| Research Article | ||
Open Vet J. 2023; 13(6): 697-704 Open Veterinary Journal, (2023), Vol. 13(6): 697-704 Original Research Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from PakistanMuhammad Riaz1, Nasreen Nasreen2, Adil Khan2* and Mourad Ben Said3,41Zoology Division, Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan 2Department of Zoology, Abdul Wali Khan University, Khyber Pakhtunkhwa, Pakistan 3Department of Basic Sciences, Higher Institute of Biotechnology of Sidi Thabet, University of Manouba, Manouba, Tunisia 4Laboratory of Microbiology, National School of Veterinary Medicine of Sidi Thabet, University of Manouba, Manouba, Tunisia *Corresponding Author: Adil Khan. Department of Zoology, Abdul Wali Khan University, Khyber Pakhtunkhwa, Pakistan. Email: dradilkhan [at] bkuc.edu.pk Submitted: 25/03/2023 Accepted: 07/05/2023 Published: 06/06/2023 © 2023 Open Veterinary Journal
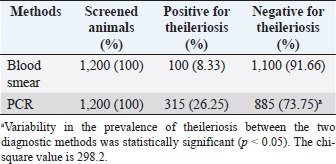
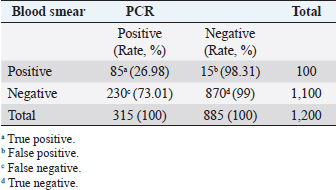
AbstractBackground: Ovine and caprine theileriosis is a tick-borne hemoprotozoan disease, caused by Theileria spp., responsible for heavy economic losses in terms of high mortality and morbidity rates. Diagnosis of ovine theileriosis is primarily based on clinical symptoms, microscopic screening of stained blood smears, and lymph node biopsy smears, but the limitations of these detection methods against Theileria spp. infection limits their specificity. Aim: To overcome these limitations, the current study reports the differential diagnosis of theileriosis through a blood smear examination and polymerase chain reaction (PCR) in small ruminants from Pakistan. Methods: The study was conducted on 1,200 apparently healthy small ruminants (737 sheep and 463 goats). First, blood smears were screened for the presence of Theileria piroplasms in red blood cells. Second, PCR amplification based on 18S rRNA gene was performed by using primers specific to Theileria spp. Results: Out of the 1,200 samples of examined blood smears, 100 animals (8.33%) were found positive for Theileria species, which showed intra-erythrocytic bodies in the form of dot and comma shapes. Amplification of the isolated DNA from randomly collected blood samples of 737 sheep and 463 goats showed that an amplicon size of 1,098 bp was positive for Theileria spp. In total, 315 out of the 1,200 small ruminants examined in this study were found positive for Theileria spp. DNA through PCR amplification. Notably, out of the 885 blood samples negative by PCR amplification, only 15 blood samples were found positive by the blood smear test. Conversely, 230 blood samples that tested negative in the smear technique produced a specific band through PCR amplification. Overall, the sensitivity and specificity rates were 26.98% and 98.31% for the blood smear method and 73.01% and 100% for the PCR assay, respectively. Conclusion: Our finding suggests that PCR is the gold standard method compared to the conventional method of smear examination for the diagnosis of ovine and caprine theileriosis in Pakistan. Keywords: Ovine and caprine theileriosis, Differential diagnosis, Microscopic screening, PCR assay, Sheep and goats, Pakistan. IntroductionHemoprotozoan diseases are a significant threat to the livestock sector, affecting 80% of the world's ruminants and causing major losses in the dairy industry worldwide (Kohli et al., 2014). Among these diseases, Theileriosis is a tick-borne illness caused by the genus Theileria (Demessie and Derso, 2015) and characterized by distinctive organelles known as the apical complex (Gul et al., 2015). Theileria is obligate intracellular protozoan parasites belonging to the phylum Apicomplexa and order Piroplasmida, infecting both wild and domestic ruminants and spreading through ixodid ticks. The completion of their life cycle involves both vertebrate and invertebrate hosts, making it a significant problem in tropical and subtropical regions of the world (Sitotaw et al., 2014). Theileriosis continues to pose a significant constraint for small ruminants in Asia, as well as parts of Africa and Southern Europe (Mehlhorn and Schein, 1984). Recent studies have highlighted the continued prevalence and impact of this disease on domestic ruminant production, especially compared to cattle. In China, for example, a study by Yang et al. (2022) found that Theileria infections were common among goats, while a recent study by Rahravani et al. (2023) reported a high rate of Theileria spp. infection among small ruminants in Iran. These findings underscore the need for increased attention and management of Theileriosis in small ruminant populations. Sheep and goats can be infected by six different species of Theileria (Razmi and Yghfoori, 2013). Among these, Theileria lestoquardi, Theileria luwenshuni, and Theileria uilenbergi are highly pathogenic, while Theileria separata, Theileria ovis, and Theileria recondite cause less severe disease in these animals (Razmi et al., 2003; Taha et al., 2013). Theileria lestoquardi, which causes malignant ovine theileriosis, can lead to several clinical signs in small ruminants, such as fever, anemia, lethargy, icterus, and hemoglobinurea (Sayin et al., 2009). Theileriosis is mainly spread through the bites of ixodid ticks (Durrani et al., 2012). Countries like Pakistan, Bangladesh, and India have a suitable climate for the growth and spread of ixodid tick species that can transmit the disease (Irshad et al., 2010). For instance, Rhipicephalus microplus, Rhipicephalus turanicus, Rhipicephalus annulatus, and Hyalomma anatolicum are some of the tick species known to transmit Theileria parasites (Aung et al., 2022; Norouzi et al., 2022; Rahravani et al., 2023). The prevalence and distribution of these tick species in different regions can vary and affect the incidence of theileriosis in small ruminants (Kumar et al., 2023; Prajapati et al., 2023). Theileriosis is typically diagnosed in acute cases through clinical symptoms and thin smears of blood and lymph nodes stained with Giemsa and Wright. Interestingly, Sallam et al. (2023) developed an enhanced methodology for classifying subtypes of acute lymphoblastic leukemia (ALL) using peripheral blood smear images. Their methodology includes image preprocessing, feature extraction, and the use of enhanced grey wolf pptimization algorithm to select the most important features characterizing the blood cells' histology. This algorithm achieves a high degree of accuracy, precision, and sensitivity in identifying the subtypes of ALL. However, clinical symptoms and thin smears are not useful for identifying carrier animals due to their low rate of parasitemia (Aktas et al., 2006). In cases of mixed infections, microscopy cannot confirm the presence of Theileria species, and therefore, the PCR assay has become the preferred technique for detecting ovine and caprine Theileria piroplasms for epidemiological analysis (Aktas et al., 2005). The OIE recommends the use of serological tests, such as the indirect fluorescent antibody test (IFAT), to detect circulating antibodies against Theileria piroplasms. However, the IFAT test's specificity is limited due to the cross-reactivity of other piroplasms with the antibodies (Nourollahi-Fard et al., 2012). It is worth noting that long-term carrier animals, considered serologically negative, may be positive for Theileria species and can infect susceptible animals (Altay et al., 2012). This underscores the importance of using accurate and specific tests for detecting Theileria piroplasms to prevent their spread to susceptible animals. Accurate and rapid detection of Theileria and Babesia species in carrier animals is crucial for controlling the spread of these pathogens. While traditional diagnostic methods such as microscopy and serology have limited sensitivity and specificity, a diagnostic method that offers higher accuracy and faster turnaround time is needed. Fortunately, the polymerase chain reaction (PCR) has emerged as a sensitive and specific molecular assay for detecting many pathogens, including several Theileria and Babesia species in carrier animals (Lew et al., 1997). PCR offers several advantages over traditional methods, including the ability to detect low levels of pathogens and the potential to identify multiple species in a single assay. Additionally, PCR can be performed in as little as one hour (Chauhan et al., 2015), making it a valuable tool for timely diagnosis and effective control of Theileria and Babesia infections. In parasitological investigations, the conventional diagnosis method for identifying parasites is light microscopy, which relies on their morphological characteristics. However, this technique has limitations, particularly in differentiating between piroplasms, making it less practical for use (Quintao-Silva and Ribeiro, 2003; Shayan and Rahbari, 2005). Molecular techniques, such as PCR, have recently been employed for diagnosing hemoprotozoa that cause parasitic diseases. Unlike the conventional technique, PCR is not influenced by environmental conditions, and animals that recover from acute or primary Theileria infection remain positive for the protozoan and act as reservoirs, contributing to the transmission of theileriosis in other sheep and goats (Cacci et al., 2000). Thus, an accurate diagnosis of theileriosis in asymptomatic carrier animals is crucial for implementing effective control programs. Despite the availability of molecular techniques, it is still important to compare their sensitivity and specificity with the conventional microscopy method for the detection of Theileria piroplasms in small ruminants in Pakistan. This study aims to assess the diagnostic performance of PCR amplification and microscopic smear techniques, as this will aid in developing more effective control strategies for theileriosis. The findings of this study are important for veterinarians, farmers, and policymakers as they provide critical information about the accuracy of different diagnostic methods for theileriosis. By identifying asymptomatic carrier animals, control measures can be implemented to prevent the transmission of the disease, ultimately reducing the economic impact of theileriosis on small ruminant production. Materials and MethodsBlood samplingAbout 1,200 blood samples were gathered from randomly chosen herds of seemingly healthy small ruminants in Multan district, Pakistan (Fig. 1), for the purpose of detecting the presence of theileriosis. The blood samples were drawn into labeled glass tubes, with each tube having a reference number and the species of the corresponding small ruminant. Screening for Theileria infection was carried out using both blood smear microscopy and PCR amplification methods. The samples were then stored in a sterilized container and kept at −20°C in the laboratory for subsequent processing. Microscopic examinationBlood smears were prepared in the field, following the guidelines outlined by the Tick Fever Research Center (TFRC, 1996). The smears were air-dried and fixed using methanol as a fixative. Subsequently, the smears were stained with Giemsa stain, diluted with water, and allowed to rest for approximately 30 minutes. To eliminate any excess stains, the smears were washed with water 3–4 times and air-dried. Detection of Theileria piroplasms in infected red blood cells was accomplished using a single drop of cedar wood oil, and the slides were examined under an oil immersion lens (Fig. 2). Morphological features, as defined by Soulsby (1982) and Urquhart et al. (1988), were used to identify Theileria piroplasms. DNA extractionFor DNA extraction from blood samples, an inorganic method was used (Shaikh et al. 2004). Briefly, 750 µl of blood and 750 µl of TE buffer (10 mM Tris-HCl, 2 mM EDTA, pH 8.0) were taken in an Eppendorf tube, mixed by vortex, and then centrifuged at 13,000 rpm for 5 minutes. The supernatant was discarded and a washing step was repeated 2–3 times to remove hemoglobin from the white blood cell pellet. A total of 600 ml of Tris-NaCl-EDTA (TNE) buffer (10 mM Tris HCl, 2 mM EDTA, 400 mM NaCl), 20 μl of 10% sodium dodecyl sulfate (SDS), and 1 µl Proteinase K was added for protein digestion after incubation overnight at 37°C. For the precipitation of digested proteins, 1 ml of 5 M NaCl was added, shaken vigorously, and chilled on ice for 15 minutes. An equal volume of chilled isopropanol was added to a new Eppendorf tube containing supernatant; the DNA fiber was appeared after shaking the mixture in the Eppendorf tube. The DNA fiber was pelleted after centrifugation at 13,000 rpm for 5 minutes. A washing step was performed on the DNA pellet by using 70% ethyl alcohol and finally, the DNA was dried and then dissolved in TE buffer for further use. The DNA quality was evaluated by analysis with a spectrophotometer at 260/280 nm density constant and gel electrophoresis method by using a 1% concentration agarose gel. PCR amplificationExtracted DNA was used for PCR amplification, by using primer set 989F: 5′-AGTTTCTGACCTATCAG-3′ and 990R: 5′-TTGCCTTAAACTTCCTTG-3′, of a 1,098 bp fragment of the small subunit rRNA gene of Theileria genus as described by Allsopp et al. (1993). The PCR reaction mixture comprising 5 µl template DNA (50–150 ng), 5 µl of 10× PCR buffer (100 mM Tris–HCl (pH 9) 500 mM KCl, 1% Triton X-100), 5 µl 50 Mm MgCl2, 6 µl of all 4 dNTPs, 4 µl each primer (Penicon) at a concentration of 10 pmol/µl, 2 U of Taq DNA polymerase (Vivantis) and 20.5 µl distilled water for total final reaction mixture of 50 µl. The PCR amplification was done using BIORAD thermal cycler. PCR tubes were incubated at 94°C for 5 minutes to denature genomic DNA, followed by 45 cycles at 94°C for 1 minute for DNA denaturation, 60°C for 1 minute for primer annealing, and 72°C for 1 minute for elongation. The PCR reaction was ended with a final extension step at 72°C for 7 minutes. An agarose gel with a concentration of 1.5% was used for the separation of the amplified PCR product. The PCR product was envisioned by using ethidium bromide under a UV-illuminator and compared with a 100–2,000 bp DNA ladder (Vivantis) (Fig. 3).
Fig. 1. Map of the Pakistani studied region. (A) Map of Pakistan showing Punjab province. (B) Map of Punjab showing the Multan district.
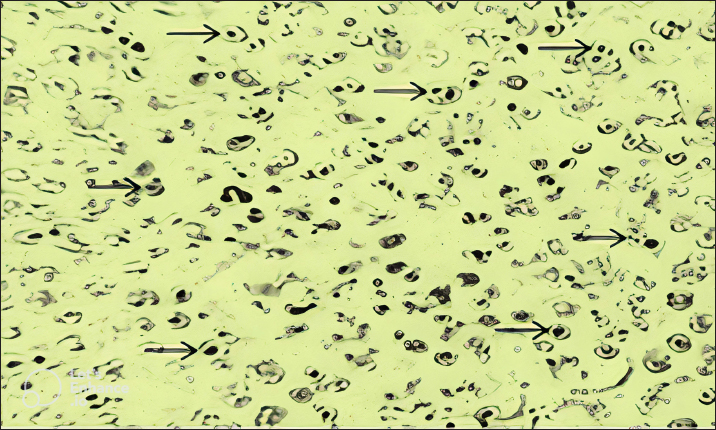
Fig. 2. Blood smears showing infection of red blood cells of sheep with Theileria schizonts.
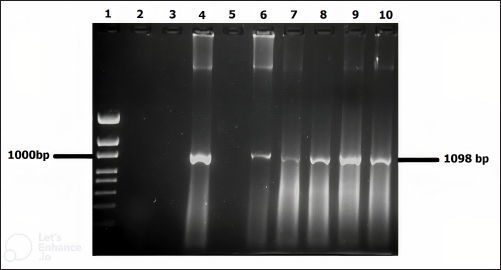
Fig. 3. Molecular detection of Theileria spp. by PCR amplification of a partial sequence (1,098 bp) of 18S rRNA gene. Lines 1: 1kb ladder; lines 2 and 3: PCR results of negative controls by using free nuclease water and Theileria spp. negative control, respectively, line 4: PCR result of positive control by using Theileria spp. positive control, line 5: PCR product of a field sample negative to Theileria spp., and lines 6–10: PCR products of field samples positive to Theileria spp. Sensitivity and specificity of PCR and microscopyThe sensitivity and specificity values for the PCR amplification diagnosis method were estimated according to Anderson et al. (1980) by using the following correlations: Sensitivity=true positive/true positive + false negative × 100. Specificity=true negative/true negative + false positive × 100. Ethical approvalEthical Research Committee of the Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan approved all the experimental procedures and protocols applied in this study. ResultsInfections caused by Theileria spp. were detected in the blood smears of animals, where intra-erythrocytic bodies with dot and comma shapes indicated the presence of Theileria schizonts (Fig. 2). Out of 1,200 samples of examined blood smears, 100 (8.33%) animals were positive for Theileria species (Fig. 2). Amplifications of the isolated DNA from randomly collected blood samples of 737 sheep and 463 goats showed an amplicon size of 1,098 bp which is considered positive for Theileria spp. (Fig. 3). Out of the 1,200 small ruminants examined by PCR in this study, Theileria spp. DNA was detected in 315 animals (26.25%) (Table 1). A total of 230 blood samples were detected negative in smear technique had produced specific band by PCR amplification while, out of 885 blood samples negative by PCR amplification, only 15 blood samples were found positive by a blood smear test. The sensitivity and specificity were 26.98% and 98.31% for the blood smear method, and 73.01% and 100% for the PCR test, respectively (Table 2). DiscussionOvine and caprine theileriosis is a tick-borne hemoprotozoan disease that is caused by Theileria spp. and is responsible for causing significant economic losses due to high mortality and morbidity rates. The diagnosis of ovine theileriosis is primarily based on clinical symptoms, microscopic screening of stained blood smears, and lymph node biopsy smears. However, these detection methods have limitations against Theileria spp. infection that restricts their specificity. Therefore, to overcome these limitations, the current study reports the differential diagnosis of theileriosis in small ruminants from Pakistan using both blood smear examination and PCR Table 1. Comparison between blood smear examination and PCR amplification.
A total of 1,200 blood samples from small ruminants were randomly examined to diagnose theileriosis. The prevalence rate was found to be 26.25% by PCR, compared to 8.33% by microscopy. Of the 230 blood samples that were negative in the smear technique, specific band production was observed by PCR amplification. Conversely, out of the 885 blood samples negative by PCR amplification, only 15 were found positive by blood smear test. The sensitivity and specificity of the blood smear method were 26.98% and 98.31%, respectively, while the PCR test had a sensitivity and specificity of 73.01% and 100%, respectively. These findings indicate that PCR amplification is a more sensitive method than microscopy for diagnosing Theileria piroplasms in infected small ruminants (Table 1). Giemsa staining is a commonly used method for identifying ovine and caprine theileriosis worldwide due to its simplicity and affordability. However, microscopy is not sensitive enough to diagnose carrier animals with low parasitemia. Serological tests are also not reliable since Theileria antibodies disappear quickly, leading to negative results in carrier animals with low parasitemia (Aktas et al., 2006). These animals could potentially spread Theileria piroplasms through the animal-tick life cycle. Therefore, PCR-based molecular diagnosis is better for understanding the status of Theileria spp. infection. Several studies have reported that PCR assay is more specific and sensitive than microscopy (Oliveira-Sequeira et al., 1995; Kirvar et al., 1998; Martin-Sanchez et al., 1999; Nagore et al., 2004; Altay et al., 2005; Altay et al., 2008; Taha et al., 2010). This method allows for direct, accurate, and sensitive identification of piroplasms even in carrier small ruminants with a low parasitemia level that show no symptoms of Theileria infection. Table 2. 2 × 2 contingency for blood smear examination and PCR.
Previous studies on ovine and caprine Theileria spp. infection have shown higher prevalence rates when diagnosed by PCR compared to microscopy. For example, in Turkey, Altay et al. (2005) reported rates of 54.03% and 19.35%, while in Iran, Heidarpour et al. (2009) reported rates of 56% and 21%, Heidarpour et al. (2010) reported rates of 60% and 22.27%, Yaghfoori et al. (2013) reported rates of 76% and 46%, and Jalali et al. (2014) reported rates of 89% and 69.7%. However, differences in prevalence rates could be due to various factors such as differences in diagnostic methods, the number of animals tested, sampling procedures, parasitemia and immunity levels in infected animals, as well as abiotic factors such as bioclimatic and ecological conditions, and host management. These factors can influence the spread of ticks and tick-borne diseases and therefore cause variations in prevalence rates (Kivaria, 2006; Zangana and Naqid, 2011). The difference in Theileria infection rates found using microscopy and PCR can be attributed to the limited sensitivity of microscopy to detect low levels of parasitemia in carrier animals (Aktas et al., 2007; Altay et al., 2007). Microscopy can only diagnose up to 1 infected cell per 10,000 cells by investigating at least 100–200 fields, and at least 0.5 µl of blood is required for such examination (Mosqueda et al., 2012). On the other hand, PCR is more sensitive and can detect even a single infected cell in 107 erythrocytes, which is equivalent to a blood parasitemia of 0.00001% (Altay et al., 2007). Due to this limitation, carrier animals with low levels of parasitemia may not be detected by microscopy, whereas PCR can detect the presence of the parasite even in carrier animals without any symptoms of Theileria infection. Results of this study indicate that small ruminants like sheep and goats could act as a host for Theileria spp. infection and they may have a lower level of parasitemia that could go unnoticed by the traditional microscopy technique. If left untreated, the infection can cause anemia, weight loss, reduced milk production, and even death in the infected small ruminants. However, treating the suspected animals with unrelated drugs, especially antibiotics, can lead to unnecessary medication and residues in milk and dairy products that may ultimately result in the rejection of their products in the international market (Chauhan et al., 2015). During this study, specific primers were used to identify the Theileria genus, which amplified a 1,098 bp fragment of the 18S rRNA gene in 315 blood samples obtained from sheep and goats (Fig. 3). The PCR technique used for the molecular diagnosis of blood samples proved to be more sensitive and specific compared to the traditional smear screening method. This technique is especially useful for identifying subclinical infections of theileriosis in small ruminants. In field conditions, subclinical theileriosis infections in small ruminants were not detected by microscopy but were picked up by PCR, indicating its effectiveness as a molecular diagnostic tool for carrier small ruminants. ConclusionIn conclusion, the study highlights that the diagnosis of ovine and caprine theileriosis in Pakistan should shift from symptom-based detection to DNA-based identification. The use of PCR amplification based on the 18S rRNA gene is a more reliable and accurate method for detecting Theileria spp. compared to the conventional method of blood smear examination. This shift from symptom-based detection to DNA-based identification can provide a significant improvement in the diagnosis of Theileria spp., reduce drug wastage, and curtail the development of drug resistance. Therefore, the use of PCR should be implemented as the gold standard method for the diagnosis of ovine and caprine theileriosis in Pakistan. AcknowledgmentsThe authors would like to thank all veterinarians for helping with sample collection. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsM.R. designed this study, collected samples and epidemiological data, and performed the molecular and microscopic diagnosis. M.R. and M.B.S performed the statistical analysis. M.R. and M.B.S. wrote the manuscript, and N.N., A.K., and MBS edited it. M.B.S finalized the manuscript and all the authors approved the final version. ReferencesAktas, M., Altay, K. and Dumanli, N. 2005. Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 133, 277–281. Aktas, M., Altay, K. and Dumanli, N. 2006. PCR-based detection of Theileria ovis in Rhipicephalus bursa adult ticks. Vet. Parasitol. 140, 259–263. Aktas, M., Altay, K. and Dumanli, N. 2007. Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Vet. Parasitol. 147, 161–165. Allsopp, B. A., Baylis, H. A., Allsopp, M. T., Cavalier-Smith, T., Bishop, R. P., Carrington, D. M., Sohanpal, B. and Spooner, P. 1993. Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitol. 107, 157–165. Altay, K., Aydin, M. F., Uluisik, U., Aktas, M. and Dumanli, M. 2008. Use of multiplex PCR for the diagnosis of Theileria annulata and Theileria buffeli. Turk. Parazitol. Derg. 120, 1–3. Altay, k., Dumanli, N., Patricia, J., Holman, B. and Aktas, M. 2005. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet. Parasitol. 127, 99–104. Altay, K., Dumanli, N. and Aktas, M. 2007. Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Vet. Parasitol. 147, 161–165. Altay, K., Dumanli, N. and Aktas, M., 2012. A study on ovine tick-borne hemo-protozoan parasites (Theileria and Babesia) in the East Black Sea Region of Turkey. Parasitol. Res. 111, 149–153. Anderson J.F., Magnarelli, L.A. and Sulzer, A.J. 1980. Canine babesiosis: Indirect fluorescent antibodies test for a North American isolate of Babesia gibsoni. Am. J. Vet. Res. 41, 2102–2105. Aung, A., Kaewlamun, W., Narapakdeesakul, D., Poofery, J. and Kaewthamasorn, M. 2022. Molecular detection and characterization of tick-borne parasites in goats and ticks from Thailand. Ticks Tick Borne Dis. 13, 101938. Cacci, S., Camma, C., Onuma, M. and Severini, C. 2000. The b-tubulin gene of Babesia and Theileria parasites is an informative marker for species discrimination. Int. J. Parasitol. 30, 1181–1185. Chauhan, H.C., Patel, B.K., Bhagat, A.G., Patel, M.V., Patel, S.I., Raval, S.H., Panchasara, H.H. Shrimali, M.D., Patel, A.C. and Chandel, B.S. 2015. Comparison of molecular and microscopic technique for detection of Theileria annulata from the field cases of cattle. Vet. World. 8, 1370–1374. Demessie, Y. and Derso, S., 2015. Tick borne hemoparasitic diseases of ruminants: a review. Adv. Biol. Res. 9, 210–224. Durrani, S., Khan, Z, Khattack, M.R.M., Andleeb, M., Ali, M., Hameed, M., Taqddas, A., Faryal, M., Kiran, S., Anwar, H., Riaz, M., Sajjid, M., Sheikh, R.S. and Iqbal, F. 2012. A comparison the presence of Theileria ovis by PCR amplification of their SSU RNA gene in small ruminants from two provinces of Pakistan. Asi. Pac. J. Trop. Dis. 2, 43–47. Gul, N., Ayaz, S., Gul, I., Adnan, M., Shams, S. and Akbar, N. 2015. Tropical theileriosis and east coast fever in cattle: present, past and future perspective. Int. J. Curr. Micro. App. Sci. 4, 1000–1018. Heidarpour, B.M., Haddadzadeh, H.R., Kazemi, B., Khazraiinia, P., Bandehpour, M. and Aktas, M. 2009. Molecular identification of ovine Theileria species by a new PCR RFLP method. Vet. Parasitol. 161, 171–177. Heidarpour, B.M., Khazraiinia, P. and Haddadzadeh, H.R.B., 2010. Identification of Theileria spp. in sheep in the eastern half of Iran using nested PCR- RFLP and microscopic techniques. Iran. J. Vet. Res. 11, 262–265. Irshad, N., Qayyum, M., Hussain, M. and Khan, M.Q. 2010. Prevalence of tick infestation and theileriosis in sheep and goats. Pak. Vet. J. 30, 178–180. Jalali, S.M., Khaki, Z., Kazemi, B.D., Rahbari, M.S., Shayan, P., Bandehpour, M. and Yasini, S.P. 2014. Molecular detection and identification of Theileria species by PCR-RFLP method in sheep from Ahvaz, Southern Iran. Iran. J. Parasitol. 9, 99–106. Kirvar, E., Ilhan, T., Katzer, F., Wilkie, G., Hooshmand-Rad, P. and Brown, D. 1998. Detection of Theileria lestoquardi (hirci) in ticks, sheep, and goats using the polymerase chain reaction. Ann. NY. Acad. Sci. 849, 52–62. Kivaria, F.M. 2006. Estimated direct economic costs associated with tick-bornediseases on cattle in Tanzania. Trop. Anim. Health Prod. 38, 291–299. Kohli, S., Atheya, U.K. and Thapliyal, A. 2014. Prevalence of theileriosis in cross- bred cattle: its detection through blood smear examination and polymerase chain reaction in Dehradun district, Uttarakhand, India. Vet. World 7, 168–171. Kumar, R., Moudgil, P., Gupta, R., Jhandai, P., Sharma, M. and Jindal, N. 2022. Molecular investigations on outbreaks of ovine theileriosis among sheep and goats in Haryana, India. Trop. Anim. Health Prod. 54, 368. Lew, A.E., Dalrymple, B.P., Jeston, P.J. and Bock, R.E. 1997. PCR methods for the discrimination of Babesia bovis isolates. Vet. Parasitol. 71, 223–237. Martin-Sanchez, J., Viseras, J., Androher, F.J. and Garcia- Fernandez, P. 1999. Nested polymerase chain reaction for detection of Theileria annulata and comparison with conventional diagnostic techniques: its use in epidemiology studies. Parasitol. Res. 85, 243–245. Mehlhorn, H. and Schein, E. 1984. The piroplasms: life cycle and sexual stages. Adv. Parasitol. 23, 37–103. Mosqueda, J., Olvera-Ramirez, A., Aguilar-Tipacamu, G. and Canto, G.J. 2012. Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 19, 1504–1518. Nagore, D., Garcia-Sanmartin, J., Garcia-Perez, A.L., Juste, R.A. and Hurtado, M.A. 2004. Detection and identification of equine Theileria and Babesia species by reverse line blotting: epidemiological survey and phylogenetic analysis. Vet. Parasitol. 123, 41–54. Norouzi, M., Dayer, M.S. and Ghaffarifar, F. 2022. Molecular detection and characterisation of Theileria in hard ticks of small ruminants in Zarrin Dasht County, Southern Iran. Vet. Med. Sci. 9, 372–379. Nourollahi-Fard, S.R., Khalili, M. and Aminzadeh. A. 2008. Prevalence of antibodies to Neospora caninum in cattle in Kerman province, South East Iran. Vet. Arch. 78, 253–259. OIE, 2014. Manual of diagnostic tests and vaccines for terrestrial animals, 7th ed. Paris, France, Office International Des Epizooties, Vol. 1–2.. Oliveira-Sequeira, T.C.G., Oliveira, M.C., Araujo, J.P. and Amarante. A.F. 2005. PCR-based detection of Babesia bovis and Babesia bigemina in their natural host Boophilus microplus and cattle. Int. J. Parasitol. 35, 105–111. Prajapati, A., Prajapati, B., Patel, A., Chauhan, P., Das, B., Raval, S., Suthar, A., Sutaria, T., Chaudhari, R.K., Patel, P., Chauhan, V. and Patel, R. 2023. Molecular identification and genetic characterization of Theileria and Anaplasma infection in sheep and goat of North Gujarat, India. Parasitol Res. 122, 1427–1433. Quintao-Silva, M.G. and Ribeiro, M.F. 2003. Infection rate of Babesia sp. sporokinetes in engorged Boophilus microplus from an area of enzootic stability in the State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 98, 9991–1002. Rahravani, M., Moravedji, M., Mostafavi, E., Mozoun, M.M., Zeeyaie, A.H., Mohammadi, M., Seyfi, H., Adhami, G., Esmaeili, S. and Ameri, M. 2023. Clinical, hematological and molecular evaluation of piroplasma and Anaplasma infections in small ruminants and tick vectors from Kurdistan province, western Iran. Res. Vet. Sci. 159, 44–56. Razmi, G. and Yaghfoori. S. 2013. Molecular surveillance of Theileria ovis, Theileria lestoquardi and Theileria annulata infection in sheep and ixodid ticks in Iran. Onderstep. J. Vet. Res. 80, 635–639. Razmi, G.R., Naghibia, A., Aslanib, M.R. and Hossein, H. 2003. An epidemiological study on Babesia infection in small ruminants in Mashhad Suburb, Khorasan Province, Iran. Kore. J. Parasitol. 50, 39–44. Sallam, N.M., Saleh A.I., Ali H.A. and Abdelsalam M.M. 2023. An efficient EGWO algorithm as feature selection for B-ALL diagnoses and its subtypes classification using peripheral blood smear images. Alexandria Eng. J. 68, 39–66. Sayin, F., Nalbantoglu, S., Bayram, B.A., Çkakmak, A. and Karer, Z. 2009. Epidemiological studies on sheep and goat Vheilåria infection. Ank. Univ. Vet. Fak. Derg. 56, 126–129. Shaikh, R.S., Ramzan, K., Nazil, S., Khan, S.N. and Riazuddin, S. 2004. A new locus for non syndromic deafness DFNB maps to chromosomes 11p13-p12. Am. J. Med. Genet. Anal. 138, 392–395. Shayan, P. and Rahbari, S. 2005. Simultaneous differentiation between Theileria spp. and Babesia spp. on stained blood smear using PCR. Parasitol. Res. 97, 281–286. Sitotaw, T., Regassa, F., Zeru, F. and Kahsay, A.G. 2014. Epidemiological significance of major hemoparasites of ruminants in and around Debre-Zeit, Central Ethiopia. J. Parasit. Vec. Biol. 6, 16–22. Soulsby, E.J. 1982. Helminths arthropods and Protozoa of domesticated animals, 7th ed. London, UK: Bailliere Tindalland Cassel Ltd, pp: 579–581. Taha, K.M., Salih, D.A., Ali, A.M., Omer, R.A. and El Hussein, A.M. 2013. Naturally occurring infections of cattle with Theileria lestoquardi and sheep with Theileria annulata in the Sudan. Vet. Parasitol. 191, 143–145. TFRC (Tick Fever Research Center). 1996. Tick fever and disease diagnosis. Queensland, Australia, Department of Primary Industries. Urquhart, G.M., Armour, J. Duncan, J.L., Dunn, A.M. and Jennings, F.W. 1996. Veterinary parasitology, 2nd ed. Oxford, UK: Blackwell Science Ltd, pp: 246–249. Yaghfoori, S., Razmi, G.R. and Heidarpour, M. 2013. Molecular detection of Theileria spp. in sheep and vector ticks in Fasa and Kazeroun areas, Fars Province. Iran. Arch. Razi. Instit. 68, 159–164. Yang, L., Wang, J.H., Upadhyay, A., Zhao, J.G., Huang, L.Y., Liao, C.H. and Han, Q. 2022. Identification of Theileria spp. and investigation of hematological profiles of their infections in goats in Hainan Island, China. Parasite 29, 13. Zangana, I.K. and Naqid, I.A. 2011. Prevalence of piroplasmosis (Theileriosis and Babesiosis) among goats in Duhok Governorate. AL-Anbar J. Vet. Sci. 4, 50–57. | ||
| How to Cite this Article |
| Pubmed Style Riaz M, Nasreen N, Khan A, Said MB. Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan. Open Vet J. 2023; 13(6): 697-704. doi:10.5455/OVJ.2023.v13.i6.4 Web Style Riaz M, Nasreen N, Khan A, Said MB. Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan. https://www.openveterinaryjournal.com/?mno=147047 [Access: July 03, 2025]. doi:10.5455/OVJ.2023.v13.i6.4 AMA (American Medical Association) Style Riaz M, Nasreen N, Khan A, Said MB. Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan. Open Vet J. 2023; 13(6): 697-704. doi:10.5455/OVJ.2023.v13.i6.4 Vancouver/ICMJE Style Riaz M, Nasreen N, Khan A, Said MB. Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan. Open Vet J. (2023), [cited July 03, 2025]; 13(6): 697-704. doi:10.5455/OVJ.2023.v13.i6.4 Harvard Style Riaz, M., Nasreen, . N., Khan, . A. & Said, . M. B. (2023) Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan. Open Vet J, 13 (6), 697-704. doi:10.5455/OVJ.2023.v13.i6.4 Turabian Style Riaz, Muhammad, Nasreen Nasreen, Adil Khan, and Mourad Ben Said. 2023. Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan. Open Veterinary Journal, 13 (6), 697-704. doi:10.5455/OVJ.2023.v13.i6.4 Chicago Style Riaz, Muhammad, Nasreen Nasreen, Adil Khan, and Mourad Ben Said. "Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan." Open Veterinary Journal 13 (2023), 697-704. doi:10.5455/OVJ.2023.v13.i6.4 MLA (The Modern Language Association) Style Riaz, Muhammad, Nasreen Nasreen, Adil Khan, and Mourad Ben Said. "Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan." Open Veterinary Journal 13.6 (2023), 697-704. Print. doi:10.5455/OVJ.2023.v13.i6.4 APA (American Psychological Association) Style Riaz, M., Nasreen, . N., Khan, . A. & Said, . M. B. (2023) Differential diagnosis of theileriosis through blood smear examination and polymerase chain reaction in small ruminants from Pakistan. Open Veterinary Journal, 13 (6), 697-704. doi:10.5455/OVJ.2023.v13.i6.4 |