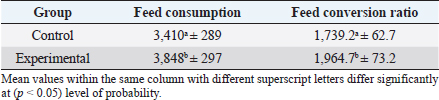| Original Article | ||
Open Vet J. 2021; 11(4): 780-788 Open Veterinary Journal, (2021), Vol. 11(4): 780–788 Original Research Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentationZiyad T. BenMahmoud1*, Bashir M. Sherif1, and Awatef M. Elfituri21Department of Animal Production, Faculty of Agriculture, University of Tripoli, Tripoli, Libya 2Department of Animal Production, Faculty of Agriculture, Misurata University, Misurata, Libya *Corresponding Author: Ziyad T. BenMahmoud. Department of Animal Production, Faculty of Agriculture, University of Tripoli, Tripoli, Libya. Email: ziadtaher [at] yahoo.com Submitted: 13/09/2021 Accepted: 20/11/2021 Published: 29/12/2021 © 2021 Open Veterinary Journal
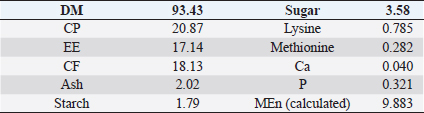
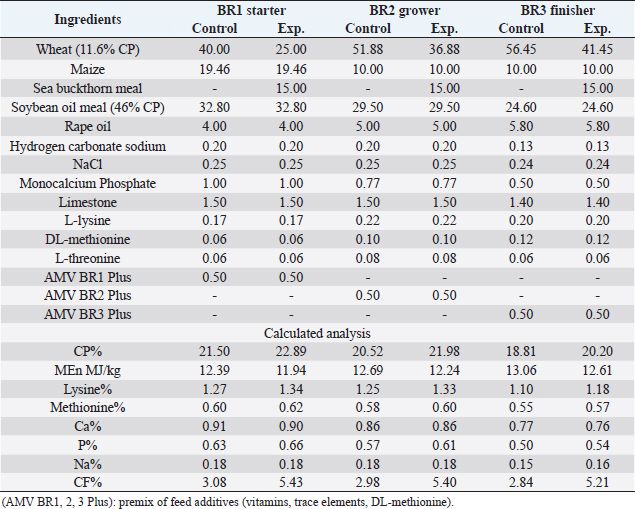
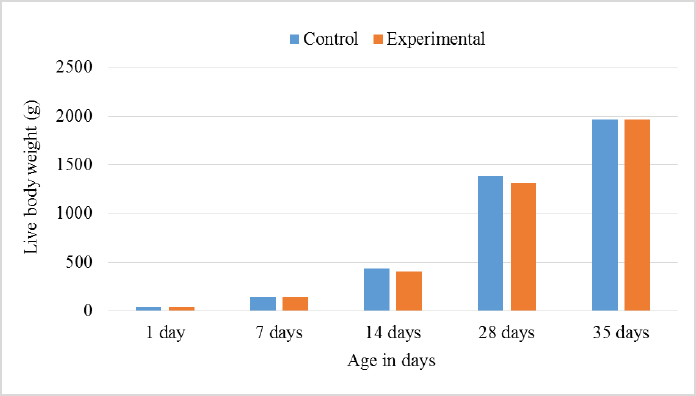
AbstractBackground: Improving poultry products is a constant research topic as the poultry industry aims to provide healthy products to consumers at economic prices and achieve financial profits for breeders. Therefore, recent research has resorted to finding cheap natural sources as alternatives to traditional feed and antibiotics. Aim: The experiment was conducted to study the effects of sea buckthorn fruit residues meal on broilers‘ performance and skin pigmentation. Method: A total of 700 broiler chicks (ROSS 308) were allotted into two groups, and each group comprises 350 birds divided into 10 replicants, 35 birds to each replicant. The experimental group was fed diets where 15% of the wheat was replaced by sea buckthorn residues meal. The control group was fed diets without any color additive. Feed and water were provided ad-libitum. Results: The sea buckthorn fruit residues contained 21% crude protein and metabolisable energy calculated was 9.88 MJ/kg. The live body weight was significantly lower than the experimental group (p < 0.05) on days 14 and 28. The feed conversion ratio was significantly poorer in the experimental group compared with the control group on days 35 of age (p < 0.05). The mortality was higher in the experimental group than in the control group. The DSM Broiler Fan assessed skin color. The skin of the broilers was significantly more yellowish in the experimental group than the control group (103.08 vs. 102.38 scales). The pigmentation of the skin was higher in males than females. Conclusion: The use of sea buckthorn residues in poultry feeding negatively affected the overall performance rate. Mightily, its use in diets depends mainly on the price of feed ingredients. Thus, improving the skin color and biological value of broiler meat can determine its usefulness in broiler feed. Keywords: Sea buckthorn residues, Broilers, Performance, Skin pigmentation. IntroductionSea buckthorn (Hippophae rhamnoides L.) has been used for several years in different forms, mainly in human nutrition, as a health supplement, due to the content of bioactive substances. It has been examined in recent years as a supplement to animal nutrition (Krejcarová et al., 2015). The reason for this is the assumption that the products of animals fed on Sea buckthorn will be enriched with valuable bioactive substances. Sea buckthorn is a hardy, deciduous shrub bearing small yellow to orange-red berries. It grows widely in Europe, central Asia, and temperate regions of South Asia, India, and China, ranging in altitude of a minimum of 60 m above sea level (ASL) to a maximum of 5,200 m ASL. Sea buckthorn is rich in carotenoids, xanthophylls, phenolics, and flavonoids and has a higher content of essential oils (Yang et al., 2000; Singh et al., 2006). The fruit and leaves are rich in nutrients and bioactive components such as vitamins (Kudritskaya et al., 1989; Zadernowski et al., 2003; Luhua et al., 2004; Ranjith et al., 2006), amino acids (Yushipitsina et al., 1988; Repyakh et al., 1990) lipids (Goncharova and Glushenkova, 1993; Ul´chenko et al., 1995; Bekker and Giuschenkova, 1997) sugars and acids (Yang, 2009), and flavonoids (Häkkinen et al., 1999). According to certain studies, sea buckthorn contains antioxidants such Carotene (vitamin A), Ascorbate (vitamin C), Tocopherol (vitamin E), and Glutathione (Geetha et al., 2002a, 2002b; Chawla et al., 2007; Püssa et al., 2007; Dorhoi et al., 2006; Geetha et al., 2009). Sea buckthorn decreases enzyme activity or hormonal status damage caused by immobilization or cold-hypoxia-restraint (Krylova et al., 2000; Saggu and Kumar, 2007). Shao et al. (2002) observed that colorants in sea buckthorn fruits are mainly composed of nine carotenoids. High content of carotenoids, xanthophylls, phenolic, and flavonoids in sea buckthorn are present (Yang et al., 2000; Singh et al., 2006). There are two sources of oil in sea buckthorn fruit: the seed contains 10%–15% (w/w) oil, and the pulpy fruit parts surrounding the seed contain 29%–48% oil. Both pulp and seed oils from sea buckthorn vary in vitamin E content depending on whether derived from seed oil (64.4 to 92.7 mg/100 g seed), juice oil (216 mg/100 g berry), or from the pulp after juice and seed removal (481 mg/100 g berry). Carotenoids also vary depending upon the source of the oil (Li, 2002). The seed cake left after oil extraction contained a higher level of crude protein (CP) (27% to 33.2%) and CF (15.0% to 21.9%) reported (Kaushal and Sharma, 2011). According to (Sharma, 2010) the dry matter (DM), CP, ether extract (EE), crude fiber (CF), total ash, nitrogen-free extract, calcium (Ca), phosphorus (P), and metabolizable energy (ME) values of sea buckthorn cake as 90.06%, 26.00%, 4.50%, 14.00%, 2.50%, 53.0%, 0.75%, 1.25% and 12.159 MJ/kg (2,906 kcal/kg), respectively. The leaves, seeds, and fruit residues of the sea buckthorn have the potential as a feed material for livestock and poultry. There are very little data on the use of sea buckthorn residues in poultry feeding. This experiment was conducted to determine the effect of a higher proportion of sea buckthorn fruit residues in the broiler diets on their performance and skin color. Material and MethodsThis experiment was conducted at the International Testing of Poultry-Ústrašice, Czech Republic. A total of 700 broiler chicks (Ross 308) was allotted into two groups, and each group was comprised of 350 birds divided into 10 replicants, 35 birds on each replicant. The chicks got housed in an air-conditioned hall on a deep litter of wood shavings. Feed and water were provided ad-libitum, and standard management practices of a commercial were applied. An automatic nipple drinker was used, and tube feeders were filled manually. The chicks were vaccinated against coccidiosis by LIVACOX T® applied to the water on day 7 of age. The lighting program for broilers was as follows: (day 1 to 7: 23 hours of light and 1 hour of darkness; day 8 to 32: 18 hours of light and 6 hours of darkness; day 33 to 35: 23 hours of light and 1 hour of darkness). The sea buckthorn fruits residue are berries from which the juice and part of oil were extracted by pressing, then dried. Proximate analyses of the sea buckthorn fruits residue were performed: DM, EE, CF, Ash – gravimetric methods; CP – Kjehldahl method; 82 starch and sugars – polarimetry; Ca and P – colorimetric; lysine and methionine – liquid chromatography. The nitrogen-corrected metabolizable energy (MEn) of the sea buckthorn fruits residue was estimated using the equation provided by the European Federation of Branches of the World‘s Poultry Association‘s Subcommittee Energy of Working Group No. 2 Nutrition (Zelenka et al., 1999). The analytical composition of sea buckthorn fruit residues is shown in Table 1. The chicks were fed (Table 2) a starter diet (BR1) from day 1 to 14, a grower diet (BR2) from day 15 to 28, and a finisher diet (BR3) from day 29 to 35 of age. The control group was without color additives supplement in the diets. In the experimental group of broilers, 15% of wheat in the diets got replaced by sea buckthorn fruit residues. 15% of sea buckthorn fruit residues in broiler diets increased contents of CP slightly, lysine and methionine, and slightly reduced content ME in experimental diets. However, the content of CF in the experimental diets with 15% of sea buckthorn fruit residues increased above the generally recommended level (Table 2). On days 7 and 14 of age, the chicks were weighed without fasting; on day 28 and 35 of age, all chicks were weighed individually after 12-hour fasting. The average weight of broiler chickens was determined in males and females and both sexes together. Consumption and feed conversion were determined on day 35 of age. The mortality of chicks was recorded daily. The skin color was determined with eight birds from each box (four males + four females). Skin color was assessed by the Dutch State Mines (DSM) Broiler Fan, expressed in a 1780–7880 scale. The data were subjected to analysis of variance followed by Duncan’s range test (analysis of variance). Ethical approvalThis study was approved by the Graduate School of the Czech University of Life Science in Prague, Faculty of Tropical AgriSciences, Department of Animal Sciences and Food Processing. All animal welfare protocols were followed. ResultsResults of the chemical composition of sea buckthorn residue meal in Table 1 showed that the DM was 93.43%, CP was 20.87%, EE was 17.14%, CF was 18.13%, Ash was 2.02%, and metabolic energy (MEn) was 9.883 kJ/kg. The results presented in Table 3 and Figure 1 revealed no significant differences (p < 0.05) in live body weights at 1 and 7 and 35 days of age between the broilers of the control group and the broilers fed on (15%) sea buckthorn fruit residue. On the other hand, the results at 14 and 28 days of age showed a significant (p < 0.05) increase in live body weights in the control group compared with the experimental group of broilers. Table 1. Chemical composition of sea buckthorn residue meal (%) and MEn (kJ/kg).
Table 2. Composition of the diets (%) and calculated analysis.
Table 3. Effect of Sea buckthorn on live body weight of broilers (X ± S.D).
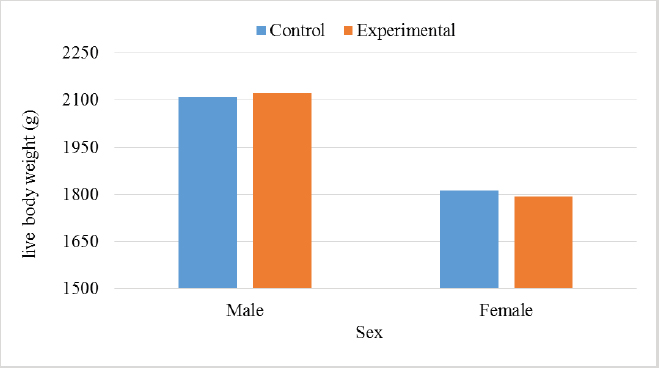
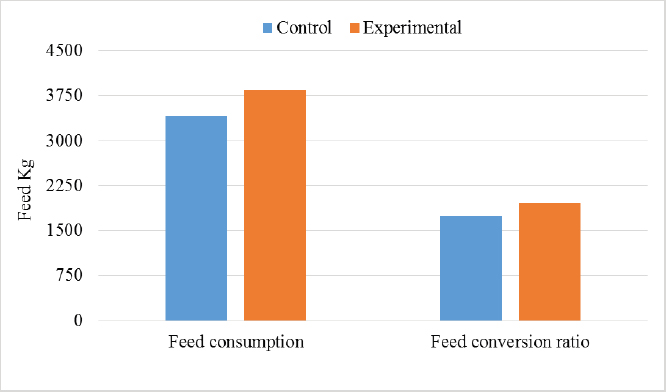
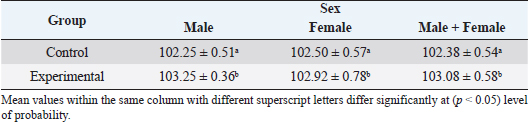
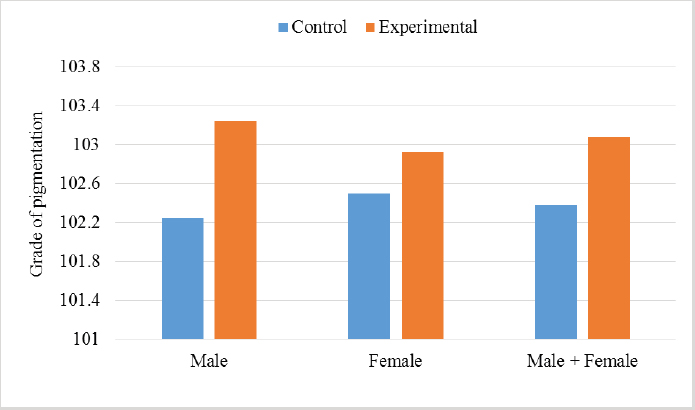
In the analysis of the results in Table 4 and Figure 2 to evaluate the effect of adding the sea buckthorn fruit residues on sex, it was found that there were no significant differences between males and females of the control group compared with males and females of the experimental group (2,109.0 & 1,812.4 g vs. 2 123.8 & 1 794.0 g), respectively. Furthermore, at the age of 35 days, numerically, the males were heavier, and the females were lighter in the experimental group than their counterparts in the control group. It was clear from Table 5 and Figure 3 that the results revealed an increase (p < 0.05) in the rate of feed consumption in broilers that fed on the sea buckthorn fruit residues compared to broilers of the control group (384 vs. 3,410 g) respectively. The results also indicated a remarkable increase (p < 0.05) in the food conversion ratio, where it was higher in the experimental group compared with the control group (1,964.7 vs. 1,739.2 g/kg), respectively. The natural pigments of sea buckthorn fruit residues were significantly efficient (p < 0.05) at increasing skin pigmentation in the experimental group (103.08) compared with the control group (102.38) as presented in Table 6 and Figure 4. In addition, the skin pigmentation of the males and females was significantly higher in the experimental group than in the control group.
Fig. 1. Effect of Sea buckthorn on live body weight of broilers. Table 4. Effect of Sea buckthorn on live body weight of broilers (male and female) on 35 days (X ± S.D).
Fig. 2. Effect of Sea buckthorn on live body weight of broilers (male and female) on 35 days. Table 5. Effect of Sea buckthorn on feed consumption (g/ bird) and feed conversion ratio (g/kg) in broilers on 35 days of age (X ± S.D).
Fig. 3. Effect of Sea buckthorn on feed consumption (g/bird) and feed conversion ratio (g/kg) in broilers on 35 days of age. Table 6. Effect of Sea buckthorn on skin color determination (X ± S.D).
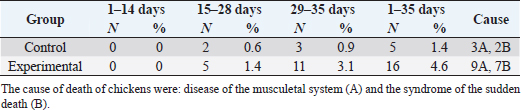
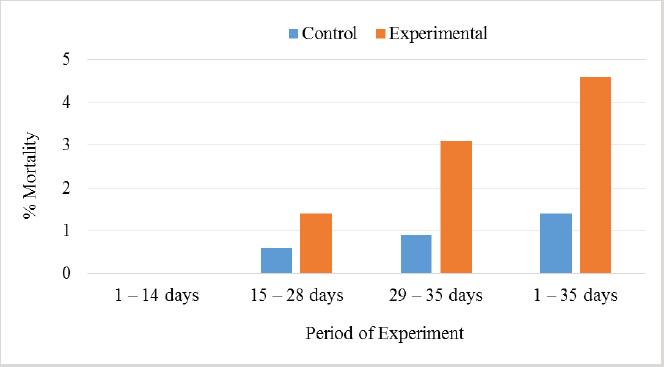
In Table 7 and Figure 5, the results showed that during the first 2 weeks, there were no cases of death in the birds of the entire experiment. Still, at the beginning of the third week, death cases appeared in the experimental and control groups. Although the number of dead birds in the experimental group was higher, the increased mortality rate was statistically insignificant. At the end of the experiment recorded 5 dead birds in the control group (1.4%) and 16 dead birds in the experimental group (4.6%). DiscussionThe chemical analysis of the sea buckthorn fruit residue showed lower CP content than reported (Sharma, 2010) – 26%, or (Kaushal and Sharma, 2011) – 27.7%–33% for sea buckthorn oil cake. Also calculated MEn were less compared with ME of sea buckthorn oil cake reported (Sharma, 2010): 9,883 kJ/kg versus 2,906 kcal/kg (12,159 kJ/kg). The results of chemical analysis were more in agreement with data reported by Singh (2012) for sea buckthorn fruit residues (CP 18.3%, EE 11.6%, CF 12.7%, lysine 0.84%, but differed in content of methionine (methionine + cysteine 0.06%), Ca 0.19% and P 0.15% (Table 1). The chemical composition of wheat grain is about (DM 85.9%–87.5%, CP 10.4%–13.2%, EE 0.91%–2.26%, CF 1.49%–2.36%, Ash 1.45%–1.85%, ME 15.7–16.4 MJ/kg (Barteczko et al., 2009). Therefore, the sea buckthorn fruit residues are a decent replacement where it is higher in DM, CP, EE, ash, and CF. The results of our study are similar to (Stef et al., 2012) that reported that the addition of dried sea buckthorn berry to feeds of broilers (ROSS 308) did not significantly improve the body weight. In addition, Solcan et al. (2011) reported that the broiler chicks who received sea buckthorn oil had a better body weight gain. Likely, higher CF content and lower metabolic energy of sea buckthorn fruit residues in the diets could be the cause of chickens’ lower live body weight. Indeed, although the high variability in weight gain of poultry fed on a diet containing 15% of the sea buckthorn fruit residues compared to the control group chickens, it is very important that the final weight of the poultry was similar (1,960.7 vs. 1,958.9 g). In other words, adding sea buckthorn fruit residues had no negative effect on the final body weight of broilers. In contrast, a negative effect was evident on the feed conversion ratio, and this is consistent with what was concluded by Stef et al. (2009, 2012), who indicated that the small addition of dried sea buckthorn berries (2%) to chicken diets broiler (ROSS 308) did not significantly improve feed efficiency. Otherwise, according to several articles (Yang and Kallio, 2005; Singh et al., 2006; Seglina, 2007), the fruits of sea buckthorn are rich in fats and fatty acids (omega-6) and (omega-3). In addition, they have beneficial effects on feed conversion ratio, thigh muscle quality, and breast muscle in broilers (Zollitsch et al., 1997; Crespo and Esteve-Garcia, 2001; Azman et al., 2005; Stobdan et al., 2013). In addition, the beneficial effect of the sea buckthorn flavones on poultry has been observed by some researchers, and according to (Li et al., 2008; Zhao et al., 2012), the supplementation of flavones from sea buckthorn to broilers significantly increased their growth, conversion of feeds, meat quality, carcass percentage apparent digestibility of dietary CP and Ca. As mentioned above, it is possible that higher content of CF and lower content of ME in the sea buckthorn fruit residues in the experimental diets (Table 2) could adversely affect the feed conversion ratio in the experimental group broilers. The results also indicated an increase in the rate of feed consumption in the experimental group. Therefore, the use of buckthorn as a substitute for wheat depends on their prices. as high wheat prices may make the use of buckthorn residues very economically feasible despite the increase in feed consumption that may result from its use. The increase in feed consumption is also a good indicator of poultry palatability of sea buckthorn in the feed. This may open the way for the use of sea buckthorn in feed under heat stress, resulting in lower feed consumption rates in birds. In terms of skin pigmentation, broilers fed on diets containing 15% sea buckthorn residues demonstrated that their skin significant were more yellowish than the control group. This alteration in skin color results from natural yellow pigments of sea buckthorn fruit, where Yang et al. (2000) reported that sea buckthorn is rich in carotenoids, xanthophylls, phenolics, and flavonoids. our findings agreed with the previous study (Ben-Mahmoud et al., 2014), where birds fed on diets with 5% of sea buckthorn fruit residue meal. In addition, (Li et al., 2008) mentioned that the broiler diets supplemented with 0.1% and 0.2% flavones of sea buckthorn significantly increased the meat color of breast and thigh muscle. It is probably dependent on the content of oil in the residues of the sea buckthorn after pressing because carotenoids are bound to the oil content in seeds and fruit pulp, as described by Beveridge et al. (1999). The mortality rate of broilers was numerically higher in the experimental group compared to the control group (Fig. 2); however, both groups were in an acceptable mortality rate. In the whole period of the study, 5 birds were dead in the control group and 16 birds dead in the experimental group. The cause of death was a disease of the musculetal system and the sudden death syndrome through the veterinary examination. So, based on the causes of death of the broilers in the experimental group, it is obvious that the mortality rate was not caused by sea buckthorn fruit residues in the diets.
Fig. 4. Effect of Sea buckthorn on skin pigmentation. Table 7. Effect of Sea buckthorn on mortality rate of broilers.
Fig. 5. Effect of Sea buckthorn on mortality rate of broilers. ConclusionIt was concluded that replacing 15% of wheat with sea buckthorn fruit residues did not negatively affect the broilers‘ final weight and increased the pigmentation of the broiler‘s skin. On the other hand, it negatively affected the feed conversion ratio and, to a lesser extent, the mortality rate. Indeed, although it seems evident that replacing 15% of wheat with sea buckthorn fruit residues in broiler feeds caused a deterioration of the feed conversion ratio and thus the economic viability of raising broiler, the possibility of offsetting the high costs of breeding by increasing the biological quality of broilers is a probable matter and needs more research in this regard. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAzman, M.A., Cerci, I.H. and Birben, N. 2005. Effects of various dietary fat sources on performance and body fatty acid composition of broiler chickens. Turkish J. Vet. Anim. Sci. 20, 811–819. Barteczko, J., Augustyn, R., Lasek, O. and Smulikowska, S. 2009. Chemical composition and nutritional value of different wheat cultivars for broiler chickens. J. Anim. Feed Sci. 18, 124–131. Bekker, N.P. and Giushenkova, A.I. 1997. Neutral lipids of the bark of Hippophae rhamnoides branches. Chem. Nat. Comp. 29, 493. Ben-Mahmoud, Z., Mohamed, M.S., Bláha, J., Lukešová, D. and Kunc, P. 2014. The effect of sea buckthorn (Hippophae rhamnoides L.) residues in compound feeds on the performance and skin color of broilers. Indian J. Anim. Res. 48(6), 548-555. Beveridge, T., Li, T.S.C., Oomah, B.D. and Smith, A. 1999. Sea buckthorn products: manufacture and composition. J. Agr. Food Chem. 47, 3480–3488. Chawla, R., Arora, R., Singh, S., Sagar, R.K., Sharma, R.K., Kumar, R., Sharma, A., Gupta, M.L., Singh, S., Prasad, J., Khan, H.A., Swaroop, A., Sinha, A.K., Gupta, A.K., Tripathi, R.P. and Ahuja, P.S. 2007. Radioprotective and antioxidant activity of fractionated extracts of berries of Hippophae rhamnoides. J. Med. Food 10, 101–109. Crespo, N. and Esteve-Garcia, E. 2001. Dietary fatty acid profile modifies abdominal fat deposition in broiler chickens. Poult. Sci. 80, 71–78. Dorhoi, A., Dobrean, V., Zahan, M. and Virag, P. 2006. Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother. Res. 20, 352–358. Geetha, S., Sai-Ram, M., Sharma, S.K., Ilavazhagan, G., Banerjee, P.K. and Sawhney, R.C. 2009. Cytoprotective and antioxidant activity of seabuckthorn (Hippophae rhamnoides L.) flavones against tert-butyl hydroperoxide-induced cytotoxicity in lymphocytes. J. Med. Food 12, 151–158. Geetha, S., Sai-Ram, M., Singh, V., Ilavazhagan, G. and Sawhney, R.C. 2002a. Anti-oxidant and immunomodulatory properties of sea buckthorn (Hippophae rhamnoides)-an in vitro study. J. Ethnopharmacol. 79, 373–378. Geetha, S., Sai-Ram, M., Singh, V., Ilavazhagan, G. and Sawhney, R.C. 2002b. Effect of sea buckthorn on sodium nitroprusside-induced cytotoxicity in murine macrophages. Biomed. Pharmacother. 56, 463–467. Goncharova, N.P. and Glushenkova, A.I. 1993. Lipids of the leaves of Central Asian forms of sea buckthorn. Chem. Nat. Comp. 33, 797–798. Häkkinen, S.H., Kärenlampi, S.O., Heinonen, I.M., Mykkänen, H.M. and Törrönen, A.R. 1999. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agr. Food Chem. 47, 2274–2279 Kaushal, M. and Sharma, P.C. 2011. Nutritional and antimicrobial property of sea buckthorn seed oil. J. Sci. Indust. Res. 70, 1033–1036. Kudritskaya, S.E., Zagorodskaya, L.M. and Shishkina, E.E. 1989. Carotenoids of the sea buckthorn, variety Obil‘naya. Chem. Nat. Comp. 25, 724–725. Krejcarová, J., Straková, E., Suchý, P., Herzig, I. and Karásková, K. 2015. Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities - a review. Acta Vet. Brno. 84(3), 257–268. Krylova, S.G., Konovalova, O.N. and Zueva, E.P. 2000. Correction by common sea buckthorn bark and sprout extracts of hormonal and metabolic disturbances during stress in rats. Eksp. Klin. Farmakol. 63, 70–73. Li, T.S.C. 2002. Product development of sea buckthorn. In Trends in new crops and new uses. Eds., Janick. J. and Whipkey, A. Alexandria, VA: ASHS Press, pp: 393–398. Li, Y., Fu, J., Wang, B., Wang, Y. and Shan, A. 2008. Effect of flavones of Sea buckthorn on carcass characteristics and meat quality of arbor acres broilers. Chinese J. Anim. Vet. Sci. 39(9), 1217–1223. Luhua, Z., Ying, T., Zhengyu, Z. and Guangji, W. 2004. Determination of alpha-tocopherol in the Traditional Chinese Medicinal preparation Sea buckthorn oil capsule by non- supplementation can alleviate negative effects of heat stress on egg production, egg quality, and digestibility of nutrients and egg yolk mineral concentrations of Japanese quails. Res. Vet. Sci. 73, 307–312. Püssa, T., Pällin, R., Raudsepp, P., Soidla, R. and Rei, M. 2007. Inhibition of lipid oxidation and dynamics of polyphenol content in mechanically deboned meat supplemented with sea buckthorn (Hippophae rhamnoides) berry residues. Food Chem. 107, 714–721. Ranjith, A., SarinKumar, K., Venugopalan, V.V., Arumughan, C., Sawhney, R.C. and Singh, V. 2006. Fatty acids, tocols, and carotenoids in pulp oil of three sea buckthorn species (Hippophae rhamnoides, H. salicifolia, and H. tibetana) grown in the Indian himalayas. J. Am. Oil Chem. Soc. 83, 359–364. Repyakh, S.M., Kargapol‘tsev, A.P., Chuprova, N.A. and Yushipitsina, G.G. 1990. Amino acid composition and biological value of proteins of the woody verdure of sea buckthorn. Chem. Nat. Comp. 26, 110–111. Saggu, S. and Kumar, R. 2007. Possible mechanism of adaptogenic activity of sea buckthorn (Hippophae rhamnoides) during exposure to cold, hypoxia and restraint (C-H-R) stress induced hypothermia and post stress recovery in rats. Food Chem. Toxicol. 45, 2426–2433. Seglina, D. 2007. Sea buckthorn fruits and their processing products. Jelgava, Latvia: Latvia University of Agriculture. Sharma, P.C. 2010. Evaluation of sea buckthorn leaves and cake as protein replacer for efficient broiler production. MVSc Thesis, Deparment of Animal Nutrition, CSK Himachal Pradesh Krishi Vishvavidyalaya, Palampur, India. Shao, T.H., Ping, L., Jing, W. and Quing, W. 2002. Qualitative study on oil soluble yellow colorants in sea buckthorn fruits. J. Northeast Agr. Uni. India 303(3), 285–290. Singh, V., Yang, B., Kallio, H., Bala, M., Sawhney, R.C., Gupta, R.K., Morsel, J.T., Lu, R. and Tolkachev, O.N. 2006. Sea buckthorn (Hippophaë L.)-a multipurpose wonder plant. In Vol. II: biochemistry and pharmacology. Ed., Singh, V. New Delhi, India: Daya Publishing House, 600 p. Singh, V. 2012. Harnessing sea buckthorn resources of Himalayas to provide nutritional and health security to India. Bombay Technol. 62, 139–156. Solcan. C., Gogu, M. and Solcan, G. 2011. Protective effect of sea buckthorn (Hippophae rhamnoides L.) oil against ochratoxicosis in chickens. Bull. UASVM Vet. Med. 68(1), 350–358. Stef, L., Dumintrescu, G., Drinceanu, D., Stef, D., Mot, D., Julean, C., Tetileanu, R. and Corcionivoschi, N. 2009. The effect of medicinal plants and plant extracted oils on broiler duodenum morphology and immunological proile. Romanian Biotechnol. Lett. 14(4), 4608–4616. Stef, D.S., Stef, L., Mot, D., Pop, C. and Hegedus, M.G. 2012. The effect of medicinal plants on broilers immunological profile and productive performance. J. Food Agr. Environ. 10(1), 434–437. Stobdan, T., Korekar, G. and Srivastava, B.R. 2013. Nutritional attributes and health application of sea buckthorn (Hippophae rhamnoides L)-a review. Curr. Nutr. Food Sci. 9(2), 151–165. Ul‘chenko, N.T., Zhmyrko, T.G., Glushenkova, A.I. and Murdakhaev, Y.M. 1995. Lipids of Hippophae rhamnoides pericarp. Chem. Nat. Comp. 31, 565–567. Yang, B. 2009. Sugars, acids, ethyl β-D-glucopyranose and a methyl inositol in sea buckthorn (Hippophae rhamnoides) berries. J. Food Chem. 112, 89–97. Yang, B.R., Kalimo, K.O., Tahvonen, R.L., Mattila, L.M., Katajisto, J.K. and Kallio, H.P. 2000. Effect of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on the fatty acid composition of skin glycerophospholipids of patients with atopic dermatitis. J. Nutr. Biochem. 11, 338–340. Yang, B. and Kallio, H.P. 2005. Lipophilic components of Sea buckthorn (Hippophae rhamnoides L.) seeds and berries. In Sea buckthorn (Hippophae L.): a multipurpose wonder plant. Ed., Singh, V. New Delhi, India: Daya Publishing House, pp: 70–97. Yushipitsina, G.G., Chuprova, N.A. and Repyakh, S.M. 1988. Fractionation and amino acid composition of proteins of the woody verdure of sea buckthorn. Chem. Nat. Comp. 24, 348–350. Zadernowski, R., Naczk, M. and Amarowicz, R. 2003. Tocopherols in sea buckthorn (Hippophaë rhamnoides L.) Berry oil. J. Am. Oil Chem. Soc. 80, 55–58. Zelenka, J., Heger, J. and Zeman, L. 1999. The need of nutrients and nutritive value of feeds for poultry. In Commission for farm animal nutrition. Brno, Czech: Czech Academy of Agricultural Sciences, pp: 63. Zhao, W., Chen, X., Yan, C., Liu, H., Zhang, Z., Wang, P., Su, J. and Yao, L.Y. 2012. Effect of Sea buckthorn leaves on inosine monophosphate and adenylosuccinatelyase gene expression in broilers during heat stress. Asian-Aust. J. Anim. Sci. 25(1), 92–97. Zollitsch, W., Knaus, W., Alchinger, F. and Lettner, F. 1997. Effects of different dietary fat sources on performance and carcass characteristics of broiler. Anim. Feed Sci. Technol. 66, 63–73. | ||
| How to Cite this Article |
| Pubmed Style Sherif BM, Benmahmoud ZT, Elfituri AM. Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation. Open Vet J. 2021; 11(4): 780-788. doi:10.5455/OVJ.2021.v11.i4.31 Web Style Sherif BM, Benmahmoud ZT, Elfituri AM. Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation. https://www.openveterinaryjournal.com/?mno=124293 [Access: July 06, 2025]. doi:10.5455/OVJ.2021.v11.i4.31 AMA (American Medical Association) Style Sherif BM, Benmahmoud ZT, Elfituri AM. Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation. Open Vet J. 2021; 11(4): 780-788. doi:10.5455/OVJ.2021.v11.i4.31 Vancouver/ICMJE Style Sherif BM, Benmahmoud ZT, Elfituri AM. Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation. Open Vet J. (2021), [cited July 06, 2025]; 11(4): 780-788. doi:10.5455/OVJ.2021.v11.i4.31 Harvard Style Sherif, B. M., Benmahmoud, . Z. T. & Elfituri, . A. M. (2021) Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation. Open Vet J, 11 (4), 780-788. doi:10.5455/OVJ.2021.v11.i4.31 Turabian Style Sherif, Bashir M., Ziyad T. Benmahmoud, and Awatef M. Elfituri. 2021. Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation. Open Veterinary Journal, 11 (4), 780-788. doi:10.5455/OVJ.2021.v11.i4.31 Chicago Style Sherif, Bashir M., Ziyad T. Benmahmoud, and Awatef M. Elfituri. "Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation." Open Veterinary Journal 11 (2021), 780-788. doi:10.5455/OVJ.2021.v11.i4.31 MLA (The Modern Language Association) Style Sherif, Bashir M., Ziyad T. Benmahmoud, and Awatef M. Elfituri. "Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation." Open Veterinary Journal 11.4 (2021), 780-788. Print. doi:10.5455/OVJ.2021.v11.i4.31 APA (American Psychological Association) Style Sherif, B. M., Benmahmoud, . Z. T. & Elfituri, . A. M. (2021) Effect of partial replacing of wheat by sea buckthorn (Hippophae rhamnoides L.) fruit residues in broiler diets on performance and skin pigmentation. Open Veterinary Journal, 11 (4), 780-788. doi:10.5455/OVJ.2021.v11.i4.31 |