| Short Communication | ||
Open Vet. J.. 2023; 13(4): 495-500 Open Veterinary Journal, (2023), Vol. 13(4): 495–500 Short Communication Cytotoxicity assay as a potential alternative method for animal testing for batch release of Italian fish autogeneous vaccinesAntonella Di Paolo*†, Lucia Anzalone†, Martina Pellegrini, Giulio Severi and Monica CagiolaIstituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati” (IZSUM), Perugia, Italy †Authors contributed equally to this work. *Corresponding Author: Antonella Di Paolo. Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati” (IZSUM), Perugia, Italy. Email: a.dipaolo [at] izsum.it. Submitted: 21/12/2022 Accepted: 28/03/2023 Published: 25/04/2023 © 2023 Open Veterinary Journal
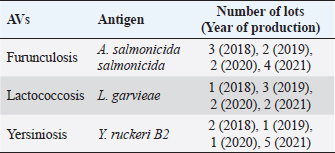
AbstractBackground: Vaccination is widely used in fish aquaculture for three primary reasons: to prevent bacterial disease spreading, minimize antibiotic use and fight antibiotic resistance. Vaccine production is an expensive and consuming process, mainly in terms of money, resources, and animals for quality control. The replace, reduce, and refine (3Rs) philosophy suggests developing and validates alternative methods to animal testing for scientific purposes, even for biologicals and vaccines. Aim: The current study explored the potential use of mouse and fish cells in the in vitro toxicity grade assessment through different methods, as an alternative assay to in vivo residual toxicity tests for autogenous fish vaccine control. Methods: BF2 and L929 cell lines were exposed to vaccine dilutions in two different ways of administration and toxicity grade was recorded by MTS assay, compared to the in vivo gold standard test. Results: Autogenous vaccines (AVs) caused no reactions in the in vivo test. In the in vitro assay, the different toxicity grade recorded was statistically significant between the cell lines adopted and the AVs way of administration. Conclusions: Data obtained represent the first application of 3Rs method to fish AVs produced in Italy, more investigations are needed to collect solid results and standardize new in vitro methods for vaccine quality control. Keywords: Autogenous vaccine, 3Rs, In vitro testing, Aquaculture, MTS. IntroductionAquaculture accounted for 46% of the total production and 52% of fish for human consumption (The State of World Fisheries and Aquaculture, 2020), also in Italy is currently an innovative sector where 30 species of fish, mollusks, and crustaceans, grouped into 5 categories, are produced (trout, alpine char, sea bass, sea bream, mussels, and clams). There are over 800 aquaculture plants with an annual fish production of 140,000 tons, constituting 40% of the national fish production and meeting 30% of the demand for fresh fish products (BUR UMBRIA, 2017). Intensive aquaculture increases the risks of severe outbreaks affecting economics and the performance of the fish companies and that cannot be resolved by massive antibiotic therapies alone. The vaccination strategy in a prudent prophylaxis system, if well supported by careful management and good zootechnical hygiene practices, minimizes the impact on the environment and results in a more productive and high-quality product. In 2017, a special plan for the protection and conservation of Umbria fish fauna (28 listed species) and sport fishing was drafted; the report clarifies the management and use of water resources related to ichthyology and fishery, guaranteeing the protection and enhancement of the fish heritage and its natural habitat (BUR UMBRIA, 2017). The most common fish vaccine types includes: bacterins (inactivated or killed whole-cell fish bacterial pathogens), live attenuated (bacteria and viruses modified so they would not progress to a disease), subunit vaccines (a small portion of fish pathogens), and finally toxoids (inactivated toxic compounds). Recombinant and DNA vaccines are also being developed with limited use because of unknown long-term effectiveness and varying regulations, respectively. Autogenous vaccines (AVs) also known as an emergency, herd-specific, or custom-made vaccines are made from inactivated bacterial or viral strains, isolated from sick animals of one herd and only usable in the same farm, or in epidemiologically correlated outbreak, following a veterinary prescription. Most importantly, AVs are not submitted to lengthy procedures to assess potency and safety, as requested for conventional vaccines usually, because of their urgent requirement (Attia et al., 2013). Since 1994, the Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati” (IZSUM) has the authorization by the Italian Ministry of Health to produce and sell AVs and auto-vaccines, and other biologicals with immunization action (Legislative Decree 287/1994). AVs play a pivotal role in bacterial disease control in aquaculture, they are produced and applied case by case if traditional vaccines are not available on the market or are not formulated as requested on farm, therefore, is the most effective method for preventing a wide range of bacterial and viral diseases. In Italy, AVs undergo quality and safety tests before being released. As stated in DL 287/1994, five mice are inoculated subcutaneously with AVs dose and are observed for 3 days. To guarantee the suitability of the product, there must be no residual reactions, neither local nor general. After the first vaccine release, the safety of the autogeneous product for all recommended uses has to be shown under field conditions in a small group of animals belonging to the farm: fishes are vaccinated and, for a period, observed for local and systemic reactions, then the remaining part of the group can be vaccinated. However, in vivo procedures needs the sacrifice of many animals and are time and resource-consuming, raising attention on the issues of manufacturing and control of AVs, therefore part of the scientific community together with the European Medicines Agency encourages member states to define alternatives to the use of animals and underlie a substantial need for faster methods able to measure in vitro reliable correlates of vaccine toxicity (EMA, 2016; Colella et al., 2020). New studies are being carried out for the evaluation of bacterial endotoxins in AVs, and till today some experiences in veterinary pharmaceutical production highlighted the potential use of in vitro tests (like MTT and LAL tests) for AVs of large and small animals before release (Di Paolo et al., 2018; Profeta et al., 2019; Colella et al., 2020), but nothing has been reported for fish AVs. Therefore, the present work evaluated the possibility to assess the toxicity of fish AVs by in vitro colorimetric assay, adopting both piscine and murine cell lines, following the Italian Legislative Decree (Legislative Decree no. 26, 2014) on protecting animals used and the replace, reduce, and refine (3Rs) philosophy for scientific purposes and veterinary medicinal products (Bruysters et al., 2017). Materials and MethodsCell culturesTwo different cell lines were tested for basal toxicity. The cell line BF2, derived from a trypsinized caudal portion of the trunk of a 1-year-old Bluegill (Lepomis macrochirus), was purchased from the European Union Reference Laboratory for fish disease in Copenhagen. The cells were maintained in minimum essential medium (MEM) (Euroclone) supplemented with 10% fetal bovine serum (FBS, Gibco); cultures were grown in a 75 cm2 culture flask and incubated at 22°C ± 2°C. The cell cultures were inspected daily, then detached using Trypsin Replacement (TrypLE Express Enzyme, Gibco) and sub-cultured for the cytotoxicity assay when a 90% confluence was attained. The cell line L929, derived from mouse fibroblasts from the connective tissue, was purchased from the Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna (biobank code BS CL 56) and cultured as described above for BF2 cells, except that the cells were maintained in the incubator at 37°C ± 2°C with 5% CO2. Fish AVsApproved AVs for salmonids against Furunculosis (Aeromonas salmonicida salmonicida) and Lactococcosis (Lactococcus garvieae) and the experimental AV against Yersiniosis (Yersinia ruckeri B2) were produced at the Pharmaceutical Unit. All the AVs were intended for intraperitoneal use. Briefly, the first bacterial isolation was performed from organs taken from symptomatic subjects on Blood agar for 24 hours at 22°C ± 2°C. A single alpha hemolytic and isolated colony was then sowed on blood agar or tryptone soy agar (TSA) to perform biochemical tests for characterization. For vaccine batch production, a single pure colony was again cultivated in TSA for 24 hours at 22°C ± 2°C, then cloned and propagated in tryptone soy broth, and kept under agitation for 48 hours at 22°C ± 2°C. All the culture media were produced at the Pharmaceutical Unit of the Institute. After purity control, the culture was inactivated by 0.05% formaldehyde (v/v) treatment for 24 hours under continuous stirring and finally adjusted at a final concentration of 1.5 × 109 CFU/ml based on the McFarland scale. For every batch before the toxicity assay, the low formaldehyde-free grade was assessed by chemical analysis (data not shown). An aliquot of each lot produced between 2018 and 2021 was tested for the in vitro assay, as listed in Table 1. Residual toxicity testAs stated in DL 287/1994, a vaccine dose 10 times (2 ml) higher than the usually adopted was administered subcutaneously to five mice weighing around 20 g. The housed animals were observed for 72 hours by the designed veterinarian, according to European Pharmacopoeia monographs and European guidelines. No animal should show notable signs of disease or die from causes because of the vaccine. Table 1. List of the samples analyzed.
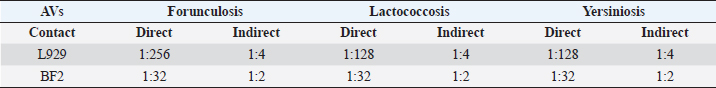
MTS assay designFor the metabolic activity assessment, two different administration methods were adopted: direct addition to cell monolayer (for 96-well) and indirect addition (for 24-well) by placing the AV on the transwell support with a porous membrane. For each plate, a 1:10 hypochlorite aqueous solution served as a positive control of cytotoxicity, whereas the negative control (NC) was represented by the culture medium with FBS. The viability was assessed after 24 hours of contact, adopting the method previously described (Mosmann, 1983). Every experiment was performed three times for each dilution, in three different sessions, to obtain normalized data. The effects of the vaccines on cell metabolic activity were expressed as a percentage by the following formula: Cell vitality=(ODs average value per dilution/NC) × 100. Finally, the first dilution that guaranteed at least 70% cell viability response (critical dilution), after a specified exposure time, was established for each AV. Direct contact experimentL929 and BF2 cells were plated at 35,000 cells/well for 24 hours with MEM with 10% FBS at 37°C 5% CO2 and 22°C, respectively. After 24 hours, the medium was replaced with AV undiluted and serially two-fold diluted in cell medium (up to 1:2,048 dilution). Finally, after more 24 hours, the medium was substituted with CellTiter 96® AQeous One Solution Cell Proliferation Assay (MTS) (Promega) (20 mg/ml) for 3 hours until a purple-colored formazan product was formed, index of vital metabolic activity. The produced formazan gives rise to a blue-violet color measured by spectrophotometric reading at 492 nm (UNI EN ISO 10993-5, 2009), using a UV-visible spectrophotometer Tecan equipped with Magellan Software. Indirect contact experimentL929 and BF2 cells were seeded at the bottom of 24-well plates (50,000 cells/well) equipped with transwell ThinCert™ (Grainer) cell culture inserts, with MEM with 10% FBS at 37°C 5% CO2 and 22°C, respectively. After the incubation period, the metabolic activity was assessed as described in the direct contact method. Statistical analysisFor descriptive purposes, the first dilution that showed 70% of cell viability on average among runs was calculated for each AV and method (cell line and way of administration). Then, to assess differences between cell lines (BF2 vs. L929) and way of administration (direct and indirect) the critical dilution was transformed into a natural logarithm for each lot by employing the non-parametric Kruskal–Wallis test. Results were presented in terms of dilutions. A p-value < 0.05 was considered statistically significant. All analyses were performed using Stata software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX, StataCorp LLC). Ethical approvalAll animals are routinely involved under the authorization of DL 287/1994 by the Italian Ministry of Health and are uniquely used for AVs safety control tests. Results and DiscussionDuring the observation period, the animals did not show local or general reactions, therefore the AVs tested were officially safe and ready for release. Cytotoxicity results are summarized in Table 2 and Figures 1 and 2. In the direct contact experiment L929 cell line mainly two different dilution values reaching 70% cell viability: 1:128 for Lactococcosis and Yersiniosis, and 1:256 for Furunculosis, whereas the dilution of 1:32 only affected 70% viability of BF2 cell in all AVs tested. In the indirect contact experiment, for the L929 fibroblasts, 70% cell viability value was found only at a 1:4 vaccine dilution, whereas BF2 showed values of metabolic activity over 70% with vaccines 1:2 diluted. The median dilution for the direct way of administration was attested between 1:64 and 1:128, while the median dilution for the indirect way of administration was 1:4 (Fig. 1) and an overall significant difference between the approaches (indirect vs. direct) was observed (p-value=0.0001). A significant difference between the cell types (BF2 vs. L929) emerged in the way of administrations (p-value=0.0001 each). The median values were 1:32 for the direct BF2-method, 1:256 for the direct L929-method, while 1:2 for the indirect BF2-method and 1:8 for the direct L929-method (Fig. 2). Driven by raising successes in disease prevention and control, there is a significant increase in vaccination against bacterial and viral infections in European aquaculture, and a proportional increase in vaccine supply to European and Italian markets. In vivo methods are still dominating the evaluation of current and new vaccines for fish. Consequently, a huge number of animals is still used during the development, licensing, and quality control of fish vaccines. Table 2. AVs critical dilutions (≥70% cell viability). The table summarises the results obtained for each type and method of administration adopted in the experiments. Overall, fish cells reached 70% viability grade at lower dilutions than mice cell line; moreover, the indirect method showed that the critical dilution for both species has been reached at lower dilutions than the direct method.
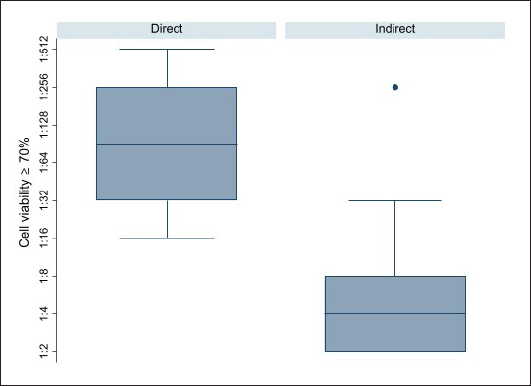
Fig. 1. Box plot of AVs critical dilutions (first dilution showing the 70% of cell viability) per way of administration. The median dilution for the direct way of administration was attested between 1:64 and 1:128, while the median dilution for the indirect way of administration was 1:4. Overall, significant difference between the approaches (indirect vs. direct) was observed (p-value=0.0001).
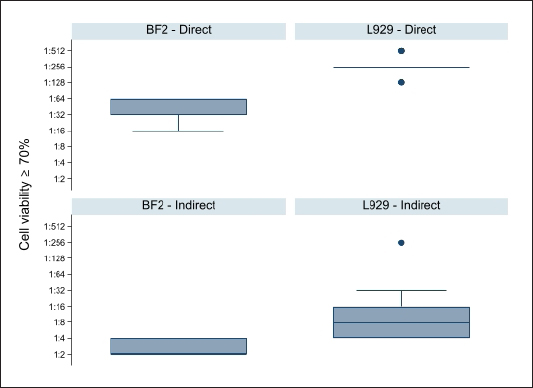
Fig. 2. Box plot of AVs critical dilutions (first dilution showing the 70% of cell viability) per cell type and way of administration. A significant difference between the cell types (BF2 vs. L929) emerged in both the way of administrations (p-value=0.0001 each). The median values were 1:32 for the direct BF2-method, 1:256 for the direct L929-method, while 1:2 for the indirect BF2-method and 1:8 for the direct L929-method. Vaccine safety control is a crucial step to release a high-quality product, requiring many funds and animals for toxicity and potency in vivo tests. The strong hints of change in the regulatory framework toward alternatives to animal testing has prompted the development of new in vitro methods in different areas. In fact, European Partnership for Alternative Approaches to Animal Testing encourages and supports the deletion of all in vivo tests from all relevant legal requirements and guidance documents (country-specific guidelines, Pharmacopoeia monographs, WHO recommendations). In vitro assays are widely used for toxicology because of the functional experimental design, easy-to-use reactive, and shorter exposure time required. On one side, cytotoxicity assays reduce management costs, lab facilities, and testing time, on the other are well accepted for ethical reasons (Roth and Henderson, 2001; EMA, 2017). However, different trends still remain and divide the public and scientific community concerning the proper application of in vitro methods to guarantee the safety and quality assurance of pharmaceuticals, biologicals, and other substances. The corresponding removal of the animal tests that these new alternatives replace is still forthcoming. There are many reasons why animal testing persists, even when there are alternatives, which have little to do with the scientific limitations of the new tests. Proponents of animal testing say that it has enabled the development of many life-saving treatments for both humans and animals, that there is no alternative method for researching a complete living organism, and that strict regulations prevent the mistreatment of animals in laboratories (Taylor, 2019). Even if the veterinary vaccine quality control sector is shifting where possible into in vitro quality control, the application and acceptance, and validation of alternative methods is difficult, according to the literature published, and even more ambitious for AVs. The dataset here presented is the first attempt to control toxicity for fish AVs in parallel with residual toxicity test, in line with 3Rs philosophy. As primary data reported, the vaccinated animals did not show local or general reactions during the observation period, highlighting the safety feature of AVs produced. Once assessed the lack of toxicity in the gold standard test, a cytotoxicity assay was performed in two ways, with the aim to verify a suitable alternative to the in vivo method that could guarantee a safe result and at the same time can reach the best results obtained in vivo. On one side, we choose to test the AVs both with mouse and fish cells, to better understand a possible and different outcomes among the species, piscine cell lines are mostly adopted for in vitro ecotoxicology assays, where the acute or chronic effect of a substance/particle is investigated (Poornavaishnavi et al., 2019). On the other side, we investigated the effect of a way of administration on the metabolic activity, trying to highlight how it could affect the safety assessment. MTS was selected among different tetrazolium compounds for several advantages: a) it is supplied as a single solution, ready to add to assay plates; b) it is faster to use (no additional steps that are usually required for MTT assays); c) there is non-radioactive waste disposal; d) the plate can be read in distinct steps for further color development; e) there is no requirement for volatile organic solvents to solubilize the formazan product (unlike MTT). MTS assay showed that BF2 cells in the 96-well directly exposed to 2-fold vaccine dilutions revealed a stronger dose-dependent increase in cell viability than L929 fibroblasts, similar results were observed when the AVs were not in direct contact with the cell population (24-well), confirming the non-toxic properties of the vaccines, with a statistical significance (p-value=0.0001), as shown in Figures 1 and 2. According to the preliminary results, we can suppose an application of the fish cell lines for the safety control of fish AVs. However, further studies are required to obtain robust data and to evaluate further cell lines as species-specific targets for the quality control of fish AVs. It is necessary to evaluate the specific sensitivity of each adopted AV and to analyze the influence of cell lines and cytotoxicity endpoint selection on the test results. As reported also by Profeta and Coll, we still not identified for sure which components of AVs handle the cytotoxicity outcome versus animal safe outcome. ConclusionThis study presents the preliminary application of in vitro cytotoxicity assay to three different Italian fish whole-cell vaccines. The experimental design assessed the cytotoxicity features of AVs and the different suitability of two different cell substrates and ways of administration. Based on these preliminary data, the piscine cell substrate exposed to AVs indirect contact obtained the best results in terms of cell viability, suggesting a peculiar specie-specific response. It is a sign that in vitro assays of toxicity can depict the toxicity profiles of bacterial vaccines. In conclusion, despite the small amount of data produced and the small sample size, the results are promising and contribute to the application of alternative methods to animal testing in veterinary vaccine quality control. This will demand proper validation trails, focused on both local and systemic toxicity in the target species, also including the currently authorized adjuvants, not easily amenable to in vitro assays. The ultimate and main purpose of our research is to replace animals involved in toxicity tests. Author contributionsConceptualization, method, formal analysis, draft preparation: A.D.P., writing—review and editing: L.A., M.P., G.S.; supervision: M.C. All authors have read and agreed to the published version of the manuscript. AcknowledgmentsThe authors are thankful to Dr Claudia Colabella and Dr Rosario Liberti for critical review, and also to Dr Andrea Felici and Dr Laura Ferroni for statistical analysis. Conflicts of interestThe authors declare no conflict of interest. FundingThis research was partially funded by the Italian Ministry of Health, grant code RFBR12017. Data availability statementThe datasets generated and analyzed during the current study are available on request from the corresponding author, A.D.P. ReferencesAttia, Y., Schmerold, I. and Hönel, A. 2013. The legal foundation of the production and use of herd-specific vaccines in Europe. Vaccine 31(36), 3651–3655. Bruysters, M.W.P., Schiffelers, M.J., Hoonakker, M., Jungbaeck, C., Ragan, I., Rommel, E., van der Stappen, T., Viviani, L., Hessel, E.V., Akkermans, A.M. and Vandebriel, R.J. 2017. Drivers and barriers in the consistency approach for vaccine batch release testing: Report of an international workshop. Biologicals 48, 1–5. BUR UMBRIA. BUR UMBRIA SERIE GENERALE PERUGIA—13 dicembre 2017. PARTE PRIMA, Sezione II ATTI DELLA REGIONE DELIBERAZIONE DELL’ASSEMBLEA LEGISLATIVA 21 novembre 2017, n. 212. Piano regionale per la tutela e la conservazione del patrimonio ittico e per la pesca sportiva—Articolo 8 della legge regionale 22 ottobre 2008, n. 15. Colella, E.M., Alborali, G.L., Dotti, S. and Amadori, M. 2020. Basic information for the development of a toxicity assay in inactivated bacterial vaccines. Res. Vet. Sci. 132, 386–392. DECRETO 17 marzo n. 287, 1994. Regolamento recante norme sulla produzione, l’impiego ed il controllo dei medicinali veterinari immunologici inattivati, aventi caratteristiche di vaccini stabulogeni ed autovaccini. Di Paolo, A., Forti, K., Anzalone, L., Corneli, S., Pellegrini, M., Severi, G. and Cagiola, M. (2018). First evaluation of endotoxins in veterinary autogenous vaccines produced in Italy by LAL assay. Biologicals 55, 71–73. EMA/CHMP/CVMP/JEG-3Rs/164002/2016. Reflection paper providing an overview of the current regulatory testing requirements for veterinary medicinal products and opportunities for implementation of the 3Rs. EMA/CMDv. 2017. Recommendations for the manufacture, control and use of inactivated autogenous veterinary vaccines within the EEA. , UK: EMA/CMDv Legislative Decree no. 26, of 4th March 2014. Implementing directive 2010/63/EU on the protection of animals used for scientific purposes. Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65(1–2), 55–63. Poornavaishnavi, C., Gowthami, R., Koigoora, S., Bramhachari, P.V. and Venkatramaiahd, N. 2019. Nickel nanoparticles induces cytotoxicity, cell morphology and oxidative stress in bluegill sunfish (BF2) cells. Appl. Surf. Sci. 483, 1174–1181. Profeta, F., Matteucci, O., Orsini, G., Sonsini, L., Lombardi, G., Capista, S., Antonucci, D., Ronchi, G.F. and Di Ventura, M. 2019. Evaluation of veterinary autogenous vaccines safety by an MTT in-vitro cytotoxicity assay. Vet. Ital. 55(4), 299–305. Roth, J.A. and Henderson, L.M. 2001. New technology for improved vaccine safety and efficacy. Vet. Clin. North Am. Food Anim. Pract. 17(3), 585–597, vii; doi:10.1016/s0749-0720(15)30008-6 Taylor, K. 2019. Chapter 24 recent developments in alternatives to animal testing. In Animal experimentation: working towards a paradigm change. Leiden, The Netherlands: Brill; doi: https://doi.org/10.1163/9789004391192_025 The State of World Fisheries and Aquaculture. 2020. Available via www.fao.org/documents/card/en/c/ca9231en | ||
| How to Cite this Article |
| Pubmed Style Di-paolo A, Anzalone L, Pellegrini M, Sveri G, Cagiola M. Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines. Open Vet. J.. 2023; 13(4): 495-500. doi:10.5455/OVJ.2023.v13.i4.12 Web Style Di-paolo A, Anzalone L, Pellegrini M, Sveri G, Cagiola M. Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines. https://www.openveterinaryjournal.com/?mno=96906 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i4.12 AMA (American Medical Association) Style Di-paolo A, Anzalone L, Pellegrini M, Sveri G, Cagiola M. Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines. Open Vet. J.. 2023; 13(4): 495-500. doi:10.5455/OVJ.2023.v13.i4.12 Vancouver/ICMJE Style Di-paolo A, Anzalone L, Pellegrini M, Sveri G, Cagiola M. Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines. Open Vet. J.. (2023), [cited January 25, 2026]; 13(4): 495-500. doi:10.5455/OVJ.2023.v13.i4.12 Harvard Style Di-paolo, A., Anzalone, . L., Pellegrini, . M., Sveri, . G. & Cagiola, . M. (2023) Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines. Open Vet. J., 13 (4), 495-500. doi:10.5455/OVJ.2023.v13.i4.12 Turabian Style Di-paolo, Antonella, Lucia Anzalone, Martina Pellegrini, Giulio Sveri, and Monica Cagiola. 2023. Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines. Open Veterinary Journal, 13 (4), 495-500. doi:10.5455/OVJ.2023.v13.i4.12 Chicago Style Di-paolo, Antonella, Lucia Anzalone, Martina Pellegrini, Giulio Sveri, and Monica Cagiola. "Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines." Open Veterinary Journal 13 (2023), 495-500. doi:10.5455/OVJ.2023.v13.i4.12 MLA (The Modern Language Association) Style Di-paolo, Antonella, Lucia Anzalone, Martina Pellegrini, Giulio Sveri, and Monica Cagiola. "Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines." Open Veterinary Journal 13.4 (2023), 495-500. Print. doi:10.5455/OVJ.2023.v13.i4.12 APA (American Psychological Association) Style Di-paolo, A., Anzalone, . L., Pellegrini, . M., Sveri, . G. & Cagiola, . M. (2023) Cytotoxicity assay as potential alternative method to animal testing for batch release of Italian fish autogenous vaccines. Open Veterinary Journal, 13 (4), 495-500. doi:10.5455/OVJ.2023.v13.i4.12 |