| Research Article | ||
Open Vet J. 2023; 13(5): 638-644 Open Veterinary Journal, (2023), Vol. 13(5): 638–644 Original Research Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, LibyaAbdulatif A. Asheg1* Mohamed F. Otman1, Imad A. Benlashehr1, El-Forjani Kraim2, Rabia A. Almashri2 and Abdulwahab M. Kammon1,31Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 2National Center of Animal Health (NCAH), Tripoli, Libya 3National Research Center for Tropical and Transboundary Diseases, Alzintan, Libya *Corresponding Author: Abdulatif Asheg. Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: A.asheg [at] uot.edu.ly Submitted: 18/02/2023 Accepted: 20/04/2023 Published: 19/05/2023 © 2023 Open Veterinary Journal
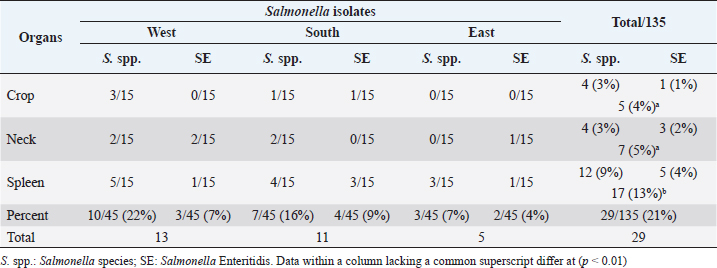
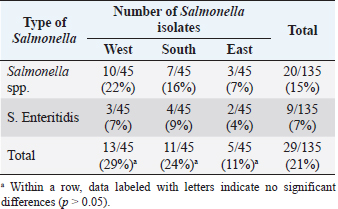
AbstractBackground: Salmonella is a leading cause of severe economic losses in poultry and foodborne illness in humans worldwide. Aim: The aim of this study was to determine the prevalence and multidrug resistance of Salmonella Enteritidis (S. Enteritidis) in several chicken abattoirs in Tripoli, Libya. The study includes the South, East, and West regions of Tripoli. Methods: Each region was assigned five slaughterhouses. Each chicken slaughterhouse was visited three times to collect samples. Five samples were taken at random from the neck skin, crop, and spleen. The total number of samples collected from all regions was 675. Bacterial isolation and identification, as well as antibiotic sensitivity testing, were performed on these samples. Results: Salmonella spp. was found to be 15% prevalent, and S. Enteritidis was found to be 7% prevalent. The south region of Tripoli had the highest S. Enteritidis (9%), while the west region had the highest Salmonella spp. (22%). Salmonella prevalence increased significantly (p < 0.01) higher in the spleen (13%) as compared with the crop (5%) and neck (7%). Based on bacterial resistance pattern, Salmonella spp. isolated from the spleen had the highest multiple antibiotic resistance (MAR) index of 0.86 in the south region followed by MAR indexes of 0.8 and 0.46 in the West and East, respectively. Conclusion: Isolation of Salmonella from the spleen may indicate chickens’ systemic infection and failure to control the most important microbe for public health. Thus, the control measures have to be revised and a national Salmonella control program should be put in place urgently. Keywords: Salmonella spp., Salmonella Enteritidis, Slaughterhouses, Broiler, Tripoli. IntroductionSalmonella is a leading cause of severe economic losses in poultry and foodborne illness in humans worldwide (Lin et al., 2014; Sallam et al., 2014). Though there are more than 2,000 different subspecies of Salmonella, few can cause serious conditions in humans and chickens (Rodpai et al., 2013). Salmonella Enteritidis (S. Enteritidis) is invasive in laying hens, and vertical transmission has been demonstrated (Cooper et al., 1989). Some strains of S. Enteritidis have been shown to cause anorexia, diarrhea, and decreased egg production in experimentally infected laying hens (Gast and Beard, 1992). Salmonella Enteritidis is also invasive in broiler chickens and is frequently isolated from the muscles of raw chicken carcasses purchased from retail outlets (Lister, 1988; Humphrey, 2000). Infection caused by Salmonella rather than S. pullorum-gallinarum (typhoid fever), the condition is known as paratyphoid fever (PT), however, signs of severe PT infection in young poultry are commonly seen with S. pullorum-gallinarum gallinarum similar to things. The presence of Salmonella in the gut, skin, and feathers of chickens can contaminate carcasses during slaughter and processing, potentially contributing to the introduction of this organism into the slaughterhouse (Paiao et al., 2013). Salmonella Enteritidis pollution is a major problem in many countries everywhere in the world. In 2018, there were 419 confirmed cases of Salmonella identical to raw chicken, including frozen breaded raw chicken products in Canada (BCCDC, 2018). Since 2014, human cases of S. Enteritidis in the European Union (EU) have increased by 3%. Over the same period, the prevalence of Salmonella in egg production increased from 0.7% to 1.21%. Salmonellosis was mentioned in 94,530 cases in the EU in 2016. Salmonella Enteritidis, the most frequent condition of Salmonella, accounts for 59% of all salmonellosis cases in the (EU) and is primarily associated with the consumption of eggs, egg products, and poultry meat (BCCDC, 2018). In Germany, 16,000 human cases of salmonellosis were reported. Salmonella Enteritidis was the most common serotype (44.4% of all isolates), as it had been in prior years (Antunes et al., 2016). Expanding resistance to antibiotics in S. Enteritidis and other Salmonella spp. is a problem leading to serious health risks worldwide (Singh et al., 2013). The reason for this problem could be due to the overuse and misuse of antibiotics in developing countries (Ikwap et al., 2014). In Libya, there were few researchers studied the prevalence of Salmonella in chicken slaughterhouses as well as in humans. Serotyping of Salmonella isolated from children with diarrhea in Zliten City resulted in the presence of S. Heidelberg and S. Enteritidis isolates (Ali et al., 2005). Therefore, the purpose of this research was to estimate the prevalence of Salmonella in poultry slaughterhouses in Tripoli, Libya’s south, west, and east regions. Material and MethodsSamplingThe survey covered the southern, eastern, and western regions of Tripoli during the 2018 period. From each region, five chickens were selected from each slaughterhouse. Each chicken abattoir was visited three times at 2-week intervals for sampling. Samples taken from each abattoir including neck skin, crops, and spleen were collected. The total number of samples from all regions was 675. All five samples were pooled prior to isolation and identification. Therefore, 135 samples were processed for isolation, identification, and antibiotic susceptibility testing. Isolation and identificationThe Salmonella isolation procedure was used as per the method of Waltman et al. (1993). The swabs were inoculated in pre-enrichment media (peptone water, Oxoid, Hampshire, UK) at 35°C–37°C for 24 hours. A loopful of the pre-enrichment medium was then inoculated in the selective-enrichment broth, Rappaport Vassiliadis (Oxoid, Hampshire, UK) at 35°C–37°C for 24 hours after collection. A loopful of the selective-enrichment broth was then streaked on the selective media, xylose lysine desoxycholate (Oxoid, Hampshire, United Kingdom) agar at 35°C–37°C for 24 hours. The morphology of the bacteria was tested by Gram stain. The isolates were then identified biochemically using a single colony selected and inoculated in triple sugar iron (Merck, Darmstadt, Germany) agar, citrate, lysine, indol, urea, and oxidase (Merck, Darmstadt, Germany). The positive colony was submitted to the National Centre for Animal Health for serotyping using slide agglutinations O1, O9, O12, and H including [f], g, m, and [p]antigens. Antimicrobial sensitivity test (the Kirby-Bauer disc method)The antibiotic susceptibility of the isolates was tested by using the disc diffusion method described by Bauer et al. (1966) with minor modifications. Fresh 3–5 colonies of the isolate were collected and suspended in 1 ml. sterile saline. The suspension was then standardized to a 0.5 McFarland standard (equivalent to approximately 1.5 × 108 CFU/ml). The prepared suspension was used as-inoculated within 15 minutes. The suspension was blotted with a sterile, non-toxic cotton swab, streaked onto a Mueller-Hinton agar plate (Merck, Darmstadt, Germany), and allowed to dry for 2–4 minutes. Antimicrobial susceptible discs (Oxoid, Hampshire, UK) were then placed in the cultures using a diffusion disc dispenser (Oxoid). Antibiotic discs tested were Ciprofloxacin, Trimethoprim, Chloramphenicol, Amoxicillin/Clavulanic acid, Sulfamethazone-Trimethoprim, Ampicillin, Gentamycin, Doxycyclin, Colistin, Neomycin, Tetracycline, Nitrofurantoin, Lincomycin, Erythromycin, and Cefuroxime. The zone of inhibition was assessed after 24 hours of incubation at 37°C to determine the degree of sensitivity (sensitive, intermediate, or resistant). The multiple antibiotic resistance (MAR) indexes were determined using the formula: a/b, where “a” is the number of antibiotics to which a particular isolate was resistant and “b” is the total number of antibiotics tested (Krumperman, 1983). Table 1. Number and percentage of Salmonella spp. and S. Enteritidis isolated from different organs and different regions.
Statistical analysisSalmonella prevalence data were subjected to Pearson’s chi-square test using Statistical Package for the Social Sciences software (SPSS Inc. Chicago, IL) to determine the significant variation, if any, among different regions (west, south, and east) and organs (crop, neck, and spleen). The value of (p < 0.01) was taken as the cut-off value to consider differences statistically significant. Ethical approvalAll work was done using international animal welfare standards. All the samples are collected from using the National Center of Animal Health (NCAH) protocol. Table 2. Prevalence of Salmonella spp. and S. Enteritidis isolated from different regions of Tripoli.
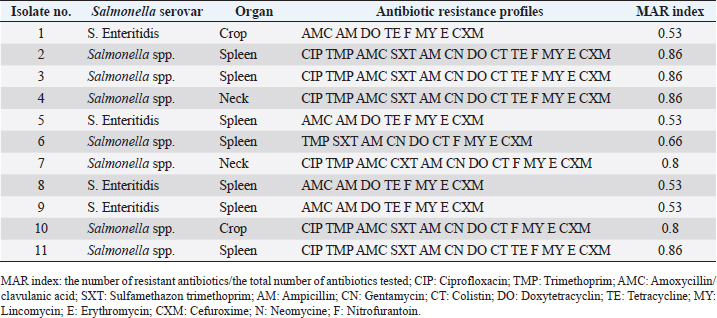
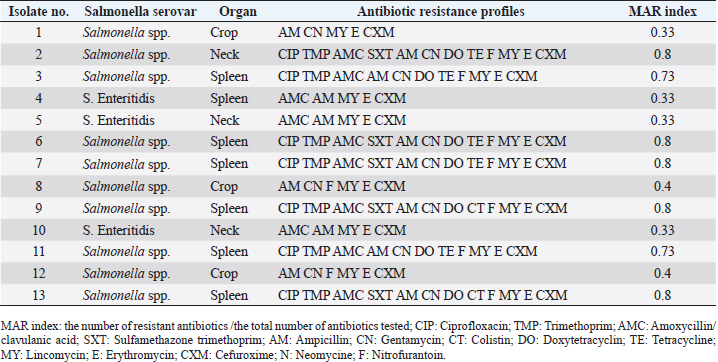
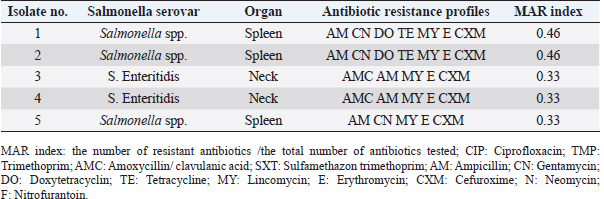
ResultsPrevalence of SalmonellaIn the current study, Salmonella spp. and S. Enteritidis were isolated from 29 out of 135 samples collected from three regions of Tripoli (Table 1). In the West region, 13 (29%) out of 45 chicken organs collected from 5 slaughterhouses, were positive for Salmonella. Among those, 3 (7%) were positive for S. Enteritidis and 10 (22%) were positive for Salmonella spp. In the south region, a total of 11 (24%) out of 45 chicken organs collected from 5 slaughterhouses, were positive for Salmonella. Among those, 4 (9%) were positive for S. Enteritidis and 7 (16%) were positive for Salmonella spp. In the East region, 5 (29%) out of 45 chicken organs collected from 5 slaughterhouses, were positive for Salmonella. Among those, 2 (4%) were positive for E. Enteritidis and 3 (7%) were positive for Salmonella spp. The overall prevalence of Salmonella isolates from different regions was 21% (Table 2). The prevalence of S. Enteritidis was 7%. The highest prevalence of S. Enteritidis (9%) was recorded in the southern region of Tripoli. Salmonella has the highest prevalence (22%). However, it is found in the western regions. Statistically, there were no significant differences (p > 0.05) in the prevalence of total Salmonella between the regions. In general, the prevalence of Salmonella was significantly (p > 0.01) higher in the spleen (13%) as compared with crop and neck where the prevalence of Salmonella in these organs was 4% and 5%, respectively. In the spleen (9%) of isolated Salmonella were Salmonella spp. and only 4% were S. Enteritidis. Antibiotic sensitivity testThe resistance pattern, Salmonella spp. isolated from the spleen had the highest MAR index value of 0.86 in the South region (Table 3) followed by a MAR index value of 0.8 in the West region (Table 4) and a MAR index value of 0.46 in the East region (Table 5). The highest MAR index of S. Enteritidis isolated from the spleen and crop was 0.53 in the South region (Table 3). Table 3. Antimicrobial resistance profiles of S. Enteritidis and Salmonella spp. isolated from chickens in the South region.
Table 4. Antimicrobial resistance profiles of S. Enteritidis and Salmonella spp. isolated from chickens in the West region.
Table 5. Antimicrobial resistance profiles of S. enteritidis and Salmonella spp. isolated from chickens in the East region.
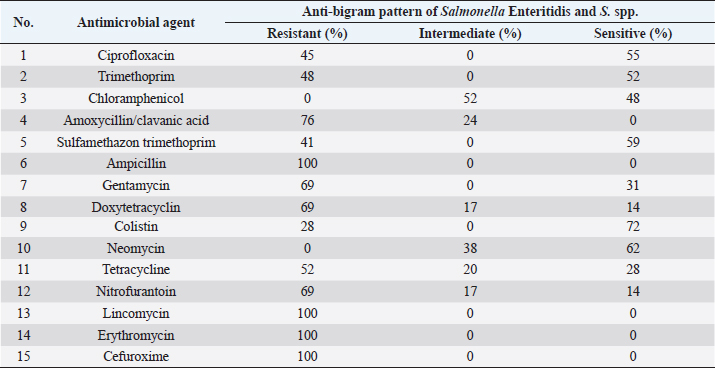
Table 6. Antimicrobial resistance pattern of S. enteritidis, and Salmonella spp. isolated from chicken organs samples tested by disc diffusion method (total of 29 isolates).
Ampicillin, Lincomycin, Erythromycin, and Cefuroxime resistance have been observed for all isolated bacteria. However, resistance to Colistin 8 (28%), and Sulfamethazon-trimethoprim 12 (41%), were found to be low. Colistin 21 (72%) had the highest sensitivity, followed by Neomycin 18 (62%), while Nitrofurantoin 4 (14%), and Doxy tetracycline 4 (14%), had the lowest (Table 6). DiscussionIn the current study, Salmonella spp. and S. Enteritidis were isolated from 29 out of 135 samples collected from three regions of Tripoli. The overall prevalence was 21%. The prevalence of Salmonella spp. was 15% whereas the prevalence of S. Enteritidis was 7%. This high prevalence might reflect poor hygienic and biosecurity measures in poultry houses, slaughterhouses, and live bird markets. Similar results were reported by Paiao et al. (2013) in Brazil and by Karim et al. (2017) in Bangladesh. A lower prevalence of Salmonella spp. (0.39%) and S. Enteritidis (1.18%) was reported in Poland (Witkowska et al., 2018). In Turkey, Goncag et al. (2005) reported a prevalence of 8.57% for S. Enteritidis in chicken carcass skins of the wing parts. In Algeria, Djeffal et al. (2018) also reported a prevalence of 8% for Salmonella spp. isolated from the skin of the chicken. However, Ramya et al. (2012) reported a higher incidence of Salmonella spp. and S. Enteritidis in chickens in India. They reported a prevalence of 64% (16 out of 25) and 56% (14 out of 25) for Salmonella spp. by polymerase chain reaction (PCR) and culture, respectively, and a prevalence of 48% (12 out of 25) for S. Enteritidis by PCR. Salmonellosis is a very important zoonotic disease in human beings causing diarrhea, nausea, abdominal pain, mild fever, chills, vomiting, prostration, headache, and malaise (Forshell and Wierup, 2006). Among the regions included in this study, the southern region of Tripoli had the highest prevalence of S. Enteritidis (9%) and the west seemed to have the highest prevalence of Salmonella spp. (22%). These two regions are known for their intensive poultry production. Studies on the extent of biosecurity measures in chicken farms in Tripoli are lacking, but poor biosecurity measures in poultry farms may be one of the causes of the spread of Salmonella. In the study by Kammon et al. (2017) the levels of biosecurity in poultry farms located in Aljabal Al-Gharbi, particularly house floors, Farm distance, the existence of cleaning products at farm entrances, use of coveralls, disposal of dead birds, and low controls of birds and rodents. 63% of poultry houses have a ground of soil and 44% of them have uncoated walls which may influence the proper cleaning and disinfection. In the current study, while visiting the slaughterhouses for sampling, low levels of biosecurity measures were observed including the absence of regular use of disinfectants, absence of coverall cloths, the presence of multiple clots of blood on walls and ground, and dirty chicken feather removing machine and cutting knives. Moreover, some slaughterhouses are located nearly to the accumulation of municipal sewage just in front of the main door. Mostly there was no program to control the flies, wild birds, and rodents. Following proper sanitation and biosecurity procedures reduces the possibility of Salmonella contamination. Sanitizing water pipes, keeping wild birds and other animals out of slaughterhouses, limiting visitor numbers to required staff, using and maintaining footbaths on a regular basis, and wearing shoe covers or special shoes. Controlling insects and rodents are a common biosecurity practice in poultry farms (Dorea et al., 2010; Van Steenwinkel, 2011). In general, the prevalence of Salmonella was significantly (p < 0.01) higher in the spleen (13%) as compared with crop and neck where the prevalence of Salmonella in these organs was 4% and 5%, respectively. In the spleen, 12 (9%) isolated Salmonella spp., and only 5 (4%) were S. Enteritidis. The prevalence of Salmonella in the spleen was 47%, 40%, and 27% in the South, West, and East regions, respectively. This result may indicate systemic infection of chickens with Salmonella. Previously, Asheg et al. (2003) confirmed the presence of S. Enteritidis in macrophage-like cells, particularly in the lamina propria of the cecum, 3–21 days after infection. S. Enteritidis colonization and migration in the chicken intestinal tract were found to be dose-dependent with the ability of macrophages to survive after phagosome/lysosome fusion (Oh et al., 1996). Salmonella Enteritidis isolates have been linked to spleen invasion in various ways (Asheg et al. 2002). Our result is in contrast to previous studies in which the crop was implicated as an important agent of carcass contamination within the processing plant (Ramirez et al., 1997). Hargis et al. (1995) found elevated levels of Salmonella in crops than in ceca during commercial evisceration. However, the localization of Salmonella in the gut, skin, and under feathers of chickens can contaminate carcasses during slaughter and processing, potentially contributing to the introduction of this organism into slaughterhouses (Paiao et al., 2013). Our study shows 100% resistance of S. Enteritidis and Salmonella spp. to ampicillin, lincomycin, erythromycin, and cefuroxime. The high and MAR indexes of 0.86 and 0.53 for Salmonella spp. and S. Enteritidis isolated from the spleen were found in the South region. Resistance to erythromycin has been reported as the most common resistance profile in retail meat production (Sallam et al., 2014). Thung et al. (2016) have found 100% resistance of Salmonella to erythromycin, 69% to gentamycin, 100% to ampicillin, 45% to ciprofloxacin, and 52% to tetracycline. In another study, Bhuvaneswari et al. (2015) reported 60.7%, 92.1%, 100%, 23.5%, and 92.1% resistance of Salmonella to erythromycin, gentamycin, ampicillin, ciprofloxacin, and tetracycline in India, respectively. Antimicrobial resistance in S. Enteritidis and other Salmonella spp. is an increasing problem leading to serious health hazards in the world (Singh et al., 2013). The reason for this problem could be due to the extensive use of antibiotics in developing countries (Ikwap et al., 2014). In contrast, our study showed that isolated S. Enteritidis was susceptible to ciprofloxacin, trimethoprim, chloramphenicol, sulfamethazon trimethoprim, gentamycin, colistin, and neomycin. In a study by Thung et al. (2016), S. Enteritidis was susceptible to trimethoprim and gentamycin. In conclusion, isolation of Salmonella from the spleen may indicate a chicken’s systemic infection and failure to control the most important microbe for public health. Thus, the control measures have to be revised and a national Salmonella control program should be put in place urgently. AcknowledgmentsThe authors would like to thank the Libyan National Center of Animal Health in Tripoli for their support in the laboratory analysis. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAli, M.B., Ghenghesh, K.S., Aissa, R.B., Abuhelfaia, A. and Dufani, M. 2005. Etiology of childhood diarrhoea in Zliten, Libya. Saudi Med. J. 26(11), 1759–1765. Antunes, P., Mourão, J. and Campos, L. 2016 Salmonellosis the role of poultry meat (review). Clin. Microbiol. Infect. 22(2), 110–121. Asheg, A., Levkut, M., Revajová, V., Ševčíková, K.Z. and Pistl, J. 2002. T lymphocyte subpopulations and B lymphocyte cells in caecum and spleen of chicks infected with Salmonella enteritidis. Acta Histochem. 104(4), 435–439. Asheg, A., Levkut, M., Revajová, V., Ševčíková, K.Z. and Pistl, J. 2003. Dynamics of lymphocyte subpopulations in immune organs of chickens infected with Salmonella enteritidis. Acta Vet. Brno 72, 359–364. Bauer, A.W., Kirby, W.M.M., Sherris, J.C. and Turck, M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 36, 493–496. BCCDC. 2018. Outbreaks of Salmonella infections linked to raw chicken, including frozen raw breaded chicken products. Available via http://www.bccdc.ca/about/news-stories/news-releases/2018/Salmonella-phn-phac (Accessed 15 November 2022). Bhuvaneswari, M., Shanmughapriya, S. and Natarajaseenivasan, K. 2015 Prevalence of multidrug-resistant (MDR) Salmonella enteritidis in poultry and backyard chicken from Tiruchirappalli, India. Microbiol. J. 5(2), 28–35. Cooper, G.L., Nicholas, R.A.J. and Bracewell, C.D. 1989. Serological and bacteriological investigation of chickens from flocks naturally infected with Salmonella enteritidis. Vet. Rec. 125, 567–572. Djeffal, S., Mamache, B., Elgroud, R., Hireche, S. and Bouaziz, O. 2018. Prevalence and risk factors for Salmonella spp. contamination in broiler chicken farms and slaughterhouses in the northeast of Algeria. Vet. World 11(8), 1102–1108. Dorea, F.C., Berghaus, R., Hofacre, C. and Cole, D.J. 2010. Survey of biosecurity protocols and practices adopted by growers on commercial poultry farms in Georgia, U.S.A. Avian Dis. 54(3), 1007–1015. Forshell, L.P. and Wierup, M. 2006. Salmonella contamination: a significant challenge to the global marketing of animal food products. Sci. Tech. Rev. OIE 25(2), 541–554. Gast, R.K. and Beard, C.W. 1992. Detection of Salmonella serogroup D-specific antibodies in the yolks of eggs laid by hens infected with Salmonella enteritidis. Poult. Sci. 70, 1273–1276. Goncag, G., Gunaydin, L. and Carli, T. 2005. Prevalence of Salmonella serogroups in chicken meat. Turk. J. Vet. Anim. Sci. 29, 103–106. Hargis, B.M., Caldwell, D.J., Brewer, R.L., Corrier, D.E. and Deloach, J.R. 1995. Evaluation of the chicken crop as a source of Salmonella contamination for broiler carcasses. Poult. Sci. 74, 1548–1552. Humphrey, T. 2000. Public-health aspects of Salmonella infection. In Salmonella in domestic animals. Eds., Wray, C. and Wray, A. New York, NY: CABI Publishing, pp: 245–263. Ikwap, K., Erume, J., Owiny, D.O., Nasinyama, G.W., Melin, L., Bengtsson, B., Lundeheim, N., Fellstrom, C. and Jacobson, M. 2014. Salmonella species in piglets and weaners from Uganda: prevalence, antimicrobial resistance, and herd-level risk factors. Prev. Vet. Med. 115, 39–47. Kammon, A., Mulatti, P., Lorenzetto, M., Ferre, N., Sharif, M., Eldaghayes, I. and Dayhum, A. 2017. Biosecurity and geospatial analysis of mycoplasma infections in poultry farms at Al-Jabal Al-Gharbi region of Libya. Open Vet. J. 7(2), 81–85. Karim R.M., Giasuddin, M., Abdus Samad, M., Mahmud, M.S., Islam, M.R., Hafizur Rahman, M. and Abu Yousuf, M. 2017. Prevalence of Salmonella spp. in poultry and poultry products in Dhaka, Bangladesh. Int. J. Anim. Biol. 3(4), 18–22. Krumperman, P.H. 1983. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 46, 165–170. Lister, S. 1988. Salmonella enteritidis infection in broilers and broiler breeders. Vet. Rec. 123, 350. Lin, D., Yan, M., Lin, S. and Chen, S. 2014. Increasing prevalence of hydrogen sulfide negative Salmonella in retail meats. Food Microbiol. 43, 1–4. Oh, Y.K., Alpuche-Aranda, C., Berthiaume, E., Jinks, T., Miller, S.I. and Swanson, J.A. 1996. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect. Immun. 64, 3877–3883. Paiao, L.F., Arisitides, G.A., Murate, S., Vilas-Bôas, G.T., Vilas-Boas, A. and Shimokomaki, M. 2013. Detection of Salmonella spp, Salmonella enteritidis and Typhimurium in naturally infected broiler chickens by a multiplex PCR-based assay. Braz. J. Microbiol. 44(1), 37–41. Ramirez, G.A., Sarlin, L.L., Caldwell, D.J., Yezak, C.R., Hume, M.E., Corrier, D.E., Deloach, J.R. and Hargis, B.M. 1997. Effect of feed withdrawal on the incidence of Salmonella in the crops and ceca of market age broiler chickens. Poult. Sci. 76, 654–656. Ramya, P., Madhavarao, T. and Rao, L.V. 2012. Study on the incidence of Salmonella enteritidis in poultry and meat samples by cultural and PCR methods, Vet. World 5(9), 541–545. Rodpai, E., Moongkarndi, P., Tungrugsasut, W., Phosannoradej, R. and Kanarat, S. 2013. Comparison of multiplex polymerase chain reaction and immunoassay to detect Salmonella spp., S. typhimurium, and Salmonella enteritidis in Thai chicken meat. Sci. Asia 39, 150–159. Sallam, K.I., Mohammed, M.A., Hassan, M.A. and Tamura, T. 2014. Prevalence, molecular identification, and antimicrobial resistance profile of Salmonella serovars isolated from retail beef products in Mansoura, Egypt. Food Contr. 38, 209–214. Singh, R., Yadav, A.S., Tripathi, V. and Singh, R.P. 2013. Antimicrobial resistance profile of Salmonella present in poultry and poultry environment in north India. Food Contr. 33, 545–548. Thung, T.Y., Mahyudin, N.A., Basri, D.F., Radzi, C.W.J., Nakaguchi, Y., Nishibuchi, M. and Radu, S. 2016. Prevalence and antibiotic resistance of Salmonella enteritidis and Salmonella Typhimurium in raw chicken meat at retail markets in Malaysia. Poult. Sci. 95, 1888–1893. Van Steenwinkel, S. 2011. Assessing biosecurity practices, movements, and densities of poultry sites across Belgium, resulting in different farm risk-groups for infectious disease introduction and spread. Prev. Vet. Med. 98(4), 259–270. Waltman, W.D., Horne, A.M. and Pirkle, C. 1993. Influence of enrichment incubation time in the isolation of Salmonella. Avian Dis. 37, 884–887. Witkowska, D., Kuncewicz, M., Żebrowska, J.P., Sobczak, J. and Sowińska, J. 2018. Prevalence of Salmonella spp. in broiler chicken flocks in northern Poland. Ann. Agric. Environ. Med. 25(4), 693–697. | ||
| How to Cite this Article |
| Pubmed Style Asheg AA, Otman MF, Benlashehr IA, Kraim E, Almashri RA, Kammon aM. Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya. Open Vet J. 2023; 13(5): 638-644. doi:10.5455/OVJ.2023.v13.i5.17 Web Style Asheg AA, Otman MF, Benlashehr IA, Kraim E, Almashri RA, Kammon aM. Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya. https://www.openveterinaryjournal.com/?mno=144111 [Access: August 31, 2024]. doi:10.5455/OVJ.2023.v13.i5.17 AMA (American Medical Association) Style Asheg AA, Otman MF, Benlashehr IA, Kraim E, Almashri RA, Kammon aM. Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya. Open Vet J. 2023; 13(5): 638-644. doi:10.5455/OVJ.2023.v13.i5.17 Vancouver/ICMJE Style Asheg AA, Otman MF, Benlashehr IA, Kraim E, Almashri RA, Kammon aM. Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya. Open Vet J. (2023), [cited August 31, 2024]; 13(5): 638-644. doi:10.5455/OVJ.2023.v13.i5.17 Harvard Style Asheg, A. A., Otman, . M. F., Benlashehr, . I. A., Kraim, . E., Almashri, . R. A. & Kammon, . a. M. (2023) Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya. Open Vet J, 13 (5), 638-644. doi:10.5455/OVJ.2023.v13.i5.17 Turabian Style Asheg, Abdulatif A., Mohamed F. Otman, Imad A. Benlashehr, El-forjani Kraim, Rabia A. Almashri, and abdulwahab M. Kammon. 2023. Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya. Open Veterinary Journal, 13 (5), 638-644. doi:10.5455/OVJ.2023.v13.i5.17 Chicago Style Asheg, Abdulatif A., Mohamed F. Otman, Imad A. Benlashehr, El-forjani Kraim, Rabia A. Almashri, and abdulwahab M. Kammon. "Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya." Open Veterinary Journal 13 (2023), 638-644. doi:10.5455/OVJ.2023.v13.i5.17 MLA (The Modern Language Association) Style Asheg, Abdulatif A., Mohamed F. Otman, Imad A. Benlashehr, El-forjani Kraim, Rabia A. Almashri, and abdulwahab M. Kammon. "Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya." Open Veterinary Journal 13.5 (2023), 638-644. Print. doi:10.5455/OVJ.2023.v13.i5.17 APA (American Psychological Association) Style Asheg, A. A., Otman, . M. F., Benlashehr, . I. A., Kraim, . E., Almashri, . R. A. & Kammon, . a. M. (2023) Prevalence of Salmonella in poultry slaughterhouses located in Tripoli, Libya. Open Veterinary Journal, 13 (5), 638-644. doi:10.5455/OVJ.2023.v13.i5.17 |