| Original Article | ||
Open Vet J. 2023; 13(3): 337-347 Open Veterinary Journal, (2023), Vol. 13(3): 337–347 Original Research Computed tomographic evaluation of heart size in clinically healthy catsAphisit Wanglerm1, Chawanwith Samachikthummakun1, Zen Kitnitchee1, Thiranan Sirinitikorn1, Sapawut Junnong1, Somchin Sutthigran2, Nan Choisunirachon2 and Chutimon Thanaboonnipat2*1Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand 2Department of Veterinary Surgery, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand *Corresponding Author: Chutimon Thanaboonnipat. Department of Veterinary Surgery, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand. Email: Chutimon.Th [at] chula.ac.th. Submitted: 09/01/2023 Accepted: 27/02/2023 Published: 15/03/2023 © 2023 Open Veterinary Journal
AbstractBackground: Computed tomography (CT) is currently one of the most useful diagnostic imaging tools for evaluating cardiac disorders in humans and animals. However, studies concerning CT and the feline heart remain scarce. Aim: To create measuring techniques for the feline heart size on CT and to determine the relationships of feline heart size observed on CT with internal parameters including age, bodyweight (BW), and sex. Methods: Four parameters observed on CT including thoracic height/width ratio (THW), relative heart area (RHA), CT vertebral heart score (ctVHS), transverse vertebral heart score (tVHS) in 1.25 mm slice thickness, and both pre- and post-contrast enhanced images were examined. Additionally, radiographic vertebral heart score (rVHS) was also evaluated. Results: THW was significantly influenced by age (p < 0.05). RHA was affected by age and gonadal status of cats (p=0.001 and p =0.016, respectively). Age also significantly affected tVHS (p =0.038). Interestingly, ctVHS was not influenced by age, sex, gonadal status, or BW. tVHS and ctVHS had significantly moderately positive correlations with rVHS (r=0.476; p=0.048 and r=0.6112; p=0.011, respectively). THW and RHA had non-significant correlations with rVHS (r=0.2642; p=0.302 and r=0.1920; p=0.455, respectively). Conclusion: CT heart size evaluation can be performed in both pre- and post-contrast enhanced images of 1.25 mm slice thickness. tVHS and ctVHS are recommended parameters for evaluating feline heart size in clinical practice. Keywords: Cats, Computer tomography, Feline heart, Transverse vertebral heart score, Vertebral heart score. IntroductionThe heart is the most vital organ of the vertebrate species that acts as a pump to propel and deliver blood throughout the body (Stephenson et al., 2017). Dysfunctions of the heart can cause serious clinical problems. Heart disease is one of the major clinical problems in cats (Freeman et al., 2017). The prevalence of feline heart disease has been noted as being 0.2% of the total number of visiting cats and 7.3% of cats were diagnosed with heart disease (Riesen et al., 2007; Michal, 2020). Feline heart disease can be categorized into congenital and acquired heart disease (Khor and Chin, 2020). The two most common types of congenital heart disease are ventricular septal defect and tricuspid valve dysplasia (Tidholm et al., 2015). Acquired cardiac diseases in cats can have several causes including primary cardiomyopathies (Sisakian, 2014) or secondarily from other diseases (Spalla et al., 2016). The most common acquired heart disease in cats in clinical practice is hypertrophic cardiomyopathy (Fuentes et al., 2020). The diagnostic methods of feline heart disease include history taking, physical examination, thoracic auscultation, thoracic radiography, electrocardiography (ECG), and echocardiogram (Fuentes et al., 2020). Additionally, the advanced diagnostic imaging modality, computed tomography (CT), is currently one of the most useful diagnostic tools for evaluating cardiac disorders in both humans (Savino et al., 2007) and cats (d’Anjou, 2018). Thoracic radiography is a routine diagnostic test for heart size evaluation (Kittleson and Cote, 2021). This technique is non-invasive, has wide availability, and is less expensive compared to other imaging modalities (Schober et al., 2007). Although thoracic radiography is readily accessible in general practice, it provides limited information for some definitive diagnoses (Henninger, 2003). There are some disadvantages of radiography such as low image resolution, and distortion and magnification effects on acquired images (Nyathi et al., 2010). Furthermore, the detail obtained from the standard radiograph normally provides only two-dimensional images that cause a superimposition of each organ (Ruderman and Flaherty, 2017). This effect could interfere with interpretation accuracy and occasionally, can make diagnosis difficult (Henninger, 2003; Bruno, 2017). Therefore, CT has been increasingly applied in veterinary clinical practice (Keane et al., 2017). CT can provide more accurate information and has several benefits over radiography (Bertolini and Angeloni, 2017). For example, CT images are not affected by the superimposition effect (Mikla and Mikla, 2014) and can be expressed as three-dimensional images with higher resolution. This allows radiologists to identify details including the size, shape, and texture of internal organs (Bertolini and Angeloni, 2017), and assist in locating lesions more precisely (d’Anjou, 2018). The combination of radiography and CT could increase the chances of finding intrathoracic lesions when compared to without (Prather et al., 2005). A recent study in dogs demonstrated that CT could assist veterinary practitioner in evaluating and diagnosing airway disorders. Furthermore, it provided information regarding heart volume and thoracic volume (Uehara et al., 2009). Since there is less information concerning feline heart size as observed on CT, the purposes of this study were, first, to create measuring techniques for feline heart size on CT. Second, to establish a reference range value of normal feline heart size on CT. Finally, to determine the relationships of feline heart size observed on CT with internal parameters such as age, bodyweight (BW), and sex. Materials and MethodsAnimalsThis study utilized the clinical, thoracic radiographs, and CT data of included client-owned cats that were presented to the Diagnostic Imaging Unit, the Small Animal Hospital, Faculty of Veterinary Science, Chulalongkorn University between February and June 2017. The data were retrieved from the hospital information system and picture archiving and communication systems (PACs). Clinical information including age, sex, breed, and BW were recorded. The exclusion criteria were incomplete clinical information, any abnormalities related to cardiocirculatory disease, abnormal feline proBNP test, incomplete information of thoracic radiographs and CT, thoracic/abdominal mass or tumors, respiratory abnormality, duration of thoracic radiographs and CT data difference of more than 1 month, cardiomegaly based on radiographic vertebral heart score (rVHS), and any thoracic vertebrae abnormalities based on radiographs and CT. All healthy cats were divided into two groups according to the age of growth plate closure (Miranda et al., 2020) which yielded a group of age less than or equal to 18 months (Gr. A) and a group aged more than 18 months (Gr. B). Thoracic radiograph evaluationThe right lateral projection of thoracic radiographs of all included cats in this study was collected as digital information and communication (DICOM) files for measuring the rVHS through DICOM viewer software (Osirix®, Geneva, Switzerland) as shown in Figure 1. The rVHS was examined by measuring the long axis (LA) and the short axis (SA) as described by Lister and Buchanan (2000). The LA and SA were manually measured using a digital caliper and the values were recorded. The LA was the distance from the ventral border of the mainstem bronchus to the cardiac apex. The SA was the widest distance of the heart perpendicular to the LA line. The LA and SA lines were transposed onto the vertebral column and the number of vertebrae beginning from the cranial end plate of the fourth thoracic vertebrae (T4) were recorded as rVHS. All radiographic measurements were performed by the same investigator using the same image archiving PACs system with the DICOM viewer software. Thoracic CT images evaluationAll CT images at both pre- and post-contrast enhancement of 1.25 mm slice thickness were evaluated using a DICOM viewer (Osirix®, Geneva, Switzerland), and a non-CT unit workstation with 2,560 × 1,440 pixels monitor. All CT images were completed using a 64 CT slice scanner (Optima 660, General Electric, Japan) in sternal positioning with 120 kVP, automated milliampere, matrix size of 512 × 512. All images were performed using multiplanar reconstruction and the soft tissue window was set [350 Hounsfield units (HU) of window width and 40 HU of window level] during evaluation to ensure the best visualization and symmetry of the thoracic structures. The measuring methods of the four parameters in this study are described as follows. On cross-sectional images at the carina level, thoracic height/width ratio (THW) was determined as described previously (Uehara et al., 2009). A manual drawing using a digital caliper from the ventral border of the vertebrae to the sternum was performed to identify the thoracic height (TH). For the thoracic width (TW), a digital caliper was used from the right to left thoracic wall at the widest part and perpendicular to the TH as shown in Figure 2. THW was then calculated. Relative heart area (RHA) was evaluated by manually drawing the outline of the heart to evaluate the heart area (Fig. 3A). For the thoracic area (Fig. 3B), manual drawing was performed as described previously (Uehara et al., 2009). Finally, RHA was then calculated as the ratio of heart area to thoracic area at the carina level. Transverse vertebral heart score (tVHS) was determined by manual drawing using a digital caliper. Heart height (HH) was the height of the heart and heart width (HW) was the widest distance perpendicular to the HH. The ratio of the sum of HH and HW to the height of the vertebral body at the carina level was then calculated as tVHS (Fig. 4). CT vertebral heart score (ctVHS) was evaluated on the sagittal plane images. The measurement method was performed in the same way as on radiography (Lister and Buchanan, 2000) (Fig. 5). All CT measurements were performed by the same experienced radiologist using the same image archiving PACs system through DICOM viewer software.
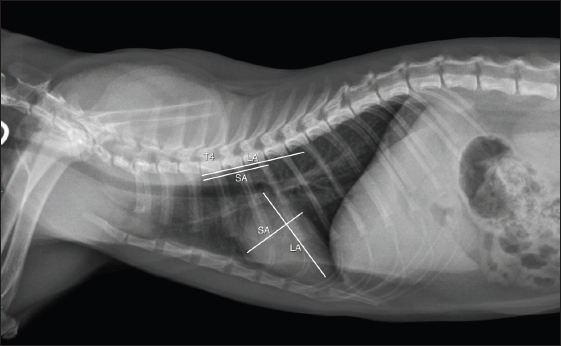
Fig. 1. rVHS measurement methods. rVHS was measured on right lateral thoracic radiograph. LA was the distance from ventral border of the mainstem bronchus to the cardiac apex. SA was the widest distance of the heart perpendicular with the LA line. LA and SA lines were transposed onto the vertebral column and the number of vertebrae beginning from the cranial end plate of the fourth thoracic vertebrae (T4) were recorded as rVHS. rVHS: radiographic vertebral heart score; LA: long axis; SA: short axis; T4: the fourth thoracic vertebrae.
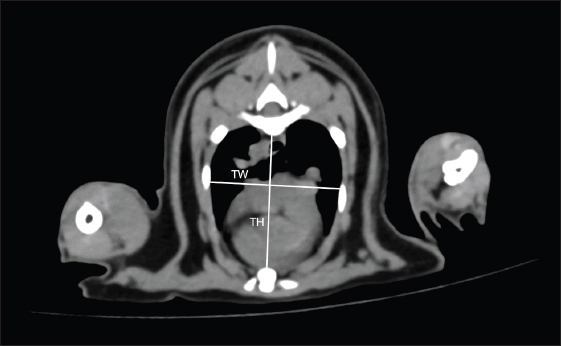
Fig. 2. THW measurement methods. THW was measured on cross-sectional CT image at the carina level. TH was distance from ventral border of the vertebrae to the sternum. TW was distance from the right to left thoracic wall at the widest part and perpendicular to TH. THW: thoracic height/width ratio; CT: computed tomography; TH: thoracic height; TW: thoracic width. Statistical analysisAll data were analyzed using statistical software (Prism 9, GraphPad®, CA, USA). The descriptive data of clinical information were expressed as mean ± standard deviation (SD) including minimum and maximum values. All data sets were analyzed using the Shapiro–Wilk test to determine the normalization of the data. All parameters were compared between age, sex, and gonadal status using the unpaired t-test and Mann–Whitney U test. The correlations and associations of each parameter with other factors such as age and BW were evaluated using Pearson’s correlation coefficient and Spearman’s correlation coefficient. A p-value less than 0.05 was considered a statistically significant difference.
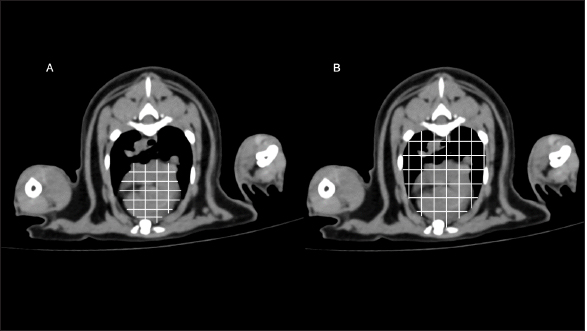
Fig. 3. RHA measurement methods. RHA was measured on cross-sectional CT image at the carina level. (A) Heart area was the area of the heart. (B) Thoracic area was the area of the thoracic cavity. RHA was calculated as the ratio of heart area to thoracic area at the carina level. RHA: relative heart area; CT: computed tomography.
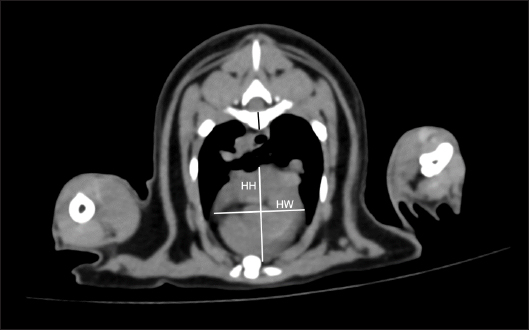
Fig. 4. tVHS measurement methods. tVHS was measured on cross-sectional CT image at the carina level. Heart height (HH) was the height of the heart. HW was the widest distance perpendicular to HH. The ratio of the sum of HH and HW to the height of the vertebral body (black line) at the carina level was then calculated as tVHS. tVHS: transverse vertebral heart score; CT: computed tomography; HH: he. Ethical approvalThis study was designed as a retrospective observational investigation. All information such as clinical demographic data, history, and all images were permitted for use by the committee of the Small Animal Hospital, Faculty of Veterinary Science, Chulalongkorn University (Approval number: 228/2563).
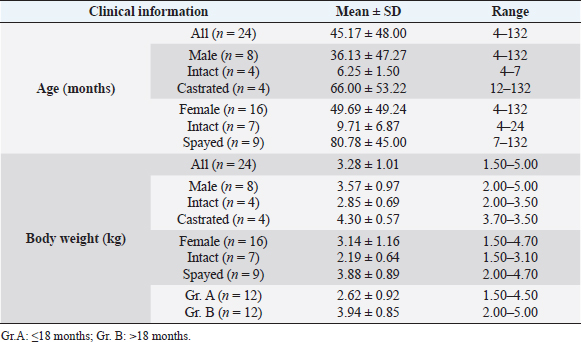
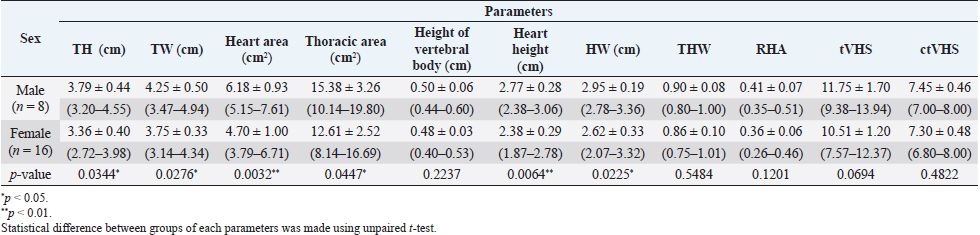
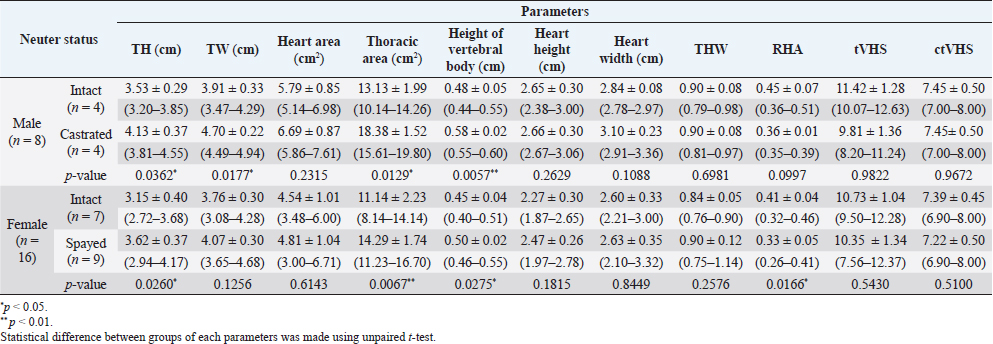
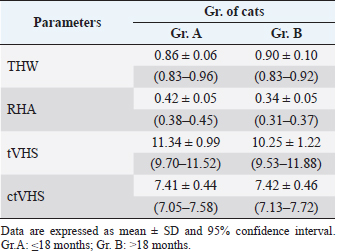
Fig. 5. ctVHS measurement methods. ctVHS was measured on the sagittal plane image. LA was the distance from ventral border of the mainstem bronchus to the cardiac apex. SA was the widest distance of the heart perpendicular with the LA line. LA and SA lines were transposed onto the vertebral column and the number of vertebrae beginning from the cranial end plate of the fourth thoracic vertebrae (T4) were recorded as ctVHS. ctVHS: computed tomography vertebral heart score; LA: long axis; SA: short axis; T4: the fourth thoracic vertebrae. ResultsClinical demographic dataThere were 24 cats that presented at the Diagnostic Imaging Unit, The Small Animal Hospital, Faculty of Veterinary Science, Chulalongkorn University and that met our inclusion criteria. There were domestic shorthair (n=19, 79.17%), American Shorthair (n=2, 8.34%), Scottish Fold (n=2, 8.34%), and Persian (n=1, 4.15%). There were eight male cats (4 castrated and 4 intact cats) and 16 female cats (9 spayed and 7 intact cats). All cats were reviewed and categorized into two groups, those aged younger or equal to 18 months (Gr. A) and those aged older than 18 months (Gr. B). Each group was composed of 12 cats. All clinical demographic information is summarized in Table 1. The BW of cats in Gr. A was significantly lower than that of cats in Gr. B ( p=0.0015). The neutered cats had significantly higher BW than the intact cats (p < 0.001). Computed tomographic (CT) heart size parametersIn this study, there was no significant difference in each parameter between pre- and post-contrast enhanced images (THW; p=0.5457, RHA; p=0.6128, ctVHS; p=0.8166, and tVHS; p=0.9148) (Table 2). Therefore, for the rest of the study, an average of pre- and post-contrast values of each parameter was used for further statistical analysis. The comparisons of each parameter between age groups which were Gr. A and Gr. B are shown in Table 3. The results indicated that cats in Gr. B had significantly higher TH, thoracic area, height of the vertebral body, and THW than cats in Gr. A (p =0.0307, p =0.0161, p =0.0061, and p =0.0414, respectively). Cats in Gr. A had significantly higher RHA and tVHS than those of Gr. B (p=0.0014 and p=0.0380, respectively). There was no significant difference in ctVHS between Gr. A and Gr. B. Regarding gender, male cats had significantly higher TH, TW, heart area, thoracic area, HH, and HW than female cats (p =0.0344, p =0.0276, p =0.0032, p =0.0447, p =0.0064, and p =0.0225, respectively) (Table 4). No significant difference of THW, RHA, tVHS, and ctVHS between male and female cats was detected. Considering gonadal status, we found that neutered cats (both male and female) had significantly higher TH, thoracic area, and height of vertebral body than intact cats (male; p =0.0362, p =0.0129, and p =0.0057, female; p =0.0260, p =0.0129, and p =0.0275, respectively) (Table 5). Neutered males also had significantly higher TW than intact males and neutered female cats had significantly lower RHA than intact females ( p=0.0166). No significant difference of THW, tVHS, and ctVHS between neutered and non-neutered cats was found in this study. The summarized reference range values of each calculated heart size parameter observed on CT in clinically healthy cats is summarized in Table 6. The correlations among heart size parametersIn this present study, cat age had significant positive correlations with TH, thoracic area, and height of the vertebral body (r =0.4979; p =0.0355, r=0.5941; p =0.0093, and r=0.4879; p=0.0400, respectively) and had a significant negative correlation with RHA ( r=−0.7228; p=0.0007). BW of cats had significant positive correlations with TH, TW, thoracic area, and height of the vertebral body (r=0.6628; p=0.0006, r =0.6514; p=0.0008, r=0.7772; p < 0.0001, and r =0.4516; p=0.0305, respectively) and had a significant negative correlation with RHA ( r=−0.5801; p=0.0036). Table 1. Clinical demographic information of all included cats in this study.
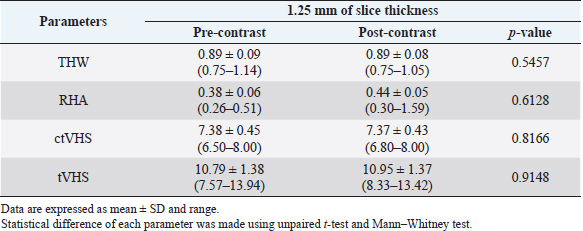
Table 2. Computed tomographic heart size parameters of all cats.
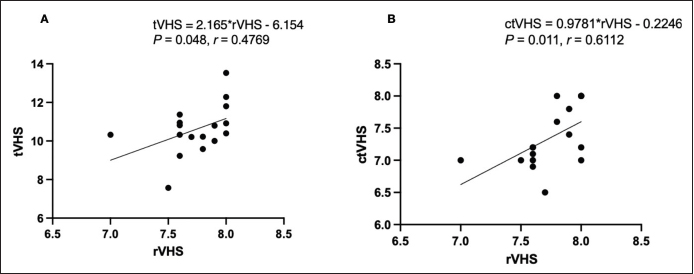
The correlations between calculated heart size parameters observed on CT and rVHS, tVHS, and ctVHS were significantly, moderately positive correlation with rVHS (r=0.4769; p=0.0480 and r =0.6112; p=0.0109, respectively) (Fig. 6). While, THW and RHA had non-significant correlation with rVHS (r=0.2642; p=0.3026 and r=0.1920; p=0.4559, respectively). DiscussionThis present study used a slice thickness of 1.25 mm for heart size evaluation based on the recommendations of a previous study as being suitable for intrathoracic structure evaluation in cats (Thammasiri et al., 2021). Additionally, several advantages of the greater slice thickness images when compared to the smaller ones have been reported, including less data storage required and greater signal-to-noise ratio (SNR) (Alshipli and Kabir, 2017). Higher SNR reflects better CT image quality. SNR is determined by slice thickness, where a greater slice thickness results in an increasing signal compared to the amount of noise in each area (Alshipli and Kabir, 2017). Accordingly, 1.25 mm of CT images can provide a comparable and high image quality and also require less data storage. Moreover, the CT-scanning time for a 1.25 mm slice thickness is faster compared to a smaller slice thickness which is suitable in clinical practice due to decreased time of radiation and anesthetization (Schwarz and O’Brien, 2011). TH, thoracic area, and THW were significantly higher in Gr. B than Gr. A. In contrast, RHA was significantly lower in Gr. B than Gr. A. These results correspond with a study in dogs (Uehara et al., 2009), in which anatomical features of the heart and thoracic cavity were influenced by body size. tVHS was significantly higher in Gr. A than in Gr. B. This may be due to the vertebral height on CT images used as a normalized parameter of tVHS. The older cats had a greater vertebral body height than younger cats due to their larger body size. Additionally, this may also be because cats in Gr. A were in the stage of incomplete bone growth as described in a previous report (Miranda et al., 2020). Table 3. CT heart size parameters between Gr. A and Gr. B of cats (mean ± SD and range).
Table 4. CT heart size parameters of male and female cats (mean ± SD and range)
Table 5. CT heart size parameters of neutered and non-neutered cats (mean ± SD and range).
Table 6. Summarized the reference range values of each calculated heart size parameter observed on CT in clinical healthy cats.
The results revealed that gender affected the thoracic cavity and also the heart size. Male cats had significantly greater TH, TW, heart area, thoracic area, HH, and HW than female cats. These results correspond with studies in dogs (Uehara et al., 2009), humans (Wooten et al., 2021), and marine mammals (Ralls and Mesnick, 2009), in which cats have a form of sexual dimorphism where the male’s body size is bigger than the female (Meachen-Samuels and Binder, 2010). It has been reported that males also have a larger heart size than females in various species such as humans (Wooten et al., 2021; Frayer and Wolpoff, 1985), cats (Meachen-Samuels and Binder, 2010), and dogs (Uehara et al., 2009). For the association of neutering status and each parameter, the results showed that neutered female cats had significantly higher TH and thoracic area than intact female cats and had significantly lower RHA than intact females. Similarly, neutered male cats had significantly higher TH, TW, and thoracic area than intact male cats. The results indicated and supported that the anatomical structures related to the heart and thoracic cavity are influenced by body size and gonadal status. These results correspond with those for human (Wooten et al., 2021) and dog (Uehara et al., 2009) studies. In dogs, it has been reported that the relative heart volume is lower in large dogs than small/medium dogs, consistent with the results of this study. Additionally, the heart volume was relative to the size of the thoracic cavity (Uehara et al., 2009). Both neutered males and females had significantly higher vertebral body height than intact males and females. This may be because most of neutered male and female cats in this study were aged more than 18 months. This study observed RHA instead of relative heart volume as in a previous study of dogs (Uehara et al., 2009). This is because the method for relative heart volume evaluation is more cumbersome and difficult than RHA assessment. Additionally, it takes a lot of time to complete relative heart volume evaluation which is not suitable for practitioners in clinical practice. Our results demonstrated that RHA can be used for heart size and thoracic cavity evaluation as relative heart volume and provides results consistent with a previous canine study (Uehara et al., 2009). The correlations between heart size parameters observed on CT and age and BW of cats showed that age and BW had significant positive correlations with TH, thoracic area, and vertebral body height. On the contrary, age had a significant negative correlation with RHA. These findings were also in agreement with previous studies in humans (Wooten et al., 2021) and dogs (Uehara et al., 2009), where a higher BW and age were related to a larger body size, which influenced heart and thoracic cavity size. Additionally, we evaluated the correlations between heart size parameters observed on CT and rVHS; the results showed that among four CT heart size parameters, tVHS and ctVHS had significant moderately positive correlations with rVHS. This could indicate that tVHS and ctVHS could be recommended parameters for evaluating feline heart size on CT images in clinical practice. Additionally, in the author’s opinion, both tVHS and ctVHS are easier to determine than other parameters in the current study. According to the nature of the retrospective study, we could not control the ECG gate and respiratory phase of cats during the CT procedure in this study. Involuntary motion from respiration or cardiac motion during image acquisition can cause artifacts in the reconstructed images (Barrett and Keat, 2004). However, in the present study, the mild motion artifact including blurring and stair-step artifact did not affect image quality and feasibility to measure all parameters. The present study used a 64-slice multidetector CT (MDCT) to scan and generate the images. Two previous reports about thoracic CT in dogs (Uehara et al., 2009) and cats (Oliveira et al., 2011) used a 16-slice MDCT in their study. Although using different MDCT scanner in each study, our study demonstrated that the results of this study correspond with a previous study in dogs (Uehara et al., 2009). Accordingly, different MDCT scanners may not affect the measured parameters, especially when following the previous study protocol. This study is the first report describing the use of CT for evaluating heart size and also providing a reference range for each CT heart size parameter in clinically healthy cats. This information could be useful for clinical practitioners and be of interest to researchers in further studies.
Fig. 6. Correlations between calculated heart size parameters observed on CT and rVHS. (A) tVHS and rVHS. (B) ctVHS and rVHS. CT: computed tomography; rVHS: radiographic vertebral heart score; tVHS: transverse vertebral heart score; ctVHS: CT vertebral heart score. The main limitation of this study was the small sample size. Additionally, we did not perform the echocardiography due to the nature of the retrospective study. However, all cats were determined as being normal before inclusion in this study. Furthermore, this study did not compare all parameters between clinically healthy cats and cats with heart disease and also could not perform and control the ECG gate and respiratory phase of cats during the CT procedure; a limitation of the retrospective study. Therefore, future studies should be considered in order to provide further information. ConclusionThis study demonstrated that CT heart size evaluation could be performed using 1.25 mm slice thickness and both pre- and post-contrast enhanced images. The anatomical structures related to the heart and thoracic cavity are associated with the age, sex, and gonadal status of cats. Among four CT heart size parameters in this study, tVHS and ctVHS are recommended for evaluating feline heart size on CT images in clinical practice. However, further studies comparing clinically healthy cats and heart disease cats should be performed to fulfill and validate the information. AcknowledgmentsThe authors would like to thank the Small Animal Hospital, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand for all support and information. FundingThis study was granted from Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand. Conflict of interestThe authors declare no conflict of interests related to this article. Author contributionsCT and NC conceived the ideas of the study; CT and NC conceived the ideas for the analysis; AW, CS, ZK, TS, SJ, SS and CT performed the analyses; AW, CS, ZK, TS, SJ, NC and CT wrote the manuscript; CT and NC were major contributors in writing the manuscript and all other authors commented on the manuscript. All authors read and approved the final manuscript. ReferencesAlshipli, M. and Kabir, N. 2017. Effect of slice thickness on image noise and diagnostic content of single-source-dual energy computed tomography. J. Physics. Conference Series, Series 851. 851, 012005; https://doi.org/10.1088/1742-6596/851/1/012005 Barrett, J.F. and Keat, N. 2004. Artifacts in CT: recognition and avoidance. Radiographics 24, 1679–1691. Bertolini, G. and Angeloni, L. 2017. Vascular and cardiac CT in small animals. In Computed tomography-advanced applications. Ed., Halefoglu, A.M. London, UK: InTechOpen, pp: 251–266. Bruno, M.A. 2017. 256 Shades of gray: uncertainly and diagnostic error in radiology. Diagnosis (Berl) 4, 149–157. d’Anjou, M.A. 2018. Principle of computed tomography and magnetic resonance imaging. In: Thrall DE, editor. Textbook of Veterinary Diagnostic Radiology, 7th ed. Elsevier saunders, pp: 71-95. Frayer, D.W. and Wolpoff, M.H. 1985. Sexual dimorphism. Annual Review of Anthropology.14(1), 429–473; https://doi.org/10.1146/annurev.an.14.100185.002241. Freeman, L.M., Rush, J.E., Stern, J.A., Huggins, G.S. and Maron, M.S. 2017. Feline hypertrophic cardiomyopathy: a spontaneous large animal model of human HCM. Cardiol. Res. 8, 139–142. Fuentes, L.V., Abbott, J., Chetboul, V., Cote, E., Fox, R.P., Haggstrom, J., Kittenson, M.D., Schober, K. and Stern, A.J. 2020. ACVM consensus statement guidelines for the classification, diagnosis and management of cardiomyopathies in cats. J. Vet. Intern. Med. 34, 1062–1077. Henninger, W. 2003. Use of computed tomography in the diseased feline thorax. J. Small Anim. Pract. 44, 56–64. Keane, M., Paul, E., Sturrock, C.J., Rauch, C. and Rutland, C.S. 2017. Computed tomography in veterinary medicine: currently published and tomorrow’s vision. In Computed tomography-advanced applications. Ed., Halefoglu, A.M. London, UK: InTechOpen, pp: 271–289. Khor, K.H. and Chin, M.X. 2020. Occurrences of heart disease in apparently healthy cats in Klang Valley. Malaysia. J. Adv. Vet. Anim. Res. 7, 501–508. Kittleson, M. and Cote, E. 2021. The feline cardiomyopathies. J. Feline Med. Surg. 23, 1009–1027. Lister, A.L. and Buchanan, J.W. 2000. Vertebral scale system to measure feline heart size in radiographs of cats. J. Am. Vet. Med. Assoc. 216, 210–214. Meachen-Samuels, J.A. and Binder, W.J. 2010. Sexual dimorphism and ontogenetic growth in the American lion and sabertoothed cat from Rancho La Brea. J. Zool. 280, 271–279. Michal, J. 2010. Congenital heart defects in cat—prevalence and survival. Available via https://stud.epsilon.slu.se/7580/17/michal_j_150202.pdf (Accessed 6 September 2022). Mikla, I.M. and Mikla, V.V. 2014. Computed Tomography. In: Mikla IM, Mikla V, editor. Medical Imaging Technology, 1st ed. Elsevier Saunders, pp: 23-28. Miranda, F.G., Souza, I.P., Viegas, F.M., Megda, T.T., Nepomuceno, A.C., Torres, R.C. and Rezende, C.M. 2020. Radiographic study of the development of the pelvis and hip and the femorotibial joints in domestic cats. J. Feline Med. Surg. 22, 476–483. Nyathi, T., Chirwa, T.F. and Van der Merwe, D.G. 2010. A survey of digital radiography practice in four South African teaching hospitals: an illuminative study. Available via http://www.biij.org/2010/1/e5 (Accessed 16 December 2021). Oliveira, C.R., Mitchell, M.A. and O'Brien, R.T. 2011. Thoracic computed tomography in feline patients without use of chemical restraint. Vet. Radiol. Ultrasound. 52, 365–376. Prather, A.B., Berry, C.R. and Thrall, D.E. 2005. Use of radiography in combination with computed tomography for the assessment of non-cardiac thoracic disease in the dog and cat. Vet. Radiol. Ultrasound 46, 114–121. Ralls, K. and Mesnick, S. 2009. Sexual dimorphism. In Eds., Perrin W.F, Wuersig B. and Thewissen J.G.M. Encyclopedia of marine mammals. Academic Press, pp: 1005–1011. Riesen, S.C., Kovacevic, A., Lombard, C.W. and Amberger, C. 2007. Prevalence of heart disease in symptomatic cats: an overview from 1998 to 2005. Euro. J. Companion Anim. Pract. 149, 65–71. Ruderman, E.M. and Flaherty, J.P. 2017. Mycobacterial infections of bones and joints. In Textbook of rheumatology, 10th ed. Eds., Kelley and Firestein. Philadelphia, PA: Elsevier Saunders, pp: 1905–1917. Savino, G., Zwerner, P., Herzog, C., Politi, M., Bonomo, L., Costello, P. and Schoepf, U.J. 2007. CT of cardiac function. J. Thorac. Imag. 22, 86–100. Schober, K.E., Maerz, I., Ludewig, E. and Stern, J.A. 2007. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: comparison with transthoracic 2-dimensional echocardiography. J. Vet. Intern. Med. 21, 709–718. Schwarz, T. and O’Brien, R. 2011. CT Acquisition Principles. In Tobias S. and Jimmy S. Veterinary Computed Tomography. Hoboken NJ: John Wiley & Sons, pp: 9–27. Sisakian, H. 2014. Cardiomyopathies: evolution of pathogenesis concepts and potential for new therapies. World J. Cardiol. 6, 478–494. Spalla, I., Locatelli, C., Riscazzi, G., Santagostino, S., Cremaschi, E. and Brambilla, P. 2016. Survival in cats with primary and secondary cardiomyopathies. J. Feline Med. Surg. 18, 501–509. Stephenson, A., Adams, J.W. and Vaccarezza, M. 2017. The vertebrate heart: an evolutionary perspective. J. Anat. 231, 787–797. Tidholm, A., Ljungvall, I., Michal, J., Haggstrom, J. and Hoglund, K. 2015. Congenital heart defects in cats: a retrospective study of 162 cats (1996-2013). J. Vet. Cardiol. 17, S215–S219. Thammasiri, N., Thanaboonnipat, C., Choisunirachon, N. and Darawiroj, D. 2021. Multi-factorial considerations for intra-thoracic lymph node evaluations of healthy cats on computed tomographic images. BMC Vet. Res. 17, 1–11. Uehara, T., Orito, K. and Fujii, Y. 2009. CT-based anatomical features of large airway and heart volume in dogs of different body size. Vet. J. 246, 21–26. Wooten, S.V., Moestl, S., Chilibeck, P., Alvero, Cruz, J.R., Mittag, U., Tank, J., Tanaka, H., Rittweger, J. and Hoffmann, F. 2021. Age-and sex-differences in cardiac characteristics determined by echocardiography in masters athletes. Front. Physiol. 11, 630148. | ||
| How to Cite this Article |
| Pubmed Style Wanglerm A, Samachikthummakun C, Kitnitchee Z, Sirinitikorn T, Junnong S, Sutthigran S, Choisunirachon N, Thanaboonnipat C. Computed tomographic evaluation of heart size in clinically healthy cats. Open Vet J. 2023; 13(3): 337-347. doi:10.5455/OVJ.2023.v13.i3.10 Web Style Wanglerm A, Samachikthummakun C, Kitnitchee Z, Sirinitikorn T, Junnong S, Sutthigran S, Choisunirachon N, Thanaboonnipat C. Computed tomographic evaluation of heart size in clinically healthy cats. https://www.openveterinaryjournal.com/?mno=137873 [Access: September 01, 2024]. doi:10.5455/OVJ.2023.v13.i3.10 AMA (American Medical Association) Style Wanglerm A, Samachikthummakun C, Kitnitchee Z, Sirinitikorn T, Junnong S, Sutthigran S, Choisunirachon N, Thanaboonnipat C. Computed tomographic evaluation of heart size in clinically healthy cats. Open Vet J. 2023; 13(3): 337-347. doi:10.5455/OVJ.2023.v13.i3.10 Vancouver/ICMJE Style Wanglerm A, Samachikthummakun C, Kitnitchee Z, Sirinitikorn T, Junnong S, Sutthigran S, Choisunirachon N, Thanaboonnipat C. Computed tomographic evaluation of heart size in clinically healthy cats. Open Vet J. (2023), [cited September 01, 2024]; 13(3): 337-347. doi:10.5455/OVJ.2023.v13.i3.10 Harvard Style Wanglerm, A., Samachikthummakun, . C., Kitnitchee, . Z., Sirinitikorn, . T., Junnong, . S., Sutthigran, . S., Choisunirachon, . N. & Thanaboonnipat, . C. (2023) Computed tomographic evaluation of heart size in clinically healthy cats. Open Vet J, 13 (3), 337-347. doi:10.5455/OVJ.2023.v13.i3.10 Turabian Style Wanglerm, Aphisit, Chawanwith Samachikthummakun, Zen Kitnitchee, Thiranan Sirinitikorn, Sapawut Junnong, Somchin Sutthigran, Nan Choisunirachon, and Chutimon Thanaboonnipat. 2023. Computed tomographic evaluation of heart size in clinically healthy cats. Open Veterinary Journal, 13 (3), 337-347. doi:10.5455/OVJ.2023.v13.i3.10 Chicago Style Wanglerm, Aphisit, Chawanwith Samachikthummakun, Zen Kitnitchee, Thiranan Sirinitikorn, Sapawut Junnong, Somchin Sutthigran, Nan Choisunirachon, and Chutimon Thanaboonnipat. "Computed tomographic evaluation of heart size in clinically healthy cats." Open Veterinary Journal 13 (2023), 337-347. doi:10.5455/OVJ.2023.v13.i3.10 MLA (The Modern Language Association) Style Wanglerm, Aphisit, Chawanwith Samachikthummakun, Zen Kitnitchee, Thiranan Sirinitikorn, Sapawut Junnong, Somchin Sutthigran, Nan Choisunirachon, and Chutimon Thanaboonnipat. "Computed tomographic evaluation of heart size in clinically healthy cats." Open Veterinary Journal 13.3 (2023), 337-347. Print. doi:10.5455/OVJ.2023.v13.i3.10 APA (American Psychological Association) Style Wanglerm, A., Samachikthummakun, . C., Kitnitchee, . Z., Sirinitikorn, . T., Junnong, . S., Sutthigran, . S., Choisunirachon, . N. & Thanaboonnipat, . C. (2023) Computed tomographic evaluation of heart size in clinically healthy cats. Open Veterinary Journal, 13 (3), 337-347. doi:10.5455/OVJ.2023.v13.i3.10 |