| Research Article | ||
Open Vet J. 2023; 13(7): 894-902 Open Veterinary Journal, (2023), Vol. 13(7): 894-902 Original Research In vitro toxicity of combination of amitraz and carvacrol on Demodex canisSina Fereydooni1, Farnoosh Arfaee1*, Mohammad Reza Youssefi2, Fatemeh Zahra Gharib3 and Mohaddeseh Abouhosseini Tabari41Department of Clinical Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran 2Department of Veterinary Parasitology, Babol Branch, Islamic Azad University, Babol, Iran 3Department of Veterinary Clinical Sciences, Babol Branch, Islamic Azad University, Babol, Iran 4Faculty of Veterinary Medicine, Amol University of Special Modern Technologies, Amol, Iran *Corresponding Author: Farnoosh Arfaee. Department of Clinical Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran. Email: f.arfaee [at] srbiau.ac.ir Submitted: 09/05/2023 Accepted: 25/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
AbstractBackground: Canine generalized demodicosis is an inflammatory skin disease, which usually has time-consuming and frustrating treatments. Aim: The present study aimed to evaluate the acaricidal activity of carvacrol as a green drug and a combination of carvacrol and amitraz on Demodex canis mites and investigate mites' survival lifetime. Methods: Mite samples were collected from a dog affected by demodicosis and had been diagnosed with deep skin scrapings. The motility of mites was examined by using optical microscopy. Samples were tested with 5% of carvacrol, the combination of Carvacrol 5% + Amitraz 0.05%, Amitraz 0.05% as a positive control, and mineral oil as a negative control. The interval time between the adding the test solutions and the moment the last mite ceased was defined as the survival time in the samples and the killing times of mites in each group were compared with each other. Results: It was determined that after administration of a 5% concentration of carvacrol, lethal effects were faster than the combination solution of Carvacrol 5% + Amitraz 0.05%, and the survival times in the control groups were longer than in both treatment groups. Conclusion: Carvacrol, as one of the most important bioactive terpenes, had the most effective in vitro miticidal activity, and it seems that carvacrol alone or in combination with amitraz can be suggested as a possible therapy in the treatment of canine demodicosis. Keywords: Canine demodicosis, Carvacrol, Amitraz, Mite, Skin. IntroductionDemodex canis is a parasitic mite of the skin of dogs and a small number of mites may be a normal commensal of the skin (Ravera et al., 2013; Patra et al., 2019). Mites are transmitted from the dam to the puppies a few days after birth by direct skin contact (Ravera et al., 2013). The proliferation of mites can cause severe inflammatory skin disease, with the clinical signs of hair loss, erythema, comedones, follicular papules to pustules and scales on the affected areas, and also can cause secondary skin bacterial infections (Jekl et al., 2006; Fourie et al., 2007). Deep skin scrapings are the choice diagnostic method for canine demodicosis which multiple scrapings of approximately 1 cm2 of lesions should be performed with mineral oil until capillary oozing, then gathered debris transferred to a microscopy slide and observed by 4× and 10× magnification (Mueller et al., 2017). The acetate tape impression by squeezing on the suspect-affected areas of the skin is another diagnostic procedure that has been reported as a good and sensitive diagnostic method (Pereira et al., 2012). Hair trichograms and skin biopsies are the other methods of mite detection (Mueller et al., 2020). The approved mainstay treatment for demodicosis is consisted of the application of an aqueous dilution of amitraz as a leave-on rinse, for decades, in many countries. Amitraz as a diamide in the formamidine group had shown variable efficacy in the treatment of demodicosis in many studies (Folz et al., 1978; Mueller, 2004). Monoamine oxidase inhibitors and alpha 2-adrenergic agonist activity of amitraz can cause acaricidal activity by disrupting the transmission of nerve impulses (Hugnet et al., 1996; Rhodes, 2004). Amitraz solution as a rinse is recommended at the concentration of 0.025%–0.05%, once weekly to every 2 weeks for canine demodicosis treatment (Kwochka et al., 1985; Hugnet et al., 2001). Despite the variable efficacy of amitraz in the treatment of canine demodicosis, some adverse effects such as depression, ataxia, skin irritations, pruritus, polydipsia, hypotension, bradycardia, hyperglycemia, vomiting, and diarrhea have been reported in dogs associated with amitraz poisoning (Miller et al., 2013). Macrocyclic lactones, such as ivermectin, doramectin, and moxidectin, demonstrated proper efficacy in the treatment of canine generalized demodicosis. These off-label drugs had the potential for toxicity, especially in collie breeds, because these dogs are mutated for (MDR-1) multidrug resistance mutation 1 (P-glycoprotein deficiency) (Paterson et al., 2009; Mueller, 2012). Milbemycin oxime is another drug that is licensed in some countries for the treatment of canine demodicosis, but some adverse effects reported in dogs homozygous for the MDR-1 mutation (Holm, 2003; Barbet et al., 2009). Another treatment protocol for canine demodicosis is the use of the combination of amitraz + metaflumizone, amitraz + fipronil + methoprene, and moxidectin + imidacloprid, these spot-on products provide convenient and safer treatment with different rates of efficacy (Heine et al., 2005; Mueller et al., 2009; Fourie et al., 2007, 2013;). Recently, isoxazoline class ectoparasiticides including fluralaner, sarolaner, lotilaner, and afoxolaner introduced to veterinary medicine for the treatment of canine demodicosis, with excellent therapeutic results in published data (Fourie et al., 2015; Beugnet et al., 2016; Six et al., 2016; Snyder et al., 2017). Although adverse reactions in treatment with isoxazolines are uncommon to rare, some gastrointestinal reactions such as anorexia, vomiting, and diarrhea without blood, and neurological symptoms such as seizure have been reported (Rohdich et al., 2014; Gaens et al., 2019; Muller et al., 2020). Herbal medicines have the potential to be introduced as effective and less toxic alternative drugs because of their few side effects, low incidence of resistance, low expense, and availability especially in developing countries (Etewa et al., 2011; Castro et al., 2018). Although the skin stratum corneum prevents enhancing any component to defend against infections, sometimes therapeutic methods need to achieve to the inner layers to have able to increase the plasma concentration and eliminate the microorganisms (Sapra et al., 2008). The factors that increase the skin barrier penetration include surfactants (Shokri et al., 2001), fatty acids/esters (Kanikkannan et al., 2000)), solvents (Okabe et al., 1994), and terpenes (Lane, 2013). One of the most effective of these terpenoids is carvacrol a role as a penetration enhancer and also has anti-parasitic effects such as dermal demodicosis (Sapra et al., 2008). Carvacrol is a monoterpene phenolic bioactive compound of various medical plant's essential oils, especially the Labiatae family, including Origanum, Satureja, Tymbra, Thymus, and Coridothymus species (Jayakumar et al., 2012; Sajed et al., 2013). Carvacrol demonstrated a majority of pharmacological properties, including anti-inflammatory, anti-bacterial, anti-fungal, anti-oxidant, and anti-cancer in many articles (Baranauskaite et al., 2017; Barnwal et al., 2017; Allaoua et al., 2018; Vinciguerra et al., 2018). By the way, carvacrol was reported to have insecticidal and acaricidal activity against agricultural, stored products, and medical arthropod pests (Ahn et al., 1998). Also, carvacrol is considered an attractive and safe food additive in many countries because of its low toxicity and low cost of production. European Union Food Improvement Agents and Joint FAO/WHO Expert Committee on Food Additives, have classified carvacrol as a safe flavoring agent in human consumption (Marchese et al., 2018; Sharifi-Rad et al., 2018; National Center for Biotechnology Information, 2020). Previous studies have reported that different herbal essential oils and their bioactive components of them, especially tea tree oil have great acaricidal efficacy against mites of the Demodex genus (Demodex folliculorum and Demodex brevis) in humans (Gao et al., 2005; Savla et al., 2019; Akkucuk and Kaya, 2022). Also, there was just one article that evaluates the in vitro acaricidal effect of tea tree oil as a herbal essential oil on D. canis mites (Neves et al., 2020). Despite thyme oil and carvacrol as the most important active ingredients, it was evaluated for the killing of D. folliculorum and D. brevis in humans and shown great and more effective mite killing in comparison with tea tree oil, black seed oil, St. John's Wort oil, and sage oil (Akkucuk and Kaya, 2022). The efficacy of carvacrol against the D. canis has never been evaluated. Therefore, the present study aimed to assess the in vitro acaricidal effects of Carvacrol 5%, Carvacrol 5% + Amitraz 0.05% (1:1 mixtures), Amitraz 0.05%, and mineral oil, and evaluate the killing times of each solution on D. canis mites. Although it is very important to treat skin diseases in dogs as soon as possible, demodicosis due to clinical symptoms such as hair loss, skin lesions, skin crusts, and pyoderma can be difficult in terms of social behavior and patient management for the owners. Material and MethodsSample collectionIn the present study, Demodex mites were collected from an approximately 2-year old intact male mongrel dog with a natural infestation of Demodex spp. The dog had the clinical signs of generalized demodicosis such as hair loss, erythema, crusts, and follicular casts, and had positive deep skin scrapings with a high Demodex mites load. The dogs also had not been treated previously with topical or systemic ectoparasiticides at least 12 weeks before the study. The collecting of mites from the lesions was done by using adhesive tape strips instead of a scraping test to decrease the erosions that may occur by doing a lot of deep skin scraping tests and animal welfare (Pereira et al., 2012). At first, suspected lesions of the skin were squeezed properly to extrude the mites from hair follicles to the surface of the skin and then the sticky surface of the tapes, measuring 6 × 2.5 cm was pressed onto the squeezed suspected lesions. The tapes contained samples mounted on a microscope glass slide directly and examined under light microscopes at 10× and 40× magnification to find slides that contained at least five adult and evidently mobile mites. The standardization of mite numbers in each slide was not possible because of the erratic distribution of the mites on each suspected lesion. The movement of chelicerae and tarsi of Demodex mites in microscopy was the choice criterion for selecting samples containing live mites (Gao et al., 2005; Tighe et al., 2013). The 20 samples containing viable live mites were chosen for the in vitro test study and divided into four groups. Carvacrol (≥98%), amitraz (≥99%), and mineral oil, as an internal standard purchased from Sigma–Aldrich (Stein-heim, Germany). All the study solutions were prepared on the day of the study. The carvacrol at the concentration of 5% was prepared by diluting the carvacrol 98% with the Taurine solution. The amitraz was prepared at the concentration of 0.05% by diluting 4 ml of the 12.5% amitraz into one liter of distilled water. Equal volume of carvacrol 5% solution and amitraz 0.05% solution diluted to obtain the 1:1 mixtures. The mineral oil was used in its original concentration by the manufacturer. Experimental proceduresThis presented study was conducted based on the procedures followed in other studies with similar objectives (Walton et al., 2004; Tighe et al., 2013; Neves et al., 2020). Twenty selected samples were randomly allocated to one of four study groups consisting of five samples in each group. Group 1 was tested with carvacrol (means 5%), group 2 was tested with 1:1 ratio mixtures of Carvacrol + Amitraz (means 5% and 0.05%, respectively) to study the potential synergistic effect, group 3 as a positive control was tested with amitraz (means 0.05%), and group 4 as a negative control were tested with mineral oil. Different solutions of the respective substances were prepared in the volume of 200 μl for each sample and the adhesive tapes with the mite samples were detached from the glass slides in each group carefully. Then the substances were applied on the entire surface of the glass slides, and the detached adhesive tapes were placed onto the glass slides to make good contact with the Demodex mites and respective substances. Toxicity assessmentThe samples were observed under the light microscope. The movement of the body and legs of the mite was observed continuously until all mites died and also the parameter of changes in the aspect of mite bodies was observed continuously for 24 hours. The initial microscopy was done at a magnification of 10× and mites were followed by zigzag line microscopy observation for 5 minutes at 5-minute intervals thereafter. After the time that mites lost their motility and were suspected death of each mite, the magnification of 40× was employed to observe the motility or lack of motility of chelicerae and tarsi of the mites continuously for 1 minute during the time of study to ensure whether a mite was alive or dead (Gao et al., 2005; Tighe et al., 2013; Neves et al., 2020). The survival time was defined as the time between when the application of the solution was added to the study group and the moment that the last mite movement in the sample ceased. The times that the last mite in each sample died were recorded as considered the total time required for the extermination of the mites in the treated samples and the average survival times in each group were compared with each other. The procedure was followed after confirmation of the last death, and all mites were observed under microscopy every 30 minutes to determine the gradual deformation and shrinkage of the Demodex mite's body. As in the previous study, the temperature of the laboratory was in the range of 25°C–27°C, and the average humidity was approximately 75%, both temperature and humidity in the laboratory were measured every 60 minutes with a standard thermometer and hygrometer (Zhao et al., 2005, 2009, 2011). Ethical approvalThe study was performed under review and guidelines by the ethics committee of animal welfare and approved by the ethics committee of Islamic Azad University of Tehran, Science, and Research branch. ResultsIn group 1, contact with carvacrol made a primary agitation of the mites suddenly after contact, and the movement of mite's body became slower between 5 and 15 minutes after contact with the solution. The death of mites was confirmed by the progressive slowing and stopping of the movements of the chelicerae and tarsi and registered between 15 and 20 minutes in the Carvacrol group (Table 1). Table 1. Results of in vitro test of D. canis mites, in contact with different treatments.
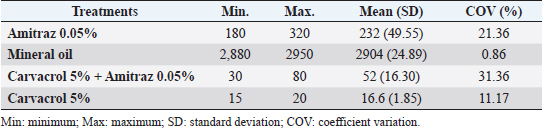
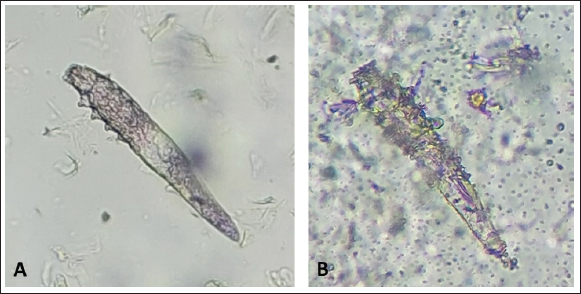
After the death of each mite by contact with the Carvacrol 5%, clarification of the opisthosomas cuticle and mild opisthosomal wrinkling was observed in the group 1 sample (Fig. 1). In group 2, after adding the Carvacrol + Amitraz, a primary agitation of the mites was followed by slowing the movement of the mites between 20 and 40 minutes. The death of the mites occurred between 30 and 80 minutes after application (Table 1). In group 2, clarification of the opisthosomas was observed after the death of each mite by contact with the Carvacrol + Amitraz (Fig. 2).
Fig. 1. (A) Alive mite in the carvacrol group before treatment. (B) Dead and clarification of the opisthosomas cuticle in mites were also followed by the microscopic observation at the magnification of 40 × after 20 minutes of carvacrol use on D. canis.
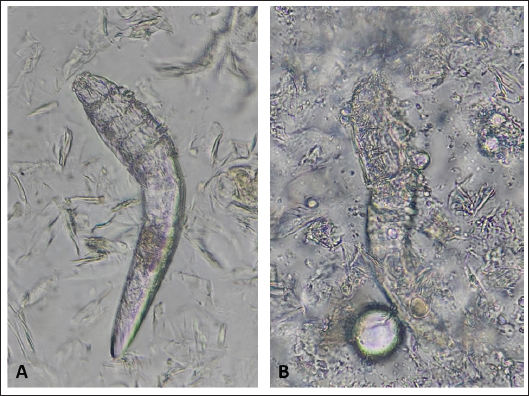
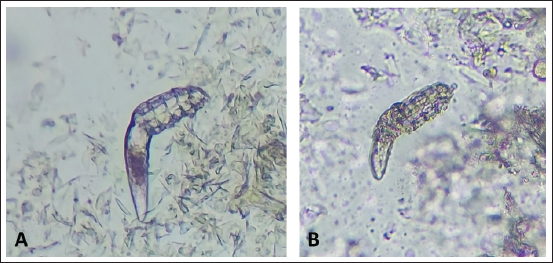
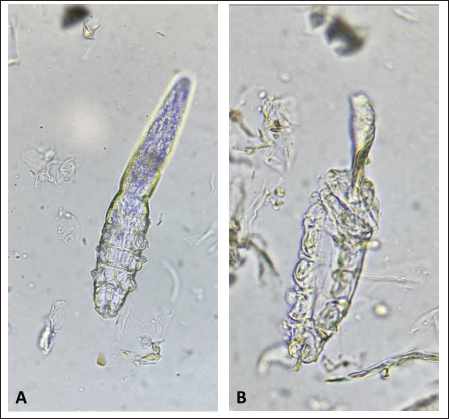
Fig. 2. (A) Alive mite in combination group before treatment. (B) Dead and clarification of the opisthosomas cuticle in mites were also followed by the microscopic observation at the magnification of 40 × after 80 minutes Combination of carvacrol + amitraz use on D. canis. In group 3, amitraz caused the death of mites between 180 and 320 minutes, and in group 4, the survival time of the mites after application of the mineral oil was more than 24 hours (Table 1). In group 3, contact of mites with the amitraz caused complete wrinkling of the opisthosoma and clarification of the opisthosomal cuticle (Fig. 3). In the mineral oil group, complete wrinkling of the opisthosoma and clarification of the opisthosomal cuticle were observed after the death of mites (Fig. 4). The mineral oil group had the best survival time (2,950 minutes) of all other groups. The lowest time observed in the Amitraz group (180 minutes) was higher than the maximum time to last mite death in both Carvacrol (20 minutes) and Amitraz + Carvacrol (80 minutes) groups. Carvacrol had the most effective in vitro miticidal activity. It was determined that miticidal activity in the Carvacrol group was faster than the Carvacrol + Amitraz group, and it could be the result of the lower volume of Carvacrol in the Carvacrol + Amitraz group.
Fig. 3. (A) Alive mite in amitraz group before treatment. (B) Dead, opisthosoma wrinkling, and clarification of the opisthosomas cuticle in mites were also followed by the microscopic observation at the magnification of 40 × after 320 minutes of amitraz use on D. canis.
Fig. 4. (A) Alive mite in mineral oil group before treatment. (B) Dead, opisthosoma wrinkling, and clarification of the opisthosomas cuticle in mites were also followed by the microscopic observation at the magnification of 40 × after 24-hour mineral oil use on D. canis. DiscussionThese days, synthetic acaricides are becoming increasingly problematic due to some health problems and the expense of these products, especially in developing countries. There are a lot of treatment options for canine generalized demodicosis but many of them are not licensed for the treatment and may have poor efficacy and narrow safety margin (Perego et al., 2019). Therefore, novel resources including plant essential oils and plant-derived bioactive compounds could have the potential to be suggested as an alternative treatment for Demodex spp. The initial result of the study demonstrated that carvacrol as a phenolic monoterpene was effective against D. canis and it could be widely used in demodicosis like amitraz without critical side effects. The combination of Carvacrol + Amitraz also had a reasonable effect against demodicosis, and in the belief of authors, mites removal from the skin with this mixture (1:1) could be more beneficial. Skin-penetrating enhancement of carvacrol could induce a synergistic implication on perilous demodicosis. Although the clinical effect of carvacrol was better than the mixture solution group, it is based on the volume of solution that became half in the mixture group and did not express the carvacrol superiority over than mixture group. Both treatment groups had better outcomes than the mineral oil (negative group) and Amitraz 0.05% (positive group), respectively. Our data are consistent with the reports of Akkucuk and Kaya (2022) concerning investigating the effects of some essential oils, especially thyme oil on D. folliculorum, where the results showed that 1% concentration of thyme oil had a more effective killing time than 5% concentration of tea tree oil and sage oil, and a significant difference was found ( p < 0.0001). Carvacrol has been identified as the most prevalent active ingredient of thyme essential oil at a concentration of 59.93%, analyzed by gas chromatography–mass spectrometry method in this study (Akkucuk and Kaya, 2022). Numerous studies evaluated in vitro and in vivo acaricidal activity of several essential oils against D. folliculorum and D. brevis with different numbers of enrolled mites and different efficacy (Gao et al., 2005, 2012; Kim et al., 2011; Zhao et al., 2011; Koo et al., 2012; Tighe et al., 2013). A study on the in vitro sensitivity of D. canis to the different concentrations of tea tree oil was conducted by Neves et al. (2020), and the results showed that the higher concentration of the oil (100%), had more quickly lethal effects on mites and also lowest concentration (3/13%) killed mites faster than the positive control group (amitraz 0/05%). This was the first in vitro study of using essential oils on D. canis. We conducted some of this study procedure such as sampling and microscopy following our recent study. In another study, Li et al. (2021) revealed that carvacrol, eugenol, and geraniol possess significant ovicidal activities against Sarcoptes scabiei eggs and at the test concentration of 5%, the ovicidal activity of carvacrol, eugenol, and geraniol was 100%, 100%, and 91.7%, respectively. The median effective concentration to obtain 50% egg mortality (EC50) was 0.5%, 0.9%, and 2.0% for carvacrol, eugenol, and geraniol, respectively. The microscopic images of eggs after each treatment indicated that terpenes may act by penetrating through the aeropyles on the egg surface (Li et al., 2021), it seems that opisthosomal cuticle of D. canis mites clarified and wrinkled after contact with the carvacrol by the same manner. The ovicidal activity of carvacrol also has been reported in castor bean tick, Ixodes ricinus (Acari: Ixodidae) (Tabari et al., 2017). Carvacrol also showed acaricidal activity in a contact assay, with LC50 of 0.26% at 24 hours and in a fumigation bioassay, killed all Psoroptes ovis mites within 50 minutes of exposure (Chen et al., 2019). The present study demonstrated that the combination of Carvacrol + Amitraz had efficient miticidal activity but in comparison with carvacrol, this combination showed a longer time in killing all D. canis mites. It can be due to the lesser volume of carvacrol in the combination of Carvacrol + Amitraz. In this study, amitraz was prepared at a concentration of 0.05% and selected as a positive control because it is an FDA-approved treatment for canine demodicosis and also it is a topical agent that can be combined with other acaricides or insecticides. Therefore, amitraz was chosen as the positive control. However, amitraz showed acaricidal action with less variation between replicates, but the killing times of mites in the amitraz group were longer than both carvacrol 5% and the combination of Carvacrol + Amitraz groups. Mineral oil has no proven acaricidal action. In the in vitro study of Akkucuk and Kaya (2022) mean survival time of D. folliculorum in contact with mineral oil was about 13,596 ± 3,827 minutes. In the present study, mites in the mineral oil group were alive for more than 24 hours, but after 48 hours, some of them were dead due to dehydration and lack of nutrition. Carvacrol, in a concentration of 5%, shows satisfactory acaricidal effects on D. canis mites in this in vitro study and we do not observe prominent synergistic effects in the combination with Carvacrol + Amitraz. In addition, it is the first time that carvacrol as a monoterpenoid found in herbs evaluated against D. canis mites in combination with amitraz as a selected acaricidal treatment and alone. However, different concentrations of carvacrol in different severity of demodicosis can be a good suggestion for further studies, but carvacrol can be introduced as a reliable and economical herbal product for the control and treatment of canine demodicosis. Conflict of interestThe authors declare that they have no competing interests. All data relevant to the study are included in the article. The authors have no conflict with data availability. Authors' contributionsManuscript writing, preparation, data collection, and sampling performed by Sina Fereydooni. Farnoosh Arfaee reviewed the manuscript and data availability and correctness. Laboratory works and microscopic evaluation during the survey managed by Mohammad Reza Youssefi. Fateme Zahra Gharib reviewed the manuscript and emend the references. Pharmacological assessments and preparation of carvacrol, amitraz, and mineral oil approved by Mohaddeseh Abouhosseini Tabari. FundingThe authors would like to acknowledge everyone who played a role in this academic accomplishment. The specific grant for this research was provided by the Science and Research Branch of Islamic Azad University of Tehran and no additional funding was used in this study. Availability of dataAll data released from this manuscript were obtained by the mentioned authors and available in this article. ReferencesAhn, Y.J., Lee, S.B., Lee, H.S. and Kim, G.H. 1998. Insecticidal and acaricidal activity of carvacrol and β-thujaplicine derived from thujopsis dolabrata var. hondai Sawdust. J. Chem. Ecol. 24, 81–90. Akkucuk, S. and Kaya, O.M. 2022. Can the thyme oil be an alternative treatment for human demodicosis?. Res. square Mustafa Kemal Uni., pp: 1–19. Allaoua, M., Etienne, P., Noirot, V., Carayon, J.L., Tene, N., Bonnafé, E. and Treilhou, M. 2018. Pharmacokinetic and antimicrobial activity of a new carvacrol-based product against a human pathogen, Campylobacter jejuni. J. Appl. Microbiol. 125(4), 1162–1174 Barbet, J.L., Snook, T., Gay, J.M. and Mealey, K.L. 2009. ABCB1-1 Delta (MDR1-1 Delta) genotype is associated with adverse reactions in dogs treated with milbemycin oxime for generalized demodicosis. Vet. Dermatol. 20, 111–114. Baranauskaite, J., Kubiliene, A., Marksa, M., Petrikaite, V., Vitkevičius, K., Baranauskas, A. and Bernatoniene, J. 2017. The influence of different oregano species on the antioxidant activity determined using HPLC postcolumn DPPH method and anticancer activity of carvacrol and rosmarinic acid. Biomed. Res. Int. 18, 7. Barnwal, P., Vafa, A., Afzal, S.M., Shahid, A., Hasan, S.K. and Alpashree Sultana, S. 2017. Benzo(a)pyrene induces lung toxicity and inflammation in mice: prevention by carvacrol. Hum. Exp. Toxicol. 37, 752–761. Beugnet, F., Halos, L., Larsen, D. and de Vos, C. 2016. Efficacy of oral afoxolaner for the treatment of canine generalized demodicosis. Parasite. 23, 14. Castro, K.N.C., Canuto, K.M., Brito, E.S., Costa-Júnior, L.M., Andrade, I.M., Magalhães, J.A. and Barros, D.M.A. 2018. In vitro efficacy of essential oils with different concentrations of 1,8-cineole against Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet. 27(2), 203–210. Chen, Z., Mol, W., Vanhecke, M., Duchateau, L. and Claerebout, E. 2019. Acaricidal activity of plant-derived essential oil components against psoroptes ovis in vitro and in vivo. Parasit. Vectors 12(1), 425. Etewa, S.E. and Abaza, S.M. 2011. Herbal medicine and parasitic diseases. Parasitol. Unit. J. 4(1), 3–14. Folz, S.D., Geng, S., Nowakowski, L.H. and Conklin, R.D. 1978. Evaluation of a new treatment for canine scabies and demodicosis. J. Vet. Pharm. Ther. 1, 199–204. Fourie, J.J., Liebenberg, J.E., Horak, I.G., Taenzler, J., Heckeroth, A.R. and Frénais, R. 2015. Efficacy of orally administered fluralaner (BravectoTM) or topically applied imidacloprid/moxidectin (Advocate®) against generalized demodicosis in dogs. Parasit. Vectors. 8(1), 187. Fourie, L.J., Dumont, P., Halos, L., Beugnet, F. and Pollmeier, M. 2013. Efficacy of a topical application of certifect ® (fipronil 6.26% w/v, amitraz 7.48% w/v, (S)- methoprene 5.63% w/v) for the treatment of canine generalized demodicosis. Parasite. 20, 46. Fourie, L., Kok, D., du Plessis, A. and Rugg, D. 2007. Efficacy of a novel formulation of metaflumizone plus amitraz for the treatment of demodectic mange in dogs. Vet. Parasitol. 150, 268–274. Gaens, D., Rummel, C., Schmidt, M., Hamann, M. and Geyer, J. 2019. Suspected neurological toxicity after oral application of fluralaner (Bravecto®) in a kooikerhondje dog. BMC Vet. Res. 15(1), 283. Gao, Y.Y., Di, Pascuale, M.A., Li, W., Baradaran-Rafii, A., Elizondo, A., Kuo, C.L., Raju, V.K. and Tseng, S.C. 2005. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br. J. Ophthalmol. 89(11), 1468–1473. Gao, Y.Y., Xu, D.L., Huang, L.J., Wang, R. and Tseng, S.C. 2012. Treatment of ocular itching associated with ocular demodicosis by 5% tea tree oil ointment. Cornea. 31(1), 14–17. Heine, J., Krieger, K., Dumont, P. and Hellmann, K. 2005. Evaluation of the efficacy and safety of imidacloprid 10% plus moxidectin 2.5% spot–on in the treatment of generalized demodicosis in dogs: results of a European field study. Parasitol. Res. 97(Suppl. 1), S89–S96. Holm, B.R. 2003. Efficacy of milbemycin oxime in the treatment of canine generalized demodicosis: a retrospective study of 99 dogs (1995–2000). Vet. Dermatol. 14, 189–195. Hugnet, C., Buronrosse, F., Pineau, X., Cadoré, J.L., Lorgue, G. and Berny, P.J. 1996. Toxicity and kinetics of amitraz in dogs. Am. J. Vet. Res. 57(10), 1506–1510. Hugnet, C., Bruchon-Hugnet, C., Royer, H. and Bourdoiseau, G. 2001. Efficacy of 1.25% amitraz solution in the treatment of generalized demodicosis (eight cases) and sarcoptic mange (five cases) in dogs. Vet. Dermatol. 12, 89–92. Jayakumar, S., Madankumar, A., Asokkumar, S., Raghunandhakumar, S., Gokula Dhas, K., Kamaraj, S., Divya, M.G. and Devaki, T. 2012. Potential preventive effect of carvacrol against diethylnitrosamineinduced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 360, 51–60. Jekl, V., Hauptman, K., Jeklova, E. and Knotek, Z. 2006. Demodicosis in nine prairie dogs (Cynomys ludovicianus). Vet. Dermatol. 17, 280–283. Kanikkannan, N., Kandimalla, K., Lamba, S.S. and Singh, M. 2000. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Cur. Med. Chem. 7(6), 593–608. Kim, J.H., Chun, Y.S. and Kim, J.C. 2011. Clinical and immunological responses in ocular demodicosis. J. Korean Med. Sci. 26(9), 1231–1237. Koo, H., Kim, T.H., Kim, K.W., Wee, S.W., Chun, Y.S. and Kim, J.C. 2012. Ocular surface discomfort and Demodex: effect of tea tree oil eyelid scrub in Demodex blepharitis. J. Korean Med. Sci. 27(12), 1574–1579. Kwochka, K.W., Kunkle, G.A. and Foil, C.S. 1985. The efficacy of amitraz for generalized demodicosis in dogs: A study of two concentrations and frequencies of application. Comp. Cont. Edu. 7, 8–17. Lane, M.E. 2013. Skin penetration enhancers. Int. J. Pharm. 447(1–2), 12–21. Li, M., Liu, S., Yin, Z., Bernigaud, C., Guillot, J. and Fang, F. 2021. Activity of terpenes derived from essential oils against Sarcoptes scabiei eggs. Parasit. Vectors 14, 600. Marchese, A., Arciola, C.R., Coppo, E., Barbieri, R., Barreca, D., Chebaibi, S., Sobarzo-Sánchez, E., Nabavi, S.F., Nabavi, S.M. and Daglia, M. 2018. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies, and bio-inspired anti-infective materials. Biofouling. 34, 630–656. Miller, W.H.Jr., Griffin, C.E. and Campbell, K.L. 2013. Muller and Kirk’s small animal dermatology, 7th ed. St Louis, MO: Elsevier Mosby, pp: 304–315. Mueller, R.S. 2012. An update on the therapy of canine demodicosis. Compend. Contin. Educ. Vet. 34(4), E1–4. Mueller, R., Meyer, D., Bensignor, E. and Sauter-Louis, C. 2009. Treatment of canine generalized demodecosis with a ‘‘spot-on’’ formulation containing 10% moxidectine and 2.5% imidacloprid (Advocate, Bayer Healthcare). Vet. Dermatol. 20, 441–446. Mueller, R.S. 2004. Treatment protocols for demodicosis: an evidence based review. Vet. Dermatol. 15, 75–89. Mueller, R.S. and Bettenay, S.V. 2017. Scraping, fine-needle aspiration and biopsy of skin and subcutaneous tissues. Textbook of veterinary internal medicine, 8th ed. St Louis, MO: Elsevier, pp: 42–345. Mueller, R.S., Rosenkrantz, W., Bensignor, E., Karas-Tezcza, J., Paterson, T. and Shipstone, M.A. 2020. Diagnosis and treatment of demodicosis in dogs and cats clinical consensus guidelines of the world association for veterinary dermatology. Vet. Dermatol. 31, 4–e2. National Center for Biotechnology Information. 2020. PubChem Compound Summary for CID. Carvacrol. 10364 Neves, R.C.S.M., Barros, L.A., Mendes, S.M.C., Amorim, T.I.S.W.A., Ferraz, V.P., Mateus, L.A.F., Leite J.D. and Ferreira, A.M. 2020. The sensitivity of Demodex canis (Acari: Demodicidae) to the essential oil of Melaleuca alternifolia – an in vitro study. Braz. J. Vet. Parasitol. 29(3), e005220. Okabe, H., Suzuki, E., Saitoh, T., Takayama, K. and Nagai, T. 1994. Development of novel transdermal system containing d-limonene and ethanol as absorption enhancers. J. Cont. Rel. 32(3), 243–247. Paterson, T., Halliwell, R., Fields, P., Lanza Louw, M., Louw, J., Ball, G., Pinckney, R. and McKibben, J. 2009. Treatment of canine generalized demodecosis: a blind, randomized clinical trial comparing the efficacy of advocate (Bayer Animal Health) with ivermectin. Vet. Dermatol. 20, 447–455. Patra, G., Behera, P., Ghosh, S., Mohanta, D., Borthakur, S.K., Biswas, P., Kumar, A. and Debbarma, A. 2019. Molecular characterization of chitin synthase gene of Demodex canis from Mizoram, India. Acta. Parasitol. 64(1), 57–62. Pereira, A.V., Pereira, S.A., Gremião, I.D.F., Campos, M.P. and Ferreira, A.M.R. .2012. Comparison of acetate tape impression with squeezing versus skin scraping for the diagnosis of canine demodicosis. Aust. Vet. J. 90(11), 448–450. Perego, R., Spada, E., Foppa, C. and Proverbio, D. 2019. Critically appraised topic for the most effective and safe treatment for canine generalized demodicosis. BMC Vet. Res. 15, 17. Ravera, I., Altet, L., Francino, O., Sánchez, A., Roldán, W., Villanueva, S., Bardagí, M. and Ferrer, L. 2013. Small Demodex populations colonize most parts of the skin of healthy dogs. Vet. Dermatol. 24(1), 168–e37. Ravera, I., Altet, L., Francino, O., Sanchez, A., Roldan, W., Villanueva, S. and Ferrer, L. 2013. Small Demodex populations colonize most parts of the skin of healthy dogs. Vet. Dermatol. 24, 168–170. Rhodes, K.H. 2004. Demodicosis. The 5-minute veterinary consult clinical companion: small animal dermatology. Philadelphia, PA: Lippincott Williams & Wilkins, pp: 203–209. Rohdich, N., Roepke, R.K. and Zschiesche, E. 2014. A randomized, blinded, controlled and multi-centered field study comparing the efficacy and safety of bravectoTM (fluralaner) against frontlineTM (fipronil) in flea- and tick-infested dogs. Parasit. Vectors 7(1), 83. Sajed, H., Sahebkar, A. and Iranshahi, M. 2013. Zataria multiflora Boiss. (Shirazi thyme)—an ancient condiment with modern pharmaceutical uses. J. Ethnopharmacol. 145, 686–698. Savla, K., Le, J.T. and Pucker, A.D. 2019. Tea tree oil for Demodex blepharitis. Cochrane Syst. Rev. 6(6), CD013333. Sapra, B., Jain, S. and Tiwary, A.K. 2008. Percutaneous permeation enhancement by terpenes: mechanistic view. The AAPS J. 10(1), 120–132. Sharifi-Rad, M. 2018. Carvacrol and human health: a comprehensive review. Phytother. Res. 32, 1675–1687. Shokri, J., Nokhodchi, A., Dashbolaghi, A., Hassan-Zadeh, D., Ghafourian, T. and Jalali, M.B. 2001. The effect of surfactants on the skin penetration of diazepam. Int. J. Pharm. 228(1–2), 99–107. Six, R.H., Becskei, C., Mazaleski, M.M., Fourie, J.J., Mahabir, S.P., Myers, M.R. and Slootmans, N. 2016. Efficacy of sarolaner, a novel oral isoxazoline, against two common mite infestations in dogs: Demodex spp. and Otodectes cynotis. Vet. Parasitol. 222, 62–66. Snyder, D.E., Wiseman, S. and Liebenberg, J.E. 2017. Efficacy of lotilaner (Credelio™), a novel oral isoxazoline against naturally occurring mange mite infestations in dogs caused by Demodex spp. Parasit. Vectors 10(1), 532. Tabari, M.A., Youssefi, M.R., Maggi, F. and Benelli, G. 2017. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 245, 86–91. Tighe, S., Gao, Y.Y. and Tseng, S.C.G. 2013. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Transl. Vis. Sci. Technol. 2(7), 2. Vinciguerra, V., Rojas, F., Tedesco, V., Giusiano, G. and Angiolella, L. 2018. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Natu. Pro. Res. 33(22), 3273–3277. Walton, S.F., McKinnon, M., Pizzutto, S., Dougall A., Williams, A. and Currie, B.J. 2004. Acaricidal activity of Melaleuca alternifolia (tea tree) oil: in vitro sensitivity of Sarcoptes scabiei var hominis to terpinen-4-ol. Arch. Dermatol. 140(5), 563–566. Zhao, Y., Guo, N., Zheng, X., Yang, S., Zhang, M., Zhang, L. and Wu, K. 2005. Observations on morphology and the survival temperature range of Demodex folliculorum. Acta. Entomol. Sin. 48(5), 754–758. Zhao, Y.E., Guo, N. and Wu, L.P. 2011. Influence of temperature and medium on viability of Demodex folliculorum and Demodex brevis (Acari: demodicidae). Exp. Appl. Acarol. 54(4), 421–425. Zhao, Y.E., Guo, N. and Wu, L.P. 2009. The effect of temperature on the viability of Demodex folliculorum and Demodex brevis. Parasitol. Res. 105(6), 1623–1628. | ||
| How to Cite this Article |
| Pubmed Style Fereydooni S, Arfaee F, Youssefi MR, Gharib FZ, Tabari MA. In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis. Open Vet J. 2023; 13(7): 894-902. doi:10.5455/OVJ.2023.v13.i7.11 Web Style Fereydooni S, Arfaee F, Youssefi MR, Gharib FZ, Tabari MA. In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis. https://www.openveterinaryjournal.com/?mno=152381 [Access: May 09, 2024]. doi:10.5455/OVJ.2023.v13.i7.11 AMA (American Medical Association) Style Fereydooni S, Arfaee F, Youssefi MR, Gharib FZ, Tabari MA. In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis. Open Vet J. 2023; 13(7): 894-902. doi:10.5455/OVJ.2023.v13.i7.11 Vancouver/ICMJE Style Fereydooni S, Arfaee F, Youssefi MR, Gharib FZ, Tabari MA. In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis. Open Vet J. (2023), [cited May 09, 2024]; 13(7): 894-902. doi:10.5455/OVJ.2023.v13.i7.11 Harvard Style Fereydooni, S., Arfaee, . F., Youssefi, . M. R., Gharib, . F. Z. & Tabari, . M. A. (2023) In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis. Open Vet J, 13 (7), 894-902. doi:10.5455/OVJ.2023.v13.i7.11 Turabian Style Fereydooni, Sina, Farnoosh Arfaee, Mohammad Reza Youssefi, Fatemeh Zahra Gharib, and Mohaddeseh Abouhosseini Tabari. 2023. In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis. Open Veterinary Journal, 13 (7), 894-902. doi:10.5455/OVJ.2023.v13.i7.11 Chicago Style Fereydooni, Sina, Farnoosh Arfaee, Mohammad Reza Youssefi, Fatemeh Zahra Gharib, and Mohaddeseh Abouhosseini Tabari. "In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis." Open Veterinary Journal 13 (2023), 894-902. doi:10.5455/OVJ.2023.v13.i7.11 MLA (The Modern Language Association) Style Fereydooni, Sina, Farnoosh Arfaee, Mohammad Reza Youssefi, Fatemeh Zahra Gharib, and Mohaddeseh Abouhosseini Tabari. "In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis." Open Veterinary Journal 13.7 (2023), 894-902. Print. doi:10.5455/OVJ.2023.v13.i7.11 APA (American Psychological Association) Style Fereydooni, S., Arfaee, . F., Youssefi, . M. R., Gharib, . F. Z. & Tabari, . M. A. (2023) In vitro toxicity of combination of Amitraz and Carvacrol on Demodex canis. Open Veterinary Journal, 13 (7), 894-902. doi:10.5455/OVJ.2023.v13.i7.11 |