| Short Communication | ||
Open Vet J. 2022; 12(6): 1027-1034 Open Veterinary Journal, (2022), Vol. 12(6): 1027–1034 Short Communication Inhibition of African swine fever virus replication by β-glucanHa Thi Thanh Tran1†, Anh Duc Truong1†, Nhu Thi Chu1, Hoai Nam Vu1, Huyen Thi Nguyen1, Tinh Nguyen2, Fatimah Siti2, Hans Lee2, Alexander De Leon2, Andrew G. Yersin3 and Hoang Vu Dang1*1Department of Biochemistry and Immunology, National Institute of Veterinary Research, 86 Truong Chinh, Dong Da, Hanoi 100000, Vietnam 2Kemin Animal Nutrition and Health, Asia Pacific 12 Senoko Drive, 758200 Singapore 3Kemin Industries, Inc. Des Moines, IA 50317, USA †Both authors contributed equally to this work Submitted: 15/09/2022 Accepted: 22/11/2022 Published: 21/12/2022 *Corresponding Author: Hoang Vu Dang. Department of Biochemistry and Immunology, National Institute of Veterinary Research, Hanoi, Vietnam. Email: dangnivr [at] yahoo.com © 2022 Open Veterinary Journal
AbstractBackground: African swine fever (ASF) is one of the most important diseases in pigs because of its effects on all ages and breeds. To date, commercial vaccines and drugs for the prevention of ASF are lacking in the market and the survival of African swine fever virus (ASFV) in various environmental, farm, and or feed matrices has allowed the virus to remain, causing new outbreaks in the pig population. Besides biosecurity and animal husbandry management practices, the improvement of the host immune responses is critical to control, managing, and preventing ASF. Aim: In this study, we investigated the protective role of β-glucan against ASFV infection using a porcine alveolar macrophage (PAM) model. Methods: The effects of β-glucan on cell proliferation were evaluated by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The potential effects of β-glucan against a field ASFV strain isolated in Vietnam were further examined by real-time PCR and hemadsorption assays. The interferon (IFN)-α and interleukin (IL)-6 protein production induced by β-glucan was determined using a sandwich enzyme-linked immunosorbent assay. Results: Our results demonstrated that the β-glucan additive possessed an immune stimulus factor against ASFV. Specifically, protection of PAMs against ASFV infection in vitro was observed at 12 hours (p < 0.05) at the tested doses (30 and 50 µg/ml) as induced by incubation with β-glucan for 2 hours. These effects remained until 24 hours after post-infection. Additionally, at a high dose (50 µg/ml), pre-treatment with the β-glucan statistically increased the expression levels of IFNα and IL-6 when compared to untreated groups or only ASFV infection. Conclusion: Together, these findings indicated that the β-glucan may protect the host against ASFV infection via the multiple cellular immune mechanisms. Keywords: African swine fever virus, β-glucan, Cytokine. IntroductionAfrican swine fever (ASF) is one of the most important diseases in pigs because of its effects on all ages and breeds and is reported to the World Health Organization for Animal Health (OIE). In Asia, the first ASF outbreak was reported in China in 2018 and the ASF outbreaks were reported in Mongolia, Vietnam, Cambodia, North Korea, Laos, Myanmar, Philippines, South Korea, Timor-Leste, Indonesia, Papua New Guinea, India, and Malaysia (Vergne et al., 2020). African swine fever virus (ASFV) is highly virulent, and both the morbidity and mortality can be up to 100%, depending on the virulence of the virus, the host, and the transmission cycles (Kapoor et al., 2017). To date, the vaccines and drugs for the prevention of ASF are lacking in the market and the survival of ASFV in various environmental, farm, and or feed matrices has allowed the virus to remain to cause new outbreaks in the pig population (Cubillos et al., 2013; Kapoor et al., 2017). Besides biosecurity and animal husbandry management practices, the improvement of the host immune responses is critical to control, manage and prevent ASF. β1,3-D-glucans (β-glucans) are naturally occurring glucose polymers forming structural components of cell walls of several species and are active biologically as immunomodulators or biological response modifiers (Moorlag et al., 2020; Iswarya et al., 2022; Wang et al., 2022). The function of glucans has been well identified and characterized in vivo and in vitro and includes antiviral activities, inhibition of cancer growth, induction of cell proliferation, and reduction of stress or cholesterol level (Anusuya and Sathiyabama, 2015; Dos Santos et al., 2019; Krishnan et al., 2022). Recently, the major function of glucans has been reported as the inhibition of pathogens such as leptospira, mycobacterium, influenza infection, and cancer because it enhances the proliferation and phagocytotic capacity of macrophages, as well as the oxidative burst of monocytes, macrophages, and neutrophils (Hetland et al., 1998; Wang et al., 2019; Moorlag et al., 2020; Peymaeei et al., 2020). In addition, the role of glucans was reported to affect the immune system by induced cytokines expression which is primarily associated with host responses to infectious diseases (Nemoto et al., 1994; Soltys and Quinn, 1999). The research demonstrated that treatment with glucan induced the proliferation of dendritic and macrophages cells, and produced chemokine and cytokines such as interferons (IFNs), tumor necrosis factor (TNF)-α, interleukin (IL)-10, IL-6, IL-12, IL-1β, IL-12, CXCL2 via binding and activation of dectin-1 (a type II transmembrane protein receptor), activated the associated toll-like receptor (TLR) signaling, JAK-STAT signaling, and also NF-κβ signaling pathways (Soltys and Quinn, 1999; Wouters et al., 2002; Harada et al., 2006; Ikeda et al., 2007; Mochizuki et al., 2013; Su et al., 2020). Furthermore, glucan can activate the immune system to produce IFNs which induces the antimicrobial functions of macrophages by increasing pinocytosis and phagocytosis, and enhancing the host inhibition of microbial infections (Soltys and Quinn, 1999; Fan et al., 2006; Werner et al., 2009). In addition, the cell-surface major histocompatibility complex (MHC) class I, class II and related genes have been shown to be upregulated by IFN-γ and IFN-α induction, which increased the cytotoxic T-cell and activation of CD4+ and CD8+ T cells, which play important roles in the host response to infectious diseases (Konopski et al., 1994; Harada et al., 2006). In this study, we evaluated the ability of β-glucan to inhibit the ASFV and produce IFN-α and IL-6 in primary porcine alveolar macrophages (PAMs). Materials and MethodsExperimental designThe β-glucan was provided by Kemin Industries, Singapore and the chemical structures of β-glucan are shown in Figure (1A). To evaluate the potential effects of β-glucan on ASFV infection and cytokine production, the cells were pre-treated with 30 and 50 µg/ml of glucan for 2 hours and then spiked with the ASFV/Vietnam/Pig/HN02 virus strain (p72 genotype II, CD2v serotype 8, I73R/I329L variant I and A179L/A137R variant 2 (Tran et al., 2021). The final concentration of ASFV was 103 hemadsorption (HAD)50/ml. Negative controls were inoculated with phosphate-buffered saline (Sigma-Aldrich), while positive controls were inoculated with 103 HAD50 of ASFV. The samples from the experimental and control groups were collected at 12, 24, or 36 hours post-inoculation (hpi).
Fig. 1. (A) Chemical structures of β-glucan. (B) The effects of β-glucan on PAMs cell at the cell viability by MTT assay. The data are presented as the mean ± SEM of three independent experiments indicating the significant differences between the control and treatment groups were determined by a one-way analysis of variance. a: p < 0.05. Cell proliferation was measured by MTT assay: briefly, PAMs were harvested, counted by hemocytometer, and diluted with medium, yielding a concentration of 106 cells/ml. From this cell suspension, 100 μl was pipetted into 96-well microtiter plates (Nunc, Denmark) and incubated for 24 hours in a 5% CO2 incubator at 37°C. Cells were then treated with 30 and 50 µg/ml of glucan. After adding the glucan, a new medium was added to make the final volume of 200 μl per well. The plate was then incubated in a 5% CO2 incubator at 37°C for 24, 48, 72, 96, and 120 hours for determining the cell proliferation by MTT kit (Abcam, Waltham, MA) according to the manufacturer’s protocols. The HAD assay was performed as described previously (Thanh et al., 2021). The HAD assay was observed for 5 days, and 50% HAD doses (HAD50) were calculated by using the method of Reed and Muench (1938). The genomic DNA of ASFV in the supernatant was detected by the real-time PCR targeting the ASFV p72 gene as recommended by OIE (2012). The IFN-α and IL-6 production induced by β-glucan was also assessed in this study. After 12- or 24-hour treatment, the supernatant was collected, and the cytokine production was determined using a sandwich enzyme-linked immunosorbent assay (ELISA). The cytokines were further analyzed using the porcine IFN-α and IL-6 ELISA kit according to the manufacturer’s instructions (Invitrogen, Waltham, MA). Statistical analysisStatistical analysis was performed using IBM SPSS software (SPSS 23.0 for Windows; IBM, Chicago, IL). A p-value < 0.05 was considered to be statistically significant. Differences among the groups were tested by Duncan’s multiple comparison methods. Ethical approvalThe study was conducted in compliance with the institutional rules for the care and use of laboratory animals and using a protocol approved by the Ministry of Agriculture and Rural Development (MARD) Vietnam (TCVN 8402:2010). Results and Discussionβ-glucan has been demonstrated to enhance the cell proliferation and differentiation of various cells such as macrophage cells (RAW 264.7 cell, THP-1 cell), or T cells (Xiao et al., 2004; Muramatsu et al., 2014; Dos Santos et al., 2019). Several research studies have demonstrated that various forms of β-glucan can be antiviral, antitumor, or antibacterial activities (Xiao et al., 2004; Maity et al., 2019; Wang et al., 2019, 2022; Liu et al., 2021; Krishnan et al., 2022). Furthermore, β-glucan can be used as an immunomodulator, particularly acting as a stimulator of cytokine production in vitro and in vivo (Werner et al., 2009; Maity et al., 2019; Wang et al., 2019). We investigated if the β-glucan would or could affect ASFV growth and the production of important immune system cytokines such as IFNα and IL-6 in PAMs. In this study, β-glucan can enhance PAM cell proliferation as evidenced in two different doses of β-glucan when compared with the untreated control group (Fig. 1B). The rate of survival of PAMs treatment with β-glucan was highest at 50 µg/ml β-glucan, especially at 120-hour post-treatment, compared to un-treatment and 30 µg/ml β-glucan. Our results demonstrated that 30 or 50 µg/ml β-glucan can enhance cell survival in a dose-dependent manner and these effects remained until 120 hours compared with the control. Recently, several reports demonstrated that β-glucan can inhibit the growth of several pathogens such as leptospira, influenza, mycobacteria, and tuberculosis (Hetland et al., 1998; Wang et al., 2019; Moorlag et al., 2020). However, the potential effects of β-glucan against ASFV infection in the host remained unclear. To evaluate the role of β-glucan against ASFV infection, two doses of β-glucan (30 and 50 µg/ml) were used to show efficacy against a field ASFV strain isolated in Vietnam at 103 HAD50/ml. As shown in Figure 2A, PAMs pre-incubated with β-glucan for 2 hours reduced significantly ASFV invasion at 12 hours post-infection compared with the control group. These effects remained statistically until 24 hours and then reached normal levels at 36 hours post-infection. Moreover, the titer of ASFV confirmed by HAD assay showed that the titer of ASFV was significantly reduced at 12 hours post-infection when compared to the positive control. Specifically, the titer of the virus at 12 hours post-infection was 102.16 HAD50/ml for 50 µg/ml of β-glucan and 102.40 HAD50/ml for 30 µg/ml of β-glucan, when compared to a positive control (102.67 HAD50/ml) with p < 0.05 (Fig. 2B). A modest effect at living viral levels was noted at 24 hours post-infection in this study. Similarly, the antiviral activity of β-glucan inhibiting the pathogens on macrophages has been demonstrated for influenza A virus, mycobacteria, tuberculosis, and herpes simplex virus 1 and indicated that β-glucan significantly decreased the pathogen growth and induced the cytokines expression through activating the JAK-STAT and TLR signaling pathways, especially produce type I IFN genes (Harada et al., 2006; Wang et al., 2019; Moorlag et al., 2020; Lopes et al., 2021; Perveen et al., 2021; Krishnan et al., 2022; Pan et al., 2022). The findings from our study have shown that β-glucan protected the host against the ASFV invasion in PAMs.
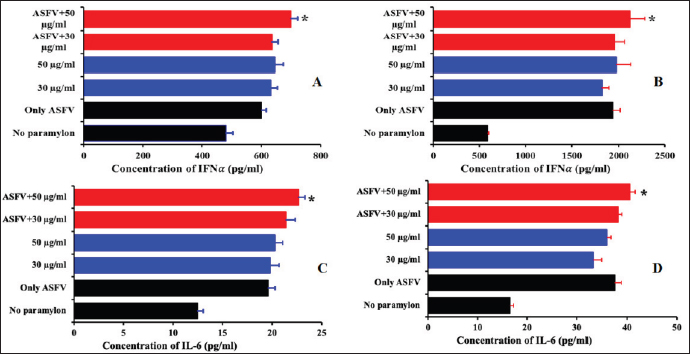
Fig. 2. The antiviral activity of β-glucan against PAMs. PAMs were pre-treated with 30 µg/ml and 50 µg/ml β-glucan, and exposed to 103 HAD50/ml ASFV for 12, 24, or 36 hours. The viral DNA of ASFV in the supernatants was collected and then real-time PCR amplification of the p72 gene was performed to detect the presence of viral DNA in the PAMs treated with β-glucan. (A) Real-time PCR amplification of the p72 gene was performed to detect the presence of viral DNA at 12, 24, and 36 hours post-infection in the PAMs treated with β-glucan for 2 hours and then infected with 103 HAD50/ml ASFV; and (B) Titer of ASFV in the PAMs pre-treated with 30 or 50 µg/ml β-glucan in 2 hours and then exposed to 103 HAD50/ml ASFV at 12 and 24 hours post-treatment. The data were presented as the mean ± SEM of three independent experiments. *: p < 0.05. It has been indicated previously that β-glucans are active as an immunomodulator and regulate both innate and adaptive immunity (Ishibashi et al., 2005; Amphan et al., 2019; Dos Santos et al., 2019). The ability of β-glucans in the innate immune system to recognize and respond to pathogen infection plays an important role in controlling the infection (Hetland et al., 2000; Guselle et al., 2007; Bayrak et al., 2008; Chen et al., 2020; Lee et al., 2020; Moorlag et al., 2020; Krishnan et al., 2022). The ability of β-glucans in the innate immunity system was inducement of cytokines and immune-related genes against pathogen infection such as IFNs, IL-1β, IL-12, IL-6, TNF-α, IL-10, and TLR2/6 through activated and associated signaling pathways such as NF-κB, MAPK, TLR, JAK-STAT (Muramatsu et al., 2014; Udayangani et al., 2017; Wang et al., 2019; Moorlag et al., 2020; Peymaeei et al., 2020; Liu et al., 2021; Pan et al., 2022). The research suggests that β-glucans may involve activation pathways and influence the innate immune system in response to pathogen infection. Additionally, IFNα and IL-6 play an important role in fighting viral infections. IL-6 is a regulatory cytokine that activates natural killer, T helper 1 (Th1), and Th17 cells to produce IFN and reduce apoptosis and cytolysis (Ikeda et al., 2007). Therefore, IFNs plays an important role in activating the process of phagocytosis and improving protection against intracellular viral infections (Mochizuki et al., 2013; Udayangani et al., 2017; Dos Santos et al., 2019; Wang et al., 2019; Liu et al., 2021; Lopes et al., 2021; Pan et al., 2022). A recent study showed that ASFV infection at the doses of 1 and 0.1 MOI for 24 hours significantly increased IFNα production in PAMs when compared to control in PAMs (Li et al., 2021; Wang et al., 2021), suggesting the protective role of type I IFN in response to ASFV infection. Additionally, the activation of this IFN expression may inhibit ASFV infection through the cGAS/STING signal pathway (Li et al., 2021; Wang et al., 2021). Our results showed that the production of IFNα and IL-6 were significantly increased in both treated groups (30 μg/ml or 50 μg/ml) at 12 hours and remained until 24 hours when compared with the control groups (p < 0.05) (Fig. 3). As expected, ASFV infection at 103 HAD50/ml induced a significant increase in the production of these cytokines. Interestingly, at the high dose of this chemical (50 μg/ml), a statistical enhancement of IFN-α and IL-6 secretion induced by β-glucan was observed when compared to only ASFV infection in these experiments (p < 0.05). The results indicated that the high dose of β-glucan enhanced significantly the IFN-α and IL6 production in PAMs in response to ASFV infection. The findings suggested the potential protective application of β-glucan in which this additive may exert its preventive effects against ASFV invasion by activating IFNα and IL6.
Fig. 3. Cytokine production by the PAMs infected with ASFV. PAMs were pre-treated with 30 or 50 µg/ml β-glucan for 2 hours and then exposed to 103 HAD50/ml ASFV for 12 (A, C) and 24 hours (B, D). The data are presented as the mean ± SEM of three independent experiments. *: p < 0.05. ConclusionOur results pose an interesting question about the protective effect and mode actions of β-glucan in response to ASFV infection. The results from this study showed that β-glucan may exert its effects to reduce/inhibit ASFV invasion in vitro via the cellular immune mechanisms in a dose-dependent manner. Additionally, these findings suggest that β-glucan is a good candidate to improve the host immune systems against ASFV circulating in Vietnam. AcknowledgmentsThis work was supported by the Ministry of Science and Technology to Hoang Vu Dang (Project No. ĐTĐL.CN-75/19) and IAEA (Reducing the Incidence and Impact of Transboundary Animal and Zoonotic Disease – the project No. VIE5023) for the research facility. The author also would like to thank Kemin Industries for providing the funding and in-kind resources necessary to complete this project. Authors’ contributionHTTT, ADT, and HVD conceived and designed the experiments. HTTT, ADT, NTC, HTN, HNV, and ADT performed the experiments. HTTT, ADT, TN, FS, HL, AL, AGY, and HVD analyzed the data. HVD and KL contributed the reagents, materials, and analytical tools. HTTT, ADT, TN, FS, HL, AL, AGY, and HVD wrote the manuscript. All authors read and approved the final manuscript. Conflict of interestThe authors declare that they have no competing interests. ReferencesAmphan, S., Unajak, S., Printrakoon, C. and Areechon, N. 2019. Feeding-regimen of beta-glucan to enhance innate immunity and disease resistance of Nile tilapia, Oreochromis niloticus Linn., against Aeromonas hydrophila and Flavobacterium columnare. Fish Shellfish Immunol. 87, 120–128. Anusuya, S. and Sathiyabama, M. 2015. Beta-D-glucan nanoparticle pre-treatment induce resistance against Pythium aphanidermatum infection in turmeric. Int. J. Biol. Macromol. 74, 278–282. Bayrak, O., Turgut, F., Karatas, O.F., Cimentepe, E., Bayrak, R., Catal, F., Atis, O., Akcay, A. and Unal, D. 2008. Oral beta-glucan protects kidney against ischemia/reperfusion injury in rats. Am. J. Nephrol. 28, 190–196. Chen, C., Hua, D., Shi, J., Tan, Z., Zhu, M., Tan, K., Zhang, L. and Huang, J. 2021. Porcine immunoglobulin Fc fused P30/P54 protein of african swine fever virus displaying on surface of S. Cerevisiae elicit strong antibody production in swine. Virol. Sin. 36(2), 207–219. Cubillos, C., Gomez-Sebastian, S., Moreno, N., Nun ez, M.C., Mulumba-Mfumu, L.K., Quembo, C.J., Heath, L., Etter, E.M., Jori, F., Escribano, J.M. and Blanco, E. 2013. African swine fever virus serodiagnosis: a general review with a focus on the analyses of African serum samples. Virus Res. 173, 159–167. Dos Santos, J.C., Barroso de Figueiredo, A.M., Teodoro Silva, M.V., Cirovic, B., de Bree, L.C.J., Damen, M., Moorlag, S., Gomes, R.S., Helsen, M.M., Oosting, M., Keating, S.T., Schlitzer, A., Netea, M.G., Ribeiro-Dias, F. and Joosten, L.A.B. 2019. Beta-glucan-induced trained immunity protects against Leishmania braziliensis infection: a crucial role for IL-32. Cell Rep. 28, 2659–2672. e2656. Fan, H., Williams, D.L., Breuel, K.F., Zingarelli, B., Teti, G., Tempel, G.E., Halushka, P.V. and Cook, J.A. 2006. Gi proteins regulate lipopolysaccharide and Staphylococcus aureus induced cytokine production but not (1--> 3)-beta-D-glucan induced cytokine suppression. Front Biosci. 11, 2264–2274. Guselle, N.J., Markham, R.J. and Speare, D.J. 2007. Timing of intraperitoneal administration of beta-1,3/1,6 glucan to rainbow trout, Oncorhynchus mykiss (Walbaum), affects protection against the microsporidian Loma salmonae. J. Fish Dis. 30, 111–116. Harada, T., Kawaminami, H., Miura, N.N., Adachi, Y., Nakajima, M., Yadomae, T. and Ohno, N. 2006. Cell to cell contact through ICAM-1-LFA-1 and TNF-alpha synergistically contributes to GM-CSF and subsequent cytokine synthesis in DBA/2 mice induced by 1,3-beta-D-glucan SCG. J. Interferon Cytokine Res. 26, 235–247. Hetland, G., Lovik, M. and Wiker, H.G. 1998. Protective effect of beta-glucan against mycobacterium bovis, BCG infection in BALB/c mice. Scand. J. Immunol. 47, 548–553. Hetland, G., Ohno, N., Aaberge, I.S. and Lovik, M. 2000. Protective effect of beta-glucan against systemic Streptococcus pneumoniae infection in mice. FEMS Immunol. Med. Microbiol. 27, 111–116. Ikeda, M., Minari, J., Shimada, N., Numata, M., Sakurai, K. and Shinkai, S. 2007. Complex formation between cationic beta-1,3-glucan and hetero-sequence oligodeoxynucleotide and its delivery into macrophage-like cells to induce cytokine secretion. Org. Biomol. Chem. 5, 22192224. Ishibashi, K., Yoshida, M., Nakabayashi, I., Shinohara, H., Miura, N.N., Adachi, Y. and Ohno, N. 2005. Role of anti-beta-glucan antibody in host defense against fungi. FEMS Immunol. Med. Microbiol. 44, 99–109. Iswarya, A., Anjugam, M., Gopi, N., Shanthi, S., Govindarajan, M., Alharbi, N.S., Kadaikunnan, S., Alharbi, M.S., Sivakamavalli, J. and Vaseeharan, B. 2022. Beta-1,3-glucan binding protein-based silver nanoparticles enhance the wound healing potential and disease resistance in Oreochromis mossambicus against Aeromonas hydrophilla. Microb. Pathog. 162, 105360. Kapoor, R., Sharma, B. and Kanwar, S.S. 2017. Antiviral phytochemicals: an overview. biochem physiol: open access 06. Konopski, Z., Seljelid, R. and Eskeland, T. 1994. IFN-gamma inhibits internalization of soluble aminated beta-1,3-D-glucan by macrophages and thereby down-regulates the glucan induced release of TNF-alpha and IL-1 beta. Scand. J. Immunol. 40, 57–63. Krishnan, R., Jang, Y.S. and Oh, M.J. 2022. Beta glucan induced immune priming protects against nervous necrosis virus infection in sevenband grouper. Fish Shellfish Immunol. 121, 163–171. Lee, P.T., Liao, Z.H., Huang, H.T., Chuang, C.Y. and Nan, F.H. 2020. Beta-glucan alleviates the immunosuppressive effects of oxytetracycline on the non-specific immune responses and resistance against Vibrio alginolyticus infection in Epinephelus fuscoguttatus x Epinephelus lanceolatus hybrids. Fish Shellfish Immunol. 100, 467–475. Li, J., Song, J., Kang, L., Huang, L., Zhou, S., Hu, L., Zheng, J., Li, C., Zhang, X., He, X., Zhao, D., Bu, Z. and Weng, C. 2021. pMGF505-7R determines pathogenicity of African swine fever virus infection by inhibiting IL-1beta and type I IFN production. PLoS Pathog. 17, e1009733. Liu, Y., Liu, X., Yang, L., Qiu, Y., Pang, J., Hu, X., Dong, Z., Liu, Z. and Jin, X. 2021. Adjuvanticity of beta -glucan for Vaccine Against Trichinella spiralis. Front Cell Dev. Biol. 9, 701708. Lopes, J.L., Quinteiro, V.S.T., Wouk, J., Darido, M.L., Dekker, R.F.H., Barbosa-Dekker, A.M., Vetvicka, V., Cunha, M.A.A., Faccin-Galhardi, L.C. and Orsato, A. 2021. Sulfonated and carboxymethylated beta-glucan derivatives with inhibitory activity against herpes and Dengue Viruses. Int. J. Mol. Sci. 22(20), 11013. Maity, G.N., Maity, P., Choudhuri, I., Bhattacharyya, N., Acharya, K., Dalai, S. and Mondal, S. 2019. Structural studies of a water insoluble beta-glucan from Pleurotus djamor and its cytotoxic effect against PA1, ovarian carcinoma cells. Carbohydr. Polym. 222, 114990. Mochizuki, S., Morishita, H. and Sakurai, K. 2013. Macrophage specific delivery of TNF-alpha siRNA complexed with beta-1,3-glucan inhibits LPS-induced cytokine production in a murine acute hepatitis model. Bioorg. Med. Chem. 21, 2535–2542. Moorlag, S., Khan, N., Novakovic, B., Kaufmann, E., Jansen, T., van Crevel, R., Divangahi, M. and Netea, M.G. 2020. Beta-glucan induces protective trained immunity against Mycobacterium tuberculosis infection: a key role for IL-1. Cell Rep. 31, 107634. Muramatsu, D., Kawata, K., Aoki, S., Uchiyama, H., Okabe, M., Miyazaki, T., Kida, H. and Iwai, A. 2014. Stimulation with the Aureobasidium pullulans-produced beta-glucan effectively induces interferon stimulated genes in macrophage-like cell lines. Sci. Rep. 4, 4777. Nemoto, J., Ohno, N., Saito, K., Adachi, Y. and Yadomae, T. 1994. Analysis of cytokine mRNAs induced by the administration of a highly branched (1-->3)-beta-D-glucan, OL-2. Biol. Pharm. Bull. 17, 948–954. OIE. 2012. African swine fever. In: Manual of diagnostic tests and vaccines for terrestrial animals. Pan, Y., Li, J., Xia, X., Wang, J., Jiang, Q., Yang, J., Dou, H., Liang, H., Li, K. and Hou, Y. 2022. Beta-glucan-coupled superparamagnetic iron oxide nanoparticles induce trained immunity to protect mice against sepsis. Theranostics 12, 675–688. Perveen, S., Yang, L., Zhou, S., Feng, B., Xie, X., Zhou, Q., Qian, D., Wang, C. and Yin, F. 2021. Beta-1,3-glucan from Euglena gracilis as an immunostimulant mediates the antiparasitic effect against Mesanophrys sp. on hemocytes in marine swimming crab (Portunus trituberculatus). Fish Shellfish Immunol. 114, 28–35. Peymaeei, F., Sadeghi, F., Safari, E., Khorrami, S., Falahati, M., Roudbar Mohammadi, S. and Roudbary, M. 2020. Candida albicans beta-glucan induce anti- cancer activity of mesenchymal stem cells against lung cancer cell line: an in-vitro experimental study. Asian Pac. J. Cancer Prev. 21, 837–843. Reed, L.J. and Muench, H. 1938. A simple method of estimaing fifty percent endpoints. Am J Epidemiol 27, 493–497. Soltys, J. and Quinn, M.T. 1999. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with beta-(1,6)-branched beta-(1,3)-glucan. Infect. Immun. 67, 244–252. Su, C.H., Lu, M.K., Lu, T.J., Lai, M.N. and Ng, L.T. 2020. A (1-->6)-branched (1-->4)-beta-d-glucan from Grifola frondosa Inhibits Lipopolysaccharide-Induced Cytokine Production in RAW264.7 Macrophages by Binding to TLR2 Rather than Dectin-1 or CR3 Receptors. J. Nat. Prod. 83, 231–242. Thanh, T.H.T., Duc, T.A., Viet, L.D., Van, H.T., Thi, N.C., Thi, C.N., Thi, N.H. and Vu, D.H. 2021. Rapid identification for serotyping of African swine fever virus based on the short fragment of the EP402R gene encoding for CD2-like protein. Acta Veterinaria 71, 98–106. Tran, H.T.T., Truong, A.D., Dang, A.K., Ly, D.V., Nguyen, C.T., Chu, N.T., Hoang, T.V., Nguyen, H.T. and Dang, H.V. 2021. Circulation of two different variants of intergenic region (IGR) located between the I73R and I329L genes of African swine fever virus strains in Vietnam. Transbound. Emerg. Dis. 68, 2693–2695. Udayangani, R.M.C., Dananjaya, S.H.S., Fronte, B., Kim, C.H., Lee, J. and De Zoysa, M. 2017. Feeding of nano scale oats beta-glucan enhances the host resistance against Edwardsiella tarda and protective immune modulation in zebrafish larvae. Fish Shellfish Immunol. 60, 72–77. Vergne, T., Guinat, C. and Pfeiffer, D.U. 2020. Undetected circulation of African swine fever in wild boar, Asia. Emerg. Infect. Dis. 26, 2480–2482. Wang, J., Jin, Z., Zhang, W., Xie, X., Song, N., Lv, T., Wu, D. and Cao, Y. 2019. The preventable efficacy of beta-glucan against leptospirosis. PLoS Negl. Trop. Dis. 13, e0007789. Wang, X., Ji, Y., Jin, D., Qi, J., Hou, X., Zhao, W., Zhou, S., Zhang, C., Luo, Y., An, P. and Luo, J. 2022. Natural polysaccharide beta-glucan protects against Doxorubicin-induced cardiotoxicity by suppressing Oxidative stress. Nutrients 14. Wang, Y., Kang, W., Yang, W., Zhang, J., Li, D. and Zheng, H. 2021. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: a review. Front Immunol. 12, 715582. Werner, J.L., Metz, A.E., Horn, D., Schoeb, T.R., Hewitt, M.M., Schwiebert, L.M., Faro-Trindade, I., Brown, G.D. and Steele, C. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182, 4938–4946. Wouters, I.M., Douwes, J., Thorne, P.S., Heederik, D. and Doekes, G. 2002. Inter- and intraindividual variation of endotoxin- and beta(1 --> 3)-glucan-induced cytokine responses in a whole blood assay. Toxicol. Ind. Health 18, 15–27. Xiao, Z., Trincado, C.A. and Murtaugh, M.P. 2004. Beta-glucan enhancement of T cell IFNgamma response in swine. Vet. Immunol. Immunopathol. 102, 315–320. | ||
| How to Cite this Article |
| Pubmed Style Duc TA, Tran HTT, Chu NT, Nguyen HT, Dang HV, Vu NH, Lee H, Siti F, Nguyen T, Leon AD, Yersin AG. Inhibition of African swine fever virus replication by β-glucan. Open Vet J. 2022; 12(6): 1027-1034. doi:10.5455/OVJ.2022.v12.i6.31 Web Style Duc TA, Tran HTT, Chu NT, Nguyen HT, Dang HV, Vu NH, Lee H, Siti F, Nguyen T, Leon AD, Yersin AG. Inhibition of African swine fever virus replication by β-glucan. https://www.openveterinaryjournal.com/?mno=113453 [Access: June 30, 2025]. doi:10.5455/OVJ.2022.v12.i6.31 AMA (American Medical Association) Style Duc TA, Tran HTT, Chu NT, Nguyen HT, Dang HV, Vu NH, Lee H, Siti F, Nguyen T, Leon AD, Yersin AG. Inhibition of African swine fever virus replication by β-glucan. Open Vet J. 2022; 12(6): 1027-1034. doi:10.5455/OVJ.2022.v12.i6.31 Vancouver/ICMJE Style Duc TA, Tran HTT, Chu NT, Nguyen HT, Dang HV, Vu NH, Lee H, Siti F, Nguyen T, Leon AD, Yersin AG. Inhibition of African swine fever virus replication by β-glucan. Open Vet J. (2022), [cited June 30, 2025]; 12(6): 1027-1034. doi:10.5455/OVJ.2022.v12.i6.31 Harvard Style Duc, T. A., Tran, . H. T. T., Chu, . N. T., Nguyen, . H. T., Dang, . H. V., Vu, . N. H., Lee, . H., Siti, . F., Nguyen, . T., Leon, . A. D. & Yersin, . A. G. (2022) Inhibition of African swine fever virus replication by β-glucan. Open Vet J, 12 (6), 1027-1034. doi:10.5455/OVJ.2022.v12.i6.31 Turabian Style Duc, Truong Anh, Ha Thi Thanh Tran, Nhu Thi Chu, Huyen Thi Nguyen, Hoang Vu Dang, Nam Hoai Vu, Hans Lee, Fatimah Siti, Tinh Nguyen, Alexander De Leon, and Andrew G Yersin. 2022. Inhibition of African swine fever virus replication by β-glucan. Open Veterinary Journal, 12 (6), 1027-1034. doi:10.5455/OVJ.2022.v12.i6.31 Chicago Style Duc, Truong Anh, Ha Thi Thanh Tran, Nhu Thi Chu, Huyen Thi Nguyen, Hoang Vu Dang, Nam Hoai Vu, Hans Lee, Fatimah Siti, Tinh Nguyen, Alexander De Leon, and Andrew G Yersin. "Inhibition of African swine fever virus replication by β-glucan." Open Veterinary Journal 12 (2022), 1027-1034. doi:10.5455/OVJ.2022.v12.i6.31 MLA (The Modern Language Association) Style Duc, Truong Anh, Ha Thi Thanh Tran, Nhu Thi Chu, Huyen Thi Nguyen, Hoang Vu Dang, Nam Hoai Vu, Hans Lee, Fatimah Siti, Tinh Nguyen, Alexander De Leon, and Andrew G Yersin. "Inhibition of African swine fever virus replication by β-glucan." Open Veterinary Journal 12.6 (2022), 1027-1034. Print. doi:10.5455/OVJ.2022.v12.i6.31 APA (American Psychological Association) Style Duc, T. A., Tran, . H. T. T., Chu, . N. T., Nguyen, . H. T., Dang, . H. V., Vu, . N. H., Lee, . H., Siti, . F., Nguyen, . T., Leon, . A. D. & Yersin, . A. G. (2022) Inhibition of African swine fever virus replication by β-glucan. Open Veterinary Journal, 12 (6), 1027-1034. doi:10.5455/OVJ.2022.v12.i6.31 |