| Case Report | ||
Open Vet J. 2023; 13(8): 1032-1036 Open Veterinary Journal, (2023), Vol. 13(8): 1032-1036 Case Report Phacoemulsification in a chinchilla (Chinchilla lanigera)Natthanet Sritrakoon1, Ladawan Areevijittrakul2, Nuttatida Nimitchaiyapong1, Natruree Khamchomphu2 and Taksaon Duangurai2,3*1Ophthalmology Unit, Kasetsart University Veterinary Teaching Hospital, Bangkok, Thailand 2Exotic Unit, Kasetsart University Veterinary Teaching Hospital, Bangkok, Thailand 3Department of Companion Animal Clinical Sciences, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand *Corresponding Author: Taksaon Duangurai. Department of Companion Animal Clinical Sciences, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand. Email: taksaon.du [at] ku.th Submitted: 15/03/2023 Accepted: 16/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
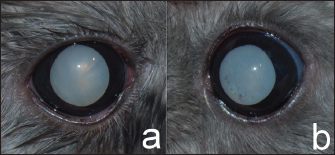
AbstractBackground: A cataract is one of the underlying causes of blindness in animals. Phacoemulsification is the standard procedure in cataract surgery for humans and animals. This procedure has been used to restore vision in cataracts in a variety of animals. However, this technique is difficult in very small animals, such as rodents, due to their small eyes. Case Description: A 4-year-old male domestic chinchilla was presented with cloudiness in the lenses for 1 month. The ophthalmic examination revealed cataracts (oculus uterque: both eyes). Positive dazzle reflex oculus sinister; left eye (OS) and negative reflex oculus dexter; right eye (OD) were noted. The electroretinography was low amplitude OS whereas a flat waveform presented OD. In this case, cataract surgery was performed using phacoemulsification without intraocular lens implantation OS. Postoperative, the chinchilla was alert and could jump on and jump off the ledge in a house. When the veterinarian approached closely to OS, the chinchilla displayed an erect body posture and open eyes, whereas the chinchilla was ignored when the veterinarian doing the same OD. The chinchilla was alert and had improved vision observe by this chinchilla can jump on and jump off the ledge in his house throughout the follow-up period 18 months later. Conclusion: In this chinchilla, phacoemulsification was successfully performed and resulted in better overall vision. The chinchilla was alert and could jump on and jump off the ledge in a house after cataract surgery throughout the follow-up period of 18 months. Keywords: Cataract, Chinchilla, Chinchilla lanigera, Phacoemulsification. IntroductionChinchillas (Chinchilla lanigera) are in the order Rodentia, suborder Hystricomorpha, and are a popular option as pets (Müller et al., 2010). Like most animals, chinchillas can get eye diseases. Normal ophthalmic examinations of chinchillas have been investigated (Lima et al., 2010; Müller et al., 2010). Problems of ophthalmic diseases in chinchillas have been reported, such as corneal injuries (Müller and Eule, 2014), anterior lens luxation, and cataracts (Müller and Eule, 2014). Lenticular changes have been reported in many animals (Barnett, 1985; Narfström, 1999; Fife et al., 2006; Rainwater et al., 2015; Sanchez et al., 2018). In chinchillas, lenticular changes have been reported, such as prominent lens sutures, cataract, and nuclear sclerosis (Müller et al., 2010). Prominent lens sutures were mainly observed in very young chinchillas, whereas nuclear sclerosis was observed in chinchillas with a mean age of 7.2 years (Müller et al., 2010). Furthermore, cataracts occurred at the median age of 8 years in chinchillas (range: 24–144 months) (Müller and Eule, 2014). To date, there have been no published reports of cataract surgery in rodents due to the small size of their eyeballs. Therefore, cataract surgery was a challenge in the current case involving a chinchilla. This report presents the success of phacoemulsification without intraocular lenses (IOL) implantation in a chinchilla. Case DetailsHistory and ophthalmic examinationA 4-year-old male domestic chinchilla presented with cloudy lenses for 1 month. Ophthalmic examination revealed that there was a mature cataract oculus dexter (OD) and a hypermature cataract oculus sinister (OS). Menace response was negative oculus uterque (OU). Dazzle reflex responses of OS and OD were positive and negative, respectively. Pupillary light reflex was positive OU. The conjunctiva, cornea, anterior chamber, and iris were normal OU using a slit lamp biomicroscope (Kowa SL-17 Portable Slit Lamp Biomicroscope; Kowa Co. Ltd.; Tokyo, Japan). Intraocular pressures (IOPs) were measured OU using a rebound tonometer (Icare®TonoVet; Icare Finland Oy; Helsinki, Finland) and were normal values (8 mmHg OD; 7 mmHg OS). Fundoscopy could not be performed due to the cloudy lenses OU. In this case, the definitive diagnosis was mature cataract OD and hypermature cataract OS (Fig. 1). The treatment was performed using phacoemulsification without intraocular lens implantation OS. However, OD was not advised to treat with surgery due to the dazzle reflex OD being negative. Application of 0.5% ketorolac tromethamine (Acular®; Allergan Pharmaceuticals Ireland; Westport, Ireland) was prescribed to control lens-induced uveitis (LIU) OU every 24 hours. The results of the pre-operative physical examination were normal. The hematology and blood chemistry were evaluated and were within normal limits. B-scan ocular ultrasonography (Logiq E9; GE Healthcare; WI, USA) with topical 0.5% tetracaine hydrochloride (Alcon®; Alcon-Couvreur; Puurs, Belgium) was evaluated with no evidence of retinal detachment OU. Pre-operative medications were prescribed with topical 1% prednisolone acetate and 0.3% ofloxacin (ExopredTM; Piramal Pharma Limited; Madhya Pradesh, India) OS every 8 hours for 3 days before surgery. Before surgery, topical 1% tropicamide (Mydriacyl®; Alcon-Couvreur; Puurs, Belgium) was applied every 15 minutes for 30 minutes to mydriasis. The chinchilla was anesthetized with 8% sevoflurane (Sevo; Singapore Pharmawealth Lifesciences, Inc.; Laguna, the Philippines) in the induction chamber and maintained with 2% sevoflurane with a face mask. Electroretinography (ERG) using a (handheld multispecies electroretinograph model 2000; Ocuscience LLC; MO, USA) based on the QuickRetCheck protocol was performed immediately before surgery. The ERG values are presented as a flat graph OD and low amplitude OS. With high light-intensity (10 cd.s/m2) stimulation, the b-wave amplitude was 3.8 µv and the implicit time was 1.3 ms OS. Based on the result of the ophthalmic reflex, ERG values, and the anatomy of the eye size, phacoemulsification without IOL implantation was performed OS.
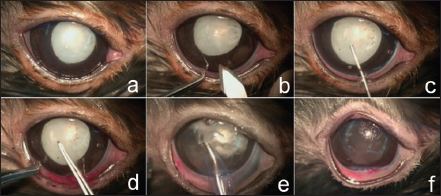
Fig. 1. Mature cataracts OD (a) and hypermature cataract OS (b) presented after mydriasis. The conjunctiva, cornea, anterior chamber, and iris OU were normal. Surgical managementThe surgical area was sterilized with 1:50 diluted povidone-iodine solution before starting surgery OS (Fig. 2a). Then, 5 mg/kg marbofloxacin (Marbocyl®; Vetoquinol; Lure, France) and 4.4 mg/kg carprofen (Rimadyl®; Inovat Industria Farmaceutica Ltda; Sao Paulo, Brazil) were administered subcutaneously. The position during surgery of this chinchilla was lateral recumbency because the globe of the chinchilla was lateral. The corneal incision was created at the 11 o’clock position of the clear cornea near the limbus with a 2.8 mm slit-angled knife (Mani®Ophthalmic Knife; Mani, Inc.; Tochigi, Japan), as shown in Figure 2b. A viscoelastic substance containing 2% sodium hyaluronate (Viscovet; AJL Ophthalmic, S.A.; Álava, Spain) was applied to the anterior chamber to maintain the anterior chamber and protect the corneal endothelium during surgery (Fig. 2c). The 25 gauge needle was inserted in the anterior chamber to scrape a tear for the initial opening in the anterior lens capsule. Additionally, a capsulorhexis was performed using the Utrata capsulorhexia forceps together with the intraocular scissors (Fig. 2d). Furthermore, a one-handed phacoemulsification (Centurian; Alcon® Surgical; TX, USA) technique using balanced torsional phaco tip was performed (Fig. 2e). During the phacoemulsification period, iris prolapse was found at the incision site due to the anterior chamber being shallow and the iris position was anterior to the cornea. The cataract is quite hard to remove. Thus, the settings of the phacoemulsification were an average of 48.2% longitudinal power with an average 58% torsional amplitude of phaco power. The torsional mode with a rotational movement was performed to remove lens materials and the U/S total time was 2.56 minutes. After the phacoemulsification, the posterior lens capsule was cleaned with the irrigation/aspiration mode. The balanced salt solution (BSSTM, Alcon Laboratories, Inc., TX, USA) was added with 2 ml of 1 mg/ml epinephrine bitartrate (Adrenaline, Atlantic Laboratories Corporation Ltd., Samut Prakan, Thailand) and 0.1 ml (25,000 I.U./5 ml) of heparin sodium (Nuparin, Troikaa Pharmaceuticals Ltd., Gujarat, India) per 500 ml of fluid was used to irrigation during and after phacoemulsification to prevent fibrin formation in the anterior chamber. Then, a remaining viscoelastic substance containing sodium hyaluronate was removed from the anterior chamber and capsular bag. The procedure was terminated after the corneal incisions had been sutured with a simple, interrupted pattern using 9/0 polyglycolic acid suture material (PGA; FSSB Chirurgische Nadeln GMBH; Jestetten, Germany), and were reinflated with the balanced salt solution (Fig. 2f).
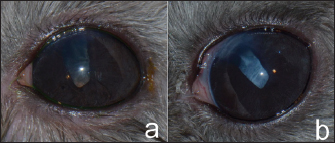
Fig. 2. Cataract surgery was performed. Hypermature cataract with mydriasis was presented and eye washed before surgery (a). Cornea was incised using a slit-angled knife (b). Sodium hyaluronate was injected into the anterior chamber (c). Capsulorhexis was performed with Urata capsulorhexis forceps (d). Phacoemulsification was performed using a one-handed technique (e). Posterior capsular cataract was left and the corneal wounds were sutured using 9/0 polyglycolic acid suture material (f). Postoperative medications were prescribed orally with 5 mg/kg marbofloxacin every 24 hours (Marbocyl®; Vetoquinol; Lure, France) and 0.3 mg/kg meloxicam every 24 hours (Melox®; Siam Bheasach Co., Ltd.; Bangkok, Thailand) for 1 week. In the postoperative period, topical 1% prednisolone acetate in combination with 0.3% ofloxacin every 8 hours and 3 mg/ml sodium hyaluronate (Hialid®0.3; Santen Pharmaceutical Co., Ltd.; Ishikawa, Japan) every 8 hours were administered OS for 4 weeks. Ketorolac tromethamine 0.5% was used every 24 hours OD continuously to control LIU. An Elizabethan collar was applied around the neck to protect the eyes from rubbing by the patient for 1 week. OutcomeTwo days after surgery, the chinchilla could comfortably open both eyes. However, mild corneal edema and conjunctivitis were evident in OS. The IOPs OD and OS were 7 and 8 mmHg, respectively. Two weeks postoperatively, the chinchilla was more alert when compared to before surgery and could jump on and jump off the ledge in his house. Ophthalmic examination revealed the corneal wound had been sealed, and lens capsule opacity and the iris trap at the surgical site were presented OS (Fig. 3a). The clear cornea and cataract were presented OD. In the ophthalmic examination room, when the veterinarian approached closely OS, the chinchilla displayed a held-up head, open eyes, erect ears, and immobile positioned forward indicating alertness or fear, whereas the chinchilla was ignored when the veterinarian doing the same OD. At 4 weeks after surgery, the chinchilla was more alert when compared to before surgery and could jump on and jump off the ledge in the new environments in his house. At 6 weeks postoperatively, the examination of OS presented posterior capsular opacity (Fig. 3b). The IOPs were within the normal limit OU (range: 5–9 mmHg OU) during follow-up times. The chinchilla maintained alertness and his vision improved by this chinchilla could jump on and jump off the ledge without bumping any objects in his house. It was noticed that the chinchilla could able jump on and jump off the ledge and jump past the objects without bumping new objects in his areas and his vision was adequate for life throughout 18 months postoperatively.
Fig. 3. Two weeks postoperatively, lens capsule opacity and iris trap at the surgical site were presented OS (a). Six weeks postoperatively, posterior capsular opacity had increased OS (b). DiscussionExotic pets have become more popular over the years. Chinchillas are one of the favorite small exotic pets (Müller et al., 2010). In general, the lens of the chinchilla is large compared to the eyeball, measuring 8.5–8.7 mm on its longest axis, with up to 50% of the axial globe length (Detwiler, 1949; Lima et al., 2010; Holmberg, 2013). The lens shape is biconvex and almost spherical (Detwiler, 1949). The retina of the chinchilla has an anangiotic pattern with physiological cupping of the optic disc (Peiffer and Johnson, 1980). Visual acuity in chinchilla is poor because of low numbers of retina and cone cells, less than 30° of stereopsis, and minimal accommodation caused by the lack of the ciliary muscle (Holmberg, 2013). Cataract in chinchillas is possibly caused by a congenital disorder, aging, anterior lens luxation, or diabetes mellitus (Müller and Eule, 2014). Thus, serum blood glucose should be evaluated in chinchillas that present with unilateral or bilateral cataracts, because diabetic cataracts have been reported in chinchillas (Müller and Eule, 2014). In the chinchilla reported in the current case, a blood glucose test was evaluated to rule out the diabetes mellitus, with the value (187 mg/dl) being within normal limits (53–249 mg/dl) (Mans and Donnelly, 2021). Before cataract surgery, ocular B-scan ultrasonography and ERG are routinely performed (Wilkie and Colitz, 2009). ERG is used to examine retinal function before cataract surgery in animals. In their study, Sandalon et al. (2019) reported that ERGs of chinchillas revealed very low amplitudes with cone and rod retina being observed, compared to mice and rats. In a study of ERG in six chinchillas, mean (±SD) photopic b-wave amplitudes were 10.2 (±2.9) for 10 cd*s/m2 flash intensities. The corresponding b-wave mean implicit times were 43.6 (±5.0) milliseconds (Sandalon et al., 2019). In the current study, the chinchilla had negative dazzle reflex OD and positive dazzle reflex OS. The ERG showed a flat graph OD and low amplitude OS, suggesting that retinal function in the right eye was not suitable for treatment using cataract surgery. Phacoemulsification has been performed in many animal species, including small mammals, such as rabbits (Sanchez et al., 2018). Otherwise, there has been no published case of cataract surgery in rodents such as guinea pigs, degus, prairie dogs, rats, mice, hamsters, and gerbils, presumably due to their small eyes. Cataract surgery is an elective procedure in animals (Wilkie and Colitz, 2009). Where cataract surgery could not be performed, long-term monitoring is required regarding cataract complications, such as LIU, secondary glaucoma, retinal detachment, and lens luxation (Wilkie and Colitz, 2009). In the current case, cataract surgery was not performed OD due to a presumed problem with retinal function, though these complications had not been seen in this chinchilla OD for 18 months. Despite substantial advances in cataract surgery over the years, many post-operative complications persist, which have reduced the success rate of the phacoemulsification procedure. In dogs, post-operative complications of phacoemulsification include postoperative ocular hypertension, endophthalmitis, posterior capsular opacification (PCO), glaucoma, and retinal detachment (Wilkie and Colitz, 2009). Thus, postoperative IOPs were monitored to rule out glaucoma in the current case. The IOPs for this chinchilla were within normal limits throughout the monitoring period. Iris prolapse is an intraoperative complication of cataract surgery that occurs through the incision when the surgeon opens the cornea to the anterior chamber during the phacoemulsification period where the iris is positioned anteriorly to the cornea and there is a shallow anterior chamber (Lima et al., 2010). This complication has been reported in rabbits (Holmberg, 2013). Thus, the surgeon must be careful during surgery. In the current case, the iris synechia at the surgical site and anisocoria was presented after surgery. PCO is the most common complication of cataract surgery in many species, such as dogs (Sigle and Nasisse, 2006), humans (Konopińska et al., 2021), rabbits (Wallentin et al., 2002), and birds (Rainwater et al., 2015). PCO is caused by the proliferation and migration of residual lens epithelial cells across the posterior capsule, resulting in decreased vision after cataract surgery (Sigle and Nasisse, 2006; Konopińska et al., 2021). PCO has not been eliminated due to its multifactorial etiology. The incidence levels of PCO in short-term complications (3 months) and long-term complications (1–2 years) in dogs were 35% and 69%, respectively (Sigle and Nasisse, 2006). In rabbits, PCO was reported on day 56 postoperatively (Wallentin et al., 2002) which was much faster than in humans. PCO in humans was reported at 11.8%, 20.7%, and 28.4% after surgery in the first, third, and fifth years, respectively (Konopińska et al., 2021). PCO presented in 44.4% (8/18) of birds that had undergone cataract removal (Rainwater et al., 2015). PCO can limit vision (Konopińska et al., 2021). In the current case with a chinchilla, mild PCO was presented after 2 weeks of the post-phacoemulsification period and increased to moderate PCO in 6 weeks post-operatively. However, the surgery substantially improved the quality of life for the chinchilla, as his vision was noticeably improved and the chinchillas had a normal life as he can jump on and jump off during the follow-up period. Phacoemulsification without IOL implantation was reported in many animals may be because of intra-operative complications such as posterior capsular tears, too large anterior capsulorhexis, lack of an appropriate commercial IOL, cost of creating custom-IOL implants, or owner declined (Fife et al., 2006; Montiani-Ferreira et al., 2010; Klein et al., 2011: Rainwater et al., 2015; Sigmund et al., 2019; Fenollosa-Romero et al., 2020). The postoperative aphakic eye may result in far-sighted or hyperopia (Davidson et al., 1993; Bigelbach, 1994). The visual image would be minimized and blurry. However, in the reported the aphakic animals, the visions were functionally acceptable after surgery (Fife et al., 2006; Montiani-Ferreira et al., 2010; Sigmund et al., 2019). However, this chinchilla’s vision was improved and restored with normal behavior, even though the surgery was taken only one eye without IOL implantation, due to he can jump on and jump off in the new environments in his house, and the owner was happy and said that the chinchilla had a good quality of life more than before surgery. ConclusionIn conclusion, this report presented successful phacoemulsification in a chinchilla. The vision outcome was good with mild complications. The chinchilla presented improved quality of vision throughout the follow-up postoperative period for 18 months. AcknowledgmentsThe authors thank Dr. Andrew Warner for English copyediting of this article. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis study was supported by Kasetsart University Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Kasetsart University, which provided surgical equipment for this case. Data availabilityAll the data are presented in figures directly in this article. Authors contributionsNatthanet S., DVM, D.T.B.V.M, performed the surgical management and follow-up case, and wrote the article. Ladawan A. DVM, performed follow-up case management. Nuttatida N., and Natruree K., assisted the surgical management. Taksaon D., DVM, Ph.D., D.T.B.V.M, performed the medical management and revised the manuscript. ReferencesBarnett, K.C. 1985. The diagnosis and differential diagnosis of cataract in the dog. J. Small. Anim. Pract. 26, 305–316. Bigelbach, A. 1994. For or against lens implantation in dogs. Vet. Quart. 16, 58–59. Davidson, M.G., Murphy, C.J., Nasisse, M.P., Hellkamp, A.S., Olivero, D.K., Brinkmann, M.C. and Campbell, L.H. 1993. Refractive state of aphakic and pseudophakic eyes of dogs. Am. J. Vet. Res. 54, 174–177. Detwiler, S.R. 1949. The eye of the chinchilla, Chinchilla lanigera. J. Morphol. 84, 123–144. Fenollosa-Romero, E., Jeanes, E., Freitas, I., Enache, A.E., Lockhart, R., Fleming, L., Knott, T.N.L., Dawson, C., Smith, K. and Busse, C. 2020. Outcome of phacoemulsification in 71 cats: a multicenter retrospective study (2006-2017). Vet. Ophthalmol. 23, 141–147. Fife, T.M., Gemensky-Metzler, A.J., Wilkie, D.A., Colitz, C.M., Bras, I.D. and Klages, D.C. 2006. Clinical features and outcomes of phacoemulsification in 39 horses: a retrospective study (1993-2003). Vet Ophthalmol. 9, 361–368. Holmberg, B.J. 2013. Ophthalmology of exotic pets. In Slatter’s fundamentals of veterinary ophthalmology, 5th ed. Eds., Maggs, D.J., Miller, P.E. and Ofri, R. St. Louis, MO: Elsevier, pp: 445–461. Klein, H.D., Krohne, S.G., Moore, G.E. and Stiles, J. 2011. Postoperative complications and visual outcomes of phacoemulsification in 103 dogs (179 eyes): 2006–2008. Vet. Ophthalmol. 14, 114–120. Konopińska, J., Młynarczyk, M., Dmuchowska, D.A. and Obuchowska, I. 2021. Posterior capsule opacification: a review of experimental studies. J. Clin. Med. 10, 2847. Lima, L., Montiani-Ferreira, F., Tramontin, M., dos Santos, L.L., Machado, M., Lange, R.R. and Abil Russ, H.H. 2010. The chinchilla eye: morphologic observations, echobiometric findings and reference values for selected ophthalmic diagnostic tests. Vet. Ophthalmol. 13, 14–25. Mans, C. and Donnelly, T.M. 2021. Chinchillas. In Ferrets, rabbits, and rodents: clinical medicine and surgery, 4th ed. Eds., Quesenberry, K.E., Orcutt, C.J., Mans, C. and Carpenter, J. W. St. Louis, MO: Elsevier, pp: 298–322. Montiani-Ferreira, F., Lima, L., Bacellar, M., D’Otaviano Vilani, R.G., Fedullo, J.D. and Lange, R.R. 2010. Bilateral phacoemulsification in an orangutan (Pongopygmaeus). Vet. Ophthalmol. 13, 91–99. Müller, K. and Eule, J.C. 2014. Ophthalmic disorders observed in pet chinchillas (Chinchilla lanigera). J. Exot. Pet. Med. 23, 201–205. Müller, K., Mauler, D.A. and Eule, J.C. 2010. Reference values for selected ophthalmic diagnostic tests and clinical characteristics of chinchilla eyes (Chinchilla lanigera). Vet. Ophthalmol. 13, 29–34. Narfström, N. 1999. Hereditary and congenital ocular disease in the cat. J. Feline. Med. Surg. 1, 135–141. Peiffer, R.L. and Johnson, P.T. 1980. Clinical ocular findings in a colony of chinchillas (Chinchilla laniger). Lab. Anim. 14, 331–335. Rainwater, K.L., Sykes, J.M. and Sapienza, J.S. 2015. Retrospective investigation of cataract management in avian species in a zoologic collection. J. Zoo. Wildl. Med. 46, 858–869. Sanchez, R.F., Everson, R., Hedley, J., Dawson, C., Lam, R., Priestnall, S.L., Garcia de Carellan, A., de Miguel, C. and Seymour, C. 2018. Rabbits with naturally occurring cataracts referred for phacoemulsification and intraocular lens implantation: a preliminary study of 12 cases. Vet. Ophthalmol. 21, 399–412. Sandalon, S., Boykova, A., Ross, M., Obolensky, A., Banin, E. and Ofri, R. 2019. Contrary to popular belief, chinchillas do not have a pure rod retina. Vet. Ophthalmol. 22, 93–97. Sigle, K.J. and Nasisse, M.P. 2006. Long-term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995-2002). J. Am. Vet. Med. Assoc. 228, 74–79. Sigmund, A.B., Jones, M.P., Ward, D.A. and Hendrix, D.V.H. 2019. Long-term outcome of phacoemulsification in raptors-a retrospective study (1999-2014). Vet. Ophthalmol. 22, 360–367. Wallentin, N., Lundgren, B., Holmén, J.B. and Lundberg, C. 2002. Development of posterior capsule opacification in the rabbit. Ophthalmic. Res. 34, 14–22. Wilkie, D.A. and Colitz, C.M.H. 2009. Update on veterinary cataract surgery. Curr. Opin. Ophthalmol. 20, 61–68. | ||
| How to Cite this Article |
| Pubmed Style Sritrakoon N, Areevijittrakul L, Nimitchaiyapong N, Khamchomphu N, Duangurai T. Phacoemulsification in a chinchilla (Chinchilla lanigera). Open Vet J. 2023; 13(8): 1032-1036. doi:10.5455/OVJ.2023.v13.i8.10 Web Style Sritrakoon N, Areevijittrakul L, Nimitchaiyapong N, Khamchomphu N, Duangurai T. Phacoemulsification in a chinchilla (Chinchilla lanigera). https://www.openveterinaryjournal.com/?mno=146482 [Access: July 03, 2025]. doi:10.5455/OVJ.2023.v13.i8.10 AMA (American Medical Association) Style Sritrakoon N, Areevijittrakul L, Nimitchaiyapong N, Khamchomphu N, Duangurai T. Phacoemulsification in a chinchilla (Chinchilla lanigera). Open Vet J. 2023; 13(8): 1032-1036. doi:10.5455/OVJ.2023.v13.i8.10 Vancouver/ICMJE Style Sritrakoon N, Areevijittrakul L, Nimitchaiyapong N, Khamchomphu N, Duangurai T. Phacoemulsification in a chinchilla (Chinchilla lanigera). Open Vet J. (2023), [cited July 03, 2025]; 13(8): 1032-1036. doi:10.5455/OVJ.2023.v13.i8.10 Harvard Style Sritrakoon, N., Areevijittrakul, . L., Nimitchaiyapong, . N., Khamchomphu, . N. & Duangurai, . T. (2023) Phacoemulsification in a chinchilla (Chinchilla lanigera). Open Vet J, 13 (8), 1032-1036. doi:10.5455/OVJ.2023.v13.i8.10 Turabian Style Sritrakoon, Natthanet, Ladawan Areevijittrakul, Nuttatida Nimitchaiyapong, Natruree Khamchomphu, and Taksaon Duangurai. 2023. Phacoemulsification in a chinchilla (Chinchilla lanigera). Open Veterinary Journal, 13 (8), 1032-1036. doi:10.5455/OVJ.2023.v13.i8.10 Chicago Style Sritrakoon, Natthanet, Ladawan Areevijittrakul, Nuttatida Nimitchaiyapong, Natruree Khamchomphu, and Taksaon Duangurai. "Phacoemulsification in a chinchilla (Chinchilla lanigera)." Open Veterinary Journal 13 (2023), 1032-1036. doi:10.5455/OVJ.2023.v13.i8.10 MLA (The Modern Language Association) Style Sritrakoon, Natthanet, Ladawan Areevijittrakul, Nuttatida Nimitchaiyapong, Natruree Khamchomphu, and Taksaon Duangurai. "Phacoemulsification in a chinchilla (Chinchilla lanigera)." Open Veterinary Journal 13.8 (2023), 1032-1036. Print. doi:10.5455/OVJ.2023.v13.i8.10 APA (American Psychological Association) Style Sritrakoon, N., Areevijittrakul, . L., Nimitchaiyapong, . N., Khamchomphu, . N. & Duangurai, . T. (2023) Phacoemulsification in a chinchilla (Chinchilla lanigera). Open Veterinary Journal, 13 (8), 1032-1036. doi:10.5455/OVJ.2023.v13.i8.10 |