing interests or personal relationships that could influence the work reported in this manuscript. ReferencesAbdel Shafy, H.I., Farid, M.R. and Shams El-Din, A.M. 2016. Water-hyacinth from Nile river: chemical contents, nutrient elements and heavy metals. Egypt. J. Chem. 59(2), 131–143. Abdulganiyu, M.A., Aremu, A., Bisi, A.A. and Abdulmojeed I.T. 2013. Growth performance and nutrient digestibility of pullet chicks fed graded levels of Water Hyacinth [Eichhornia crassipes (Martius) Solms-Laubach] meal diets at the starter phase (0-8 weeks). Int. J. Adv. Res. 1(9), 46–54. American Public Health Association, American Water Works Association, Water Environment Federation. 2012. Standard methods for the examination of water and wastewater. American Water Work Association Publications 2012, 22nd ed. Washington, DC: American Public Health Association, American Water Works Association, Water Environment Federation. American Public Health Association, American Water Works Association, Water Environment Federation. 2017. Standard methods for the examination of water and wastewater. In American Water Work Association Publications, 23rd ed. Eds., Rice, E.W., Baird, R.B. and Eaton, A.D. American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC. Anastopoulos, I., Karamesouti, M., Mitropoulos, A.C. and Kyzas, G.Z. 2017. A review for coffee adsorbents. J. Mol. Liq. 229, 555–565. Ansari, A., Gill, S., Khan, F. and Ghauri, N. 2014. Phytoremediation systems for the recovery of nutrients from eutrophic waters. Eutrophication: causes, consequences and control. pp: 239–248. Applegate, T.J. and Angel, R. 2014. Nutrient requirements of poultry publication: history and need for an update. Appl. Poult. Res. 23(3), 567–575. Chai, T.T., Ngoi, J.C. and Wong, F.C. 2013. Herbicidal potential of Eichhornia crassipes leaf extract against Mimosa pigra and Vigna radiata. Int. J. Agric. Biol. 15(5), 835‒842. Che, C., Shen, C., Li, S., Cai, Z., Man, R. and Wand, X. 2020. Effect of Echinacea purpurea extract given in drinking water on performance, slaughter variables, and meat quality of broilers. ES Food Agroforestry 2, 42–49. Dar, S.H., Kumawat, D.M., Singh, N. and Wani, K.A. 2011. Sewage treatment potential of water hyacinth (Eichhornia crassipes). Res. J. Environ. Sci. 5(4), 377–385. Deng, Q., Chen, L., Wei, Y., Li, Y., Han, X., Liang, W., Zhao, Y., Wang, X. and Yin, J. 2018. Understanding the association between environmental factors and longevity in Hechi, China: a drinking water and soil quality perspective. Int. J. Environ. Res. Public Health 15(10), 2272. Dumaup, H.J.J. and Ampode, K.M.B. 2020. Inclusion of water hyacinth meal in broiler chicken diets: potential on the production performance and cell-mediated immunity. Int. J. Biosci. 17(6), 469–479. Elshikh, M., Ahmed, S., Funston, S., Dunlop, P., McGaw, M., Marchant, R. and Banat, I.M. 2016. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 38(4), 1015–1019. Guna, V., Ilangovan, M., Anantha Prasad, M.G. and Reddy, N. 2017. Water hyacinth: a unique source for sustainable materials and products. ACS Sustain. Chem. Eng. 5(6), 4478–4490. Hartmann, J., Van der Aa, M., Wuijts, S., De Roda Husman, A.M. and Van der Hoek, J.P. 2018. risk governance of potential emerging risks to drinking water quality: analysing current practices. Environ. Sci. Policy 84, 97–104. Indulekha, V.P., Thomas, C.G. and Anil, K.S. 2019. Utilization of water hyacinth as livestock feed by ensiling with additives. Indian J. Weed Sci. 51(1), 67–71. Jacobs, L., Persia, M.E., Siman-Tov, N., McCoy, J., Ahmed, M., Lyman, J. and Good, L. 2020. Impact of water sanitation on broiler chicken production and welfare parameters. J. Appl. Poult. Res. 29(1), 258–268. Jianbo, L.U., Zhihui, F.U. and Zhaozheng, Y.I.N. 2008. Performance of a water hyacinth (Eichhornia crassipes) system in the treatment of wastewater from a duck farm and the effects of using water hyacinth as duck feed. J. Environ. Sci. 20(5), 513–519. Kim, S.K. and Lee, J.H. 2016. Biofilm modeling systems. Korean J. Microbiol. 52(2), 125–139. Kong, F., Xiong, K., and Zhang, N. 2014. Determinants of farmers' willingness to pay and its level for ecological compensation of poyang lake wetland, china: a household-level survey. Sustainability 6(10), 6714–6728. Li, P. and Qian, H. 2018a. Water resource development and protection in loess areas of the world: a summary to the thematic issue of water in loess. Environ. Earth Sci. 77(24), 1–11. Li, P., Qian, H. and Zhou, W. 2017a. Finding harmony between the environment and humanity: an introduction to the thematic issue of the Silk Road. Environ. Earth Sci. 76(3), 105. Long, D.T., Pearson, A.L., Voice, T.C., Polanco-Rodríguez, A.G., Sanchez-Rodríguez, E.C., Xagoraraki, I., Concha-Valdez, F.G., Puc-Franco, M., Lopez-Cetz, R. and Rzotkiewicz, A.T. 2018. Influence of rainy season and land use on drinking water quality in a karst landscape, State of Yucatán, Mexico. Appl. Geochem. 98, 265–277. Maes, S., Vackier, T., Nguyen Huu, S. Heyndrickx, M., Steenackers, H., Sampers, I., Raes, K., Verplaetse, A. and Reu, K.D. 2019. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 19, 77. Magor, K.E., Miranzo Navarro, D., Barber, M.R.W., Petkau, K., Fleming-Canepa, X., Blyth, G.A.D. and Blaine, A.H. 2013. Defense genes missing from the flight division. Dev. Comp. Immunol. 41(3), 377–388. Maharjan, P. 2016. Development of a biofilm model for evaluating poultry drinking water sanitation procedures. University of Arkansas. Available via https://www.proquest.com/docview/1796849054?pq-origsite=gscholar&fromopenview=true Maharjan, P., Clark, T., Kuenzel, C., Foy, M.K. and Watkins, S. 2016. On farm monitoring of the impact of water system sanitation on microbial levels in broiler house water supplies. J. Appl. Poult. Sci. 25(2), 266–271. Mahmood, T., Malik, S.A. and Hussain, S.T. 2010. Biosorption and recovery of heavy metals from aqueous solutions by Eichhornia crassipes (water hyacinth) ash. Bioresoures 5(2), 1244–1256. Mishra, S. and Maiti, A. 2017. The efficiency of Eichhornia crassipes in the removal of organic and inorganic pollutants from wastewater: a review. Environ. Sci. Pollut. Res. 24, 7921–7937. Mudgal, V., Madaan, N. and Mudgal, A. 2010. Heavy metals in plants phytoremediation: plants used to remediate heavy metal pollution. Am. J. Agric. Biol. Sci. 1(1), 40–46. Murray, P.R., Rosenthal, K.S. and Pfaller, M.A. 2015. Medical microbiology, 8th ed. Philadelphia, PA: Elsevier Health Sciences. National Research Council (NRC). 1994. Nutrient requirements for poultry, 9th ed. New York, NY: National Research Council. Palansooriya, K.N., Yang, Y., Tsang, Y.F., Sarkar, B., Hou, D., Cao, X., Meers, E., Rinklebe, J., Kim, K.H. and Ok, Y.S. 2020. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: a review. Crit. Rev. Environ. Sci. Technol. 50(6), 549–611. Pellegrini, M.O.O., Horn, C.N. and Alemida, R.F. 2018. Total evidence phylogeny of Pontederiaceae (Commelinales) sheds light on the necessity of its recircumscription and synopsis of Pontederia L. PhytoKeys 108, 25–83. Priya, E.S. and Selvan, P.S. 2017. Water hyacinth (Eichhornia crassipes)—an efficient and economic adsorbent for textile effluent treatment—a review. Arab. J. Chem. 10(2), S3548–S3558. Raval, N.P., Shah, P.U. and Shah, N.K. 2016. Adsorptive removal of nickel (II) ions from aqueous environment: a review. J. Environ. Manage. 179, 1–20. Robinson, T., McMullan, G., Marchant, R. and Nigam, P. 2001. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 77(3), 247–255. Rufchaei, R., Nedaei, S., Hoseinifar, S.H., Hassanpour, S., Golshan, M. and Bourani, M.S. 2021. Improved growth performance, serum and mucosal immunity, haematology and antioxidant capacity in pikeperch (Sander lucioperca) using dietary water hyacinth (Eichhornia crassipes) leaf powder. Aquac. Res. 52, 2194–2204. Saha, P., Shinde, O. and Sarkar, S. 2017. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediation. 19(1), 87–96. Sarika, D., Singh, J., Prasad, R., Vishan, I., Varma, V.S. and Kalamdhad, A.S. 2014. Study of physico-chemical and biochemical parameters during rotary drum composting of water hyacinth. Int. J. Recycl. Org. Waste in Agric. 3(3), 9. Sarkar, M., Rahman, A.K.M.L. and Bhoumik, N.C. 2017. Remediation of chromium and copper on water hyacinth (E. crassipes) shoot powder. Water Resour. Ind. 17, 1–6. Shah, R.A., Kumawat, D.M., Singh, N., and Khursheeed A.W. 2010. Water hyacinth (Eichhornia crassipes) as a remediation tool For dye-effluent pollution. Int. J. Netw. Secur. 1(2), 172–178. Sharma, N.K., Aggarwal, A., Saini and Yadav, A. 2016. Beyond biocontrol: water hyacinth-opportunities and challenges. J. Environ. Sci. Technol 9(1), 26–48. Soliman, E.S. and Hassan, R.A. 2017. Evaluation of superphosphate and meta-bisulfide efficiency in litter treatment on productive performance and immunity of broilers exposed to ammonia stress. Adv. Anim. Vet. Sci. 5(6), 253–259. Soliman, E.S. and Hassan, R.A. 2019. Impact of lighting color and duration on productive performance and Newcastle disease vaccination efficiency in broiler chickens. Vet. World 12(7), 1052–1059. Soliman, E.S. and Abdallah, M.S. 2020a. Assessment of biosecurity measures in broiler's farms in the Suez Canal area—Egypt using a seasonal prevalence of Salmonellosis. Vet. World 13(4), 622–632. Soliman, E.S. and Hassan, R.A. 2020b. Influence of housing floor on air quality, growth traits, and immunity in broiler chicken farms. Adv. Anim. Vet. Sci. 8(9), 997–1008. Soliman, E.S., Reddy, P.G., Sobieh, M.A.A., Busby, H. and Rowe, S. 2009a. Epidemiological surveillance on environmental contaminants in poultry farms. Int. J. Poult. Sci. 8(2), 151–155. Soliman, E.S., Sobieh, M.A.A., Ahmad, Z.H., Hussein, M.M., AbdelLatiff, H. and Moneim, A.A. 2009b. Seasonal epidemiological surveillance on bacterial and fungal pathogens in broiler farms in Egypt. Int. J. Poult. Sci. 8(8), 720–727. Soliman, E.S., Hamad, R.T. and Ahmed, A. 2017. Prophylactic and immune modulatory influences of Nigella sativa Linn. in broilers exposed to biological challenge. Vet. World 10(12), 1447–1455. Soliman, E.S., Sallam, N.H. and Abouelhassan, E.M. 2018. Effectiveness of poultry litter amendments on bacterial survival and Eimeria oocyst sporulation. Vet. World 11(8), 1064–1073. Soliman, E.S., Ali, A.A. and Gafaar, R.E.M. 2021a. Impact of heating systems on air and litter quality in broiler houses, performance, behavior, and immunity in broiler chickens. Adv. Anim. Vet. Sci. 9(2), 301–314. Soliman, E.S., Hamad, R.T. and Abdallah, M.S. 2021b. Preventive antimicrobial action and tissue architecture ameliorations of Bacillus subtilis in challenged broilers. Vet. World 14(2), 523–536. Soliman, E.S., Hamad, R.T. and Hassan, R.A. 2021c. Moderations in performance, immunity, tissue architecture, and vaccine viability induced by water magnetization in broiler farms. Vet. World 14(6), 1695–1710. Soliman, E.S., Hassan, R.A. and Farid, D.S. 2021d. The efficiency of natural-ecofriendly clay filters on water purification for improving performance and immunity in broiler chickens. Open Vet. J. 11(3), 483–499. Soliman, E.S., Hassan, R.A. and Farid, D.S. 2022. Nile Tilapia bones as eco-friendly alternative in water treatment and enhancing performance and immunity in broiler chickens. Open Vet. J. 12(6), 1000–1017. SPSS. 2020. Statistical Packages of Social Sciences. IBM SPSS Statistics for Windows, Version 27.0. IBM Corp, Armonk, NY. Available via https://www.ibm.com/support/pages/spss-statistics-210-available-download Srivastava, A., Chahar, V., Sharma, V., Swain, K.K., Hoyler, F., Murthy, G.S., Scherer, U.W., Rupp, H., Knolle, F., Maekawa, M. and Schnug, E. 2019. Study of toxic elements in river water and wetland using water hyacinth (Eichhornia crassipes) as pollution monitor. Glob. Challenge 3(6), 1800087. Su, F., Wu, J. and He, S. 2019. Set pair analysis (SPA)-Markov chain model for groundwater quality assessment and prediction: a case study of Xi'an City, China. Hum. Ecol. Risk Assess. 25, 158–175. Szczepanik, B. 2017. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: a review. Appl. Clay Sci. 141, 227–239. Thapa, G., Das, D., Gunupuru, L.R. and Tang, B. 2016. Endurance assessment of Eichhornia crassipes (Mart.) Solms, in heavy metal contaminated site–a case study,” Cogent. Environ. Sci. 2(1), 1215280. Thrusfield, M. and Christley, R. 2018. Veterinary epidemiology, 4th ed. London, UK: Blackwell Science Ltd., Chapter 11, pp: 214–256. Tian, R. and Wu, J. 2019. Groundwater quality appraisal by improved set pair analysis with game theory weightage and health risk estimation of contaminants for Xuecha drinking water source in a loess area in Northwest China. Hum. Ecol. Risk Assess. 25, 132–157. Twongo, T. 2019. Growing impact of water hyacinth on nearshore environments on Lakes Victoria and Kyoga (East Africa). In The limnology, climatology and paleoclimatology of the East African Lakes, 1st ed. Routledge, pp: 633–642. Van der Wielen, P.W.J.J. and Lut, M.C. 2016. Distribution of microbial activity and specific microorganisms across sediment size fractions and pipe wall biofilm in a drinking water distribution system. Water Sci. Technol. Water Supply 16(4), 896–904. Victor, K.K., Séka, Y., Norbert, K.K.T.A., Celestin, B. 2016. Phytoremediation of wastewater toxicity using water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes). Int. J. Phytoremediation 18(10), 949–955. Vishwakarma, C.A., Sen, R., Singh, N., Singh, P., Rena, V., Rina, K. and Mukherjee, S. 2018. Geochemical characterization and controlling factors of chemical composition of spring water in a part of Eastern Himalaya. J. Geol. Soc. India 92, 753–763. Wei, I.T.A., Jamali, N.S. and Ting, W.H.T. 2019. Phytoremediation of palm oil mill effluent (POME) using Eichhornia crassipes. J. Appl. Sci. Process Eng. 6(1), 340–354. Wenwei, W., Ang, L., Konghuan, W., Lei, Z., Xiaohua, B., Kun-zhi, L., Ashraf, M.A. and Limei, C. 2016. The physiological and biochemical mechanism of nitrate-nitrogen removal by water hyacinth from agriculture eutrophic wastewater. Braz. Arch. Biol. Technol. 59, e16160517. Wimalarathne, H.D.A. and Perera, P.C.D. 2019. Potentials of water hyacinth as livestock feed in Sri Lanka. Indian J. Weed Sci. 51(2), 101–105. Wuijts, S., Driessen, P.P.J. and Van Rijswick, H.F.M.W. 2018. Governance conditions for improving quality drinking water resources: the need for enhancing connectivity. Water Resour. Manag. 32, 1245–1260. | ||
| How to Cite this Article |
| Pubmed Style Soliman ES, Hassan RA, Farid DS. Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens. Open Vet. J.. 2023; 13(4): 451-465. doi:10.5455/OVJ.2023.v13.i4.7 Web Style Soliman ES, Hassan RA, Farid DS. Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens. https://www.openveterinaryjournal.com/?mno=92710 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i4.7 AMA (American Medical Association) Style Soliman ES, Hassan RA, Farid DS. Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens. Open Vet. J.. 2023; 13(4): 451-465. doi:10.5455/OVJ.2023.v13.i4.7 Vancouver/ICMJE Style Soliman ES, Hassan RA, Farid DS. Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens. Open Vet. J.. (2023), [cited January 25, 2026]; 13(4): 451-465. doi:10.5455/OVJ.2023.v13.i4.7 Harvard Style Soliman, E. S., Hassan, . R. A. & Farid, . D. S. (2023) Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens. Open Vet. J., 13 (4), 451-465. doi:10.5455/OVJ.2023.v13.i4.7 Turabian Style Soliman, Essam S., Rania A. Hassan, and Doaa S. Farid. 2023. Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens. Open Veterinary Journal, 13 (4), 451-465. doi:10.5455/OVJ.2023.v13.i4.7 Chicago Style Soliman, Essam S., Rania A. Hassan, and Doaa S. Farid. "Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens." Open Veterinary Journal 13 (2023), 451-465. doi:10.5455/OVJ.2023.v13.i4.7 MLA (The Modern Language Association) Style Soliman, Essam S., Rania A. Hassan, and Doaa S. Farid. "Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens." Open Veterinary Journal 13.4 (2023), 451-465. Print. doi:10.5455/OVJ.2023.v13.i4.7 APA (American Psychological Association) Style Soliman, E. S., Hassan, . R. A. & Farid, . D. S. (2023) Eichhornia crassipes from wastes to valuable products in water purification and influence on growth and impregnability in overwhelmed broiler chickens. Open Veterinary Journal, 13 (4), 451-465. doi:10.5455/OVJ.2023.v13.i4.7 |