| Original Article | ||
Open Vet J. 2023; 13(2): 218-224 Open Veterinary Journal, (2023), Vol. 13(2): 218–224 Original Research Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogsRyutaro Yoshikawa1*, Fumitaka Yoshikawa2, Sho Goto1, Ryota Iwasaki1 and Takashi Mori1,2,31Animal Medical Centre, Faculty of Applied Biological Sciences, Gifu University, Gifu, Japan 2Laboratory of Veterinary Clinical Oncology, Joint Department of Veterinary Medicine, Gifu University, Gifu, Japan 3Center for Highly Advanced Integration of Nano and Life Sciences (G-CHAIN), Gifu University, Gifu, Japan *Corresponding Author: Ryutaro Yoshikawa. Animal Medical Centre, Faculty of Applied Biological Sciences, Gifu University, Gifu, Japan. Email: yoshikawaryutaro [at] gmail.com Submitted: 26/12/2022 Accepted: 28/01/2023 Published: 17/02/2023 © 2023 Open Veterinary Journal
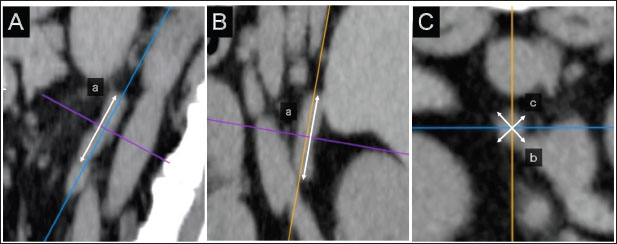
AbstractBackground: Computed tomography (CT) is useful for evaluating the anatomical position of the adrenal gland and the presence of adrenal tumor (AT) metastasis or vascular invasion from ATs. Aim: To determine a weight-independent reference for adrenal gland size in normal dogs using CT. Methods: The medical records database of Gifu University was searched for data collected from April 2010 to December 2015 for records of dogs that underwent abdominal CT. The CT images were retrospectively analyzed using a Digital Imaging and Communications in Medicine viewer. The ratios of the minor axes of the adrenal glands to the height of the spinal cavity were investigated. Results: In total, 939 dogs were included. The left and right adrenal minor axes showed a moderate positive correlation with body weight (right: r=0.61, p < 0.05; left: r=0.54, p < 0.05). The L4 spinal cavity height showed a strong positive correlation with body weight (r=0.82, p < 0.05). The left and right adrenal minor axis/L4 spinal cavity ratio did not correlate with body weight (right: r=0.02, p=0.53; left: r=−0.082, p < 0.05). The 95% confidence intervals of the adrenal minor axis/L4 spinal cavity ratios were as follows: right: 0.5–1.3 and left: 0.5–1.4. Conclusion: These results indicate that the adrenal minor axis/L4 spinal cavity ratio can be used as an index of adrenal gland size that is not affected by body weight. Patients in whom the adrenal minor axis/L4 spinal cavity ratio exceeds the upper limit (right 1.3, left 1.4) may have adrenal swelling. Keywords: Adrenal size, Body weight, Canine, Computed tomography, Novel index. IntroductionUltrasound is widely used in veterinary medicine to evaluate the adrenal gland (McGreevy et al., 2005). However, the presence of other organs, such as the kidney, and gas or fluid in the pylorus, duodenum, and colon may limit the visualization of the entire adrenal gland (Mogicato et al., 2011). As an alternative method for assessing the adrenal gland, computed tomography (CT) features high accuracy in diagnosing adrenal malignancies, allowing the simultaneous evaluation of the pituitary gland (Robertson, 2003; Bertolini et al., 2006; Schultz et al., 2009; Gregori et al., 2015), visualizes the entire abdominal cavity, and enables the objective assessment of the adrenal gland without obstructions by other organs, gases, or fluids in the gastrointestinal tract (Mogicato et al., 2011). In addition, CT is useful for evaluating the anatomical position of the adrenal gland and the presence of AT metastasis or vascular invasion from ATs (Schultz et al., 2009; Gregori et al., 2015). The development of diagnostic imaging technology has improved the identification of adrenal accidental tumors, the incidence of which is reportedly 4%–9% (Holmes et al., 2006, 2007; Gregori et al., 2015). Despite the low incidence of adrenal accidental tumors, their clinical significance has not been fully elucidated (Colliard et al., 2006). This report defines the swelling criterion as 8 mm, but does not consider the animals’ body size. A previous study has reported a significant correlation between adrenal volume and body weight in normal dogs (Bertolini et al., 2006), whereas other previous studies have suggested that 25%–59% of dogs are overweight, and their weights are affected by their nutritional status and metabolic disorders (Robertson, 2003; Holmes et al., 2006, 2007; Mogicato et al., 2011; Baum et al., 2016). Standard values divided by weight can lead to inaccurate assessments of dogs’ weight. We hypothesized that the development of a weight-independent reference value for adrenal gland size can improve the accuracy of diagnosing adrenal accidental tumors. This study aimed to establish a weight-independent standard for adrenal gland size in normal dogs using CT. Materials and MethodsCase selectionThe medical records database of Gifu University was searched for data regarding dogs that underwent abdominal CT from April 2010 to December 2015. Owing to the retrospective nature of the study, the need for owner consent was waived. Dogs were considered to have normal adrenal glands if they met the following criteria: (1) not diagnosed with hyperadrenocorticism (pituitary-dependent hyperadrenocorticism, AT) or pheochromocytoma, (2) no suspected hyperadrenocorticism (alkaline phosphatase level that is above the normal range, polydipsia, incidence of hepatomegaly, swelling of the abdomen, and/or skin abnormality), and (3) no suspected AT (adrenal calcification, cysts, and morphological abnormalities on CT images). Case informationThis study included 939 dogs with a median weight of 8.9 kg (1.4–40.2 kg) and a median age of 12.1 years (8 months to 18.4 years). Of them, 294, 165, 255, and 225 were intact male, castrated male, intact female, and spayed female dogs, respectively. The breeds (number and percentage) included were as follows: Miniature Dachshunds (200, 21.3%), mixed-breed dogs (103, 11.0%), Pembroke Welsh Corgis (88, 9.4%), Labrador Retrievers (78, 8.3%), Golden Retrievers (58, 6.2%), Chihuahuas (45, 4.8%), Poodles (36, 3.8%), Shiba Inus (35, 3.7%), Shih Tzus (28, 3.0%), Papillons (27, 2.9%), Yorkshire Terriers (23, 2.4%), French Bulldogs (22, 2.3%), Shetland Sheepdogs (22, 2.3%), Miniature Schnauzers (19, 2.0%), Malteses (14, 1.5%), Border Collies (14, 1.5%), Beagles (13, 1.4%), Pugs (13, 1.4%), and Jack Russell Terriers (11, 1.2%). The remaining 90 dogs comprise 1–8 members of 33 other breeds. In all 939 dogs included in the study, no symptoms of suspected hyperadrenocorticism (alkaline phosphatase level that is above the normal range, polydipsia, incidence of hepatomegaly, swelling of the abdomen, and/or skin abnormality) were found. In addition, imaging findings on CT scan that were suspicious for pituitary-dependent hyperadrenocorticism or AT (calcification, cysts, or morphologic abnormalities in the adrenal glands on CT images) were not observed in all patients. Dogs diagnosed with hyperadrenocorticism (pituitary-dependent hyperadrenocorticism, AT) and pheochromocytoma on various tests were excluded from inclusion. CT imageCT images were acquired using a 16-detector row CT unit (Alexion TSX-034A, Canon Medical Systems, Tochigi, Japan.). The technical parameters for CT were as follows: rotation time, 0.75 s; slice thickness, 2 mm; field of view, 160–340 mm; matrix dimensions, 512 × 512; reconstruction interval, 0.5–1 mm; detector pitch, 15; collimator pitch, 0.94; X-ray tube potential, 120 kVp; and X-ray tube current, variable milliamperage determined by x-, y-, and z-axis dose modulation. An intravenous iodine-based contrast medium (Omnipaque 300, Daiichi Sankyo, Tokyo, Japan) was administered using a power injector at a dose of 450 mg/kg into a cephalic catheter. After unenhanced scanning, post-contrast images were acquired 30–60 seconds after the initiation of contrast medium injection. CT images were retrospectively analyzed using a Digital Imaging and Communications in Medicine viewer (OsiriX Imaging Software, version 8.0, OsiriX Pixmeo, Geneva, Switzerland). Adrenal gland evaluation on CTIn the sagittal and dorsal sections, the crosshairs were adjusted to be parallel to the maximum diameter of the adrenal gland (Fig. 1A and B). The crosshairs showed the slice position in the other two panels of Figure 1. A transverse section that includes the maximum diameter was created; the maximum diameter parallel to the section plane was defined as the major axis, whereas the maximum diameter perpendicular to the section plane was defined as the minor axis (Fig. 1a–c). The major axis of the adrenal gland was defined as the longest dimension in the transverse plane (Fig. 1b), and the dimension of the minor axis was defined as the length perpendicular to the major axis in the same plane (Fig. 1c). The major and minor axes of the adrenal glands were measured using three-dimensional multi-planar reconstruction (3D-MPR) and a soft-tissue algorithm (window level: 40, window width: 250) (Fig. 1A–C).
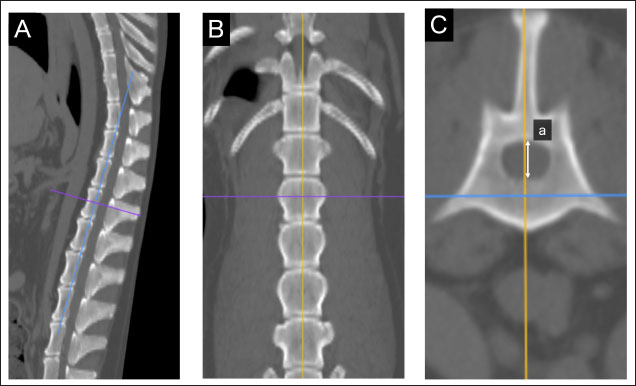
Fig. 1. Method used for measuring minor axes of the bilateral adrenal glands. In the sagittal (A) and dorsal (B) sections, the crosshairs were positioned to be parallel to the maximum diameter of the left adrenal gland (a). The crosshairs show the slice position in the other two panels. The transverse section including the maximum diameter (a) is created. (C). The maximum diameter is defined as the major axis (b) parallel to the section plane, and the maximum diameter perpendicular to (b) is defined as the minor axis (c). Spinal cavity evaluation on CTIt has been reported that the right adrenal gland is situated near the T13–L1 vertebrae, while the left adrenal gland is located near the L2 vertebra (Evans, 2013). Therefore, the T13–L5 vertebral body was selected as a candidate for the normalized site of the reference value. The spinal cavity of T13–L5, which is close to the bilateral adrenal glands, was selected as a candidate for the reference site. The T13–L5 spinal cavity was measured on 3D-MPR from CT images using the bone algorithm (window level: 300, window width: 1,500). In the sagittal and dorsal planes, the crosshairs were adjusted so they were parallel to the spinal cord. In the sagittal sections, the plane passing through the center of the dorsal edge of each vertebral body was drawn in the transverse plane (Fig. 2A–C). The height of the spinal cavity visualized in the cross-plane was recorded (Fig. 2Ca). Examination of the reference site for creating standard valuesThe bilateral adrenal minor diameters (mm) and T13–L5 spinal cavity height (mm) were used to calculate the adrenal minor axis/measurement site ratios. Scatterplots of the calculated ratios and body weights showed approximate straight lines. To compare the variation of data, the standard deviation was divided by the average. The coefficient of variation was calculated. Initially, we used 40 randomly selected animals from the case group; subsequently, we gradually increased the number of animals and searched for measurement sites with a slope close to zero (approximate straight). Association between adrenal minor axis/L4 spinal cavity ratio and body weightThe adrenal minor axis (mm) was divided by the height of the L4 spinal cavity (mm) to calculate the adrenal minor axis/L4 spinal cavity ratio. Associations among the adrenal minor axis, L4 spinal cavity height, adrenal minor axis/L4 spinal cavity ratio, and body weight were investigated using a scatter diagram, and the corresponding correlation coefficient (r) values were calculated. The 95% range was determined by percentile, and the standard range of adrenal minor axis/L4 spinal cavity ratio was estimated.
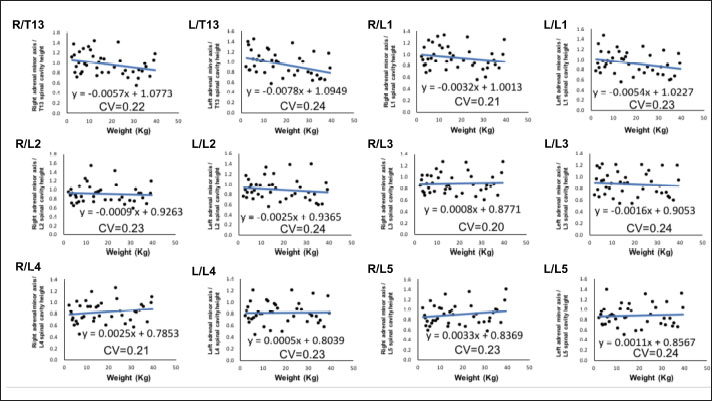
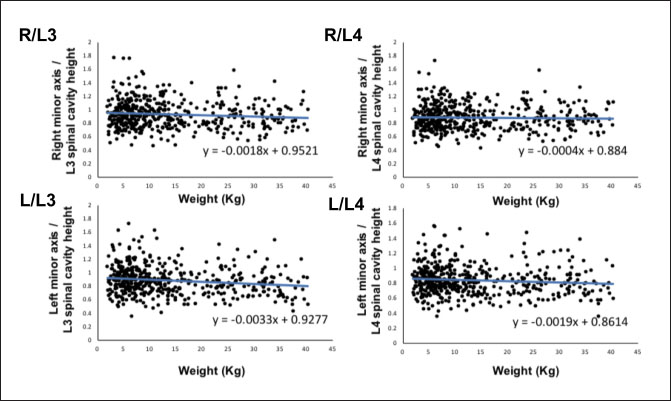
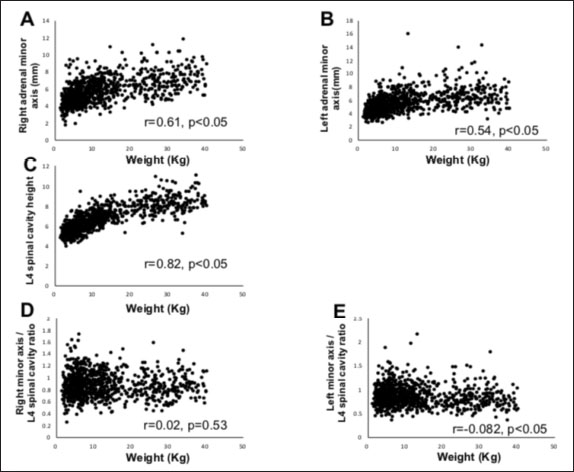
Fig. 2. Method used for measuring the height and width of the L2 spinal cavity. In the sagittal (A) and dorsal (B) sections, the crosshairs were adjusted to be parallel to the spine. In the sagittal section (A), a line passing through the center of the dorsal edge of the L2 vertebral body is depicted in the transverse section (C). The spinal cavity height (a) depicted in the transverse section (C) was measured. Statistical analysesNormality of data distribution was determined using the Shapiro–Wilk test. Spearman’s rank correlation test was used to calculate r. All statistical analyses were performed using Excel 2013 (Microsoft Corp, Redmond, Washington, USA) and EZR (Saitama Medical Center, Saitama, Japan) statistical software (Kanda, 2013). p values ≤0.05 were considered statistically significant. Ethical approvalThe authors confirm that the ethical policies of the journal have been followed and that no ethical approval was required for this study. ResultsExamination of measurement siteForty randomly selected dogs were included in each group (grouping: weight vs. bilateral adrenal minor axis/T13 [right: R/T13, left: L/T13], /L1 [right: R/L1, left: L/L1], /L2 [right: R/L2, left: L/L2], /L3 [right: R/L3, left: L/L3], /L4 [right: R/L4, left: L/L4], and /L5 [right: R/L5, left: L/L5]). The T13–L5 spinal cavity height was measured, and the adrenal minor axis/measurement site ratio was calculated. The corresponding scatter diagrams, approximate expressions, and CVs are shown in Figure 3. The slopes of the approximate expressions of the heights of L3 (right: 0.008, left: –0.0016) and L4 spinal cavities (right: 0.0025, left: 0.0005) were small and were reviewed again in 474 randomly selected dogs (grouping: weight vs. right adrenal minor axis/L3 [R/L3], /L4 [R/L4], left adrenal minor axis/L3 [L/L3], /L4 [L/L4]). L3 and L4 spinal cavity heights were measured, and the adrenal minor axis/measurement site ratio was calculated. The corresponding scatter diagrams, approximate expressions, and CVs are shown in Figure 4. Approximate expressions and CVs are as follows: height of L3 spinal cavity: right y=−0.0018 x + 0.95 and 0.22, respectively, and left y=−0.0033 x + 0.93 and 0.24, respectively, and height of L4 spinal cavity: right y=−0.0004 x + 0.88 and 0.22, respectively, and left y=−0.0019 x + 0.86 and 0.24, respectively (y: adrenal minor axis [mm], x: body weight [kg]). L4 was selected as the measurement site because the corresponding slopes of approximate expression on both sides were small, and CVs were sufficiently small. Association between adrenal minor axis/L4 spinal cavity ratio and body weightThe measured values in 939 dogs were as follows (mean ± SD): right adrenal minor axis, 5.7 ± 1.5 mm; left adrenal minor axis, 5.6 ± 1.5 mm; height of L4 spinal cavity, 6.6 ± 1.3 mm; right adrenal minor axis/L4 spinal cavity ratio, 0.87 ± 0.20; and left adrenal minor axis/L4 spinal cavity ratio, 0.86 ± 0.22. All results were not normally distributed. The 95% confidence intervals of the adrenal minor axis/L4 spinal cavity ratios were as follows: right: 0.5–1.3 and left: 0.5–1.4. To examine the associations among the L4 spinal cavity height, adrenal minor axis/L4 spinal cavity ratio, and body weight, scatterplots were created for each of the left and right adrenal glands (Fig. 5). The left and right adrenal minor axes showed a moderate positive correlation with body weight (right: r=0.61, p < 0.05; left: r=0.54, p < 0.05). The L4 spinal cavity height showed a strong positive correlation with body weight (r=0.82, p < 0.05). On the contrary, the left and right adrenal minor axis/L4 spinal cavity ratio did not correlate with body weight (right: r=0.02, p=0.53; left: r=−0.082, p < 0.05).
Fig. 2. Associations between adrenal minor axis/measurement site ratio and body weight. The bilateral adrenal minor axis/T13–L5 spinal cavity height ratio was calculated using data from randomly selected 40 dogs without adrenal disease. Scatterplots of weight vs. bilateral adrenal minor axis/T13 (right: R/T13, left: L/T13), /L1 (right: R/L1, left: L/L1), /L2 (right: R/L2, left: L/L2), /L3 (right: R/L3, left: L/L3), /L4 (right: R/L4, left: L/L4), and /L5 (right: R/L5, left: L/L5). Every black dot in the scatterplots indicates each case; the approximate straight line is indicated by a straight blue line, and the formula of each approximate straight line and the corresponding CV is shown below each straight line.
Fig. 4. Associations between the adrenal minor axis/L3 or L4 spinal cavity ratio and body weight. The associations were determined using the data from 474 dogs. Scatterplots of weight vs. right adrenal minor axis/L3 spinal cavity height (R/L3), right adrenal minor axis/L4 spinal cavity height (R/L4), left adrenal minor axis/L3 spinal cavity height (L/L3), and left adrenal minor axis/L4 spinal cavity height (L/L4). Each black dot in the scatterplots indicates each case; the approximate straight line is indicated by a straight blue line, and the formula of each approximate straight line is shown. DiscussionThe results of this study suggest that the adrenal minor axis/L4 spinal cavity ratio can be used as a novel index for measuring the size of the adrenal gland without dependence on body weight (right: 0.5–1.3, left: 0.5–1.4). The upper limits of the right and left normal adrenal minor axis/L4 spinal cavity ratios were 1.3 and 1.4, respectively. The minor axes of the left and right adrenal glands showed a moderate positive correlation with body weight (right: r =0.61, left: r=0.54). These results are similar to those of previous reports that used Ultrasound (Grooters et al., 1995, 1996; de Chalus et al., 2013; Soulsby et al., 2015). By contrast, compared with the strong positive correlation between the L4 spinal cavity height and body weight (r=0.82), the correlation between adrenal minor axis and body weight was not significant. It is thought that the spinal cavity is surrounded by bone and is unaffected by the surrounding soft tissue. A previous study has also reported that the size of the spinal cavity increases with increases in body weight (Hecht et al., 2014; Seo et al., 2014). These results suggest that the size and weight of the spinal cavity may be repatriated because the adrenal glands that show an adrenal minor axis/L4 spinal cavity ratio that exceeds the upper limit (right 1.3, left 1.4) are likely to be swollen. Adrenal size may be affected by various factors, among which body weight is relatively important (Grooters et al., 1995, 1996; Boozer et al., 2005; de Chalus et al., 2013; Soulsby et al., 2015). By dividing by the L4 spinal cavity height, which strongly correlates with body weight, the adrenal minor axis/L4 spinal cavity ratio had no correlation with body weight. Because adrenal size did not change even if the body weight increased, the adrenal gland/L4 spinal cavity ratio may be an independent index of body weight.
Fig. 5. Associations between adrenal minor axis, L4 spinal cavity height, adrenal minor axis/L4 spinal cavity ratio, and body weight. The associations were determined using the data from 939 dogs without adrenal disease. Scatterplots of body weight vs. left/right adrenal minor axis, L4 spinal cavity height, or left/right adrenal minor axis/L4 spinal cavity ratio. Each black dot in the scatterplots indicates each case; correlation coefficients (r values) and p values are shown. The right adrenal minor axis (A) and left adrenal minor axis (B) show a moderate positive correlation with body weight. The L4 spinal cavity height (C) shows a strong positive correlation with body weight. The right adrenal minor axis/L4 spinal cavity ratio (D) and left adrenal minor axis/L4 spinal cavity ratio (E) show no correlation with body weight. In humans, adrenal activation is induced in patients with multiple myeloma, chronic lymphocytic leukemia, lung cancer, and other malignancies (Jenkins et al., 1999). In dogs, adrenal swelling is induced by a malignant tumor and severe illness (Kaplan et al., 1995; Jenkins et al., 1999; Sacornrattana et al., 2013). This study excluded dogs with suspected adrenal disease but included those with various other diseases. Therefore, there is a possibility that adrenal activation and inactivation were affected by these diseases (Jenkins et al., 1999). This study is subject to further limitations. First, we acknowledge the possibility of a breed bias. There may be differences in the size and morphology of the adrenal gland and spinal cavity according to breed, and the extent of effect of this potential bias on the results of this study remains unclear. Second, the dogs included in this study, although without adrenal disease, were unhealthy; the effects of different drugs used for the management of diseases on adrenal size are unknown. Future studies must determine factors that may affect adrenal size and optimal standard values for the detection of adrenal incidental tumors. In conclusion, this study measured the left and right adrenal minor axes and L4 spinal cavity height in 979 dogs without adrenal disease and calculated the adrenal minor axis/L4 spinal cavity ratio, which showed a significant correlation with body weight. Adrenal size did not change even if the body weight increased (right: r=−0.082, p < 0.05; left: r=0.02, p=0.53). Our results indicate that the adrenal minor axis/L4 spinal cavity ratio can be used as an index of adrenal gland size that is unaffected by body weight. Patients showing an adrenal minor axis/L4 spinal cavity ratio that exceeds the upper limit (right 1.3, left 1.4) may have adrenal swelling. AcknowledgmentsThe authors thank Editage (www.editage.jp) for the English language editing of this manuscript. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsConceptualization and writing the original draft: Ryutaro Yoshikawa, Fumitaka Yoshikawa, Sho Goto, Ryota Iwasaki, and Takashi Mori. Investigation: RyutaroYoshikawa and Fumitaka Yoshikawa. Data curation: Ryutaro Yoshikawa, Fumitaka Yoshikawa, Sho Goto, Ryota Iwasaki, and Takashi Mori. Statistical analysis: Ryutaro Yoshikawa and Fumitaka Yoshikawa. Writing review and editing: all authors. Supervision: Takashi Mori. ReferencesBaum, J.I., Boston, S.E. and Case, J.B. 2016. Prevalence of adrenal gland masses as incidental findings during abdominal computed tomography in dogs: 270 cases (2013-2014). J. Am. Vet. Med. Assoc. 249, 1165–1169. Bertolini, G., Furlanello, T., De Lorenzi, D. and Caldin, M. 2006. Computed tomographic quantification of canine adrenal gland volume and attenuation. Vet. Radiol. Ultrasound. 47, 444–448. Boozer, A.L., Behrend, E.N., Kemppainen, R.J., Whitley, E.M., Smith, A.N. and Busch, K.A. 2005. Pituitary-adrenal gland axis function in dogs with neoplasia. Vet. Comp. Oncol. 3, 194–202. Colliard, L., Ancel, J., Benet, J.J., Paragon, B.M. and Blanchard, G. 2006. Risk factors for obesity in dogs in France. J. Nutr. 136(7 Suppl.), 1951S–1954S. Courcier, E.A., Thomson, R.M., Mellor, D.J. and Yam, P.S. 2010. An epidemiological study of environmental factors associated with canine obesity. J. Small Anim. Pract. 51, 362–367. de Chalus, T., Combes, A., Bedu, A.S., Pey, P., Daminet, S., Duchateau, L. and Saunders, J.H. 2013. Ultrasonographic adrenal gland measurements in healthy Yorkshire terriers and labrador retrievers. Nat. Histol. Embryol. 42, 57–64. Evans, H.E. 2013. Miller’s anatomy of the dog, 4th ed. Saint Louis, MO: Saunders/Elsevier, pp: 418. Gregori, T., Mantis, P., Benigni, L., Priestnall, S.L. and Lamb, C.R. 2015. Comparison of computed tomographic and pathologic findings in 17 dogs with primary adrenal neoplasia. Vet. Radiol. Ultrasound. 56, 153–159. Grooters, A.M., Biller, D.S. and Merryman, J. 1995. Ultrasonographic parameters of normal canine adrenal glands: comparison to necropsy findings. Vet. Radiol. Ultrasound. 36, 126–130. Grooters, A.M., Biller, D.S., Theisen, S.K. and Miyabayashi, T. 1996. Ultrasonographic characteristics of the adrenal glands in dogs with pituitary-dependent hyperadrenocorticism: comparison with normal dogs. J. Vet. Intern. Med. 10, 110–115. Hecht, S., Huerta, M.M. and Reed, R.B. 2014. Magnetic resonance imaging (MRI) spinal cord and canal measurements in normal dogs. Anat. Histol. Embryol. 43, 36–41. Holmes, K.L., Morris, P.J., Abdulla, Z., Hackett, R. and Rawlings, J.M. 2007. Risk factors associated with excess body weight in dogs in the UK. J. Anim. Physiol. Anim. Nutr. 91, 166–167. Jenkins, P.J., Sohaib, S.A., Trainer, P.J., Lister, T.A., Besser, G.M. and Reznek, R. 1999. Adrenal enlargement and failure of suppression of circulating cortisol by dexamethasone in patients with malignancy. Br. J. Cancer. 80, 1815–1819. Kanda, Y. 2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458. Kaplan, A.J., Peterson, M.E. and Kemppainen, R.J. 1995. Effects of disease on the results of diagnostic tests for use in detecting hyperadrenocorticism in dogs. J. Am. Vet. Med. Assoc. 207, 445–451. McGreevy, P.D., Thomson, P.C., Pride, C., Fawcett, A., Grassi, T. and Jones, B. 2005. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet. Rec. 156, 695–702. Mogicato, G., Layssol-Lamour, C., Conchou, F., Diquelou, A., Raharison, F., Sautet, J. and Concordet, D. 2011. Ultrasonographic evaluation of the adrenal glands in healthy dogs: repeatability, reproducibility, observer-dependent variability, and the effect of bodyweight, age and sex. Vet. Rec. 168(5), 130. Robertson, I.D. 2003. The association of exercise, diet and other factors with owner-perceived obesity in privately owned dogs from metropolitan Perth, WA. Prev. Vet. Med. 58, 75–83. Sacornrattana, O., Dervisis, N.G. and McNiel, E.A. 2013. Abdominal ultrasonographic findings at diagnosis of osteosarcoma in dogs and association with treatment outcome. Vet. Comp. Oncol. 11, 199–207. Schultz, R.M., Wisner, E.R., Johnson, E.G. and MacLeod, J.S. 2009. Contrast-enhanced computed tomography as a preoperative indicator of vascular invasion from adrenal masses in dogs. Vet. Radiol. Ultrasound. 50, 625–629. Seo, E., Choi, J., Choi, M. and Yoon, J. 2014. Computed tomographic evaluation of cervical vertebral canal and spinal cord morphometry in normal dogs. J. Vet. Sci. 15, 187–193. Soulsby, S.N., Holland, M., Hudson, J.A. and Behrend, E.N. 2015. Ultrasonographic evaluation of adrenal gland size compared to body weight in normal dogs. Vet. Radiol. Ultrasound. 56, 317–326. | ||
| How to Cite this Article |
| Pubmed Style Yoshikawa R, Yoshikawa F, Goto S, Iwasaki R, Mori T. Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs. Open Vet J. 2023; 13(2): 218-224. doi:10.5455/OVJ.2023.v13.i2.10 Web Style Yoshikawa R, Yoshikawa F, Goto S, Iwasaki R, Mori T. Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs. https://www.openveterinaryjournal.com/?mno=133716 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i2.10 AMA (American Medical Association) Style Yoshikawa R, Yoshikawa F, Goto S, Iwasaki R, Mori T. Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs. Open Vet J. 2023; 13(2): 218-224. doi:10.5455/OVJ.2023.v13.i2.10 Vancouver/ICMJE Style Yoshikawa R, Yoshikawa F, Goto S, Iwasaki R, Mori T. Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs. Open Vet J. (2023), [cited July 01, 2025]; 13(2): 218-224. doi:10.5455/OVJ.2023.v13.i2.10 Harvard Style Yoshikawa, R., Yoshikawa, . F., Goto, . S., Iwasaki, . R. & Mori, . T. (2023) Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs. Open Vet J, 13 (2), 218-224. doi:10.5455/OVJ.2023.v13.i2.10 Turabian Style Yoshikawa, Ryutaro, Fumitaka Yoshikawa, Sho Goto, Ryota Iwasaki, and Takashi Mori. 2023. Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs. Open Veterinary Journal, 13 (2), 218-224. doi:10.5455/OVJ.2023.v13.i2.10 Chicago Style Yoshikawa, Ryutaro, Fumitaka Yoshikawa, Sho Goto, Ryota Iwasaki, and Takashi Mori. "Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs." Open Veterinary Journal 13 (2023), 218-224. doi:10.5455/OVJ.2023.v13.i2.10 MLA (The Modern Language Association) Style Yoshikawa, Ryutaro, Fumitaka Yoshikawa, Sho Goto, Ryota Iwasaki, and Takashi Mori. "Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs." Open Veterinary Journal 13.2 (2023), 218-224. Print. doi:10.5455/OVJ.2023.v13.i2.10 APA (American Psychological Association) Style Yoshikawa, R., Yoshikawa, . F., Goto, . S., Iwasaki, . R. & Mori, . T. (2023) Computed tomography-based evaluation for normal adrenal gland size independent of body weight in dogs. Open Veterinary Journal, 13 (2), 218-224. doi:10.5455/OVJ.2023.v13.i2.10 |